Abstract
Diabetes mellitus (DM) is a common complication of chronic pancreatitis (CP) and increases the mortality. The identification of risk factors for DM development may contribute to the early detection and potential risk reduction of DM in patients with CP.
Patients with CP admitted to Changhai Hospital (Shanghai, China) from January 2000 to December 2013 were enrolled. Cumulative rates of DM after the onset of CP were calculated by Kaplan-Meier method. Risk factors for DM development after the diagnosis of CP were identified by Cox proportional hazards regression model.
A total of 2011 patients with CP were enrolled. During follow-up (median duration, 22.0 years), 564 patients developed DM. Cumulative rates of DM 20 and 50 years after the onset of CP were 45.8% (95% confidence interval [CI], 41.8%–50.0%) and 90.0% (95% CI, 75.4%–97.7%), respectively. Five risk factors for DM development after the diagnosis of CP were identified: male sex (hazard ratio [HR], 1.51; 95% CI, 1.08–2.11), alcohol abuse (HR, 2.00; 95% CI, 1.43–2.79), steatorrhea (HR, 1.46; 95% CI, 1.01–2.11), biliary stricture (HR, 2.25; 95% CI, 1.43–3.52), and distal pancreatectomy (HR, 3.41; 95% CI, 1.80–6.44).
In conclusion, the risk of developing DM in patients with CP is not only influenced by the development of biliary stricture and steatorrhea indicating disease progression, and inherent nature of study subjects such as male sex, but also by modifiable factors including alcohol abuse and distal pancreatectomy .
INTRODUCTION
Chronic pancreatitis (CP) is a progressive disease with inflammation and destruction of the pancreatic parenchyma.1,2 Decrease in beta cells and insulin resistance contributes to diabetes mellitus (DM), which accounts for 26% to 41% in the natural course of CP.3–8 The micro- or macrovascular damage secondary to DM is a major threat for CP patients, increasing the mortality.9,10 Referred to as brittle diabetes, DM secondary to CP (type 3c DM) is characterized by frequent episodes of hypoglycemia, which is difficult to control and affects quality of life of patients with CP.11 In this sense, type 3c DM warrants much more attention nowadays.
Identification of CP patients at high risk of developing DM contributes to early detection of DM, therefore decreasing DM-associated complication rates and mortality in the long term.12 CP patients with different lifestyles, genetic background, clinical manifestations, and medical interventions may have different risks of developing DM.11,13 Several studies have confirmed alcoholism and distal pancreatectomy as independent risk factors for DM development in CP patients.3,4 Studies also showed that type 3c DM may share common risk factors with type 1 and type 2 DM.11 The identification of risk factors may contribute to risk stratification of CP patients, therefore providing better guidance on screening interval for DM monitoring, and evidence for lifestyle modifications and therapy selection.
We therefore established a large cohort with a long duration of follow-up. We aimed to determine the cumulative rates of DM after the onset of CP, and identify risk factors for DM development after the diagnosis of CP.
MATERIALS AND METHODS
Patients and Database
Since the 1990s, an electronic medical record system (Goodwill Inc, Beijing, China) has been used in Changhai Hospital (Shanghai, China), which has facilitated several studies on CP.14–18 To track changes consistently throughout the course of CP and facilitate the evaluation and study of CP, a dedicated database, Changhai Chronic Pancreatitis Database (version 2.1, Yinma Inc, Shanghai, China), was established in 2005 to collect clinical data of CP patients who were admitted to Changhai Hospital. Data from January 2000 to December 2004 were retrospectively collected according to the electronic medical record system and complemented through telephone, letter, and e-mail inquiries. Data were prospectively collected since January 2005. The following information was documented in detail: demographic data (age, sex, birthplace, etc), course of diseases and medication history, smoking and alcohol history, family history, experimental and imaging results, and treatment.
The database was set to remind the investigators to call patients for clinical checkups. Aside from visits owing to complaints of discomfort related to CP, all patients were periodically (annually at least) contacted for clinical and instrumental re-checkups. Ultrasound, computed tomography (CT), or magnetic resonance imaging (MRI) was selected as an evaluation modality during each follow-up visit. An evaluation of each revisit or evaluation via telephone inquiries for patients who did not return to Changhai Hospital was added to the database. In December 2013, we contacted all the patients in our database, except those who died or were lost to follow-up for a final evaluation. The duration of follow-up is defined as the duration from the onset of CP to the end of follow-up (December 2013), last personal contact, or death, whichever came first.
Exclusion criteria were as follows: pancreatic cancer diagnosed within 2 years after the diagnosis of CP,16,19 groove pancreatitis (GP), and autoimmune pancreatitis (AIP).20 In the present study on DM development in CP patients, the following patients were further excluded: those with a family history of insulin-dependent DM in first-/second-/third-degree relatives and those whose insulin-dependent DM was diagnosed over 2 years before the onset of CP.3,17
The study was approved by the Ethics Committee of Changhai Hospital, Second Military Medical University, Shanghai, China, according to the Helsinki Declaration. Written informed consent was obtained from all participating patients. All of the diagnostic and therapeutic modalities were carried out in accordance to the approved guidelines.
