Abstract
The study shows a promising next-generation surgical option for the correction of moderate to high ametropia. Hole implantable collamer lens (ICL), STAAR Surgical, is a posterior chamber phakic intraocular lens with a central artificial hole. As yet, however, no long-term comparison of the clinical results of the implantation of ICLs with and without such a hole has hitherto been conducted. A prospective, randomized, controlled trial was carried out in order to compare the long-term clinical outcomes of the implantation, in such eyes, of ICLs with and without a central artificial hole.
Examinations were conducted of the 64 eyes of 32 consecutive patients with spherical equivalents of −7.53 ± 2.39 diopters (D) (mean ± standard deviation) in whom implantation of a Hole ICL was performed in 1 eye, and that of a conventional ICL was carried out in the other, by randomized assignment. Before 1, 3, and 6 months, and 1, 3, and 5 years after surgery, the safety, efficacy, predictability, stability, intraocular pressure, endothelial cell density, and adverse events of the 2 surgical techniques were assessed and compared over time.
The measurements of LogMAR uncorrected and corrected distance visual acuity 5 years postoperatively were −0.17 ± 0.14 and −0.24 ± 0.08 in the Hole ICL group, and −0.16 ± 0.10 and −0.25 ± 0.08 in the conventional ICL group. In these 2 groups, 96% and 100% of eyes, respectively, were within 1.0 D of the targeted correction 5 years postoperatively. Manifest refraction changed by −0.17 ± 0.41 D and −0.10 ± 0.26 D occurred in from 1 month to 5 years in the Hole and conventional ICL groups, respectively. Only 1 eye (3.1%), which was in the conventional ICL group, developed an asymptomatic anterior subcapsular cataract.
Both Hole and conventional ICLs corrected of ametropia successfully throughout the 5-year observation period. It appears likely that the presence of the central hole does not significantly affect these visual and refractive outcomes.
Trial Registration: UMIN000018771.
INTRODUCTION
It has been demonstrated that the Visian Implantable Collamer Lens (ICLTM, STAAR Surgical, Nidau, Switzerland), a posterior chamber phakic intraocular lens, improves the correction of moderate to high ametropia over the long term.1–4 Furthermore, the toric ICL has been found to provide effective correction of high myopic astigmatism.5–8 However, in order to prevent pupillary block, either of the following is essential for this surgical technique: (1) preoperative laser iridotomy, which often involves some pain, especially in younger subjects, or (2) intraoperative peripheral iridectomy, which may prove difficult surgically because of iris hemorrhage. Moreover, some concerns still remain, for both patient and surgeon, in relation to the possibility of cataract formation, due, for example, to direct physical contact between the ICL and the crystalline lens or to malnutrition resulting from poor circulation of the aqueous humor.9–12 We have developed a new ICL with a central artificial hole (Hole ICL; KS-APTM, STAAR Surgical), which seems to be a promising next-generation surgical option in the treatment of moderate to high myopia, owing to the excellent visual performance, almost equivalent to that of a conventional ICL, that it offers, because no further peripheral iridotomies are required, and because it may reduce the risk of cataract formation.13–15 However, no long-term comparison between the clinical outcomes of implantation of ICLs with and without an artificial hole has yet been reported. The purpose of the present study was to provide prospective and intraindividual comparisons of the clinical outcomes of conventional and Hole ICL implantation in the correction of moderate to high myopia.
