Abstract
Nesidioblastosis is a rare cause of endogenous hyperinsulinemic hypoglycemia in adults. Diagnosis is often challenging and therapeutic options are scarce.
In 2009, a 46-year-old female patient presented with recurrent severe hypoglycemia and immediate recovery after glucose ingestion. Although 72-h-fasting test was positive, various imaging technologies (sonography, computed tomography, somatostatin receptor scintigraphy, dopamine receptor positron emission tomography [DOPA-PET]) were negative. Endoscopic ultrasound revealed a lesion in the pancreatic corpus, whereas selective arterial calcium stimulation test, portal venous sampling and GLP-1-receptor scintigraphy were indicative of a lesion in the pancreatic tail, which was surgically removed. The histopathologic examination revealed beta cell hyperplasia and microadenomas expressing glucagon. After surgery, the patient was free of symptoms for 6 months, after which hypoglycemic episodes recurred. After unsuccessful treatment with corticosteroids and somatostatin analogs, treatment with pasireotide, a novel somatostatin analog with high affinity to somatostatin receptor 5 and a possible side effect of hyperglycemia, was initiated (0.6 mg BID). To date, our patient has been free of severe hypoglycemic episodes ever since. Yearly repeated imaging procedures have shown no abnormities over the last 3 years.
We report for the first time that pasireotide was successfully used in the treatment of adult nesidioblastosis.
INTRODUCTION
Nesidioblastosis is a condition with diffuse hyperplasia of the pancreatic islets, leading to hyperinsulinemic hypoglycemia. It is the most important differential diagnosis to insulinoma in the adult, but only 0.5% to 5.0% of the cases with hyperinsulinemic hypoglycemia can be attributed to noninsulinoma pancreatogenous hypoglycemia syndrome (NIPHS),1,2 which is currently more often seen in patients who have undergone bariatric surgery.
To fulfill the diagnostic criteria, detection of endogenous hyperinsulinemic hypoglycemia, positive selective arterial calcium stimulation test (SACST),3 and negative imaging studies are required after exclusion of artificial causes of hypoglycemia such as inappropriate use of insulin or sulfonylurea. Nonetheless, the results of the above-mentioned exams might be inconclusive, that is, small insulinomas might not be detected in imaging studies or large hyperplastic areas showing large gradients in SACST might be interpreted as insulinoma. Thus, the final diagnosis can only be established after histopathologic examination.
In mild cases, dietary modifications (low carbohydrate diet) might be sufficient to resolve symptoms.4 Pharmacological treatment options include diazoxide, acarbose, corticosteroids, verapamil, and octreotide. In patients developing severe symptoms partial or total pancreatectomy might be required.
Here we report a case of a patient with nesidioblastosis successfully treated with pasireotide, a somatostatin analog with high affinity for somatostatin receptor 5, originally developed for the treatment of Cushing's disease. For the herein presented case, the patient has provided written informed consent for publication.
Clinical Case
In 2009, a 46-year-old woman was admitted with a blood glucose level of 38 mg/dL (2.1 mmol/L). Even upon intravenous glucose and glucose-rich diet, glucose levels did not exceed 90 mg/dL (5.0 mmol/L). The patient's history revealed fatigue, sweating, craving for sweets over the last months and weight gain of 5 kg in 1 year. Insulinoma was suspected.
By performing an oral glucose tolerance test, reactive postprandial hypoglycemia could be ruled out. A consecutive 72 hours fast showed a decline in glucose to 34 mg/dL (1.9 mmol/L) after 14 hours of fasting. At that time the insulin level was inadequately in the normal range (13.0 μU/mL, normal range: 2.0–25.0 μU/mL) and C-peptide was elevated (8.2 ng/mL, normal range: 0.78–1.89 ng/mL), indicative of autonomous insulin secretion.
