Supplemental Digital Content is available in the text
Abstract
Chronic kidney disease (CKD) is a major health problem worldwide because of the aging population and lifestyle changes. One of the important etiologies of CKD is diabetes mellitus (DM). The long-term effects of pay-for-performance (P4P) on disease progression have not been thoroughly examined.
This study is a retrospective population-based patient cohort design to examine the continuous effects of diabetes and CKD P4P interventions. This study used the health insurance claims database to conduct a longitudinal analysis. A total of 32,084 early CKD patients with diabetes were extracted from the outpatient claims database from January 2011 to December 2012, and the follow-up period was extended to August 2014. A 4-group matching design, including both diabetes and early CKD P4P interventions, with only diabetes P4P intervention, with only early CKD P4P intervention, and without any P4P interventions, was performed according to their descending intensity. The primary outcome of this study was all-cause mortality and the causes of death. The statistical methods included a Chi-squared test, ANOVA, and multi-variable Cox regression models.
A dose–response relationship between the intervention groups and all-cause mortality was observed as follows: comparing to both diabetes and early CKD P4P interventions (reference), hazard ratio (HR) was 1.22 (95% confidence interval [CI], 1.00–1.50) for patients with only a diabetes P4P intervention; HR was 2.00 (95% CI, 1.66–2.42) for patients with only an early CKD P4P intervention; and HR was 2.42 (95% CI, 2.02–2.91) for patients without any P4P interventions. The leading cause of death of the total diabetic nephropathy patient cohort was infectious diseases (34.32%) followed by cardiovascular diseases (17.12%), acute renal failure (1.50%), and malignant neoplasm of liver (1.40%).
Because the earlier interventions have lasting long-term effects on the patient's prognosis regardless of disease course, an integrated early intervention plan is suggested in future care plan designs. The mechanisms regarding the effects of P4P intervention, such as health education on diet control, continuity of care, and practice guidelines and adherence, are the primary components of disease management programs.
INTRODUCTION
Chronic kidney disease (CKD) is an important health issues in the 21st century. The incidence and prevalence of CKD are increasing with the growing aging populations and lifestyle alterations. One of the primary etiologies proceeding to CKD is diabetes mellitus (DM). According to the statistics of WHO, CKD and diabetes were ranked in the top 10 causes of death in recent years.1 Both diseases impose considerable negative impacts on human health and economic burdens. Therefore, preventive medicine and disease management programs play key roles in the current health care services. A close inter-relationship was found between diabetes and CKD. The natural disease course of diabetes has shown that the development of CKD is a major pathway for diabetes.2 The increasing trend of diabetic nephropathy was acknowledged as the primary cause for the increased incidence and prevalence of end-stage renal disease (ESRD) in Taiwan.3 Approximately 33% of the diabetic patients developed CKD in an average of 7 to 10 years.4,5 Another report documented that approximately 44% of the patients with CKD have diabetes as one of their underlying diseases.1 According to the statistics of US Renal Data System, approximately 45% to 50% of new ESRD cases resulted from diabetes.6 Furthermore, a study has demonstrated that patients with diabetes and CKD have a high 10-year mortality (31.1%) compared to the patients with diabetes alone (11.5%). Consequently, preventing disease complications is the primary goal of a health care system. Currently, many countries implemented pay for performance (P4P) systems for the care of selective diseases. The purpose of P4P mechanism for improving quality of care is to motivate healthcare providers to achieve a predetermined, benchmarked level of care quality or performance indicator with financial incentives.
Recent studies have assessed the effects of P4P programs on diabetes care from 2 perspectives in terms of practice behaviors and patient outcomes.7–10 The majority of studies reported positive effects of P4P interventions, including glucose control,11–14 blood pressure (BP) control,12,15,16 prevention of chronic diabetic complications,11,13–15 and prescription changes.16 The intervention of CKD by P4P was found in many countries, including the United Kingdom, Japan, the United States, Australia, and Taiwan.17–21 Similar to the P4P in diabetes care, the majority of the reports in the literature have demonstrated practice behavioral changes. Few studies have examined the outcome measures, such as the incidence of complications, disease progression, and mortality,22 infrequently mentioned the long-term integrated effects of P4P interventions from diabetes to CKD.