MATERIALS AND METHODS
Study Population
The protocol of the present prospective study was registered with the University Hospital Medical Information Network Clinical Trial Registry (000018771). The study covered 64 eyes of 32 consecutive patients (10 men and 22 women, mean age ± standard deviation [SD], 31.2 ± 7.6 years) in whom bilateral posterior chamber phakic implantable collamer lenses with or without a 0.36-mm central artificial holes (Hole ICL; KS-APTM, V4c and conventional ICL; V4b, STAAR Surgical) were implanted at Kitasato University Hospital in order to correct cases of both moderate to high myopia and of myopic astigmatism (manifest spherical equivalent −4.00 diopters [D] or more). A number of these subjects were introduced in our previous report documenting higher-order aberrations and contrast sensitivity after Hole ICL and conventional ICL implantation.14 Eligible patients were randomly allocated to either of 2 groups, 1 in which Hole ICLs implanted in 1 eye (the study group), and the other which received conventional ICLs in the other eye (control group), as described in our previous study. In both Hole ICL and conventional ICL groups, nontoric ICLs were selected for 18 eyes (56%) with a manifest cylinder of 1.25 D or less, and toric ICLs were used for the remaining 14 eyes (44%) that had a manifest cylinder of 1.5 D or more. Before surgery, and 1, 3, and 6 months, and 1, 3, and 5 years after surgery, the following determinations were carried out: the logarithm of the minimal angle of resolution (log MAR) of uncorrected distance visual acuity (UDVA), the log MAR of corrected distance visual acuity (CDVA), manifest refraction (spherical equivalent), intraocular pressure (IOP), endothelial cell density (except for 6 months postoperatively), and anterior chamber depth, as well as the standard slit-lamp biomicroscopic and funduscopic examinations. The horizontal white-to-white distance was measured using a scanning-slit topograph (Orbscan IIz, Bausch & Lomb, Rochester). The anterior chamber depth was also determined prospectively, using this topograph, as the distance from the corneal endothelium to the anterior surface of the crystalline lens, and also postoperatively, as the distance from the corneal endothelium to the anterior surface of the ICL. The mean keratometric readings and the central corneal thickness were measured using an autorefractometer (ARK-700A, Nidek, Gamagori, Japan) and an ultrasound pachymeter (DGH-500, DGH Technologies, Exton), respectively. The IOP was assessed using a noncontact tonometer (KT-500, Kowa, Tokyo, Japan), and the endothelial cell density was determined using a noncontact specular microscope (SP-8800, Konan, Nishinomiya, Japan). The sample size (26 to 32 eyes) in this study offered a 90.4% to 95.3% statistical power at the 5% level in order to detect a 0.10 difference in the logMAR of visual acuity, when the SD of the mean difference was 0.15. Eyes with keratoconus were excluded from the study by using the keratoconus screening test of Placido disk videokeratography (TMS-2, Tomey, Nagoya, Japan). The present prospective study received the approval of the Institutional Review Board at Kitasato University and followed the tenets of the Declaration of Helsinki. Informed consent was obtained from all patients after the nature and possible consequences of the study were described and explained.
Implantable Collamer Lens Power Calculation
ICL power calculation was performed using a modified vertex formula according to the instructions of the manufacturer (STAAR Surgical). To minimize preoperative refractive errors in all eyes, emmetropia was selected as the target refraction. The size of the ICL was also chosen following the manufacturer's instructions and was based on the horizontal corneal diameter and the anterior chamber depth determined using scanning-slit topography (Orbscan IIz).
Implantable Collamer Lens Surgical Procedure
For conventional ICL implantation, the patients underwent 2 preoperative peripheral iridotomies with a neodymium-YAG laser. For Hole ICL implantation, patients did not undergo preoperative iridotomy or intraoperative peripheral iridectomy, but on the day of surgery, they were given dilating and cycloplegic agents. After topical anesthesia, a model V4 ICL (V4c; Hole ICL or V4b; conventional ICL) was inserted through a 3-mm clear corneal incision by means of an injector cartridge (STAAR Surgical) after insertion of a viscosurgical device (OpeganTM; Santen, Osaka, Japan) into the anterior chamber. The ICL was placed in the posterior chamber, the viscosurgical device was washed out of the anterior chamber using a balanced salt solution, and a miotic agent was instilled. In order to suppress potential cyclotorsion in the supine position during the process of toric ICL implantation, the zero horizontal axis was marked preoperatively by means of a slit-lamp. The ICL was then placed in the posterior chamber and rotated by 22.5° or less by using the manipulator. All surgeries were performed by 1 experienced surgeon (K.S.). Postoperatively, steroidal and antibiotic medications (0.1% betamethasone; RinderonTM; Shionogi, Osaka, Japan; and 0.5% levofloxacin; CravitTM; Santen, Osaka, Japan, respectively) were administered topically 4 times daily for 2 weeks, and the doses were reduced gradually thereafter.