Imaging procedures including abdominal ultrasound, magnetic resonance imaging (MRI), computed tomography scan (CT scan), fluorodeoxyglucose positron emission tomography (FDG-PET), dopamine receptor positron emission tomography (DOPA-PET), octreotide receptor scintigraphy, and diagnostic laparotomy with palpation of the pancreas revealed no pathological findings.
Selective arterial calcium stimulation test (SACST) with hepatic venous sampling to determine the localization of hyperinsulinemia within the pancreas,5,6 showed a 2.1-fold, positive increase in insulin (>2)5,6 measured in the hepatic vein after calcium injection in the great pancreatic artery. In patients with NIPHS, an increase in insulin is usually observed after injection of multiple arteries, in patients with insulinoma the response would be expected to be positive in 1 artery alone.
In endoscopic ultrasound a hypoechogenic lesion in the pancreatic corpus at the height of the confluens could be located. As the results were inconclusive with regard to the localization of the lesion, portal venous sampling was additionally performed to differentiate localized (solitary insulinoma) from diffuse hyperinsulinism caused by adenomatosis, hyperplasia, and nesidioblastosis.2 It was indicative of an increased insulin secretion in the tail of the pancreas.
This was confirmed by glucagon-like peptide-1 (GLP-1) receptor scintigraphy showing an increased uptake not only in the head (physiologic) but also in the tail of the pancreas (Figure 1 A and B). Subsequently, distal pancreatectomy was performed, histopathologic examination of the pancreatic tail revealing hyperplasia of the islets of Langerhans (Figure 2).
FIGURE 1.
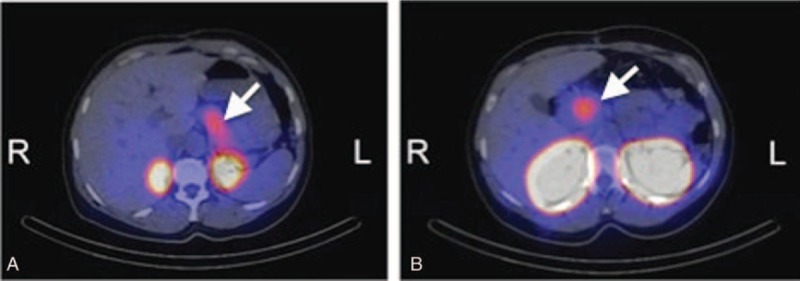
Glucagon-like peptide-1 (GLP-1) receptor scintigraphy showing an increased uptake both in the head and tail of the pancreas.
FIGURE 2.
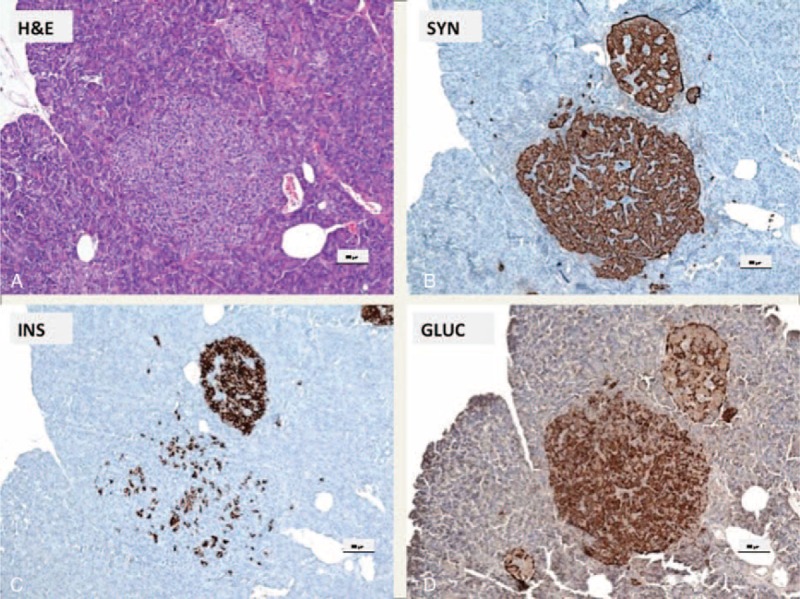
Microadenoma and enlarged LH islet: (A) H&E; (B) synaptophysin; (C) insulin; (D) glucagon. (A) Microadenoma in the vicinity of an enlarged LH islet (H&E). (B) The endocrine cells of the microadenoma and the LH islet stain with antibodies against synaptophysin. (C) The majority of the cells of the LH islet are positive, whereas most of the cells of the microadenoma are not labeled with insulin antibodies (D) but are positive with antibodies against glucagon. In contrast in the LH islet only the alpha cells are glucagon positive; scale bars indicate 100 μm.
