Abstract
Systemic inflammation and immune response were associated with prognosis of tumors. However, data was limited due to the relatively low incidence of gastrointestinal stromal tumors (GISTs). The aim of the present study was to investigate the predictive value of preoperative peripheral blood cells in prognosis of GISTs.
From September 2008 to July 2015, a total of 274 GIST patients in our department were enrolled in the present study. Clinicopathological features of GISTs were recorded. The association between preoperative peripheral blood cells and prognosis of GISTs were analyzed.
Tumor location, tumor size, mitotic index, intratumoral necrosis, and National Institutes of Health (NIH) risk category were associated with prognosis of GISTs. High neutrophil-to-lymphocyte ratio (NLR), monocyte-to-lymphocyte ratio (MLR), platelet-to-lymphocyte ratio (PLR), neutrophil-to-white blood cell ratio (NWR), monocyte-to-white blood cell ratio (MWR) and low lymphocyte-to-white blood cell ratio (LWR) was associated with poor prognosis of GISTs (76.2% vs 83.7%, P = 0.010. 70.5% vs 98.7%, P = 0.000. 65.7% vs 96.4%, P = 0.004. 78.5% vs 82.5%, P = 0.044. 73.5% vs 97.8%, P = 0.004. 76.6% vs 83.6%, P = 0.012, respectively). However, tumor size was the only independent risk factor for prognosis according to multivariate analysis (P = 0.006). Tumor location, tumor size, mitotic index, and NIH risk category were significantly correlated with the above-mentioned parameters (all P < 0.05). The prognosis of GISTs with tumor size >5 cm, high MLR, high PLR, and high MWR was significantly lower than the remnant patients (P = 0.010).
The peripheral blood routine test is convenient, reproducible, and inexpensive. High NLR, MLR, PLR, NWR, MWR, and low LWR were associated with poor prognosis of GISTs. The association between the above parameters and prognosis of GISTs may be attributed to their correlation with tumor size, mitotic index, and NIH risk category. The combination of tumor size, MLR, PLR, and MWR could further increase the predictive value of prognosis of GISTs.
INTRODUCTION
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal tumors of the gastrointestinal (GI) tract and represent 1% to 2% of all GI tumors.1 GISTs are considered to arise from the interstitial cells of Cajal (ICC), the pacemaker cells of the GI tract.2 GISTs can occur anywhere throughout the GI tract and are seen most commonly in the stomach (40–70%), followed by small intestine (20–40%), and colon and rectum (5–15%).3 Many clinicopathological features were reported as prognostic factors for GISTs, including tumor location,4 tumor size, mitotic index,5 Ki-67,6 histological types,7 gene mutations,8 and so on. However, only tumor size and mitotic index are the best prognostic indicators for determining the malignant potential of GISTs.9
Recently, it has been demonstrated that systemic inflammation could promote tumor progression and metastasis by inhibition of apoptosis and promotion of angiogenesis.10 The neutrophil-to-lymphocyte ratio (NLR) is one of the systemic inflammation markers, and it was reported that high level of NLR was associated with poor prognosis of various tumors.11 However, investigations on the prognostic value of NLR for GISTs are limited and the results are controversial.12–16
The immune response is also an important prognostic factor for GISTs. Natural killer (NK) cells expression of NKp30c isoform17 and low secretion of interferon-γ by peripheral NK cells18 both correlated with poor prognosis of GISTs. CD3+ tumor infiltrating lymphocytes were found to be highly activated in GISTs and high density of these cells were associated with a reduced relapse rate.19 However, the role of preoperative peripheral blood lymphocyte rate in the prognosis of GISTs has not been investigated until now.
Given this situation, we attempted to investigate the association between preoperative peripheral blood cells and prognosis of GISTs.
METHODS
This study was performed in the Xijing Hospital of Digestive Diseases affiliated to the Fourth Military Medical University. From September 2008 to July 2015, a total of 274 GIST patients in our department were enrolled in the present study. The inclusion criteria were listed as follows: (1) without other malignant tumor, (2) without distant metastasis, (3) without preoperative imatinib therapy, (4) with R0 resection, (5) without signs of infection. This study was approved by the Ethics Committee of Xijing Hospital, and written informed consent was obtained from all patients before surgery.
All the preoperative peripheral blood routine tests were performed within 7 days before surgery. The Blood NLR value was calculated as neutrophil count (number of neutrophils/μL) divided by the lymphocyte count (number of lymphocytes/μL). Low and high NLR values were defined with respect to sample median. The value of monocyte-to-lymphocyte ratio (MLR), platelet-to-lymphocyte ratio (PLR), neutrophil-to-white blood cell ratio (NWR), lymphocyte-to-white blood cell ratio (LWR), monocyte-to-white blood cell ratio (MWR), and platelet-to-white blood cell ratio (PWR) were calculated same as NLR.
Specimens were fixed in 10% neutral formalin immediately after resection and embedded routinely for histologic examination. Histological type and mitotic index were detected by hematoxylin and eosin stain. Immunohistochemistry was performed using the following antibodies: CD117, CD34, and DOG-1 (discovered on GIST 1).
Clinicopathological data including gender, age, blood routine test, location of tumor, tumor size, mitotic index, histological type, intratumoral hemorrhage, intratumoral necrosis, and National Institutes of Health (NIH) risk category were collected. The patients after resection were followed up through endoscopic ultrasound (EUS) and computed tomography (CT) every 6 months to evaluate tumor recurrence and distant metastasis.
Data were processed using SPSS 22.0 for Windows (SPSS Inc, Chicago, IL). Discrete variables were analyzed using the Chi-square test or Fisher's exact test. Significant predictors for survival identified by univariate analysis were further assessed by multivariate analysis using logistic regression analysis. Evaluation for disease-free survival (DFS) was obtained by the Kaplan–Meier method. The P values were considered to be statistically significant at 5% level.
RESULTS
There were 138 male (50.4%) and 136 female (49.6%). The patient age ranged from 19 to 86 years (median, 56 years; mean, 56.6 years). The most common location was stomach (71.2%), followed by small intestine (16.4%), duodenum (4.4%), colorectum (1.8%), esophagus (1.5%), and extra-gastrointestinal sites (4.7%). The tumors ranged from 0.3 to 30 cm in maximum diameter (median, 4.5 cm; mean, 5.5 cm). The mitotic index of 124 patients exceeded 5/50 high power field (HPF) (45.3%). According to NIH risk classification, 50 patients were classified as very low risk (18.2%), 74 patients were classified as low risk (27.1%), 51 patients were classified as intermediate risk (18.6%), and 99 patients were classified as high risk (36.1%). The follow up time ranged from 2 to 83 months (mean, 31.6 months; median, 30.0 months). Nineteen patients showed recurrence or metastasis; 8 patients suffered from GIST related deaths. The 1-, 3- and 5-year survival rate of DFS was 98.7%, 91.8% and 80.2%, respectively.
