Supplemental Digital Content is available in the text
Abstract
Low-density lipoprotein cholesterol (LDL-C) is frequently estimated using the empirical Friedewald equation. We compared the accuracy of the novel equation named as the 180-cell method (180-c), which estimates LDL-C using a stratification approach, to those of 9 previously suggested formulas, including the Friedewald equation.
We compared the accuracy of 10 equations by calculating intraclass correlation coefficient (ICC) and weighted kappa index in relation to direct LDL-C measurement values. Two independent populations used in the validation were the Severance Hospital LDL-C (SHL) registry (n = 164,358) and the Korea National Health and Nutrition Examination Survey (KNHANES) 2009 to 2010 (n = 3,854), each representing the hospital patient population and the general Korean population, respectively.
The 180-c and DeLong equations showed the highest ICCs, indicating the best agreement with direct LDL-C measurement. The 180-c and Chen equations showed the highest kappa indices. For the hypertriglyceridemic subpopulation from SHL, the 180-c equation showed the best agreement with direct LDL-C measurement in terms of ICC.
We compared the novel 180-c method for LDL-C estimation with 9 previous formulas in a non-US population as the first external validation. The 180-c equation, with Chen equation, appeared to be more accurate than the Friedewald equation. Although the DeLong equation showed better performance in the hypertriglyceridemic subpopulation, the 180-c equation performed appropriately in Asian population.
INTRODUCTION
Cardiovascular disease (CVD) is one of the most common causes of morbidity and mortality worldwide.1 Elevated low-density lipoprotein cholesterol (LDL-C) has been recognized as an important risk factor for CVD, with clinical trials conclusively showing that LDL-C lowering therapy can reduce the risk of CVD.2,3 For this reason, numerous clinical practice guidelines have continuously identified LDL-C as the primary target for CVD prevention and therapy.4–7 Understandably, accurate measurement of LDL-C is essential to proper utilization of clinical practice guidelines for patient care. Among various principles of LDL-C measurement, the ultracentrifugal method, which is also called as β-quantification, remains as the reference procedure because LDL-C is unaffected by the presence of chylomicrons or other triglyceride-rich lipoproteins in this method. Nevertheless, β-quantification is inadequate for routine clinical laboratory use as it is labor-intensive, time-consuming and required impractically large volume of plasma. Therefore, LDL-C is alternatively estimated using the empirical Friedewald equation in most clinical environments: LDL-C = (total cholesterol [TC]) – (high-density lipoprotein cholesterol [HDL-C]) – (triglyceride [TG]/5).8 The final term, which refers to the estimate of very low-density lipoprotein cholesterol (VLDL-C), is calculated using a fixed denominator of 5 in this equation. However, this uniformly fixed ratio has been suspected to cause incorrect results because VLDL-C is a group of various lipoproteins containing individually different proportions of TG to TC.9
Many groups have consistently evaluated the accuracy of the Friedewald equation in different ethnicities or various disease entities, and proposed alternative formulas for more precise LDL-C estimation until today. For instance, DeLong et al proposed the expression (0.16 × TG) as a more accurate estimate of VLDL-C, suggesting a fixed factor of 6 rather than 5.10 Other factor values have also been suggested in specific populations, but no fixed factor appears to be accurate under all circumstances due to high inter-individual variance in the TG:VLDL-C ratio. To date, none of proposed alternative methods have replaced the Friedewald equation in routine clinical practice, and the National Cholesterol Education Program (NCEP) still recommends the use of the original factor of 5 for estimating LDL-C.11
Recently, Martin et al have recommended a novel equation to estimate LDL-C by applying an adjustable factor for the TG:VLDL-C ratio, which was developed using a stratification approach based on the levels of TG and non-HDL-C.9 This formula was derived from the United States (US) population. However, a subsequent validation study reported that this novel equation has no clear benefit over the Friedewald calculation to make changes in medical decisions.12
Although previous studies have already compared the accuracy of several formulas to estimate LDL-C in relation to the Friedewald equation, we compared a total of 10 formulas for LDL-C calculation, including the latest novel method,9 to the direct measurement of LDL-C. Therefore, the aim of this study was to compare the 10 equations and validate the most powerful method for LDL-C estimation, using the largest cohort sample size to date. We validated the clinical utility and application of different formulas for LDL-C calculation not only in the hospitalized patients, but also in the general population.