In addition, neuroendocrine microadenomas with immunohistochemical staining for glucagon in all of the adenoma cells were present (Figure 3). Co-occurrence of adult nesidioblastosis with microadenomas composed of alpha cells (Figure 3) without elevation of serum glucagon, as measured on several occasions in the presented case, (Figure 2C) has to the best of our knowledge not been described in the literature so far. Subsequent genetic testing for multiple endocrine neoplasia type 1 (MEN-1) was negative.
FIGURE 3.
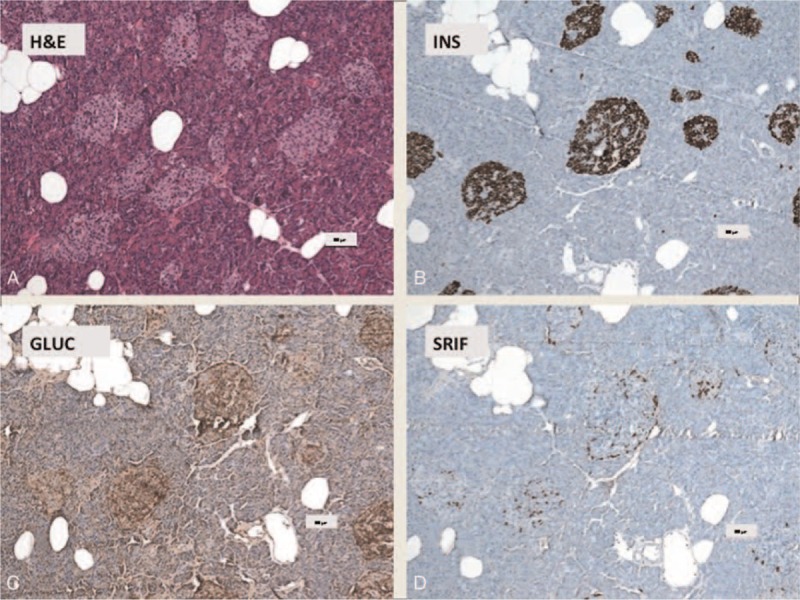
Nesidioblastosis with increased numbers enlarged of Langerhans islets (A–D): (A) H&E; (B) insulin; (C) glucagon; (D) SRIF (somatotropin release-inhibiting factor). (A) Increased number of enlarged Langerhans islets (H&E). (B) The islets consist of mainly of ß-cells (60–70%) which are labeled with antibodies directed against insulin. (C) Approximately 10% to 15% of the islet cells are decorated by antibodies against glucagon and (D) some islet cells are positive with antibodies against somatostatin. Scale bars indicate 100 μm.
During the first 6 months following distal pancreatectomy, the patient was free of hypoglycemic symptoms. After 6 months, symptoms of endogenous hyperinsulinism including hypoglycemia started to reappear. As the patient was hesitant toward any further surgical procedure, conservative medical therapy including administration of corticosteroids, everolimus, and octreotide was initiated; however, all medical treatment approaches were futile. Yearly diagnostic imaging follow-up including abdominal ultrasound, MRI, octreotide receptor scintigraphy, and DOPA-PET repeatedly showed no pathological finding.
Over time, hypoglycemia unawareness developed. Subsequently, in the beginning of 2012, the patient was equipped with a continuous glucose monitoring (CGM) system (Guardian RT, Medtronic-Minimed, Northridge, CA) to alert her timely to prevent severe hypoglycemia.