Prognostic factors for DFS in GIST patients according to univariate analysis were summarized in Table 1. The results showed that location, tumor size, mitotic index, intratumoral necrosis, NIH risk category, NLR, MLR, PLR, NWR, LWR, and MWR were associated with prognosis of GIST patients. It was worth mentioning that high NLR, MLR, PLR, NWR, and MWR were associated with poor prognosis of GISTs (76.2% vs 83.7%, P = 0.010. 70.5% vs 98.7%, P = 0.000. 65.7% vs 96.4%, P = 0.004. 78.5% vs 82.5%, P = 0.044. 73.5% vs 97.8%, P = 0.004, respectively). However, low LWR was associated with poor prognosis of GISTs (76.6% vs 83.6%, P = 0.012). Tumor size was the only independent risk factor for prognosis according to multivariate analysis (Table 2). The DFS of GISTs according to NLR, MLR, PLR, NWR, LWR, MWR, and tumor size were shown in Figures 1–6, respectively.
TABLE 1.
Prognostic Factors for DFS in GIST Patients According to Univariate Analysis
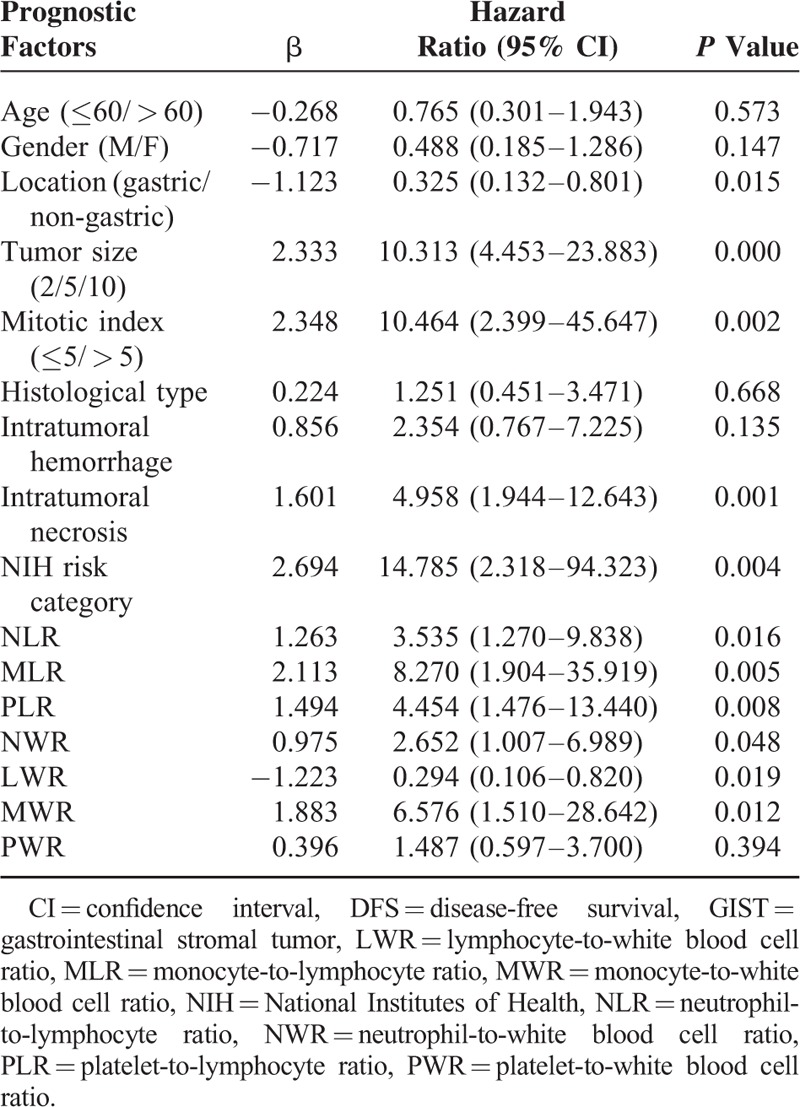
TABLE 2.
Prognostic Factors for DFS in GIST Patients According to Multivariate Analysis
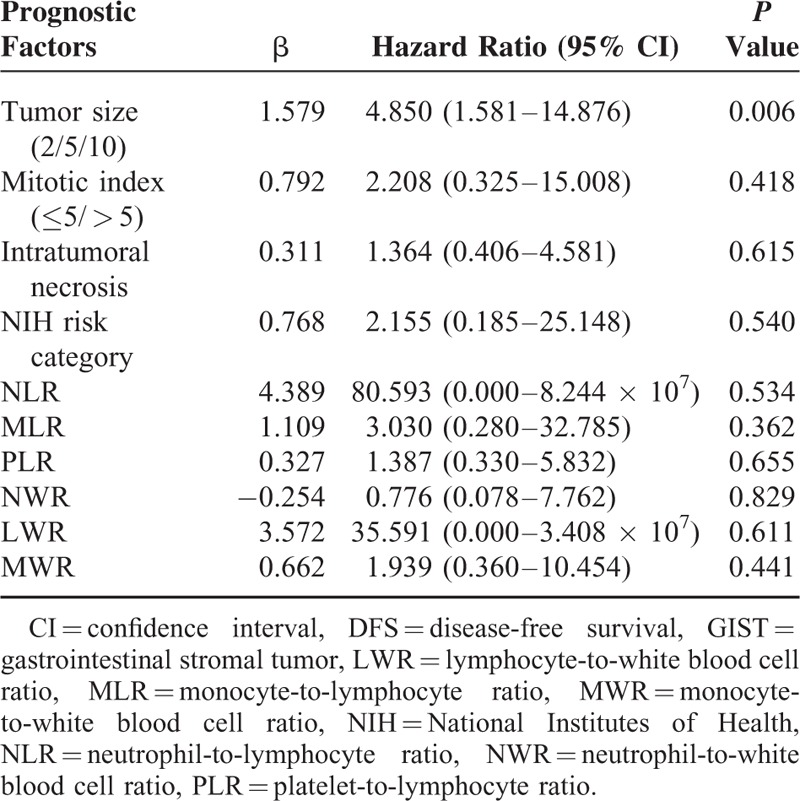
FIGURE 1.

DFS of GIST patients according to NLR and tumor size. DFS = disease-free survival, GIST = gastrointestinal stromal tumor, NLR = neutrophil-to-lymphocyte ratio.
FIGURE 6.