Definitions
The diagnosis of CP was established according to the Asia-Pacific consensus.21 Alcoholic CP was considered when alcohol intake exceeded 80 g/day for males or 60 g/day for females for at least 2 years in the absence of other causes.17,22 Hereditary CP refers to 2 first-degree relatives or ≥3 second-degree relatives, in ≥2 generations with recurrent acute pancreatitis and/or CP, for which there were no precipitating factors.5 Although it remains a controversy whether abnormal anatomy of pancreatic duct (including pancreas divisum and anomalous pancreaticobiliary junction) is a cause of CP, we defined it as an etiology.23 Patients were defined as having post-traumatic CP when there was a history of abdominal trauma with imaging evidence of pancreatic injury and subsequent ductal dilation. Hyperlipidemia is considered as an etiology when blood triglyceride is >1000 mg/dL.24 Patients with CP were considered idiopathic when none of the above causes were found.
DM was diagnosed according to the criteria of the American Diabetes Association.25 In cases of DM diagnosed within 2 years before the symptomatic onset of CP, DM was considered as the initial manifestation of painless CP, and the corresponding time of DM diagnosis was considered as that of the onset of CP.
Treatment Strategy
As a tertiary referral center, Changhai Hospital has an annual volume of >800 CP patients for diagnosis and treatment from all over China. In our center, endoscopic treatment was taken as principle methods before surgery: extracorporeal shockwave lithotripsy (ESWL)/endoscopic retrograde cholangiopancreatography (ERCP) for stone removal and main pancreatic duct drainage,15,26,27 whereas surgery, for example, pancreaticoduodenectomy, distal pancreatectomy, and surgical drainage, is a supplementary modality. For CP patients who did not experience pain, interventions were performed only when complications such as biliary stricture, pancreatic portal hypertension etc had occurred, whereas DM and/or steatorrhea was not an indication for invasive treatment of CP.
Statistical Analysis
Continuous and categorical variables were presented as mean ± standard deviation (SD) and counts (percentages), respectively. Student t test or Mann-Whitney U test was used as indicated for continuous variables. χ2 test or Fisher exact test was used as indicated for categorical variables. Cumulative rates of DM after the onset of CP were calculated by using the Kaplan-Meier method. After further exclusion of CP patients whose DM were diagnosed before/at the diagnosis of CP, risk factors for DM development after the diagnosis of CP were identified by multivariate analysis using Cox proportional hazards regression model including factors with a significance level of P < 0.10 based on univariate analysis, in which the following 15 factors were included: age at the onset of CP, age at the diagnosis of CP, male sex, alcohol abuse, smoking, abnormal anatomy of pancreatic duct, hereditary CP, pancreatic diseases in first-/second-/third-degree relatives (excluding hereditary CP), steatorrhea, pancreatic stones, biliary stricture, pancreatic pseudocysts, abdominal pain, severe acute pancreatitis, treatment strategy (conservative treatment, ERCP/ESWL, pancreaticoduodenectomy, distal pancreatectomy, surgical drainage, other surgical treatment). Hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated. Data were analyzed by using SPSS 18.0 (SPSS Inc, Chicago, IL). All statistical tests were 2-sided. Statistical analyses were conducted at a significance level of 0.05 for all analyses.
RESULTS
General Characteristics of Study Subjects
As shown in Figure 1, from January 2000 to December 2013, a total of 2287 CP patients were entered in the Changhai Chronic Pancreatitis Database. After exclusion of 134 patients, which consists of 16 patients diagnosed with pancreatic cancer within 2 years after the diagnosis of CP, 10 patients diagnosed with GP, and 108 patients diagnosed with AIP, a cohort of 2153 patients with CP was established. After further exclusion of 142 patients in the present study on DM development in patients with CP, which consists of 134 patients with a family history of insulin-dependent DM, and 8 patients whose insulin-dependent DM was diagnosed over 2 years before the onset of CP, a total of 2011 patients with CP were finally enrolled in our study.
FIGURE 1.
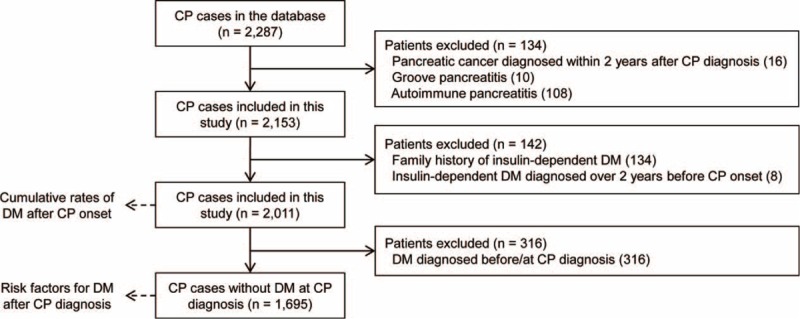
Flow diagram of patient enrollment and study design.