METHODS
Study Subjects
To compare the accuracy of LDL-C estimating equations, we used two independent population datasets; the Severance Hospital LDL-C (SHL) registry and the Korea National Health and Nutrition Examination Survey (KNHANES) 2009–2010.
The SHL registry was established from the electronic database of hospital patient records in Severance hospital for this study. Severance hospital is a 2100-bed university-affiliated teaching tertiary-level hospital in Seoul, Korea, with more than 10,000 outpatients visiting daily. From January 2008 to December 2013, we collected subjects who were aged ≥18 years and whose lipid profiles (i.e., TC, HDL-C, LDL-C, and TG) were directly measured from blood after overnight fasting. Among a total of 164,358 subjects who were analyzed in the study, the number of individuals with TG level higher than 400 mg/dL was 1481, and this subgroup was independently reanalyzed for hypertriglyceridemia in the ancillary study. The Institutional Review Board of the Yonsei University College of Medicine approved the present study project (No. 4-2015-0571).
To enhance the generalizability of validation, data from the KNHANES 2009–2010 was independently analyzed. The KNHANES is a nationwide cross-sectional study regularly conducted by the Division of Chronic Disease Surveillance, Korea Centers for Disease Control and Prevention of the Ministry of Health and Welfare.13 A total of 19,491 individuals participated in the KNHANES 2009–2010. Among these participants, we excluded individuals who were aged <18 years (n = 4659) and whose HDL-C or LDL-C was not assessed by direct measurement in the survey (n = 10,870). Unlike the SHL, subjects with TG higher than 400 mg/dL (n = 108) were excluded from the analysis to minimize biases by outliers and to properly represent the general Korean population. Finally, a total of 3854 subjects were investigated. Written informed consent was secured from all participants and the KNHANES was conducted according to ethical approval by Institutional Review Board of Korea Center for Disease Control and Prevention (No: 2009-01CON-03-2C, 2010-02CON-21-C).
Measurements of Lipid Profiles
In patients from the SHL, serum TC and TG levels were determined by enzymatic method using reagents from Sekisui Medical Corporation (Tokyo, Japan) and Roche Diagnostic (Indianapolis, IN), respectively, on a Hitachi 7600 automated chemistry analyzer (Hitachi, Tokyo, Japan). HDL-C and LDL-C levels were measured by a homogenous direct assay using reagents from Sekisui Medical Corporation on a Hitachi 7600 automated analyzer.
In the KNHANES 2009–2010, blood samples were collected from each subject after overnight fasting for more than 8 hours, refrigerated immediately, transported in cold storage to the central laboratory (Neodin Medical Institute, Seoul, Korea) and analyzed within 24 hours after transportation.14 TC and TG levels were determined by enzymatic method using reagents from Sekisui Medical Corporation. HDL-C and LDL-C levels were measured by a homogenous direct assay using reagents from Sekisui Medical Corporation. All analytes were analyzed on a Hitachi 7600 automated analyzer.
Equations for Estimating LDL-C
A total of 10 formulas for estimating LDL-C in units of mg/dL are summarized in Supplementary Table 1. These include the novel 180-cell (180-c) method9 along with equations suggested by Friedewald et al,8 Hattori et al,15 Anandaraja et al,16 Chen et al,17 Cordova,18 Teerakanchana et al,19 Ahmadi et al,20 DeLong et al,10 and Rao et al.21
Statistical Analyses
We assessed the agreement and accuracy of the 10 equations for estimating LDL-C using the direct LDL-C measurement as the reference value. Two statistical concepts were utilized for comparison of the accuracy of the 10 equations compared to the direct LDL-C measurement.