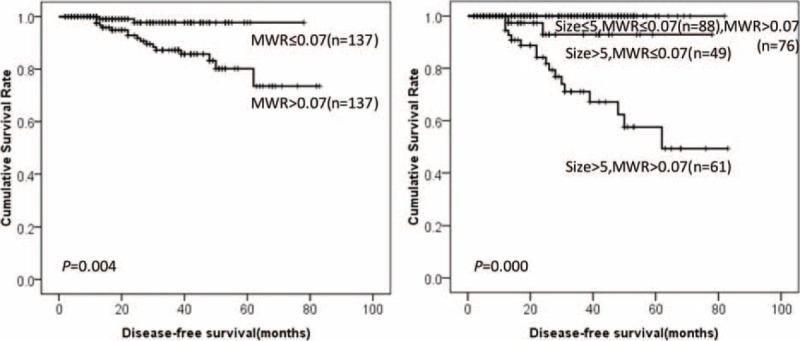
DFS of GIST patients according to MWR and tumor size. DFS = disease-free survival, GIST = gastrointestinal stromal tumor, MWR = monocyte-to-white blood cell ratio.
FIGURE 2.
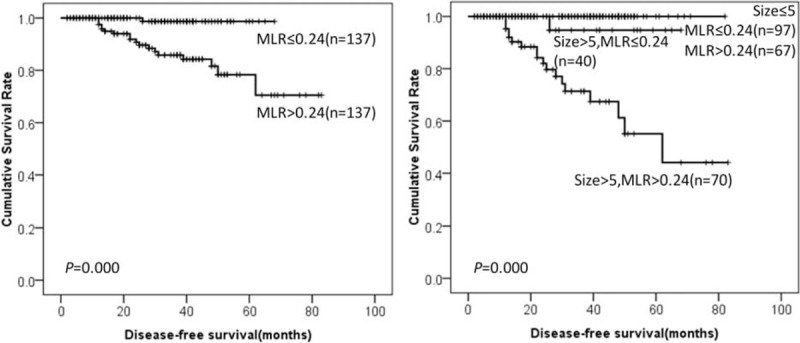
DFS of GIST patients according to MLR and tumor size. DFS = disease-free survival, GIST = gastrointestinal stromal tumor, MLR = monocyte-to-lymphocyte ratio.
FIGURE 3.
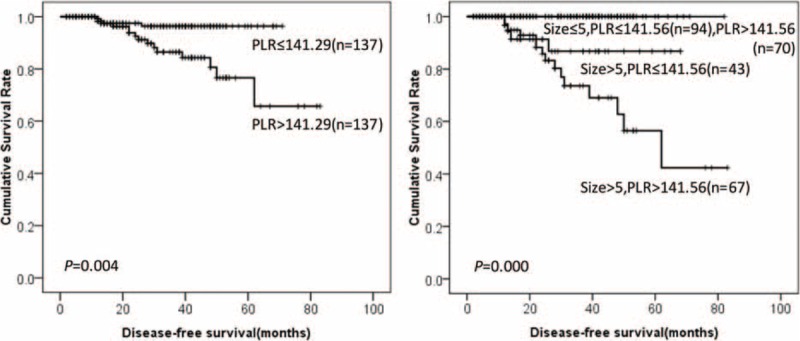
DFS of GIST patients according to PLR and tumor size. DFS = disease-free survival, GIST = gastrointestinal stromal tumor, PLR = platelet-to-lymphocyte ratio.
FIGURE 4.
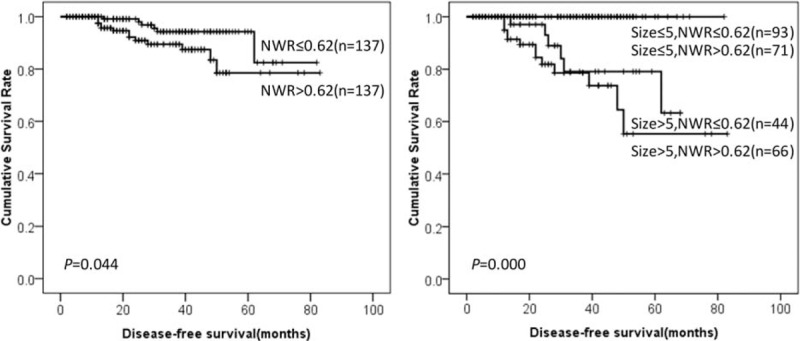
DFS of GIST patients according to NWR and tumor size. DFS = disease-free survival, GIST = gastrointestinal stromal tumor, NWR = neutrophil-to-white blood cell ratio.
FIGURE 5.
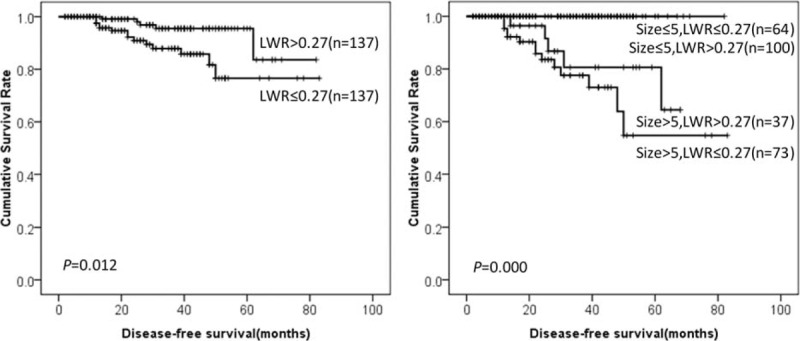
DFS of GIST patients according to LWR and tumor size. DFS = disease-free survival, GIST = gastrointestinal stromal tumor, LWR = lymphocyte-to-white blood cell ratio.