Statistical Analysis
All statistical analyses were carried out with the use of commercially available statistical software (Ekuseru-Toukei 2010, Social Survey Research Information Co., Ltd, Tokyo, Japan). One-way analysis of variance (ANOVA) was used to analyze the time courses of changes, and multiple comparisons were performed using the Dunnett test being employed for. The normality of all data samples was first checked using the Kolmogorov–Smirnov test. The use of parametric statistics was not possible, and the Wilcoxon signed-rank test was used for intergroup data comparisons between the 2 groups. Unless otherwise indicated, the results are expressed as means ± SD, and a value of P < 0.05 was considered statistically significant.
RESULTS
Study Population
The preoperative demographics of the study population are summarized in Table 1. We found no significant differences in terms of the preoperative manifest spherical equivalent (Wilcoxon signed-rank test, P = 0.85), manifest cylinder (P = 0.64), LogMAR UDVA (P = 0.27), LogMAR CDVA (P = 0.21), mean keratometric readings (P = 0.75), white-to-white distance (P = 0.38), anterior chamber depth (P = 0.06), or pachymetry (P = 0.54), between the 2 groups at 1, 3, and 6 months and 1, 3, and 5 years postoperatively. The numbers of eyes examined 1, 3, and 6 months and 1, 3, and 5 years postoperatively, were 32 (100%), 30 (94%), 30 (94%), 28 (88%), 27 (84%), and 26 (81%), respectively. All patients who were lost to the postoperative follow-up in this series had reasons unrelated to the visual and refractive outcomes of these surgical techniques.
TABLE 1.
Preoperative Patient Demographics in Eyes Undergoing Implantable Collamer Lens Implantation With and Without a Central Artificial Hole

Safety Outcomes
Five years postoperatively LogMAR CDVA was −0.24 ± 0.08 (range −0.08 to −0.30) in the Hole ICL group and −0.25 ± 0.08 (range −0.08 to −0.30) in the conventional ICL group (P = 0.46). At that time, CDVA underwent no change in 9 eyes (35%), whereas 11 eyes (42%) gained a single line, 2 (8%) increased by 2 lines, and 4 eyes (15%) lost 1 line in the Hole ICL group. However, 5 years postoperatively in the conventional ICL group, CDVA did not alter in 11 eyes (42%), whereas 12 eyes (46%) gained 1 line, 2 eyes (8%) gained 2 lines, and 1 eye (4%) lost a line (Figure 1).
FIGURE 1.
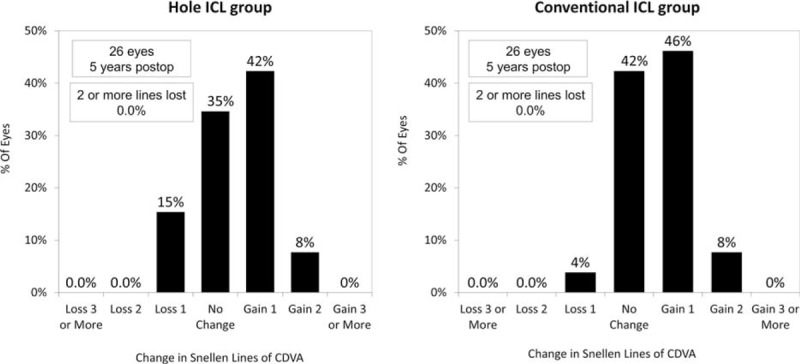
Changes in corrected distance visual acuity (CDVA) 5 years after conventional and Hole implantable collamer lens (ICL) implantation. CDVA = corrected distance visual acuity, ICL = implantable collamer lens.