Firstly, the intraclass correlation coefficient (ICC) was calculated to compare the degrees of agreement between the 10 formulas and the direct LDL-C measurement. The level of agreement was defined according to ICC value; good agreement when ICC >0.75, and moderate agreement when 0.5< ICC <0.75.22
Secondly, we calculated a weighted kappa (к) index to compare the concordance for estimating LDL-C in relation to the direct LDL-C measurement according to the LDL-C level classification guideline. Similar to the ICC values, the к index was used to define levels of agreement; good concordance when к index >0.8, and substantial concordance when 0.6< к index <0.8.23 To induce the к index from the datasets, two most commonly used clinical practice guidelines to classify LDL-C values were applied; the US guideline (<70, 70–99, 100–129, 130–159, 160–189, and ≥190 mg/dL) and the European (EU) guideline (<70, 70–99, 100–154, 155–189, and ≥190 mg/dL).6,7
Bland–Altman plots were also expressed to compare the direct measurement of LDL-C and other estimates calculated using the 10 equations.24 Categorical variables regarding concordance were compared using the χ2 test, whereas numerical variables were compared using the t-test. A P <0.05 was considered statistically significant. All statistical analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC), MedCalc version 12.7 (MedCalc software, Ostend, Belgium), R package version 3.0.2 (http://www.R-project.org), and SPSS version 20.0 for Windows (IBM Corp., Armonk, NY).
RESULTS
Characteristics of Two Independent Population Datasets—the SHL and the KNHANES 2009–2010
Table 1 summarizes the general characteristics and lipid profiles of subjects in two independent datasets; the SHL and the KNHANES 2009–2010. Among a total of 164,358 subjects in the SHL, 1,481 (0.9%) individuals showed TG level higher than 400 mg/dL. The mean age was older in the SHL than in the KNHANES 2009–2010, possibly due to the difference between the hospital registry and the general population.
TABLE 1.
Characteristics of Study Subjects in the SHL and KNHANES 2009–2010

Comparison of the Performance of the 10 Equations
The quantitative agreements according to ICC value between the direct LDL-C measurement and other LDL-C estimates using the 10 equations are presented in Table 2. Most equations except the Ahmadi and Anandaraja formulas showed ICCs >0.90 in both datasets, indicating excellent agreement with the direct LDL-C measurement. ICCs of the 180-c (0.980), DeLong (0.980), and Chen (0.977) equations were superior to that of the Friedewald equation (0.975) in the SHL. Similar patterns were observed in the KNHANES 2009–2010. Mean differences between direct LDL-C measurement and estimates of each method were less than 10 mg/dL in all equations except the Ahmadi equation, indicating that most formulas investigated in this study were comparable with the direct LDL-C measurement in terms of ICC validation. The Bland–Altman plots between LDL-C values determined by direct measurement and other methods in the SHL are depicted in Supplementary Figure 1.