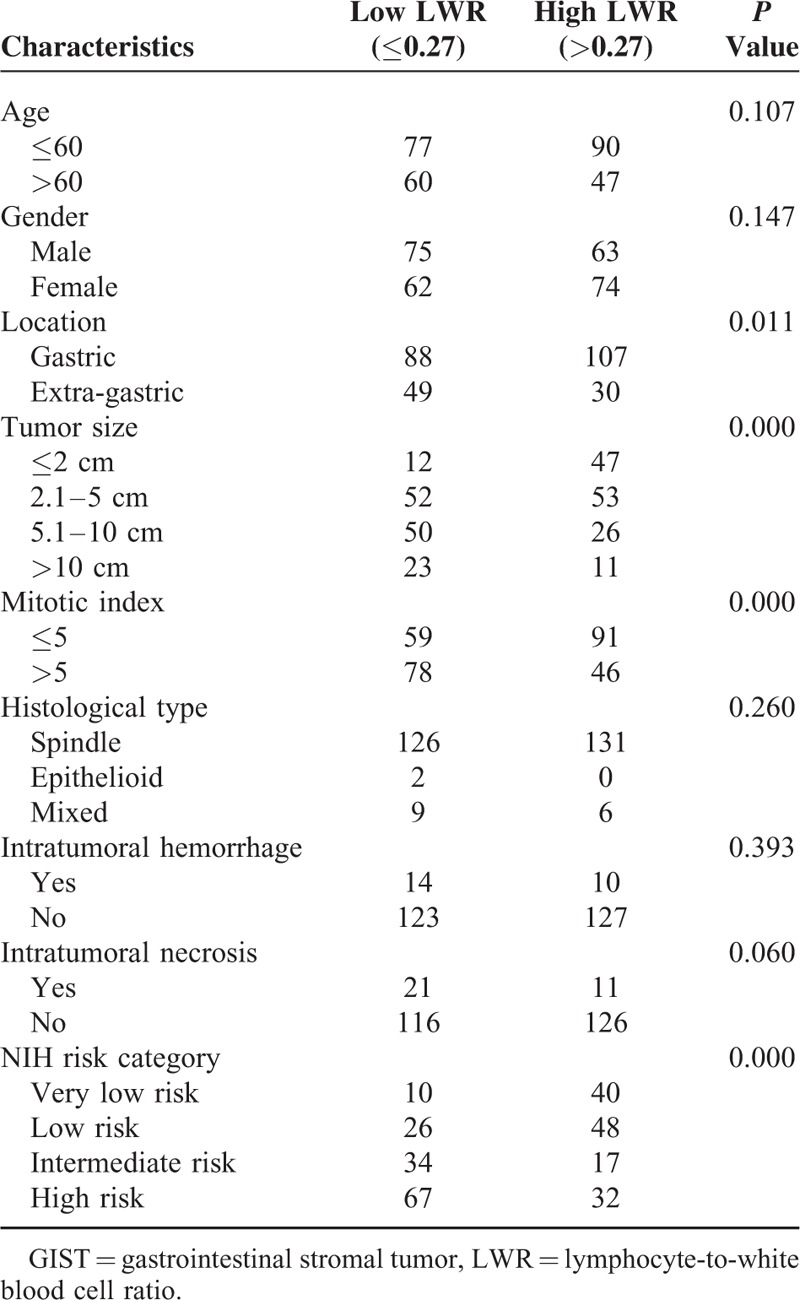
The clinicopathological features between high and low group of NLR, MLR, PLR, NWR, LWR, and MWR were analyzed and summarized in Tables 3–8, respectively. The results showed that location, tumor size, mitotic index, and NIH risk category were significantly correlated with the above-mentioned parameters (all P < 0.05). This indicated that the correlation between the above parameters and prognosis may be attributed to their correlation with tumor size, mitotic index, and NIH risk category.
TABLE 3.
Clinicopathological Features of GIST Patients Stratified by Preoperative NLR

TABLE 8.
Clinicopathological Features of GIST Patients Stratified by Preoperative MWR
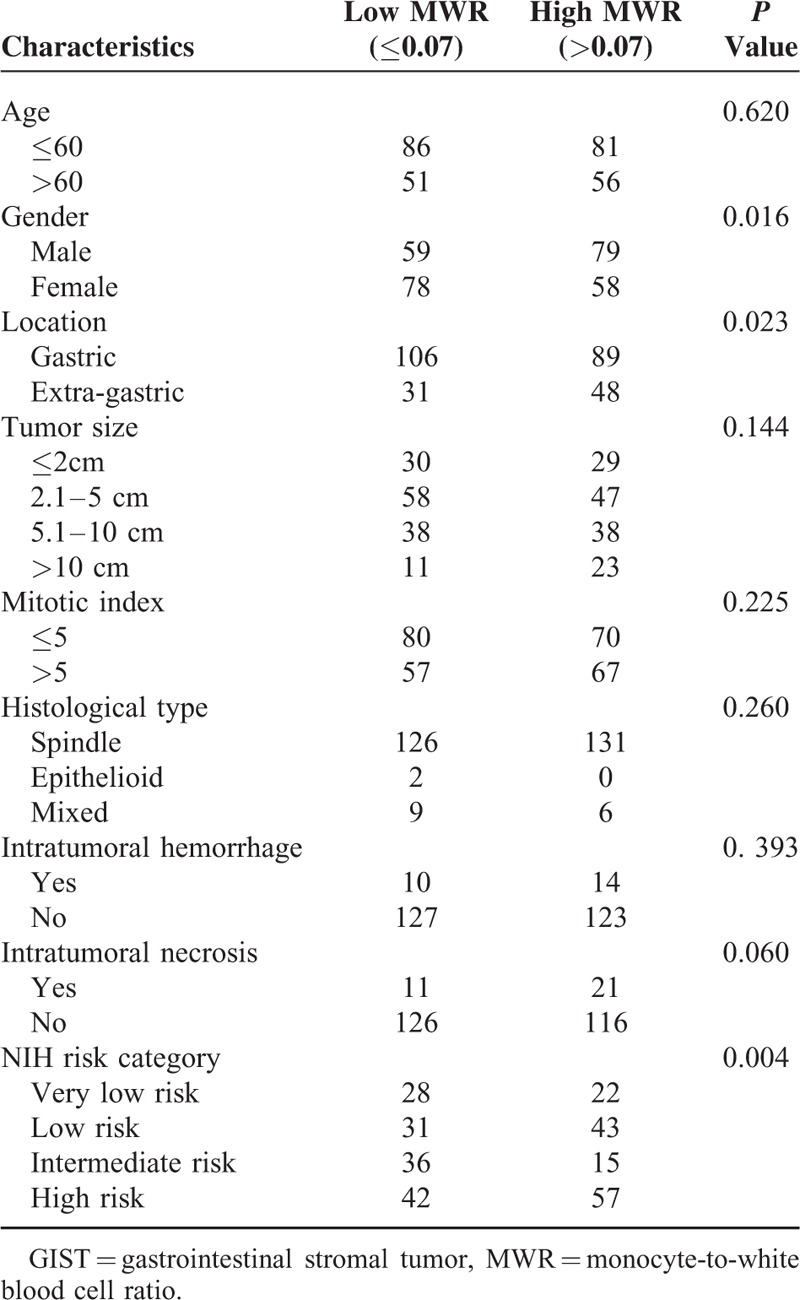
TABLE 4.