Effectiveness Outcomes
LogMAR UDVA was −0.17 ± 0.14 (range 0.15 to −0.30) in the Hole ICL group and −0.16 ± 0.10 (range, 0 to −0.30) in the conventional ICL group 5 years postoperatively (P = 0.62). The postoperative UDVAs of the Hole ICL group were 20/20 or better 1, 3, and 6 months and 1, 3, and 5 years postoperatively, in 97%, 100%, 100%, 100%, 100%, and 85% of eyes, respectively, those of the conventional ICL group being 20/20 or better after the same time intervals in 100%, 100%, 97%, 96%, 100%, and 100% of eyes, respectively (Figure 2).
FIGURE 2.
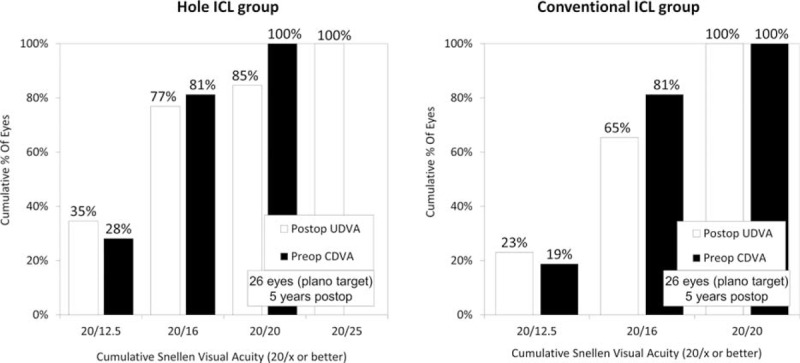
Cumulative percentages of eyes attaining specified cumulative levels of uncorrected distance visual acuity (UDVA) 5 years after conventional and Hole implantable collamer lens (ICL) implantation. ICL = implantable collamer lens, UDVA = uncorrected distance visual acuity.
Predictability
Scatter plots of the attempted versus the achieved spherical equivalent correction are shown in Figure 3. One, 3, and 6 months and 1, 3, and 5 years postoperatively, 100%, 97%, 97%, 100%, 100%, and 88% of eyes, respectively, in the Hole ICL group and 100%, 100%, 100%, 96%, 100%, and 92% of eyes, respectively, in the conventional ICL group were within ± 0.5 D of the attempted correction. One, 3, and 6 months and 1, 3, and 5 years postoperatively, 100%, 100%, 100%, 100%, 100%, and 96% of eyes, respectively, in the Hole ICL group and 100%, 100%, 100%, 100%, 100%, and 100% of eyes, respectively, in the conventional ICL group were within ± 1.0 D of the attempted correction (Figure 4).
FIGURE 3.

A scatter plot of the attempted versus the achieved manifest spherical equivalent correction 5 years after conventional and Hole implantable collamer lens (ICL) implantation. ICL = implantable collamer lens.
FIGURE 4.

Percentages of eyes within different diopter ranges of the attempted correction (spherical equivalent) 5 years after conventional and Hole implantable collamer lens (ICL) implantation. ICL = implantable collamer lens.
STABILITY
The time-course changes in the manifest spherical equivalent are shown in Figure 5. Changes in manifest refraction from 1 month to 5 years were −0.17 ± 0.41 D (range −1.25 to 0.50 D) in the Hole ICL group and −0.10 ± 0.26 D (range −0.75 to 0.25 D) in the conventional ICL group (P = 0.29).
FIGURE 5.
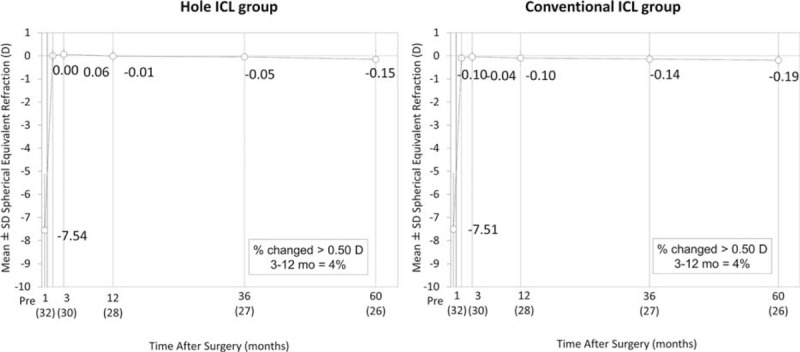
Time course of manifest spherical equivalent after conventional and Hole implantable collamer lens (ICL) implantation. ICL = implantable collamer lens.