In the attempt to alleviate the burden of disease, pasireotide 0.6 mg twice daily was initiated in November 2012. Pasireotide is a somatostatin analog with a 40-fold increased affinity to somatostatin receptor 5 compared to other somatostatin analogs and is used in the treatment of Cushing's disease and acromegaly. It is known to suppress insulin secretion and thus to cause hyperglycemia as one of its most concerning side effects.7 Pasireotide has been successfully applied in patients suffering from dumping syndrome with hypoglycemia.8 Also, treatment with pasireotide in patients with severe post Roux-en-Y gastric bypass hyperinsulinemic hypoglycemia has been suggested.9 To the best of our knowledge, pasireotide has never been used in adult nesidioblastosis so far.
After the first administration of pasireotide, glycemic levels started rising immediately, the carbohydrate requirements decreased, and the observed hypoglycemic levels were milder in their extent and mainly above the commonly accepted threshold for hypoglycemia (above 56 mg/dL [3.1 mmol/L]) (Table 1). The glycemic response to pasireotide was monitored with CGM (Figure 4). The substance was well tolerated with only mild nausea that could be successfully treated with ondansetron. When comparing the CGM values prior to and 2 weeks following initiation of pasireotide therapy a significant increase in glycemia, reduced time in hypoglycemia, and increased time in eu- and even hyperglycemia could be observed. Mean sensor glucose increased from 74 ± 16 mg/dL (4.1 ± 0.9 mmol/L) to 103 ± 23 mg/dL (5.7 ± 1.3 mmol/L).
TABLE 1.
Levels of Hypoglycemia Assessed by Continuous Glucose Monitoring the Last 14 days Before Initiation of Pasireotide as Compared to the First 14 Days Using Pasireotide
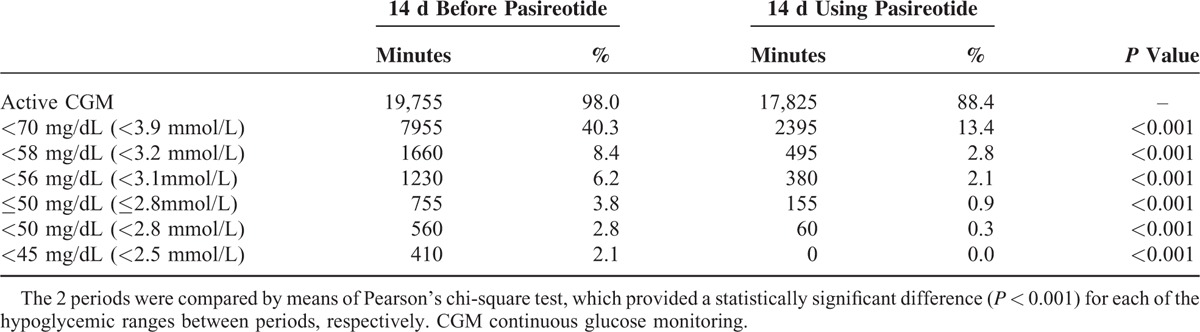
FIGURE 4.