Clinicopathological Features of GIST Patients Stratified by Preoperative MLR
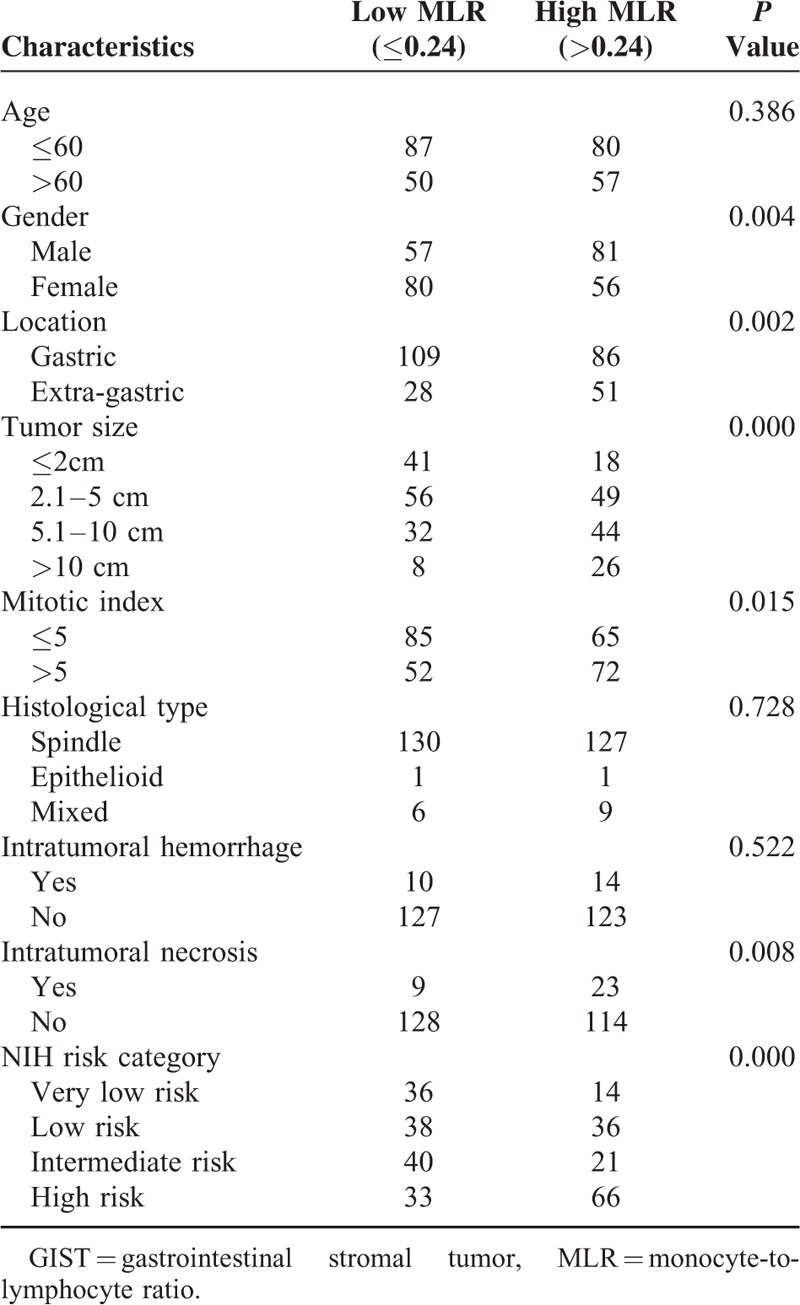
TABLE 5.
Clinicopathological Features of GIST Patients Stratified by Preoperative PLR

TABLE 6.
Clinicopathological Features of GIST Patients Stratified by Preoperative NWR
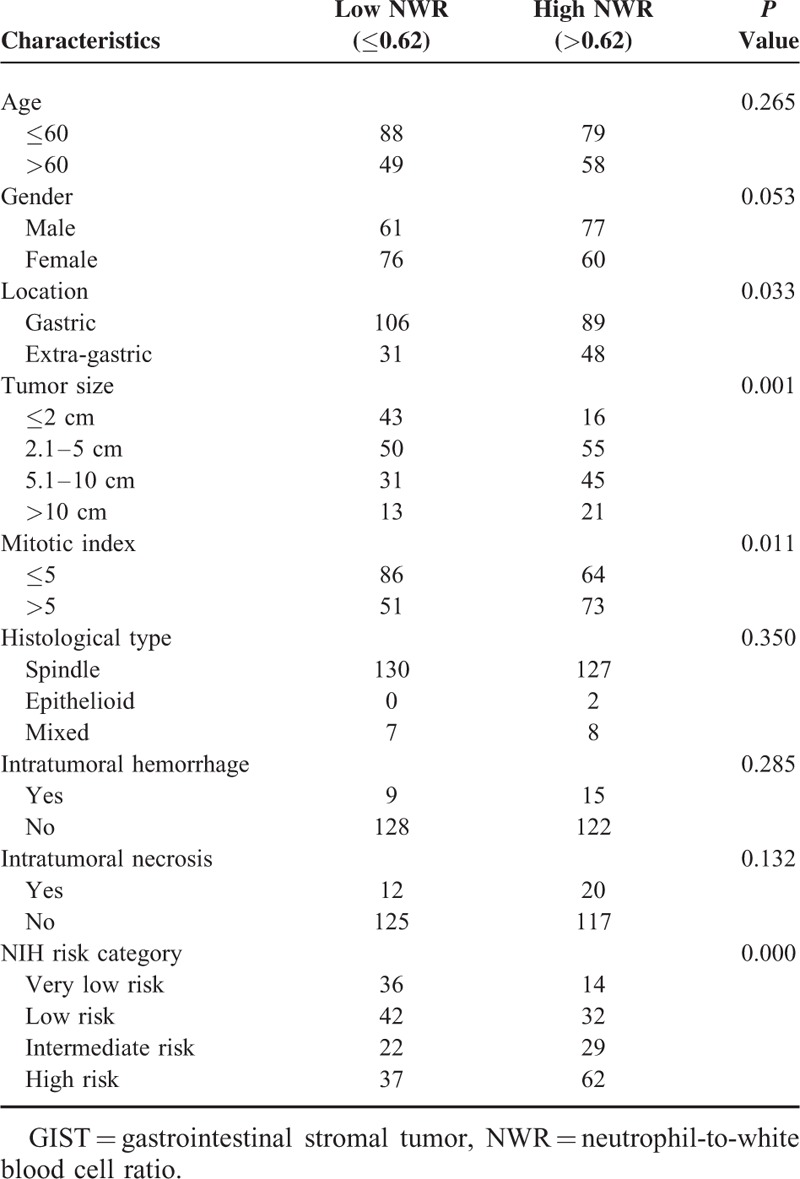
TABLE 7.
Clinicopathological Features of GIST Patients Stratified by Preoperative LWR
Among NLR, MLR, PLR, NWR, LWR, and MWR, the predictive values of MLR, PLR, and MWR are superior to NLR, NWR, and LWR. Furthermore, all the recurrence occurred in GIST patients with tumor size larger than 5 cm in our present study. Thus, GIST patients with tumor size >5 cm were divided into 4 groups—Group 1: low MLR, low PLR, and low MWR; Group 2: high MLR, low PLR, low MWR, or low MLR, high PLR, low MWR, or low MLR, low PLR, high MWR; Group 3: high MLR, high PLR, low MWR, or low MLR, high PLR, high MWR, or high MLR, low PLR, high MWR; Group 4: high MLR, high PLR, and high MWR. The prognosis of group 4 was significantly lower than Group 1, Group 2, and Group 3 (28.2% vs 100.0% vs 89.1% vs 86.3%, P = 0.010, Figure 7).
FIGURE 7.