TABLE 2.
Quantitative agreement by intraclass correlation coefficient between direct LDL-C and other equations for estimating LDL-C

Concordance According to Clinical LDL-C Classification Guidelines
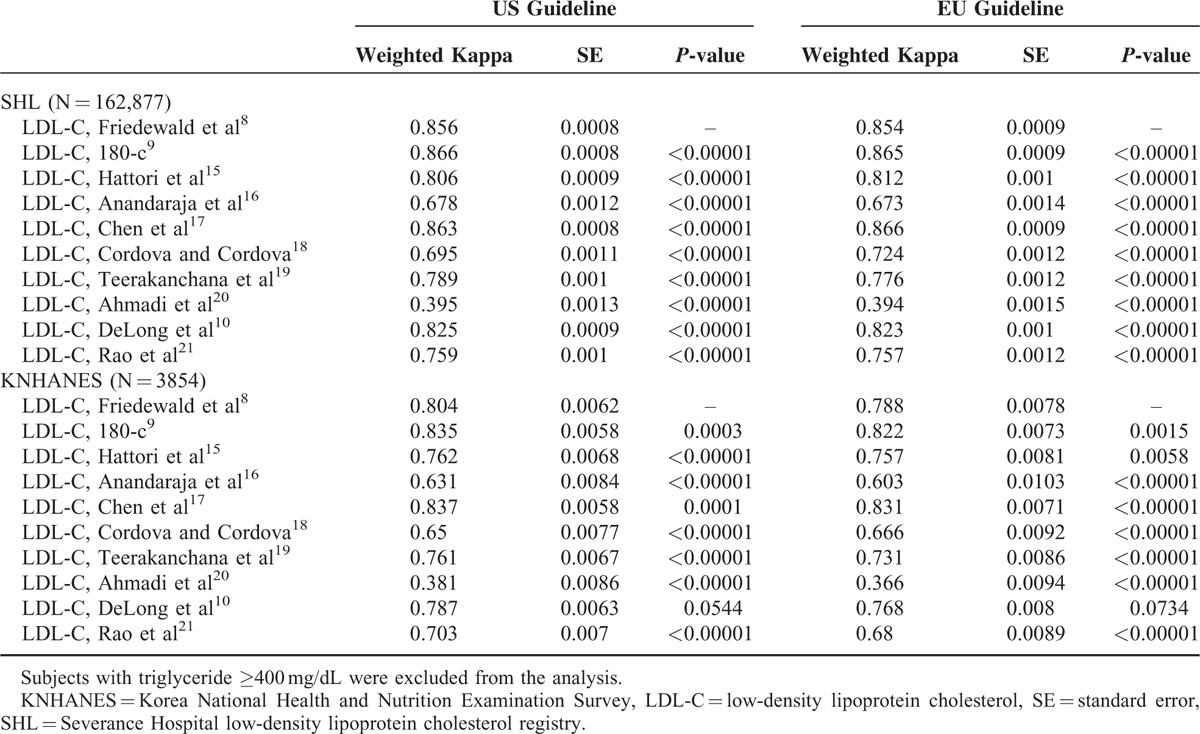
The weighted к index was used to assess the concordance between direct LDL-C measurement and other LDL-C estimates calculated using equations according to the clinical classification guidelines from the US and EU (Table 3). Only two methods showed superior concordance with direct LDL-C measurement when compared to the Friedewald equation; 180-c and Chen equations [к index: 0.866 (0.865) and 0.863 (0.866) according to US (EU) classification, respectively]. Other equations showed inferior concordance when compared to the Friedewald equation. Similar patterns were observed in the KNHANES 2009–2010.
TABLE 3.
Weighted Kappa Index: Concordance in Guideline Classification of LDL-C Levels by Friedewald versus Other Equations for Estimating LDL-C in Relation to Direct LDL-C
Comparison Between the Friedewald Equation and the 180-c Method According to LDL-C Level
Since 180-c method showed the best agreement with direct measurement among the 10 equations based on ICC and к index, concordance of direct LDL-C measurement with LDL-C estimates using the 180-c method was evaluated in comparison with the Friedewald equation (Figure 1). Interestingly, the 180-c method appeared to overestimate LDL-C values significantly more often than the Friedewald equation (12.3% vs. 9.8%; P < 0.001); however, the concordant percentage was also significantly higher in the 180-c method (84.0% vs. 82.6%; P < 0.001) (Figure 1A). After stratification by LDL-C level, both Friedewald and 180-c equations tended to overestimate LDL-C compared to direct measurement, especially in the low ranges of LDL-C (<100 mg/dL for Friedewald and < 130 mg/dL for 180-c), whereas both equations underestimated LDL-C in the high ranges (Figure 1B and C).
FIGURE 1.

Concordance of direct LDL-C with LDL-C using the Friedewald or 180-cell equations (A), and concordance according to LDL-C stratum between direct LDL-C and Friedewald (B) or 180-cell equations (C). LDL-C = low-density lipoprotein cholesterol.
Subgroup Analyses Based on LDL-C and TG Levels Using the Friedewald Equation and the 180-cell Method
Subgroup analyses were performed to measure quantitative agreement using ICC values according to the strata of LDL-C (<69, 70–99, 100–129, 130–159, and ≥160 mg/dL) and TG (<99, 100–149, 150–199, and 200–399 mg/dL) levels. In other words, direct LDL-C and TG levels were divided into 5 and 4 categories, respectively, to conduct a total of 20 comparisons in different combinations. The quantitative agreement between direct LDL-C measurement and the Friedewald or 180-cell equation were compared by means of ICC values.