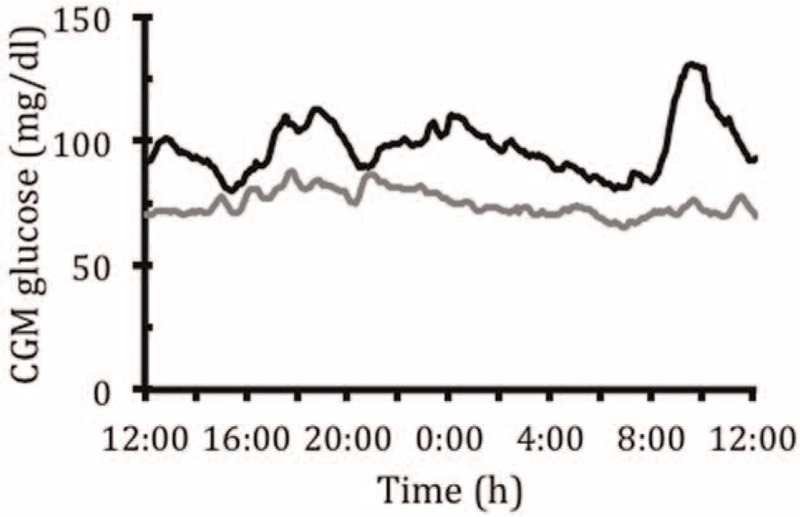
Mean sensor glucose level over a 14-day period prior to (gray line) and immediately after (black line) initiation of pasireotide treatment.
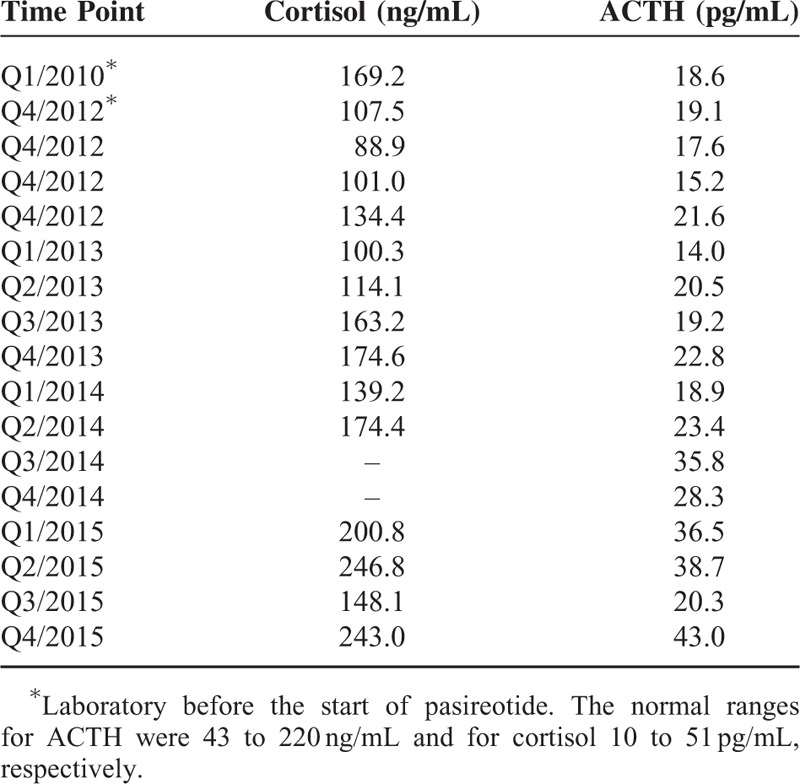
The patient has been successfully treated with pasireotide for 3 years now with marked improvement in quality of life. Pasireotide treatment is—except for intermittent mild nausea—well tolerated and the patient has not experienced any severe hypoglycemic episode ever since. ACTH and cortisol were controlled regularly and have been in the normal range (Table 2). Imaging procedures (MRI, somatostatin receptor scintigraphy, 18F-DOPA-PET) were performed in yearly intervals and showed no abnormities over time.
TABLE 2.
Levels of ACTH (Adrenocorticotropic Hormone) and Cortisol Were Monitored on a Regular Basis and Did Not Show Any Clinically Significant Abnormalites
DISCUSSION
We present a case of nesidioblastosis with microadenomas expressing glucagon with recurrent hypoglycemic episodes after partial pancreatectomy unresponsive to usual therapeutic approaches. Only initiation of therapy with pasireotide has re-established and maintained normoglycemia and a good quality of life for the affected patient for a total of 3 years.