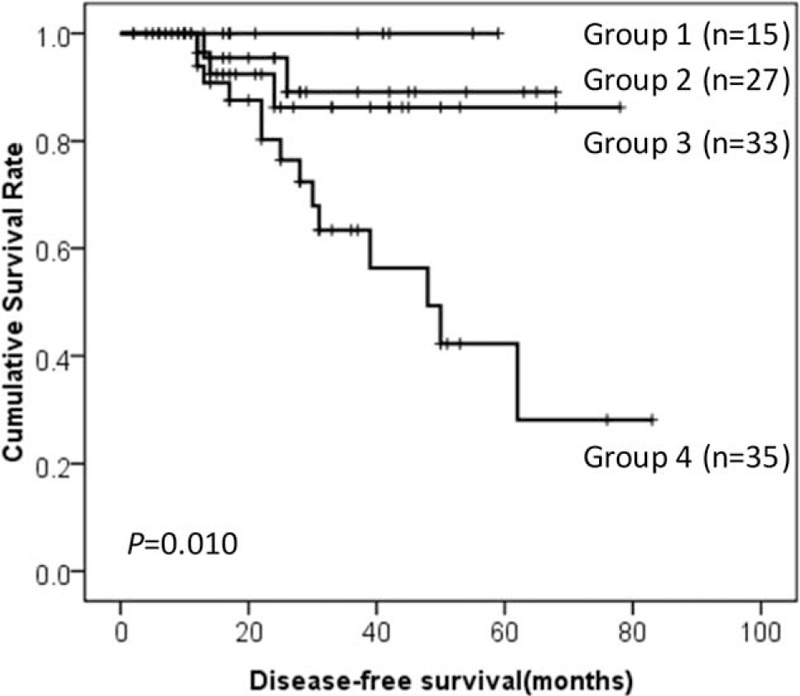
DFS of GIST patients according to the combination of tumor size, PLR, MLR, and MWR. DFS = disease-free survival, GIST = gastrointestinal stromal tumor, NLR = neutrophil-to-lymphocyte ratio, MLR = monocyte-to-lymphocyte ratio, MWR = monocyte-to-white blood cell ratio, PLR = platelet-to-lymphocyte ratio.
DISCUSSION
The peripheral blood routine test is convenient, reproducible, and inexpensive. Data about the association between peripheral blood cell and prognosis of GISTs were limited and also controversial. Therefore, we evaluated the prognostic value of peripheral blood cell for GISTs. We found that high NLR, MLR, PLR, NWR, MWR, and low LWR were associated with poor prognosis of GISTs. However, none of them was an independent risk factor for prognosis of GISTs.
Peripheral blood neutrophil is one of the markers for acute and chronic inflammation.20 For the first time, we found that high NWR was associated with poor prognosis of GISTs. The mechanism of the association between NWR and prognosis of GISTs remains unclear. It was reported that neutrophils could inhibit the immune system by suppressing the activity of NK cells, lymphocytes, and activated T cells.21–23 On the other hand, neutrophils could promote the angiogenesis and progression of tumor by producing the vascular endothelial growth factor24 and matrix metalloproteinase-9.25
Lymphocytes play critical roles in host immune response. The increased level of lymphocyte was associated with improved prognosis of a variety of tumors.26 Rusakiewicz et al reported that high densities of CD3+ tumor tissue infiltrating lymphocytes predicted progression-free survival of GISTs.19 However, the correlation between peripheral blood LWR and prognosis of GISTs has not been investigated before. Our present study showed that low LWR was associated with poor prognosis of prognosis of GISTs.
NLR could be considered as a combined marker for inflammatory and immune status in vivo. Increased level of NLR may reflect increased inflammation or/and decreased immune reaction. Thus, high level of NLR was associated with poor prognosis of various tumors.11 Previously, there were only 5 studies investigate the association between NLR and prognosis of GISTs. The results of the 5 studies were summarized in Table 9. Among them, Perez et al12 Atila et al,13 Kargin et al,15 and Goh et al16 showed that high level of NLR was correlated with poor prognosis of GISTs. No correlation between NLR and prognosis of GISTs was found in the study reported by Racz et al.14 This may attribute to the relatively small sample size of the study. In our present study, we also found that high NLR was associated with poor prognosis of GISTs.
TABLE 9.
Evaluation of Correlation Between NLR/PLR and Prognosis in GISTs
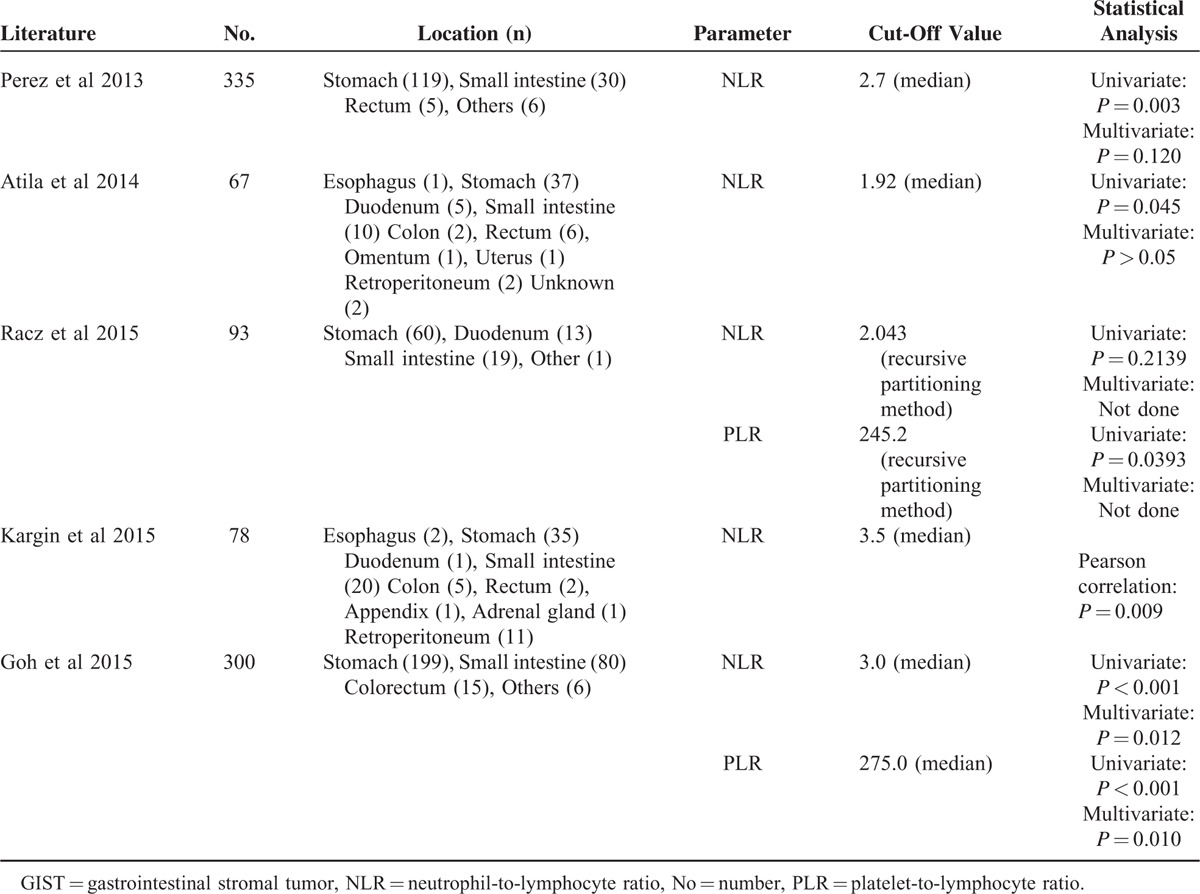
PLR has also been demonstrated as prognostic factors for several solid malignancies.14 Furthermore, superiority of PLR over NLR as an independent prognostic factor has been demonstrated in ovarian,27 colon,28 and esophageal cancer.29 However, these studies did not provide a reasonable explanation for the results. The results in our present study showed that high level of PLR was associated with poor prognosis of GISTs. This was consistent with the studies reported by Racz et al14 and Goh et al.16
Monocytes were reported to be associated with prognosis of a variety of tumors. High level of preoperative monocyte is independently predictive of poor survival of T3N0M0 rectal cancer without neoadjuvant chemoradiotherapy.30 Preoperative circulating monocyte has also been identified as an independent unfavorable prognostic parameter for survival in breast cancer patients.31 In vitro, monocyte could induce prostate cancer cell invasion through increased level of chemokine ligand 2 and nuclear factor-κB activity.32 Amedei et al reported that tumor-infiltrating T cells had the potential to express