The general characteristics of the 2011 patients with CP are presented in Table 1. The median duration from the onset of CP to the diagnosis of CP was 2.1 years (mean 4.7 years), whereas the median duration from the onset of CP to the appearance of pancreatic stones was 3.2 years (mean, 5.6 years). Idiopathic CP was most common (77.4%) in this study, whereas alcoholic CP constituted 18.3%. Among the 368 patients with a history of alcohol abuse, 116 (31.5%) became abstinent and 252 (68.5%) continued to drink alcohol during follow-up. The overall treatment strategy was classified as endotherapy (ERCP/ESWL) alone (n = 1387, 69.0%), surgery alone (n = 233, 11.6%), both endotherapy and surgery (n = 162, 8.1%), and conservative treatment (n = 229, 11.4%). In this cohort of 2011 CP patients, a total of 1492 patients received endotherapy as the first-line treatment, in which endotherapy was the only treatment option in 1387 patients, with an overall success rate of 92.1% (1278/1387), whereas endotherapy with subsequent surgery was performed in the other 105 patients, with an overall success rate of 98.1% (103/105). We lost contact with 260 patients (12.9%) during follow-up.
TABLE 1.
General Characteristics of 2011 Patients With CP
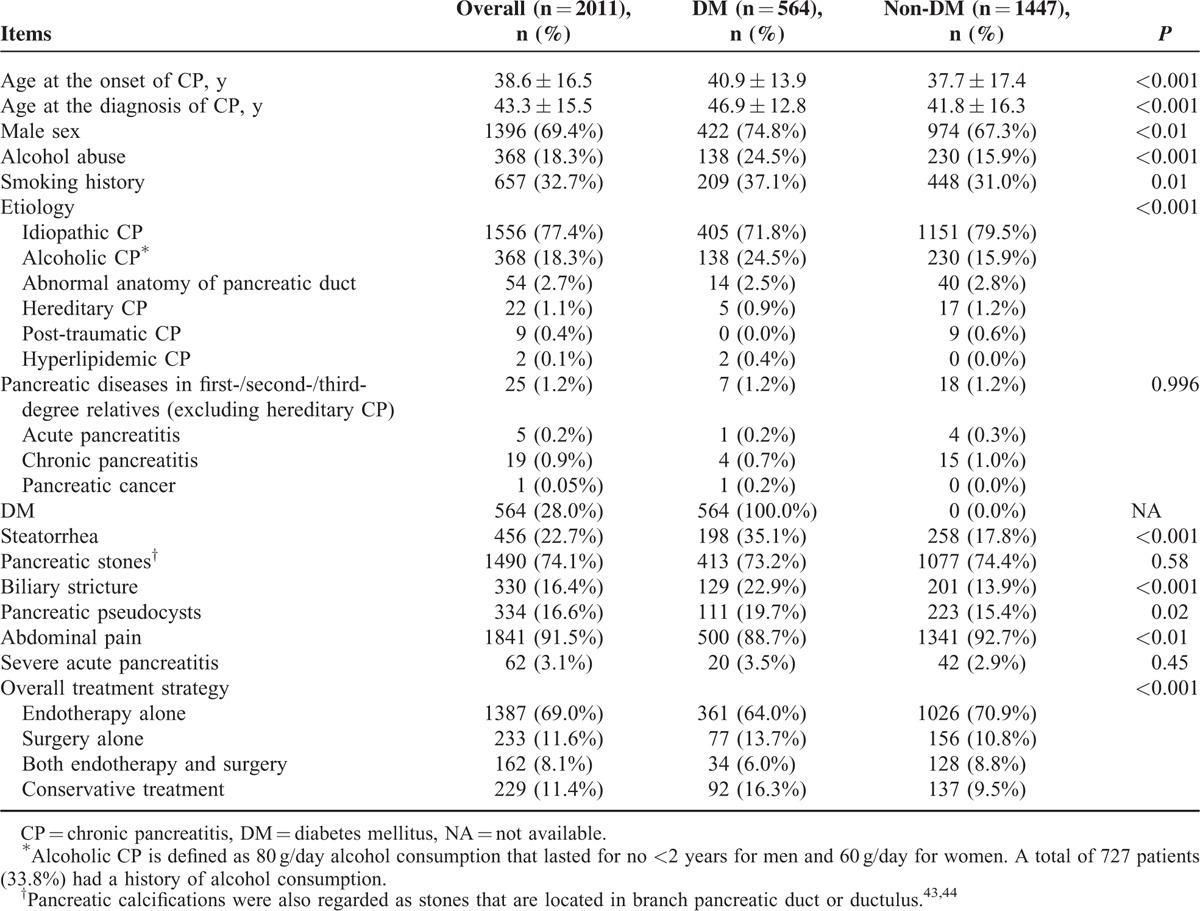
As shown in Table 1, CP patients with DM differed from those without DM in the following aspects: age at the onset of CP, age at the diagnosis of CP, male sex, alcohol abuse, smoking history, etiology, steatorrhea, biliary stricture, pancreatic pseudocysts, abdominal pain, and overall treatment strategy. No significant differences between these 2 groups were detected in terms of pancreatic diseases in first-/second-/third-degree relatives (excluding hereditary CP), pancreatic stones, and severe acute pancreatitis.