Intraocular Pressure
The time-course changes in the intraocular pressure (IOP) are shown in Figure 6. There was no significant change in the IOP in the Hole ICL group (ANOVA, P = 0.53) or in the conventional ICL group (P = 0.35). No significant increase in IOP (> 21 mm Hg) occurred in any case during the 5-year observation period.
FIGURE 6.

Time course of changes in intraocular pressure after conventional and Hole implantable collamer lens (ICL) implantation. ICL = implantable collamer lens.
Endothelial Cell Density
The time-course changes in the corneal endothelial cell density are shown in Figure 7. There was no significant change in the endothelial cell density in the Hole ICL group (ANOVA, P = 0.73) or in the conventional ICL group (P = 0.59). The mean percentage of endothelial cell loss 5 years postoperatively was 0.5 ± 5.4% and 1.2 ± 7.2% in the Hole and conventional ICL groups, respectively. No significant decrease in the endothelial cell density (> 15 %) occurred in any case 5 years postoperatively.
FIGURE 7.
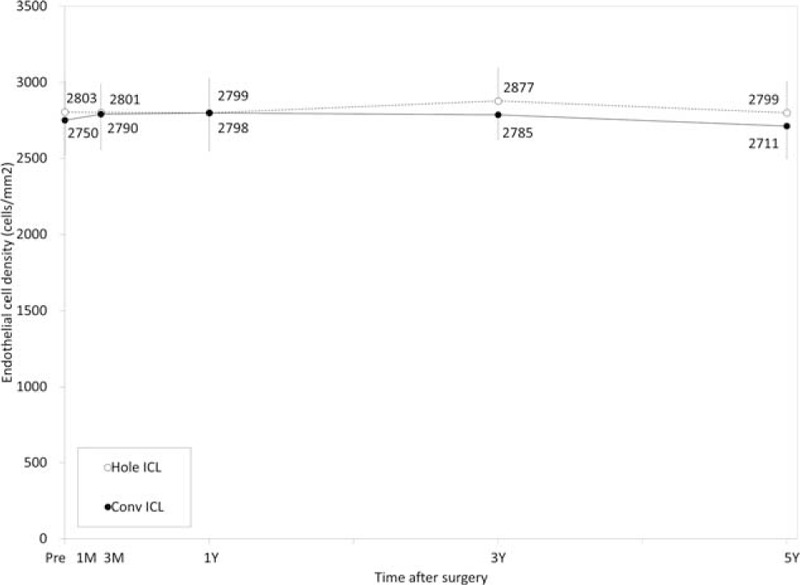
Time course of changes in endothelial cell density after conventional and Hole implantable collamer lens (ICL) implantation. ICL = implantable collamer lens.
Anterior Chamber Depth
The time-course changes in the anterior chamber depth are shown in Figure 8. There was a significant change in the anterior chamber depth in the Hole ICL group (ANOVA, P < 0.001) but not in the conventional ICL group (P < 0.001). Multiple comparisons demonstrated significant differences between measurements made before surgery and at all postoperative times (Dunnett test, P < 0.001).
FIGURE 8.

Time course of changes in anterior chamber depth after conventional and Hole implantable collamer lens (ICL) implantation. ICL = implantable collamer lens.
Secondary Surgeries / Adverse Events
There were no intraoperative complications, and all implantations were uneventful. Of the 64 eyes examined, only 1 eye (3.1%) in the conventional ICL group developed asymptomatic anterior subcapsular cataract and lost 1 line in CDVA. One eye (3.1%) developed significant axis rotation of the toric ICL (30 degrees) in the conventional ICL group. One eye (3.1%) required photorefractive keratectomy due to undercorrection in the conventional ICL group. Otherwise, neither pigment dispersion glaucoma, nor pupillary block, nor any other vision-threatening complications were observed at any time during the 5-year follow-up period.