helper function for MMP-2, MMP-9, and VEGF production by monocytes, which play an important role in angiogenesis, invasion, and metastasis.33 In our present study, we found that both high level of MWR and MLR were associated with poor prognosis of GISTs. Monocyte was considered to be the critical component of inflammation system and might directly stimulate tumor cell growth through producing proinflammatory cytokines, including tumor necrosis factor, interleukin-1, and interleukin-6.34 In addition, monocytes could differentiate into tumor-associated macrophages via cytokines and chemokines produced by tumor cells.35 Tumor-associated macrophages could promote growth, migration, and metastasis of tumor cells.36 These may, in part, explain the prognostic value of MWR and MLR in the prognosis of GISTs in our present study.
Although all the parameters mentioned above could predict the prognosis of GISTs, the predictive values were different from each other. We found that the predictive values of MLR, PLR, and MWR are superior to NLR, NWR, and LWR. Thus, we wonder whether combination of MLR, PLR, and MWR could increase the predictive value of prognosis of GISTs or not. Considering tumor size was the only independent risk factor for prognosis of GISTs, and all the recurrence occurred in GIST patients with tumor size >5 cm in our present study. Thus, we compared the prognosis of GISTs > 5 cm with high MLR, high PLR, and high MWR with the remnant GISTs > 5 cm. We found that the combination of tumor size, MLR, PLR, and MWR could further increase the predictive value of prognosis of GISTs.
There are several limitations in our present study. First, it was a retrospective study of a single center's experience. Prospective studies are needed to verify the predictive value of these parameters. Second, the sample size was not large enough and the incidence of recurrence and metastasis was relatively low, which may result in bias during analysis. Third, the cut-off value of parameters were defined with respect to sample median, which could only be obtained retrospectively. Furthermore, the cut-off values of our present and previous studies were different from each other. Thus, a reasonable cut-off value which could be used to predict prognosis of GISTs prospectively should be identified. Fourth, tumor location was an independent risk factor for prognosis of GISTs. Because of the relatively small sample size, we did not analyze the predictive value of different parameters under the condition of particular tumor locations. Fifth, we only analyze the predictive value of parameters in primary, localized and operable GISTs. Whether these parameters have a predictive value in inoperable GISTs with imatinib therapy needs further investigation.
CONCLUSIONS
The peripheral blood routine test is convenient, reproducible, and inexpensive. Data about the association between peripheral blood cell and prognosis of GISTs were limited and also controversial. Therefore, we evaluated the prognostic value of peripheral blood cell for GISTs. We found that high NLR, MLR, PLR, NWR, MWR, and low LWR were associated with poor prognosis of GISTs. The combination of tumor size, MLR, PLR, and MWR could further increase the predictive value of prognosis of GISTs.
Footnotes
Abbreviations: CI = confidence interval, CT = computed tomography, DFS = disease-free survival, DOG-1 = discovered on GIST 1, EUS = endoscopic ultrasound, GI = gastrointestinal, GIST = gastrointestinal stromal tumor, HPF = high power field, ICC = interstitial cells of Cajal, LWR = lymphocyte-to-white blood cell ratio, MLR = monocyte-to-lymphocyte ratio, MWR = monocyte-to-white blood cell ratio, NIH = National Institutes of Health, NK = natural killer, NLR = neutrophil-to-lymphocyte ratio, NWR = neutrophil-to-white blood cell ratio, PLR = platelet-to-lymphocyte ratio, PWR = platelet-to-white blood cell ratio.
FF, YT, and SL contributed equally to this study.