Overall, higher ICC values were observed in the 180-c equation than the Friedewald equation for all subgroups (Figure 2 and Supplementary Table 2). However, the differences between the two equations were not significant. Subjects with high LDL-C (≥160 mg/dL) and low TG (<150 mg/dL) levels showed the best agreement by the 180-c equation (ICC: 0.93), and the Friedewald equation (ICC: 0.92). Subjects with the lowest LDL-C (<70 mg/dL) and TG (<100 mg/dL) levels also showed comparable ICCs as determined by both equations (ICC by 180-c: 0.92, ICC by Friedewald: 0.91). Regardless of TG level, ICCs by the Friedewald or 180-c equation were >0.90 in subjects with the highest LDL-C (≥160 mg/dL), indicating very strong agreement with direct LDL-C measurement in this subpopulation. However, in subjects with LDL-C <160 mg/dL, the ICC decreased significantly with increasing TG concentration, resulting in the worst agreement with direct LDL-C measurement in individuals with TG ≥200 mg/dL (all ICCs <0.65).
FIGURE 2.

Quantitative agreement by intraclass correlation coefficient between direct LDL-C and other equations for estimating LDL-C by different strata of LDL-C and triglycerides in the SHL cohort (F, Friedewald equation;8 180-c, 180-cell equation).9 LDL-C = low-density lipoprotein cholesterol, SHL = Severance Hospital LDL-C.
Ancillary Analyses for the Hypertriglyceridemic Subpopulation
Quantitative and qualitative agreements between direct LDL-C measurement and other LDL-C estimates calculated using the different equations were assessed in subjects with TG level ≥400 mg/dL derived from the SHL. Among the 10 formulas, the 180-c equation showed the best agreement with direct LDL-C measurement (ICC: 0.82, P < 0.001), followed by Teerakanchana's equation (ICC: 0.81, P < 0.001) (Supplementary Table 3). The equations proposed by Chen, Anandaraja, and DeLong also showed better agreement with direct LDL-C measurement than did the Friedewald equation (ICC: 0.77). Supplementary Table 4 describes the concordance levels between direct LDL-C measurement and other LDL-C estimates in terms of weighted к index in subjects with TG level ≥400 mg/dL, using the same classification as in previous analyses. The DeLong equation showed the best concordance with direct LDL-C measurement [к index: 0.618 (0.617) by US (EU) classification], superior to that of the Friedewald equation. The 180-c equation resulted in inferior concordance compared to the Friedewald equation.
DISCUSSION
The international guidelines for CVD treatment continuously emphasize the importance of LDL-C assay for risk assessment and patient follow-up.4–7,25 The current NCEP Adult Treatment Panel recommendations for cardiovascular risk assessment are mostly based on early epidemiologic studies that used the Friedewald equation to estimate LDL-C.4 However, the accuracy of the Friedewald formula has been called into question based on several critical limitations including inaccuracy in patients with hypertriglyceridemia, subjects with very low TG level, and patients with liver or renal disease. Although the Friedewald equation has been traditionally believed to show high accuracy compared to β-quantification when TG <4.52 mmol/L (or 400 mg/dL), this has been also suspected with the development of new direct LDL-C assays.26 These shortcomings of the Friedewald formula have prompted the recent development of new equations for LDL-C calculation. Among the formulas developed to overcome problems of the Friedewald equation, the most up-to-date 180-cell method derived from a US population has been highlighted for possibility to be used in practice with high accuracy, which applies an adjustable factor for the TG:VLDL-C ratio using a stratification approach according to TG and non-HDL-C levels.9 This estimation method was shown to provide higher fidelity estimates than the Friedewald equation, resulting in more accurate guideline risk classification.9 However, these favorable findings are required to be externally validated in different ethnicities before being applied to other populations. Although one study12 validating the novel 180-cell method concluded that its improvement over the Friedewald equation is not sufficient to supplant the original formula, the results in this study were derived from another US population. To the best of our knowledge, this is the first study to validate a new stratification approach in an Asian population using two independent large populations: one hospital patient based cohort and one general population.
Among the 10 equations compared in the present study, all equations except the Ahmadi equation revealed ICC >0.90, suggesting that these equations are in good accordance with direct LDL-C measurement. The fact that the Ahmadi equation was derived only from the patients with high cholesterol (>250 mg/dL) could have caused this inferior ICC value compared to those of other formulas. When we analyzed к index to distinguish the most concordant equation, two most accurate methods showing the best concordance agreement according to NCEP category with direct LDL-C measurement were the 180-c method and the Chen equation [к index: 0.866 (0.865) and 0.863 (0.866) by US (EU) classification, respectively]. The Friedewald and DeLong equations followed with к index of 0.856 (0.854) and 0.825 (0.823) by US (EU) classification, respectively.