Cumulative Rates of DM After the Onset of CP
DM developed in 28.0% (564 patients) of the 2011 eligible patients after the onset of CP, with a median follow-up duration of 22.0 years (95% CI, 19.9–24.1). In detail (Figure 2), DM developed in 449, 532, 556, 561, and 564 patients at 10, 20, 30, 40, and 50 years after the onset of CP, with corresponding cumulative rates of 27.9% (95% CI, 25.6%–30.3%), 45.8% (95% CI, 41.8%–50.0%), 64.1% (95% CI, 57.1%–71.0%), 75.0% (95% CI, 65.1%–83.9%), and 90.0% (95% CI, 75.4%–97.7%), respectively.
FIGURE 2.
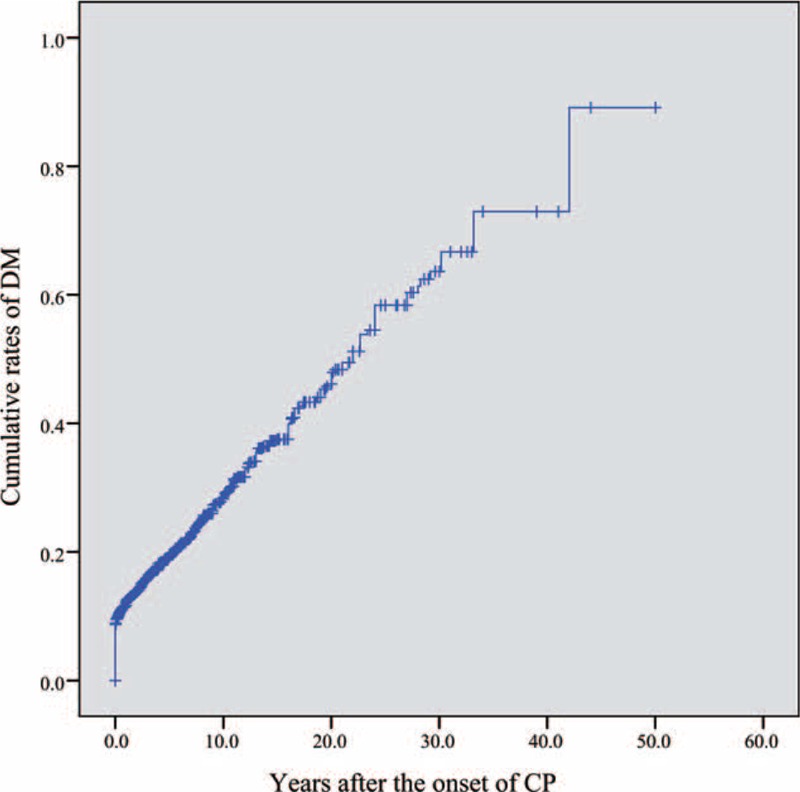
Cumulative rates of diabetes mellitus after the onset of chronic pancreatitis.
Risk Factors for DM Development After the Diagnosis of CP
Among the 2011 patients enrolled, we further excluded 316 patients who were diagnosed with DM before or at the diagnosis of CP to arrive at 1695 patients without DM at the diagnosis of CP, who were included in the risk factor analysis.
Univariate analysis of the risk factors for DM development is shown in Table 2. The risks of developing DM were significantly higher in patients with older age at the onset (HR, 1.01; 95% CI, 1.01–1.02; P < 0.001) or diagnosis (HR, 1.02; 95% CI, 1.01–1.03; P < 0.001) of CP, respectively. Male was associated with a higher risk of DM than female (HR, 1.73; 95% CI, 1.28–2.33; P < 0.001). CP patients with history of alcohol abuse or smoking carried a 2.0-fold (HR, 1.96; 95% CI, 1.49–2.57; P < 0.001) or 1.4-fold (HR, 1.37; 95% CI, 1.05–1.77; P = 0.02) increase in DM risk, respectively. CP patients with steatorrhea (HR, 1.36; 95% CI, 0.94–1.96; P = 0.10), biliary stricture (HR, 2.33; 95% CI, 1.60–3.39; P < 0.001), or pancreatic pseudocysts (HR, 1.61; 95% CI, 1.07–2.42; P = 0.02) were associated with a higher risk of developing DM than those without corresponding concomitant condition at the diagnosis of CP. Compared with CP patients receiving conservative treatment, those receiving endotherapy (ERCP/ESWL) did not benefit a decrease in subsequent DM risk (HR, 0.91; 95% CI, 0.65–1.26; P = 0.57), whereas those receiving pancreaticoduodenectomy (HR, 1.93; 95% CI, 1.21–3.06; P = 0.01) or distal pancreatectomy (HR, 3.26; 95% CI, 1.77–6.00; P < 0.001) had a significantly higher risk of developing DM.
TABLE 2.