The National Health Insurance (NHI) in Taiwan implemented serial P4P programs for diabetes care in 2001 and early CKD in 2010. The P4P programs in Taiwan provide financial incentives to healthcare providers with the goal of enhancing the medical management of patients and improving the quality of care. For more than a decade of implementation, examining the long-term integrated effects of diabetes and early CKD P4P programs with this nationwide population-based observational study has proven to be invaluable.
METHODS
This study uses the health insurance claims database to conduct a longitudinal analysis on the effects of both diabetes and early CKD P4P programs. The Taiwan NHI implemented in 1995 covers more than 99% of the population. The P4P programs of diabetes care and early CKD care were implemented in Taiwan in 2001 and 2010, respectively. There are explicit and rigorous criteria for providers to recruit patients into P4P program in Taiwan (Appendix 1). The test results of estimated glomerular filtration rate (eGFR) and urine albuminuria and creatinine ratio (UACR or urine protein and creatinine ratio [UPCR]) of each enrolled patient were examined and recorded to ensure the appropriateness and fairness of program execution. The data consisted of ambulatory care records, inpatient care records, and administrative registration files. In addition, the qualified physicians should receive at least additional 6-hour CKD care training and be awarded previously at least one of the specialties including renal, cardiology, metabolism, internal medicine, surgeon, gynecology/obstetrics, pediatrics, and family medicine. The program required physicians to report eGFR and UACR (or UPCR) of each P4P enrolled patient every 6 months. Additionally, the physicians are required to provide annual evaluations for the enrolled patients, including a management plan (such as goals, treatment, and monitoring instructions), medical history, examinations, and biochemical tests (including urine protein, urine creatinine, serum creatinine, LDL, and HbA1c). The P4P program is to reward additional health care fees of each recruited patient to the physician but not depending on the patient's renal progression.
The data from a total of 63,923 early CKD patients previously diagnosed with diabetes were extracted from the outpatient claims database from January 2011 to December 2012, and the follow-up study period was extended to August 31, 2014. The inclusion criteria for the study samples were those who were previously diagnosed as having diabetes at least 1 year before CKD and who were classified as ICD-9-CM codes 250.40, 250.42, or 250.xx with 581.9. The study included outpatients who were with at least 4 outpatient visits or more than 1 year of medical records, but excluded the subjects who developed late CKD, ESRD, or death within 30 days of CKD diagnosis or with missing information on the study variables. To improve the comparability among study groups, a propensity score matching by sex, age, DM vintage, Charlson comorbidity index (CCI) score, and baseline CKD stage was made and came out with a total of 32,084 study subjects including 8021 subjects in each participation group. This study was approved by the Internal Review Board of the research ethic committee (#103-6411B).
A 4-group design, with both diabetes and early CKD P4P interventions, with only a diabetes P4P intervention, with only an early CKD P4P intervention, and without any P4P interventions, was performed with their intensity as the independent variable. The primary outcome of this study was all-cause mortality during the follow-up years. In addition, the primary cause of death was extracted from the medical records of the NHI claim database.
The confounding factors included certain characteristics of patients (sex, age, CCI, years of DM vintage, and CKD stage) and providers (hospital accreditation level, location, physician's age, physician's sex, and years of physician specialty license awarded), and the eGFR at the baseline. The patients’ ages in years were categorized as ≤50, 50.1 to 60, 60.1 to 70, and >70. The CCI23 was applied in this study and was categorized as ≤1, 2, 3, and ≥4. The patient's DM vintage was categorized as median low (<8.64 years) or median high (≥8.64 years). The hospital accreditation levels were categorized into medical centers, regional hospitals, district hospitals, and primary care clinics. The physicians’ ages were categorized as ≤40 years, 40.1 to 50 years, and >50 years. The location of study hospitals included Taipei, northern Taiwan, central Taiwan, southern Taiwan, Kao-Ping, and eastern regions. The years of physician specialty license awarded were categorized as ≤10, 10.1 to 15, and >15.