Considering the results of several previous reports by objective third parties who focused on the validation and comparison of suggested equations for LDL-C estimation, our results highlight the superior performance of the novel 180-c method. Also our data support the good predictive performance of the Chen equation, which has been undervalued in other reports. Martins et al recently reported that the Hattori formula performed the best in hospitalized patients when compared to the Friedewald, Chen and Cordova equations.27 Although direct LDL-C measurement was used for reference value in comparisons (as in our study), the use of different reagents and instruments for lipid measurements as well as different ethnicities and populations with various health conditions might have caused the discordant findings with our results. On the contrary, Oliveira et al concluded that the Friedewald equation showed the best accuracy when compared to the Chen, Anandaraja, and Vujovic (which was not included in this study) formulas.28 Even though the reference method was different from that in our study (β-quantification in Oliveira's study), none of the equations performed adequately for hypertriglyceridemic patients, which is in line with our findings.
When we focused on the comparison of only the 180-c method and the Friedewald equation, the ICC of the 180-c method (0.980) was superior to that of the Friedewald equation (0.975) in the SHL. In all subgroups classified by different strata of TG and LDL-C levels, higher ICC values were also observed using the 180-c method compared to the Friedewald equation; however, the difference between the ICC values was small. These results suggest that the novel 180-c method can be suitably and appropriately applied in Asian populations. Furthermore, it might perform more accurately than the Friedewald equation in all ranges of TG and LDL-C.
One interesting result in this study was that the ICC values for subgroup with LDL-C >160 mg/dL consistently showed the highest values with only a slight decreasing trend as TG level increased. However, in the subgroup with LDL-C <160 mg/dL, the ICC values proportionally decreased by significant amounts as TG level increased, resulting in ICC values of 0.54 to 0.63 among the subgroup with TG level of 200 to 399 mg/dL. From the clinical perspective, this finding is important because physicians can easily use and rely on the Friedewald or 180-c equation at their discretion in order to manage patients with high level of LDL-C (>160 mg/dL). Relatively small differences between direct LDL-C measurement and LDL-C estimates calculated using the other equations might not crucially change the treatment practice among patients who are already being treated. More importantly, a relatively large bias of LDL-C estimate in individuals with a low or middle range LDL-C is of serious concern because this underestimation will delay timely prevention and appropriate treatment for dyslipidemia. This is especially true for Asian populations because large proportion of the population was reported to be unaware of high LDL-C.29
From the results of subpopulation analyses, we challenged to find out the best equation to estimate LDL-C level in patients with hypertriglyceridemia, which is the most commonly recognized factor to cause misleading estimated as determined by the Friedewald equation. Among the 7 equations that had a good level of agreement according to the ICC value, the 180-c method outperformed all other equations in estimating LDL-C compared to direct measurement. Meanwhile, the Friedewald equation was indeed inferior to 6 equations in hypertriglyceridemic conditions. When we compared the performances of the 10 equations in terms of к index, all equations except the DeLong formula showed poor concordance level, not satisfying the minimum value of 0.6. Since the DeLong equation was the only formula that demonstrated fairly good agreement and concordance level by the indices of ICC and к index, we propose that physicians consider using the DeLong equation for LDL-C prediction in patients with hypertriglyceridemia. However, it is important to realize that the performance of all the suggested equations in the hypertriglyceridemic subpopulation was worse than that of the subgroup with TG level <400 mg/dL. Therefore, we suggest that the critical limitation of the LDL-C formulas still remains in patients with hypertriglyceridemia despite the development of several new equations.