Predictors for DM Development After the Diagnosis of CP (1695 Cases)
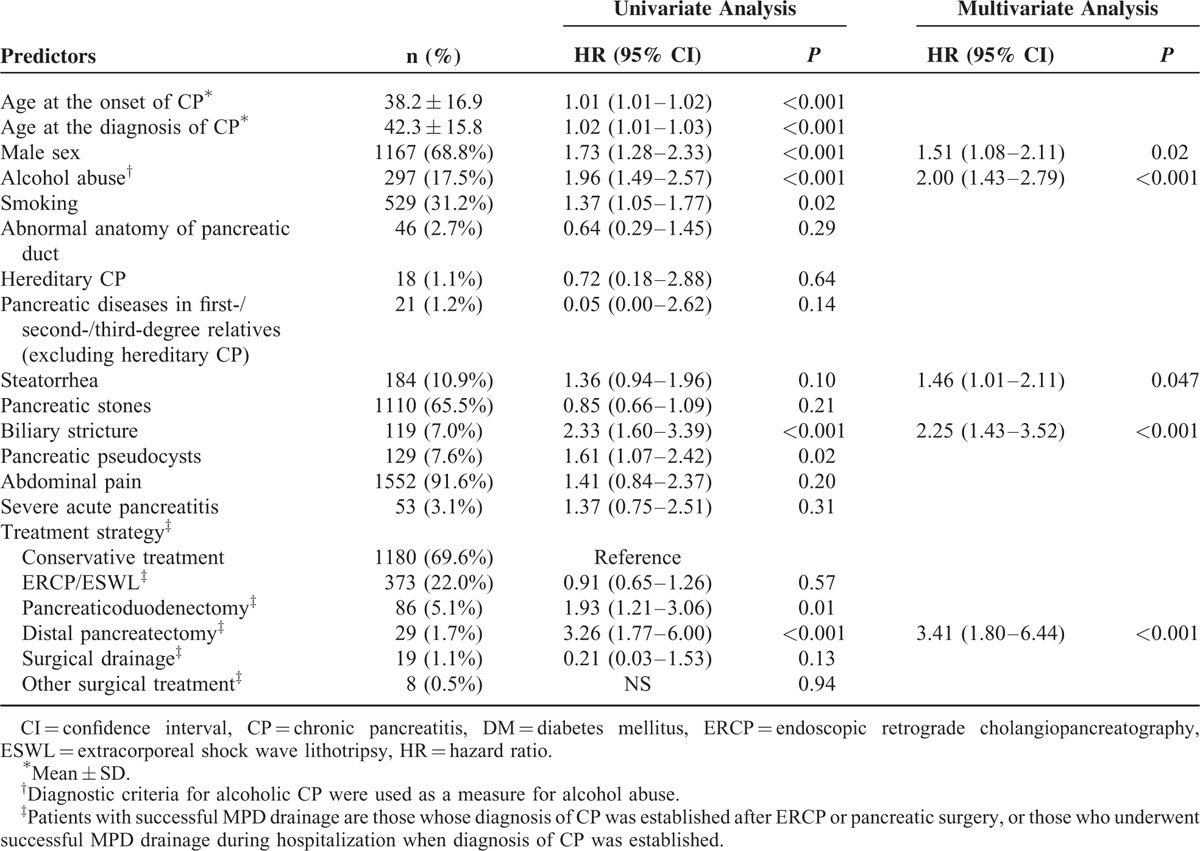
For multivariate analysis, the following 9 predictors were included in Cox proportional hazards regression model: age at the onset of CP, age at the diagnosis of CP, male sex, alcohol abuse, smoking, steatorrhea, biliary stricture, pancreatic pseudocysts, and treatment strategy. Finally, 5 independent risk factors for DM development were identified: male sex (HR, 1.51; 95% CI, 1.08–2.11; P = 0.02), alcohol abuse (HR, 2.00; 95% CI, 1.43–2.79; P < 0.001), steatorrhea (HR, 1.46; 95% CI, 1.01–2.11; P = 0.047), biliary stricture (HR, 2.25; 95% CI, 1.43–3.52; P < 0.001), and distal pancreatectomy (HR, 3.41; 95% CI, 1.80–6.44; P < 0.001).
DISCUSSION
DM is a common complication of CP, with a prevalence ranging from 26% to 41% according to previous reports.3–8 In the present study, 28% of patients with CP developed DM, which is comparable with previous studies. Our study is consistent with previous studies in risk factors such as alcoholism3,4 and distal pancreatectomy.3 Nevertheless, with recruitment of 2011 patients (4.5 times in number of our previous study in 201117) and much longer duration of follow-up (22.0 vs 7.3 years in 2011), our study identified 3 new risk factors for DM: male sex, steatorrhea, and biliary stricture.