The categorical variables were expressed as the frequency and percentage, whereas the numerical variables were displayed as the mean and standard deviation values. A Chi-squared test was conducted to examine the differences of the categorical variables among the study groups. ANOVA was used to differentiate the average of the numerical variables among the study groups. This study used multivariable Cox regression models to examine the association between the hazard of deaths from all causes/the primary causes of death and the study groups, including nonparticipation, CKD-P4P participation, DM-P4P participation, and CKD-DM-P4Ps participation. To ensure the sufficiency of sample size in analyzing each cause of death, the analysis of leading causes of death used total DM nephropathy patient cohort dataset. The model selection strategy of multivariable Cox regression analysis was based on the significance (P < 0.05) of univariate analyses. A 2-sided P < 0.05 was considered to be statistically significant in this study. The analysis was performed using SAS (SAS system for Windows, Version 9.2, SAS Institute Inc., SAS Campus Drive, Cary, NC) and SPSS 17.0 (SPSS Inc. Released 2008, SPSS Statistics for Windows, Version 17.0. SPSS Inc., Chicago, IL).
RESULTS
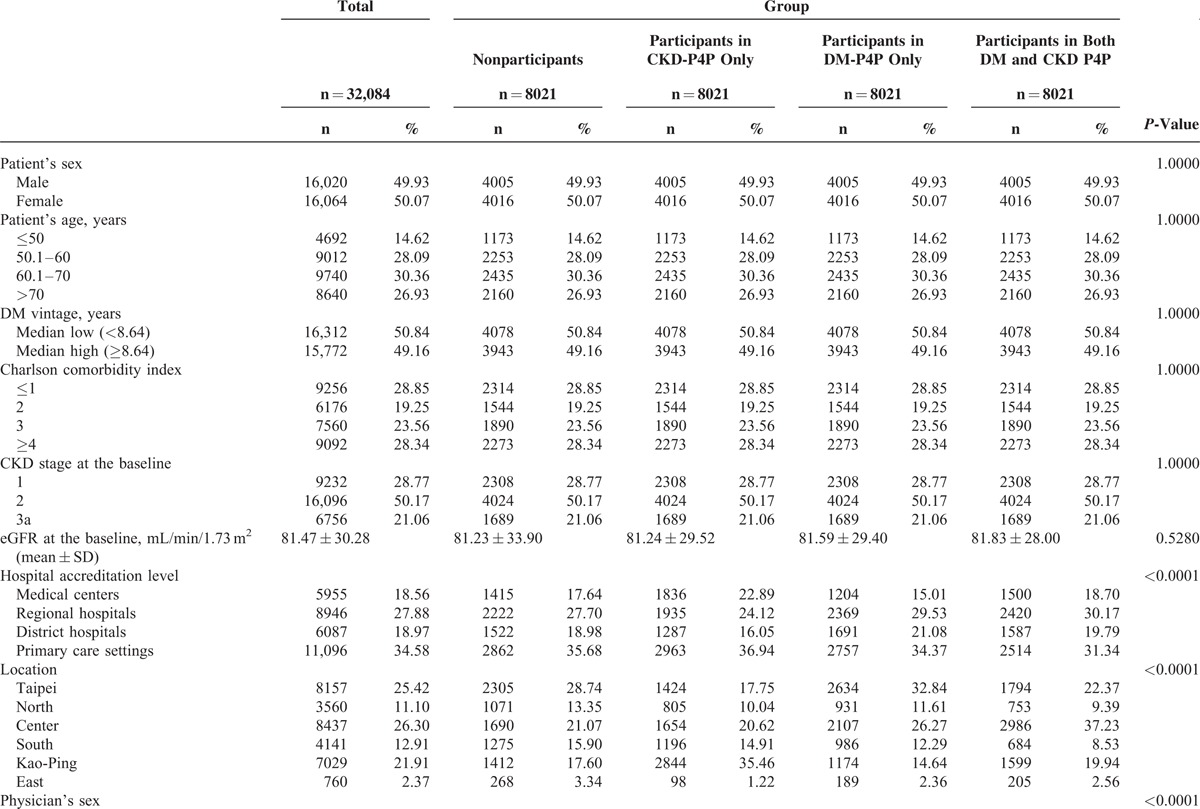
The characteristics of the study subjects and providers among the study groups, including nonparticipation, CKD-P4P participation, DM-P4P participation, and both CKD and DM P4Ps participation, are described in Table 1 . Overall, the male patients consisted of 49.93% of the 32,084 study subjects. The average age of all of the subjects was 62.69 years, with a range from 11 to 97 years. The median years of DM vintage was found to be 8.64, ranging from 1 to 13 years. Approximately 48.10% of the study subjects had a score of ≤2 in the CCI, whereas approximately 51.90% of the subjects had a CCI score greater than or equal to 4. The majority (34.58%; range, 31.34%–36.94% among the 4 study groups) of the