One limitation in this study is that we used direct homogenous LDL-C measurement as the reference value instead of β-quantification. Due to the limited clinical availability of β-quantification, direct LDL-C assays have frequently served as the reference values in several reports validating different equations for LDL-C estimation. To avoid incorrect comparisons of formulas with direct LDL-C data obtained from different methods, we used only one direct LDL-C assay (Sekisui reagent), which was reported to perform the best among seven assays when compared to β-quantification.30 Another limitation of our study is the different TC and TG assays used in two independent populations. However, a recent study reported that the use of different direct HDL assays is more likely to significantly affect the LDL-C estimates than various TC and TG methods because of the better standardization of TC and TG.31
In conclusion, we compared the novel 180-c method for LDL-C estimation with 9 previously reported formulas in a Korean population as the first external validation in a non-US population. The 180-c equation appeared to be more accurate than the most commonly used Friedewald equation, along with the Chen equation. Although the DeLong equation showed better performance in the hypertriglyceridemic subpopulation, the 180-c method might perform better in hospitalized Korean patients as well as a general Korean population without hypertriglyceridemia.
Supplementary Material
Footnotes
Abbreviations: 180-c = 180-cell method, CVD = cardiovascular disease, EU = European, HDL-C = high-density lipoprotein cholesterol, ICC = intraclass correlation coefficient, KNHANES = Korea National Health and Nutrition Examination Survey, LDL-C = low-density lipoprotein cholesterol, NCEP = National Cholesterol Education Program, SHL = Severance Hospital low-density lipoprotein cholesterol, TC = total cholesterol, US = United States, VLDL-C = very low-density lipoprotein cholesterol.
JHR and Y-hL contributed equally to this work.
Funding Sources: This study was supported by a new faculty research seed money grant of the Yonsei University College of Medicine for 2014 (2014-32-0025) and by the grant of the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (No. HI14C2476).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.McQueen MJ, Hawken S, Wang X, et al. Lipids, lipoproteins, and apolipoproteins as risk markers of myocardial infarction in 52 countries (the INTERHEART study): a case-control study. Lancet 2008; 372:224–233. [DOI] [PubMed] [Google Scholar]
- 2.Smith SC, Jr, Benjamin EJ, Bonow RO, et al. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation. Circulation 2011; 124:2458–2473. [DOI] [PubMed] [Google Scholar]
- 3.Kuklina EV, Yoon PW, Keenan NL. Trends in high levels of low-density lipoprotein cholesterol in the United States, 1999–2006. JAMA 2009; 302:2104–2110. [DOI] [PubMed] [Google Scholar]
- 4.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III). JAMA 2001; 285:2486–2497. [DOI] [PubMed] [Google Scholar]
- 5.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) final report. Circulation 2002; 106:3143–3421. [PubMed] [Google Scholar]
- 6.Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation 2004; 110:227–239. [DOI] [PubMed] [Google Scholar]
- 7.Reiner Z, Catapano AL, De Backer G, et al. ESC/EAS guidelines for the management of dyslipidaemias: the Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). Eur Heart J 2011; 32:1769–1818. [DOI] [PubMed] [Google Scholar]
- 8.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972; 18:499–502. [PubMed] [Google Scholar]
- 9.Martin SS, Blaha MJ, Elshazly MB, et al. Comparison of a novel method vs the Friedewald equation for estimating low-density lipoprotein cholesterol levels from the standard lipid profile. JAMA 2013; 310:2061–2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeLong DM, DeLong ER, Wood PD, et al. A comparison of methods for the estimation of plasma low- and very low-density lipoprotein cholesterol. The Lipid Research Clinics Prevalence Study. JAMA 1986; 256:2372–2377. [PubMed] [Google Scholar]
- 11.National cholesterol education program. Recommendations on lipoprotein measurement: From the working group on lipoprotein measurement. Bethesda (MD): NIH, national heart, lung, and blood institute; 1995. NIH publication no. 95-3044. [Google Scholar]