DISCUSSION
It was found in the present study that, during the entire 5-year follow-up period, the safety efficacy, predictability, and stability of both Hole ICL and conventional ICL implants showed favorable results in correcting moderate to high ametropia. Now that these new surgical procedures are coming into wider use, the prospective comparison of Hole ICL and conventional ICL implantation for achieving an equivalent improvement of myopia is important clinically. Our results using conventional ICL implantation were similar, or slightly superior, to those of other long-term studies (spanning 5 years or more) that deal with phakic IOL implantation.3,4,16–22 Our study of the appropriate literature shows this to be the longest study that has appeared hitherto on the visual and refractive outcomes of Hole ICL implantation. It may be said with regard to optical quality that at least in theory, the presence of the central hole degrades the optical quality of the ICL by, for example, the introduction of glare or halo. However, Shiratani et al noted similarities between the modulation transfer function (MTF), obtained using optical simulation software, of an ICL with a 1.0-mm central hole and the MTF of an unperforated ICL.10 Uozato et al also showed that the MTF of a Hole ICL and that of a conventional ICL differed only in minor and clinically negligible ways, and that ICLs with a 0.36-mm central hole at various IOL powers perform optically in vitro at a level fulfilling the ISO criteria for MTF.11 In a clinical setting, we have already demonstrated that implantation of a Hole ICL was equivalent in most characteristics to that of conventional ICL, as far as the following characteristics are concerned: the induction of higher-order aberrations,14 the contrast sensitivity function under both photopic and mesopic conditions,14 and the detailed optical parameters including intraocular forward scattering.15 Ieong et al reported that glare, halo, and other night vision symptoms frequently manifested in the early postoperative period in conventional ICL-implanted eyes, despite the high degree of satisfaction with the outcome of surgery expressed by most patients.23 It is possible that the presence of a 0.36-mm central artificial hole does not significantly affect the overall subjective or objective optical performance for clinical use. Despite a certain degree of edge glare that manifested around the artificial hole, it appeared to have no clinical significance, the edge of the myopic ICL that extended beyond the edge of the hole being far narrower than that of conventional IOLs. Moreover, we observed that the visual and refractive outcomes of Hole ICL implantation were, for practical purposes, essentially equivalent to those of conventional ICL implantation.
There are ongoing concerns about postoperative complications such as IOP increase (including pupillary block), corneal endothelial cell loss, and cataract formation, after Hole ICL implantation. We found no significant IOP rise (>21 mm Hg) or significant endothelial cell loss (>25%) in any case in the 2 groups. According to our experience, both ICL implantations appear to be safe in terms of IOP and corneal endothelial cell density, even without preoperative laser iridotomies or intraoperative peripheral iridectomy. With regard to cataract formation, we found clinically significant symptomatic cataract formation only in 1 eye (3%) which was undergoing conventional ICL implantation. Kawamorita et al demonstrated that newly developed Hole ICLs improve the circulation of aqueous humor to the anterior surface of the crystalline lens.12 However, the sample size in this study was small enough to make the detection of rare complications, such as cataract formation, difficult. In order to clarify whether the rate of cataract formation after Hole ICL implantation is significantly lower than that after conventional ICL implantation, another study, one that covers more patients, is required.
There are at least 2 limitations to this study. One is that the amounts of sample data were rather limited for the detection of rare complications such as cataract formation. However, the sample size in the present study offered ≥80% statistical power at the 5% level. Moreover, we indicated a prospective intraindividual comparative study, which provides more accurate information for comparing the clinical outcomes of the 2 surgical techniques, because the patient age and gender were identical, and because the amounts of myopic correction were closely matched. Another limitation is that some eyes were lost to follow-up owing to reasons unrelated to visual and refractive outcomes of these surgical procedures. Considering that the patients undergoing refractive surgery, whose visual acuity was good, tended to forget to come to the hospital for routine postoperative examinations, our longitudinal data may have a possible source of selection bias. A model-based analysis using, for example, a mixed model or a generalized estimating equation may be helpful for obtaining more accurate longitudinal outcomes using these surgical procedures.