Male sex was identified to be a risk factor for DM development in CP patients (HR, 1.51; 95% CI, 1.08–2.11). We also observed a positive correlation between alcohol abuse and DM occurrence in CP patients (HR, 2.00; 95% CI, 1.43–2.79). Alcoholic cause is the most common etiology of CP in western countries,28 whereas it constitutes 19% in our single-center cohort. As suggested by many previous studies, pre-existing asymptomatic pancreatic damage might be amplified and accelerated by alcohol intake, therefore increasing the risk of developing DM in CP.29
The effect of smoking on DM development in patients with CP is inconsistent among previous studies. Maisonneuve et al revealed that the risk of DM was higher in CP patients who were smokers than non-smokers, even independent of alcohol consumption.30 Experimental results revealed that smoking might lead to insulin resistance in peripheral tissues,31 and elevated level of catecholamines due to smoking might also cause insulin resistance.32 However, Imoto et al concluded that smoking did not affect DM development.33 In our study, we did not find smoking as a risk factor for increasing the risk of DM in CP, though a significant difference in DM development was only found between smokers and non-smokers in univariate analysis (P = 0.02).
Steatorrhea was identified to be associated with an increased risk of developing DM (HR, 1.46; 95% CI, 1.01–2.11). The fibro-inflammatory damage of the pancreatic parenchyma in CP causes not only DM but primarily pancreatic exocrine insufficiency (PEI).34 The close correlation between steatorrhea and DM revealed by our risk factor analysis could also be explained by the impaired incretin system caused by food maldigestion in the context of PEI, as the lower level of incretin causes decreased secretion of insulin.35,36 Moreover, as calcium is necessary for insulin secretion, fat-soluble vitamin D malabsorption and deficiency caused by steatorrhea affects calcium absorption and utilization, thus contributing to DM and poor glycemic control.37,38 The finding of a positive correlation between biliary stricture and DM in CP may attest that the onset of DM in patients with CP is mainly caused by progression of the disease.
Multivariate analysis showed that distal pancreatectomy was the only treatment option associated with an increased risk of developing DM in CP, with a 3.4-fold increase. This is in accordance with previous studies,39,40 and is further confirmed by a recent systematic review reporting a rate of 39% for new-onset DM after distal pancreatectomy.41 The relatively enriched distribution of islets in distal pancreas and the additional surgical resection based on pre-existing fibroinflammatory destruction of pancreatic parenchyma offer possible explanations.42 The finding that endoscopic treatment by ERCP/ESWL was not associated with an increased/decreased risk of DM development is also consistent with previous studies.43
Interpretation of the identified risk factors is 2-fold. First, DM monitoring (eg, fasting glucose and HbA1c) in high-risk individuals (those with one or more risk factors) should be more frequent than “average-risk” individuals (those without identified risk factors). The most recent consensus statement released at PancreasFest 2012 recommended an annual screening for DM in patients with CP.11 In the context of our findings, the screening interval for DM should be further individualized with consideration of the number of risk factors each individual has. Those with more risk factors should be screened more frequently with a screening interval <1 year.
Second, the identification of modifiable risk factors provides evidence for guiding clinical practice and patient education. For example, lifestyle modifications such as alcohol abstinence, as recommended for CP patients, have been further confirmed by identifying alcohol abuse as a risk factor for DM. The potential correlation between pancreatic exocrine insufficiency (steatorrhea) and endocrine insufficiency (DM) suggests that pancreatic enzyme supplementation may help postpone or probably reduce the risk of DM development, though further investigations are warranted. Moreover, as a strong risk factor for DM development, distal pancreatectomy should be avoided unless necessary.
There are several limitations of our study. First, the observational study design (cohort study) is inherent to selection bias,44 even though the adjusted HRs and 95% CIs were calculated in multivariate analysis using Cox regression model. Second, the inclusion of retrospective cohort introduced recall bias.44 Nevertheless, statistical analysis showed that there was no significant difference between the clinical characteristics of patients admitted before and after January 2005. In this sense, the recall bias minimally influenced the results of the study. Third, our study failed to distinguish DM secondary to CP (type 3c DM) from type 1 or type 2 DM. We had made efforts to exclude patients diagnosed with DM over 2 years before the onset of CP, and in fact, in most cases of DM occurring in patients with CP, the diagnosis is type 3c.
In conclusion, the risk of developing DM in patients with CP is not only influenced by the development of biliary stricture and steatorrhea indicating disease progression, and inherent nature of study subjects such as male sex, but also by modifiable factors including alcohol abuse and distal pancreatectomy. This provides additional suggestions and evidence for early detection and probable risk reduction of DM in patients with CP.
Footnotes
Abbreviations: AIP = autoimmune pancreatitis, CI = confidence interval, CP = chronic pancreatitis, CT = computed tomography, DM = diabetes mellitus, ERCP = endoscopic retrograde cholangiopancreatography, ESWL = extracorporeal shockwave lithotripsy, GP = groove pancreatitis, HR = hazard ratio, MRI = magnetic resonance imaging, PEI = pancreatic exocrine insufficiency, SD = standard deviation.
The authors have no funding and conflicts of interest to disclose.
JP, LX, and DW contributed equally to this work.
This study was supported by the National Natural Science Foundation of China (Grant Nos. 81422010 [ZL], 81100316 [L-HH], 81470883 [L-HH), and 81300355 [LX]), Shanghai ChenGuang Program (Grant No. 12CG40 [L-HH]), and Shanghai Health Bureau Youth Scientific Research Program (Grant No. AB83070002011019 [L-HH]) and Shanghai Outstanding Youth Doctor Training Program (Grant No. AB83030002015034 [L.HH]).