- 12.Meeusen JW, Lueke AJ, Jaffe AS, et al. Validation of a proposed novel equation for estimating LDL cholesterol. Clin Chem 2014; 60:1519–1523. [DOI] [PubMed] [Google Scholar]
- 13.Korea Centers for Disease Control and Prevention, Ministry of Health and Welfare, South Korea. Korea National Health and Nutrition Examination Survey (KNHANES). Available at : https://knhanes.cdc.go.kr/knhanes/eng/index.do Accessed 14 Jan 2015. [Google Scholar]
- 14.Lee SG, Lee YH, Kim KJ, et al. Additive association of vitamin D insufficiency and sarcopenia with low femoral bone mineral density in noninstitutionalized elderly population: the Korea National Health and Nutrition Examination Surveys 2009–2010. Osteoporos Int 2013; 24:2789–2799. [DOI] [PubMed] [Google Scholar]
- 15.Hattori Y, Suzuki M, Tsushima M, et al. Development of approximate formula for LDL-chol, LDL-apo B and LDL-chol/LDL-apo B as indices of hyperapobetalipoproteinemia and small dense LDL. Atherosclerosis 1998; 138:289–299. [DOI] [PubMed] [Google Scholar]
- 16.Anandaraja S, Narang R, Godeswar R, et al. Low-density lipoprotein cholesterol estimation by a new formula in Indian population. Int J Cardiol 2005; 102:117–120. [DOI] [PubMed] [Google Scholar]
- 17.Chen Y, Zhang X, Pan B, et al. A modified formula for calculating low-density lipoprotein cholesterol values. Lipids Health Dis 2010; 9:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Cordova CM, de Cordova MM. A new accurate, simple formula for LDL-cholesterol estimation based on directly measured blood lipids from a large cohort. Ann Clin Biochem 2013; 50 (Pt 1):13–19. [DOI] [PubMed] [Google Scholar]
- 19.Teerakanchana T, Puavilai W, Suriyaprom K, et al. Comparative study of LDL-cholesterol levels in Thai patients by the direct method and using the Friedewald formula. Southeast Asian J Trop Med Public Health 2007; 38:519–527. [PubMed] [Google Scholar]
- 20.Ahmadi SA, Boroumand MA, Gohari-Moghaddam K, et al. The impact of low serum triglyceride on LDL-cholesterol estimation. Arch Iran Med 2008; 11:318–321. [PubMed] [Google Scholar]
- 21.Rao A, Parker AH, el-Sheroni NA, et al. Calculation of low-density lipoprotein cholesterol with use of triglyceride/cholesterol ratios in lipoproteins compared with other calculation methods. Clin Chem 1988; 34:2532–2534. [PubMed] [Google Scholar]
- 22.Portney LG, Watkins MP. Foundations of Clinical Research: Applications to Practice. Vol. 2. Upper Saddle River, NJ: Prentice Hall; 2000. [Google Scholar]
- 23.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977; 33:159–174. [PubMed] [Google Scholar]
- 24.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986; 1:307–310. [PubMed] [Google Scholar]
- 25.Daniels SR, Greer FR. Lipid screening and cardiovascular health in childhood. Pediatrics 2008; 122:198–208. [DOI] [PubMed] [Google Scholar]
- 26.Tighe DA, Ockene IS, Reed G, et al. Calculated low density lipoprotein cholesterol levels frequently underestimate directly measured low density lipoprotein cholesterol determinations in patients with serum triglyceride levels <or = 4.52 mmol/l: an analysis comparing the LipiDirect magnetic LDL assay with the Friedewald calculation. Clin Chim Acta 2006; 365:236–242. [DOI] [PubMed] [Google Scholar]
- 27.Martins J, Olorunju SA, Murray LM, et al. Comparison of equations for the calculation of LDL-cholesterol in hospitalized patients. Clin Chim Acta 2015; 444:137–142. [DOI] [PubMed] [Google Scholar]
- 28.Oliveira MJ, van Deventer HE, Bachmann LM, et al. Evaluation of four different equations for calculating LDL-C with eight different direct HDL-C assays. Clin Chim Acta 2013; 423:135–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee YH, Lee SG, Lee MH, et al. Serum cholesterol concentration and prevalence, awareness, treatment, and control of high low-density lipoprotein cholesterol in the Korea National Health and Nutrition Examination Surveys 2008–2010: Beyond the Tip of the Iceberg. J Am Heart Assoc 2014; 3:446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller WG, Myers GL, Sakurabayashi I, et al. Seven direct methods for measuring HDL and LDL cholesterol compared with ultracentrifugation reference measurement procedures. Clin Chem 2010; 56:977–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Korzun WJ, Nilsson G, Bachmann LM, et al. Difference in bias approach for commutability assessment: application to frozen pools of human serum measured by 8 direct methods for HDL and LDL cholesterol. Clin Chem 2015; 61:1107–1113. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