Pasireotide is a somatostatin analog targeting 4 out of the 5 somatostatin receptor subtypes (SSTR) with the highest affinity for sst1 and sst5. The affinity for sst5 is 40-fold higher compared to octreotide.10 Pasireotide has shown to effectively inhibit ACTH and corticosterone secretion both in vitro and in vivo11,12 and has proven effective in the treatment of Cushing's disease.7,13 Although responders to pasireotide treatment showed decreased plasma ACTH and cortisol levels, increases in blood glucose and glycated haemoglobin levels were observed; 46% of the treated patients in that specific study even required initiation of diabetes therapy.13 A study in healthy volunteers also reported elevated blood glucose levels 2 to 6 hours after single doses of pasireotide (200–1200 μg) which resolved within 23 hours after dosing. The described effect was more pronounced at higher doses of 600 to 1200 μg pasireotide.14 In a mechanistic clamp study, Henry et al demonstrated that hyperglycemia induced by pasireotide administration is caused by decreased insulin secretion and incretin hormone responses, without influencing the hepatic and/or peripheral insulin sensitivity.15 From the beginning we could observe the described effects of pasireotide on glycemia14,15 in our patient in support of previous studies showing effective application of pasiretoide in dumping syndrome8 and in a patient with postprandial hyperinsulinemic hypoglycemia following Roux-en-Y gastric bypass.9 In the latter patient, pasireotide inhibited insulin and GLP-1 more efficiently than octreotide resulting in a better control of postprandial hyperinsulinemic hypoglycemia.9 Inhibition of insulin secretion by islet beta cells is mediated by somatostatin receptor 2 (SSTR2) and 5 (SSTR5). Glucagon inhibition from alpha cells is mediated almost entirely by SSTR2. As the affinity of pasireotide to SSTR5 is much higher than to SSTR2,16 pasireotide suppresses mostly insulin. Another factor in the avoidance of severe hypoglycemia achieved by pasireotide might be its suppression of GLP-1, as suggested by de Heide.9
Another effect of pasireotide rendering it successful in the treatment of neuroendocrine tumors could be its direct and indirect antitumor effects mediated via SSTR, such as apoptosis and inhibition of cell proliferation.17–19 In patients with advanced neuroendocrine tumors refractory or resistant to octreotide LAR therapy, pasireotide treatment was effective and well-tolerated in controlling symptoms of carcinoid syndrome in 27% of patients and resulted in stable disease in more than half of the patients.20
Consistent with these data, pasireotide has been well tolerated by our patient for >3 years now. According to the regularly monitored CGM signal—the availability of which being one of the strengths of this case, hypoglycemia is well controlled and the patient is in euglycemia the majority of the time. Further, as seen in the regularly performed imaging studies (MRI, somatostatin receptor scintigraphy, DOPA-PET), the discreet tracer uptake in DOPA-PET, indicating that beta cell hyperplasia is still present, has remained stable. There has also been no growth of the pancreas following distal pancreatectomy, as seen in MRI. This is in line with the tumor control described by Kvols and colleagues.20
Another unusual feature in this case was the co-occurrence of nesidioblastosis and microadenomas consisting of alpha cells, which to the best of our knowledge has not yet been reported so far in adults. Although genetic testing could rule out MEN1 syndrome, we cannot exclude that other, so far unknown mutations could play a role in the pathogenesis of the microadenomas in our patient. Finally, it may be speculated that regulatory mechanisms against the effects of nesidioblastosis could be involved in hyperplasia of alpha-cells and the formation of microadenomas; these, however, are insufficient to counteract hyperinsulinism and hypoglycemia in the present case.
Data from the patient presented here and from the case report by de Heide bring up the question as to the significance of pasireotide in the treatment of patients with NIPHS post-bariatric surgery in the future. The numbers of patients undergoing bariatric surgery are rising, consecutively causing higher rates of nesidioblastosis. Especially in these patients pasireotide might be an interesting treatment option, as re-surgery might cause numerous complications including adhesions. Further, these patients are at higher general risk due to their overweight.