patients sought medical assistance in primary care clinics. A higher percentage (36.52%) of the study patients was located in Taipei or in the northern regions. The majority of patients sought medical assistance in the primary care facility (34.58%) and with physicians who were male (84.74%), aged 40.1 to 50 years (42.17%), and were awarded specialty license for more than 15 years (58.78%). The average eGFR of the study subjects were 81.47 ± 30.28 at the baseline and showed no statistical significance among the study groups, including nonparticipation (81.23 ± 33.90), CKD-P4P participation (81.24 ± 29.52), DM-P4P participation (81.59 ± 29.40), and both CKD and DM P4Ps participation (81.83 ± 28.00) (P = 0.5280). The overall progression rate to late CKD or ESRD was 8.38%. The overall all-cause mortality was 3.37% during follow-up period and in a decreasing order of nonparticipants (4.85%), CKD-P4P participants (3.85%), DM-P4P participants (2.62%), and both DM and CKD participants (2.17%) (Table 1 ).
TABLE 1.
Distribution of the Study Variables Among the 4 Groups
A dose–response relationship was found between the P4P participation group and all-cause mortality in patients with only DM-P4P participation, with only CKD-P4P participation, and neither DM nor CKD P4P participation having multivariate-adjusted hazard ratios (HRs) of 1.22 (95% confidence interval [CI], 1.00–1.50), 2.00 (95% CI, 1.66–2.42), and 2.42 (95% CI, 2.02–2.91) in dying from any cause, respectively. Additionally, the patients with the characteristics of male (HR, 1.71; 95% CI, 1.51–1.93), old age (HR of age >70 years, 4.93; 95% CI, 3.71–6.54), higher CCI (HR of CCI ≥4 = 2.54; 95% CI, 2.11–3.06), longer DM vintage (HR of median high, 1.27; 95% CI, 1.12–1.45), medical assistance in a regional hospital (HR, 1.21; 95% CI, 1.01–1.45), and center region (HR, 1.37; 95% CI, 1.12–1.68) were at a higher risk of mortality using the multiple Cox regression model (Table 2).
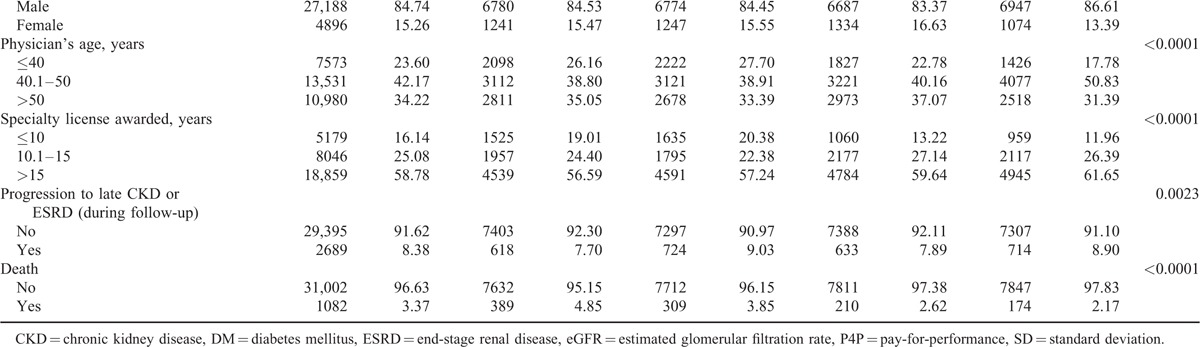
TABLE 1 (Continued).