REFERENCES
- 1.Etemad B, Whitcomb DC. Chronic pancreatitis: diagnosis, classification, and new genetic developments. Gastroenterology 2001; 120:682–707. [DOI] [PubMed] [Google Scholar]
- 2.Witt H, Apte MV, Keim V, et al. Chronic pancreatitis: challenges and advances in pathogenesis, genetics, diagnosis, and therapy. Gastroenterology 2007; 132:1557–1573. [DOI] [PubMed] [Google Scholar]
- 3.Malka D, Hammel P, Sauvanet A, et al. Risk factors for diabetes mellitus in chronic pancreatitis. Gastroenterology 2000; 119:1324–1332. [DOI] [PubMed] [Google Scholar]
- 4.Balakrishnan V, Unnikrishnan AG, Thomas V, et al. Chronic pancreatitis. A prospective nationwide study of 1,086 subjects from India. JOP 2008; 9:593–600. [PubMed] [Google Scholar]
- 5.Howes N, Lerch MM, Greenhalf W, et al. Clinical and genetic characteristics of hereditary pancreatitis in Europe. Clin Gastroenterol Hepatol 2004; 2:252–261. [DOI] [PubMed] [Google Scholar]
- 6.Ryu JK, Lee JK, Kim YT, et al. Clinical features of chronic pancreatitis in Korea: a multicenter nationwide study. Digestion 2005; 72:207–211. [DOI] [PubMed] [Google Scholar]
- 7.Rebours V, Boutron-Ruault MC, Schnee M, et al. The natural history of hereditary pancreatitis: a national series. Gut 2009; 58:97–103. [DOI] [PubMed] [Google Scholar]
- 8.Hirota M, Shimosegawa T, Masamune A, et al. The seventh nationwide epidemiological survey for chronic pancreatitis in Japan: clinical significance of smoking habit in Japanese patients. Pancreatology 2014; 14:490–496. [DOI] [PubMed] [Google Scholar]
- 9.Seicean A, Tantau M, Grigorescu M, et al. Mortality risk factors in chronic pancreatitis. J Gastrointestin Liver Dis 2006; 15:21–26. [PubMed] [Google Scholar]
- 10.Gullo L, Parenti M, Monti L, et al. Diabetic retinopathy in chronic pancreatitis. Gastroenterology 1990; 98:1577–1581. [DOI] [PubMed] [Google Scholar]
- 11.Rickels MR, Bellin M, Toledo FG, et al. Detection, evaluation and treatment of diabetes mellitus in chronic pancreatitis: recommendations from PancreasFest 2012. Pancreatology 2013; 13:336–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Selph S, Dana T, Blazina I, et al. Screening for type 2 diabetes mellitus: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med 2015; 162:765–776. [DOI] [PubMed] [Google Scholar]
- 13.Cui Y, Andersen DK. Pancreatogenic diabetes: special considerations for management. Pancreatology 2011; 11:279–294. [DOI] [PubMed] [Google Scholar]
- 14.Wang W, Liao Z, Li ZS, et al. Chronic pancreatitis in Chinese children: etiology, clinical presentation and imaging diagnosis. J Gastroenterol Hepatol 2009; 24:1862–1868. [DOI] [PubMed] [Google Scholar]
- 15.Li ZS, Wang W, Liao Z, et al. A long-term follow-up study on endoscopic management of children and adolescents with chronic pancreatitis. Am J Gastroenterol 2010; 105:1884–1892. [DOI] [PubMed] [Google Scholar]
- 16.Wang W, Liao Z, Li G, et al. Incidence of pancreatic cancer in chinese patients with chronic pancreatitis. Pancreatology 2011; 11:16–23. [DOI] [PubMed] [Google Scholar]
- 17.Wang W, Guo Y, Liao Z, et al. Occurrence of and risk factors for diabetes mellitus in Chinese patients with chronic pancreatitis. Pancreas 2011; 40:206–212. [DOI] [PubMed] [Google Scholar]
- 18.Xin L, He YX, Zhu XF, et al. Diagnosis and treatment of autoimmune pancreatitis: experience with 100 patients. Hepatobiliary Pancreat Dis Int 2014; 13:642–648. [DOI] [PubMed] [Google Scholar]
- 19.Li BR, Hu LH, Li ZS. Chronic pancreatitis and pancreatic cancer. Gastroenterology 2014; 147:541–542. [DOI] [PubMed] [Google Scholar]
- 20.Malde DJ, Oliveira-Cunha M, Smith AM. Pancreatic carcinoma masquerading as groove pancreatitis: case report and review of literature. JOP 2011; 12:598–602. [PubMed] [Google Scholar]
- 21.Tandon RK, Sato N, Garg PK. Consensus Study Group. Chronic pancreatitis: Asia-Pacific consensus report. J Gastroenterol Hepatol 2002; 17:508–518. [DOI] [PubMed] [Google Scholar]