However, further data are needed before pasireotide can be recommended as a treatment option in different scenarios of autonomous endogenous hyperinsulinemic hypoglycemia. Expectations are raised, though, after successful application of pasireotide in postprandial hyperinsulinemic hypoglycemia after Roux-en-Y gastric bypass and upon the first successful long-term treatment with pasireotide of a patient with recurrent hypoglycemic episodes due to adult nesidioblastosis presented here.
Acknowledgments
The authors would like to thank the patient for consenting publication of the case.
Footnotes
Abbreviations: ACTH = adrenocorticotropic hormone, CGM = continuous glucose monitoring, DOPA-PET = dopamine receptor positron emission tomography, GLP-1 = glucagon-like peptide-1, H&E = hematoxylin and eosin staining, LAR = long-acting release, MEN-1 = multiple endocrine neoplasia type 1, MRI = magnetic resonance imaging, NIPHS = non-insulinoma pancreatogenous hypoglycemia syndrome, SACST = selective arterial calcium stimulation test, SRIF = somatotropin release-inhibiting factor (somatostatin), SSTR = somatostatin receptor subtypes.
The authors have no conflicts of interest to disclose.
Funding: this research did not receive any funding from the public or commercial sector. Medication supply was provided to the patient for a period of 6 months free of charge by Novartis Austria until national health insurance started reimbursing.
Author contributions: VS, HS, and JKM drafted the manuscript; GT, HS, and JKM interpreted data; KH, PK, and TRP added to discussion; CL and FF interpreted data and added to discussion; all authors critically revised the article and approved the final version of the manuscript. JKM is guarantor of this work.
REFERENCES
- 1.Jabri AL, Bayard C. Nesidioblastosis associated with hyperinsulinemic hypoglycemia in adults: review of the literature. Eur J Intern Med 2004; 15:407–410.doi: S0953-6205(04)00194-3 [pii]. [DOI] [PubMed] [Google Scholar]
- 2.Fajans SS, Vinik AI. Insulin-producing islet cell tumors. Endocrinol Metab Clin North Am 1989; 18:45–74. [PubMed] [Google Scholar]
- 3.Thompson SM, Vella A, Thompson GB, et al. Selective arterial calcium stimulation with hepatic venous sampling differentiates insulinoma from nesidioblastosis. J Clin Endocrinol Metab 2015; 100:4189–4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kellogg TA, Bantle JP, Leslie DB, et al. Postgastric bypass hyperinsulinemic hypoglycemia syndrome: characterization and response to a modified diet. Surg Obes Relat Dis 2008; 4:492–499.doi: 10.1016/j.soard.2008.05.005 [doi]. [DOI] [PubMed] [Google Scholar]
- 5.Doppman JL, Chang R, Fraker DL, et al. Localization of insulinomas to regions of the pancreas by intra-arterial stimulation with calcium. Ann Intern Med 1995; 123:269–273. [DOI] [PubMed] [Google Scholar]
- 6.O'Shea D, Rohrer-Theurs AW, Lynn JA, et al. Localization of insulinomas by selective intraarterial calcium injection. J Clin Endocrinol Metab 1996; 81:1623–1627.doi: 10.1210/jcem.81.4.8636378 [doi]. [DOI] [PubMed] [Google Scholar]
- 7.Boscaro M, Ludlam WH, Atkinson B, et al. Treatment of pituitary-dependent Cushing's disease with the multireceptor ligand somatostatin analog pasireotide (SOM230): a multicenter, phase II trial. J Clin Endocrinol Metab 2009; 94:115–122.doi: 10.1210/jc.2008-1008 [doi]. [DOI] [PubMed] [Google Scholar]