In summary, our prospective randomized intraindividual comparative study supports the view that both a Hole ICL and a conventional ICL performed well in the correction of moderate to high ametropia during the 5-year follow-up period, and that Hole ICL implantation was essentially equivalent to conventional ICL implantation in terms of safety, efficacy, predictability, and stability, even without preoperative laser iridotomies or intraoperative peripheral iridectomy. It is suggested that that Hole ICL implantation is a feasible surgical option for the treatment of moderate to high ametropia.
Footnotes
Abbreviations: ANOVA = analysis of variance, CDVA = corrected distance visual acuity, D = diopter, ICL = Implantable collamer lens, IOP = Intraocular pressure, logMAR = logarithm of the minimal angle of resolution, MTF = modulation transfer function, OSI = objective scattering index, SD = standard deviation, UDVA = uncorrected distance visual acuity.
Competing interest: Dr. Shimizu is a consultant of STAAR Surgical who has assisted with the development of patented technologies. No other author has a financial or proprietary interest in any material or method mentioned.
Financial disclosure: The authors have no financial interests to report for this submission.
The study was approved by the Institutional Review Board at Kitasato University School of Medicine.
The authors were involved in the design and conduct of the study (KK, KS); collection, management, analysis, and interpretation of data (KK, AI, HK); and preparation, review, and approval of the manuscript (KK, KS, AI, HK).
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.Sanders DR, Doney K, Poco M. ICL in Treatment of Myopia Study Group. United States Food and Drug Administration clinical trial of the Implantable Collamer Lens (ICL) for moderate to high myopia: three-year follow-up. Ophthalmology 2004; 111:1683–1692. [DOI] [PubMed] [Google Scholar]
- 2.Kamiya K, Shimizu K, Igarashi A, et al. Four-year follow-up of posterior chamber phakic intraocular lens implantation for moderate to high myopia. Arch Ophthalmol 2009; 127:845–850. [DOI] [PubMed] [Google Scholar]
- 3.Alfonso JF, Baamonde B, Fernández-Vega L, et al. Posterior chamber collagen copolymer phakic intraocular lenses to correct myopia: five-year follow-up. J Cataract Refract Surg 2011; 37:873–880. [DOI] [PubMed] [Google Scholar]
- 4.Igarashi A, Shimizu K, Kamiya K. Eight-year follow-up of posterior chamber phakic intraocular lens implantation for moderate to high myopia. Am J Ophthalmol 2014; 157:532–539. [DOI] [PubMed] [Google Scholar]
- 5.Sanders DR, Schneider D, Martin R, et al. Toric implantable Collamer lens for moderate to high myopic astigmatism. Ophthalmology 2007; 114:54–61. [DOI] [PubMed] [Google Scholar]
- 6.Kamiya K, Shimizu K, Igarashi A, et al. Comparison of Collamer toric implantable contact lens implantation and wavefront-guided laser in situ keratomileusis for high myopic astigmatism. J Cataract Refract Surg 2008; 34:1687–1693. [DOI] [PubMed] [Google Scholar]
- 7.Kamiya K, Shimizu K, Aizawa D, et al. One-year follow-up of posterior chamber toric phakic intraocular lens implantation for moderate to high myopic astigmatism. Ophthalmology 2010; 117:2287–2294. [DOI] [PubMed] [Google Scholar]
- 8.Alfonso JF, Fernández-Vega L, Fernandes P, et al. Collagen copolymer toric posterior chamber phakic intraocular lens for myopic astigmatism: one-year follow-up. J Cataract Refract Surg 2010; 36:568–576. [DOI] [PubMed] [Google Scholar]