Funding: this study was supported in part by grants from the National Natural Scientific Foundation of China (NO. 31100643, 31570907, 81300301, 81572306, 81502403, XJZT12Z03).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Corless C. Gastrointestinal stromal tumors: what do we know now? Mod Pathol 2014; 27 Suppl 1:S1–S16. [DOI] [PubMed] [Google Scholar]
- 2.Miettinen M, Lasota J. Gastrointestinal stromal tumors. Gastroenterol Clin North Am 2013; 42:399–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Vogelaere K, Van Loo I, Peters O, et al. Laparoscopic resection of gastric gastrointestinal stromal tumors (GIST) is safe and effective, irrespective of tumor size. Surg Endosc 2012; 26:2339–2345. [DOI] [PubMed] [Google Scholar]
- 4.Rutkowski P, Nowecki ZI, Michej W, et al. Risk criteria and prognostic factors for predicting recurrences after resection of primary gastrointestinal stromal tumor. Ann Surg Oncol 2007; 14:2018–2027. [DOI] [PubMed] [Google Scholar]
- 5.Joensuu H, Hohenberger P, Corless CL. Gastrointestinal stromal tumour. Lancet 2013; 382:973–983. [DOI] [PubMed] [Google Scholar]
- 6.Lu C, Liu L, Wu X, et al. CD133 and Ki-67 expression is associated with gastrointestinal stromal tumor prognosis. Oncol Lett 2013; 6:1289–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haller F, Cortis J, Helfrich J, et al. Epithelioid/mixed phenotype in gastrointestinal stromal tumors with KIT mutation from the stomach is associated with accelerated passage of late phases of the cell cycle and shorter disease-free survival. Mod Pathol 2011; 24:248–255. [DOI] [PubMed] [Google Scholar]
- 8.Wang M, Xu J, Zhao W, et al. Prognostic value of mutational characteristics in gastrointestinal stromal tumors: a single-center experience in 275 cases. Med Oncol 2014; 31:819. [DOI] [PubMed] [Google Scholar]
- 9.Dematteo RP, Gold JS, Saran L, et al. Tumor mitotic rate, size, and location independently predict recurrence after resection of primary gastrointestinal stromal tumor (GIST). Cancer 2008; 112:608–615. [DOI] [PubMed] [Google Scholar]
- 10.McMillan DC. Systemic inflammation, nutritional status and survival in patients with cancer. Curr Opin Clin Nutr Metab Care 2009; 12:223–226. [DOI] [PubMed] [Google Scholar]
- 11.Templeton AJ, McNamara MG, Seruga B, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst 2014; 106:dju124. [DOI] [PubMed] [Google Scholar]
- 12.Perez DR, Baser RE, Cavnar MJ, et al. Blood neutrophil-to-lymphocyte ratio is prognostic in gastrointestinal stromal tumor. Ann Surg Oncol 2013; 20:593–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Atila K, Arslan NC, Derici S, et al. Neutrophil-to-lymphocyte ratio: could it be used in the clinic as prognostic marker for gastrointestinal stromal tumor? Hepatogastroenterology 2014; 61:1649–1653. [PubMed] [Google Scholar]
- 14.Racz JM, Cleghorn MC, Jimenez MC, et al. Predictive ability of blood neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios in gastrointestinal stromal tumors. Ann Surg Oncol 2015; 22:2343–2350. [DOI] [PubMed] [Google Scholar]
- 15.Kargin S, Cakir M, Gundes E, et al. Relationship of preoperative neutrophil lymphocyte ratio with prognosis in gastrointestinal stromal tumors. Ulus Cerrahi Derg 2015; 31:61–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goh BK, Chok AY, Allen JC, Jr, et al. Blood neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios are independent prognostic factors for surgically resected gastrointestinal stromal tumors. Surgery 2015; 159:1146–1156. [DOI] [PubMed] [Google Scholar]
- 17.Delahaye NF, Rusakiewicz S, Martins I, et al. Alternatively spliced NKp30 isoforms affect the prognosis of gastrointestinal stromal tumors. Nat Med 2011; 17:700–707. [DOI] [PubMed] [Google Scholar]
- 18.Menard C, Blay JY, Borg C, et al. Natural killer cell IFN-gamma levels predict long-term survival with imatinib mesylate therapy in gastrointestinal stromal tumor-bearing patients. Cancer Res 2009; 69:3563–3569. [DOI] [PubMed] [Google Scholar]
- 19.Rusakiewicz S, Semeraro M, Sarabi M, et al. Immune infiltrates are prognostic factors in localized gastrointestinal stromal tumors. Cancer Res 2013; 73:3499–3510. [DOI] [PubMed] [Google Scholar]
- 20.Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol 2013; 13:159–175. [DOI] [PubMed] [Google Scholar]
- 21.Kay HD, Smith DL. Regulation of human lymphocyte-mediated natural killer (NK) cell activity. I. Inhibition in vitro by peripheral blood granulocytes. J Immunol 1983; 130:475–483. [PubMed] [Google Scholar]
- 22.el-Hag A, Clark RA. Immunosuppression by activated human neutrophils. Dependence on the myeloperoxidase system. J Immunol 1987; 139:2406–2413. [PubMed] [Google Scholar]
- 23.Petrie HT, Klassen LW, Kay HD. Inhibition of human cytotoxic T lymphocyte activity in vitro by autologous peripheral blood granulocytes. J Immunol 1985; 134:230–234. [PubMed] [Google Scholar]
- 24.Tan KW, Chong SZ, Wong FH, et al. Neutrophils contribute to inflammatory lymphangiogenesis by increasing VEGF-A bioavailability and secreting VEGF-D. Blood 2013; 122:3666–3677. [DOI] [PubMed] [Google Scholar]
- 25.Bausch D, Pausch T, Krauss T, et al. Neutrophil granulocyte derived MMP-9 is a VEGF independent functional component of the angiogenic switch in pancreatic ductal adenocarcinoma. Angiogenesis 2011; 14:235–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quigley DA, Kristensen V. Predicting prognosis and therapeutic response from interactions between lymphocytes and tumor cells. Mol Oncol 2015; 9:2054–2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raungkaewmanee S, Tangjitgamol S, Manusirivithaya S, et al. Platelet to lymphocyte ratio as a prognostic factor for epithelial ovarian cancer. J Gynecol Oncol 2012; 23:265–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kwon HC, Kim SH, Oh SY, et al. Clinical significance of preoperative neutrophil-lymphocyte versus platelet-lymphocyte ratio in patients with operable colorectal cancer. Biomarkers 2012; 17:216–222. [DOI] [PubMed] [Google Scholar]
- 29.Feng JF, Huang Y, Zhao Q, et al. Clinical significance of preoperative neutrophil lymphocyte ratio versus platelet lymphocyte ratio in patients with small cell carcinoma of the esophagus. ScientificWorldJournal 2013; 2013:504365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang LN, Xiao W, OuYang PY, et al. The prognostic impact of preoperative blood monocyte count in pathological T3N0M0 rectal cancer without neoadjuvant chemoradiotherapy. Tumour Biol 2015; 36:8213–8219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wen J, Ye F, Huang X, et al. Prognostic significance of preoperative circulating monocyte count in patients with breast cancer: based on a large cohort study. Medicine (Baltimore) 2015; 94:e2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lindholm PF, Sivapurapu N, Jovanovic B, et al. Monocyte-induced prostate cancer cell invasion is mediated by chemokine ligand 2 and nuclear factor-kappaB activity. J Clin Cell Immunol 2015; 6:308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Amedei A, Munari F, Bella CD, et al. Helicobacter pylori secreted peptidyl prolyl cis, trans-isomerase drives Th17 inflammation in gastric adenocarcinoma. Intern Emerg Med 2014; 9:303–309. [DOI] [PubMed] [Google Scholar]
- 34.Ikemoto S, Sugimura K, Yoshida N, et al. TNF alpha, IL-1 beta and IL-6 production by peripheral blood monocytes in patients with renal cell carcinoma. Anticancer Res 2000; 20 (1A):317–321. [PubMed] [Google Scholar]
- 35.Mantovani A, Schioppa T, Porta C, et al. Role of tumor-associated macrophages in tumor progression and invasion. Cancer Metastasis Rev 2006; 25:315–322. [DOI] [PubMed] [Google Scholar]
- 36.Mantovani A, Allavena P, Sica A, et al. Cancer-related inflammation. Nature 2008; 454:436–444. [DOI] [PubMed] [Google Scholar]