- 22.Witt H, Sahin-Toth M, Landt O, et al. A degradation-sensitive anionic trypsinogen (PRSS2) variant protects against chronic pancreatitis. Nat Genet 2006; 38:668–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu WF. ERCP and CT diagnosis of pancreas divisum and its relation to etiology of chronic pancreatitis. World J Gastroenterol 1998; 4:150–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yadav D, Pitchumoni CS. Issues in hyperlipidemic pancreatitis. J Clin Gastroenterol 2003; 36:54–62. [DOI] [PubMed] [Google Scholar]
- 25.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2014; 37 Suppl 1:S81–S90. [DOI] [PubMed] [Google Scholar]
- 26.Li BR, Liao Z, Du TT, et al. Risk factors for complications of pancreatic extracorporeal shock wave lithotripsy. Endoscopy 2014; 46:1092–1100. [DOI] [PubMed] [Google Scholar]
- 27.Sun XT, Hu LH, Xia T, et al. Clinical Features and Endoscopic Treatment of Chinese Patients With Hereditary Pancreatitis. Pancreas 2015; 44:59–63. [DOI] [PubMed] [Google Scholar]
- 28.Cote GA, Yadav D, Slivka A, et al. Alcohol and smoking as risk factors in an epidemiology study of patients with chronic pancreatitis. Clin Gastroenterol Hepatol 2011; 9:266–273.quiz e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yadav D, Whitcomb DC. The role of alcohol and smoking in pancreatitis. Nat Rev Gastroenterol Hepatol 2010; 7:131–145. [DOI] [PubMed] [Google Scholar]
- 30.Maisonneuve P, Lowenfels AB, Mullhaupt B, et al. Cigarette smoking accelerates progression of alcoholic chronic pancreatitis. Gut 2005; 54:510–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Facchini FS, Hollenbeck CB, Jeppesen J, et al. Insulin resistance and cigarette smoking. Lancet 1992; 339:1128–1130. [DOI] [PubMed] [Google Scholar]
- 32.Cryer PE, Haymond MW, Santiago JV, et al. Norepinephrine and epinephrine release and adrenergic mediation of smoking-associated hemodynamic and metabolic events. N Engl J Med 1976; 295:573–577. [DOI] [PubMed] [Google Scholar]
- 33.Imoto M, DiMagno EP. Cigarette smoking increases the risk of pancreatic calcification in late-onset but not early-onset idiopathic chronic pancreatitis. Pancreas 2000; 21:115–119. [DOI] [PubMed] [Google Scholar]
- 34.Nyboe Andersen B, Krarup T, Thorsgaard Pedersen NT, et al. B cell function in patients with chronic pancreatitis and its relation to exocrine pancreatic function. Diabetologia 1982; 23:86–89. [DOI] [PubMed] [Google Scholar]
- 35.Ebert R, Creutzfeldt W. Reversal of impaired GIP and insulin secretion in patients with pancreatogenic steatorrhea following enzyme substitution. Diabetologia 1980; 19:198–204. [DOI] [PubMed] [Google Scholar]
- 36.Knop FK, Vilsboll T, Larsen S, et al. Increased postprandial responses of GLP-1 and GIP in patients with chronic pancreatitis and steatorrhea following pancreatic enzyme substitution. Am J Physiol Endocrinol Metab 2007; 292:E324–E330. [DOI] [PubMed] [Google Scholar]
- 37.Ford ES, Zhao G, Tsai J, et al. Associations between concentrations of vitamin D and concentrations of insulin, glucose, and HbA1c among adolescents in the United States. Diabetes Care 2011; 34:646–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boucher BJ. Vitamin D insufficiency and diabetes risks. Curr Drug Targets 2011; 12:61–87. [DOI] [PubMed] [Google Scholar]
- 39.Schnelldorfer T, Lewin DN, Adams DB. Operative management of chronic pancreatitis: longterm results in 372 patients. J Am Coll Surg 2007; 204:1039–1045.discussion 1045–7. [DOI] [PubMed] [Google Scholar]
- 40.van der Gaag NA, van Gulik TM, Busch OR, et al. Functional and medical outcomes after tailored surgery for pain due to chronic pancreatitis. Ann Surg 2012; 255:763–770. [DOI] [PubMed] [Google Scholar]
- 41.De Bruijn KM, van Eijck CH. New-onset diabetes after distal pancreatectomy: a systematic review. Ann Surg 2015; 261:854–861. [DOI] [PubMed] [Google Scholar]
- 42.Wittingen J, Frey CF. Islet concentration in the head, body, tail and uncinate process of the pancreas. Ann Surg 1974; 179:412–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Adamek HE, Jakobs R, Buttmann A, et al. Long term follow up of patients with chronic pancreatitis and pancreatic stones treated with extracorporeal shock wave lithotripsy. Gut 1999; 45:402–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Song JW, Chung KC. Observational studies: cohort and case-control studies. Plast Reconstr Surg 2010; 126:2234–2242. [DOI] [PMC free article] [PubMed] [Google Scholar]