- 9.Fujisawa K, Shimizu K, Uga S, et al. Changes in the crystalline lens resulting from insertion of a phakic IOL (ICL) into the porcine eye. Graefes Arch Clin Exp Ophthalmol 2007; 245:114–122. [DOI] [PubMed] [Google Scholar]
- 10.Shiratani T, Shimizu K, Fujisawa K, et al. Crystalline lens changes in porcine eyes with implanted phakic IOL (ICL) with a central hole. Graefes Arch Clin Exp Ophthalmol 2008; 246:719–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uozato H, Shimizu K, Kawamorita T, et al. Modulation transfer function of intraocular Collamer lens with a central artificial hole. Graefes Arch Clin Exp Ophthalmol 2011; 249:1081–1085. [DOI] [PubMed] [Google Scholar]
- 12.Kawamorita T, Uozato H, Shimizu K. Fluid dynamics simulation of aqueous humour in a posterior-chamber phakic intraocular lens with a central perforation. Graefes Arch Clin Exp Ophthalmol 2012; 250:935–939. [DOI] [PubMed] [Google Scholar]
- 13.Shimizu K, Kamiya K, Igarashi A, et al. Early clinical outcomes of implantation of posterior chamber phakic intraocular lens with a central hole (Hole ICL) for moderate to high myopia. Br J Ophthalmol 2012; 96:409–412. [DOI] [PubMed] [Google Scholar]
- 14.Shimizu K, Kamiya K, Igarashi A, et al. Intraindividual comparison of visual performance after posterior chamber phakic intraocular lens with and without a central hole implantation for moderate to high myopia. Am J Ophthalmol 2012; 154:486–494. [DOI] [PubMed] [Google Scholar]
- 15.Kamiya K, Shimizu K, Saito A, et al. Comparison of optical quality and intraocular scattering after posterior chamber phakic intraocular lens with and without a central hole (Hole ICL and Conventional ICL) implantation using the double-pass instrument. PLoS One 2013; 8:e66846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tahzib NG, Nuijts RM, Wu WY, et al. Long-term study of Artisan phakic intraocular lens implantation for the correction of moderate to high myopia: ten-year follow-up results. Ophthalmology 2007; 114:1133–1142. [DOI] [PubMed] [Google Scholar]
- 17.Silva RA, Jain A, Manche EE. Prospective long-term evaluation of the efficacy, safety, and stability of the phakic intraocular lens for high myopia. Arch Ophthalmol 2008; 126:775–781. [DOI] [PubMed] [Google Scholar]
- 18.Güell JL, Morral M, Gris O, et al. Five-year follow-up of 399 phakic Artisan-Verisyse implantation for myopia, hyperopia, and/or astigmatism. Ophthalmology 2008; 115:1002–1012. [DOI] [PubMed] [Google Scholar]
- 19.Fechner PU, Haubitz I, Wichmann W, et al. Worst–Fechner biconcave minus power phakic iris-claw lens. J Refract Surg 1999; 15:93–105. [DOI] [PubMed] [Google Scholar]
- 20.Javaloy J, Alió JL, Iradier MT, et al. Outcomes of ZB5 M angle-supported anterior chamber phakic intraocular lenses at 12 years. J Refract Surg 2007; 23:147–158. [DOI] [PubMed] [Google Scholar]
- 21.Rosman M, Alió JL, Ortiz D, et al. Refractive stability of LASIK with the Visx 20/20 excimer laser vs ZB5m phakic iol implantation in patients with high myopia (>-10.00 d): a 10-year retrospective study. J Refract Surg 2011; 27:279–286. [DOI] [PubMed] [Google Scholar]
- 22.Plainer S, Wenzl E, Saalabian AA, et al. Long-term follow-up with I-CARE phakic IOLs. Br J Ophthalmol 2011; 95:710–714. [DOI] [PubMed] [Google Scholar]
- 23.Ieong A, Hau SC, Rubin GS, et al. Quality of life in high myopia before and after implantable Collamer lens implantation. Ophthalmology 2010; 117:2295–2300. [DOI] [PubMed] [Google Scholar]