- 8.Deloose E, Bisschops R, Holvoet L, et al. A pilot study of the effects of the somatostatin analog pasireotide in postoperative dumping syndrome. Neurogastroenterol Motil 2014; 26:803–809.doi: 10.1111/nmo.12333 [doi]. [DOI] [PubMed] [Google Scholar]
- 9.de Heide LJ, Laskewitz AJ, Apers JA. Treatment of severe postRYGB hyperinsulinemic hypoglycemia with pasireotide: a comparison with octreotide on insulin, glucagon, and GLP-1. Surg Obes Relat Dis 2014; 10:e31–e33.doi: 10.1016/j.soard.2013.11.006 [doi]. [DOI] [PubMed] [Google Scholar]
- 10.Bruns C, Lewis I, Briner U, et al. SOM230: a novel somatostatin peptidomimetic with broad somatotropin release inhibiting factor (SRIF) receptor binding and a unique antisecretory profile. Eur J Endocrinol 2002; 146:707–716.doi: 146707 [pii]. [DOI] [PubMed] [Google Scholar]
- 11.Hofland LJ, van der Hoek J, Feelders R, et al. The multi-ligand somatostatin analogue SOM230 inhibits ACTH secretion by cultured human corticotroph adenomas via somatostatin receptor type 5. Eur J Endocrinol 2005; 152:645–654.doi: 152/4/645 [pii]. [DOI] [PubMed] [Google Scholar]
- 12.Silva AP, Schoeffter P, Weckbecker G, et al. Regulation of CRH-induced secretion of ACTH and corticosterone by SOM230 in rats. Eur J Endocrinol 2005; 153:R7–R10.doi: 153/3/R7 [pii]. [DOI] [PubMed] [Google Scholar]
- 13.Colao A, Petersenn S, Newell-Price J, et al. A 12-month phase 3 study of pasireotide in Cushing's disease. N Engl J Med 2012; 366:914–924.doi: 10.1056/NEJMoa1105743 [doi]. [DOI] [PubMed] [Google Scholar]
- 14.Golor G, Hu K, Ruffin M, et al. A first-in-man study to evaluate the safety, tolerability, and pharmacokinetics of pasireotide (SOM230), a multireceptor-targeted somatostatin analog, in healthy volunteers. Drug Des Devel Ther 2012; 6:71–79.doi: 10.2147/DDDT.S29125 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henry RR, Ciaraldi TP, Armstrong D, et al. Hyperglycemia associated with pasireotide: results from a mechanistic study in healthy volunteers. J Clin Endocrinol Metab 2013; 98:3446–3453.doi: 10.1210/jc.2013-1771 [doi]. [DOI] [PubMed] [Google Scholar]
- 16.Boerlin V, van der Hoek J, Beglinger C, et al. New insights on SOM230, a universal somatostatin receptor ligand. J Endocrinol Invest 2003; 26 (8 Suppl):14–16. [PubMed] [Google Scholar]
- 17.Adams RL, Adams IP, Lindow SW, et al. Inhibition of endothelial proliferation by the somatostatin analogue SOM230. Clin Endocrinol (Oxf) 2004; 61:431–436.doi: CEN2098 [pii]. [DOI] [PubMed] [Google Scholar]
- 18.Lattuada D, Casnici C, Crotta K, et al. Inhibitory effect of pasireotide and octreotide on lymphocyte activation. J Neuroimmunol 2007; 182:153–159.doi: S0165-5728(06)00392-4 [pii]. [DOI] [PubMed] [Google Scholar]
- 19.Ono K, Suzuki T, Miki Y, et al. Somatostatin receptor subtypes in human non-functioning neuroendocrine tumors and effects of somatostatin analogue SOM230 on cell proliferation in cell line NCI-H727. Anticancer Res 2007; 27 (4B):2231–2239. [PubMed] [Google Scholar]
- 20.Kvols LK, Oberg KE, O’Dorisio TM, et al. Pasireotide (SOM230) shows efficacy and tolerability in the treatment of patients with advanced neuroendocrine tumors refractory or resistant to octreotide LAR: results from a phase II study. Endocr Relat Cancer 2012; 19:657–666.doi: 10.1530/ERC-11-0367 [doi]. [DOI] [PubMed] [Google Scholar]