Distribution of the Study Variables Among the 4 Groups
We performed additional stratified analyses using patient factors, including sex, age group, CCI, years of DM vintage, and CKD stage at the baseline to examine the reliability of the associations between the participation group and the patients’ prognoses. The results demonstrated the lowest likelihood of all-cause mortality of both DM-CKD P4P participants among the 4 participation groups. The analyses of all-cause mortality showed an increasing trend among the patients with both P4Ps participation, DM P4P alone participation, CKD P4P alone participation, and nonparticipation in high risk strata, including male, high age, high CCI, high DM vintage, and high CKD stages (Figure 1).
FIGURE 1.
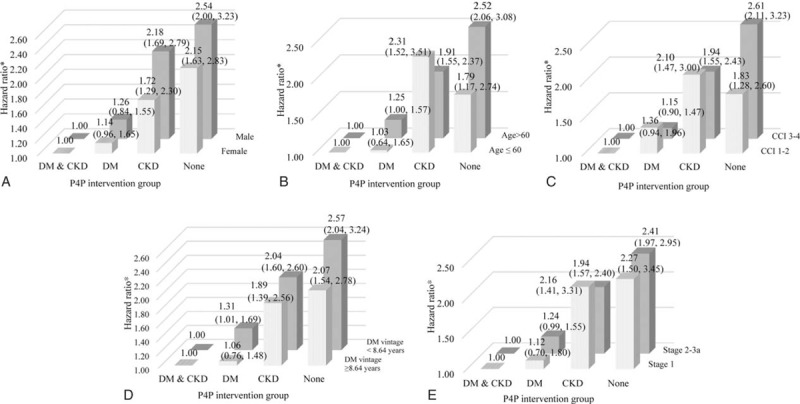
Stratified analysis of the association between the study group and all-cause mortality (A: by patient's sex; B: by patient's age group; C: by patient's CCI group; D: by patient DM vintage group; and E: by patient's CKD stage group).
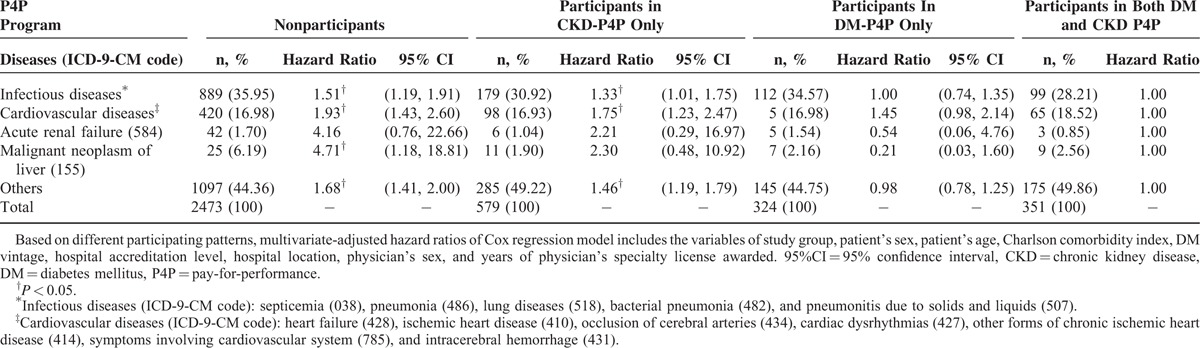
We have further investigated the primary cause of death among the total diabetic nephropathy patients. The leading cause of death was infectious diseases (34.32%) followed by cardiovascular diseases (17.12%), acute renal failure (1.50%), and malignant neoplasm of liver (1.40%). The proportion of deaths due to infectious diseases was increased by decreasing intervention strength: both DM and CKD P4P (28.21%), CKD P4P only (30.92%), DM P4P only (34.57%), and none participation (35.95%). Moreover, the hazard ratios of deaths due to infectious diseases, cardiovascular diseases, acute renal diseases, and malignant neoplasm of liver among study groups were all found to be descending according to nonparticipation, CKD P4P only, and DM P4P only/both DM and CKD P4P (Table 3).
TABLE 2.
Factors Associated With All-Cause Mortality Among Early CKD Patients
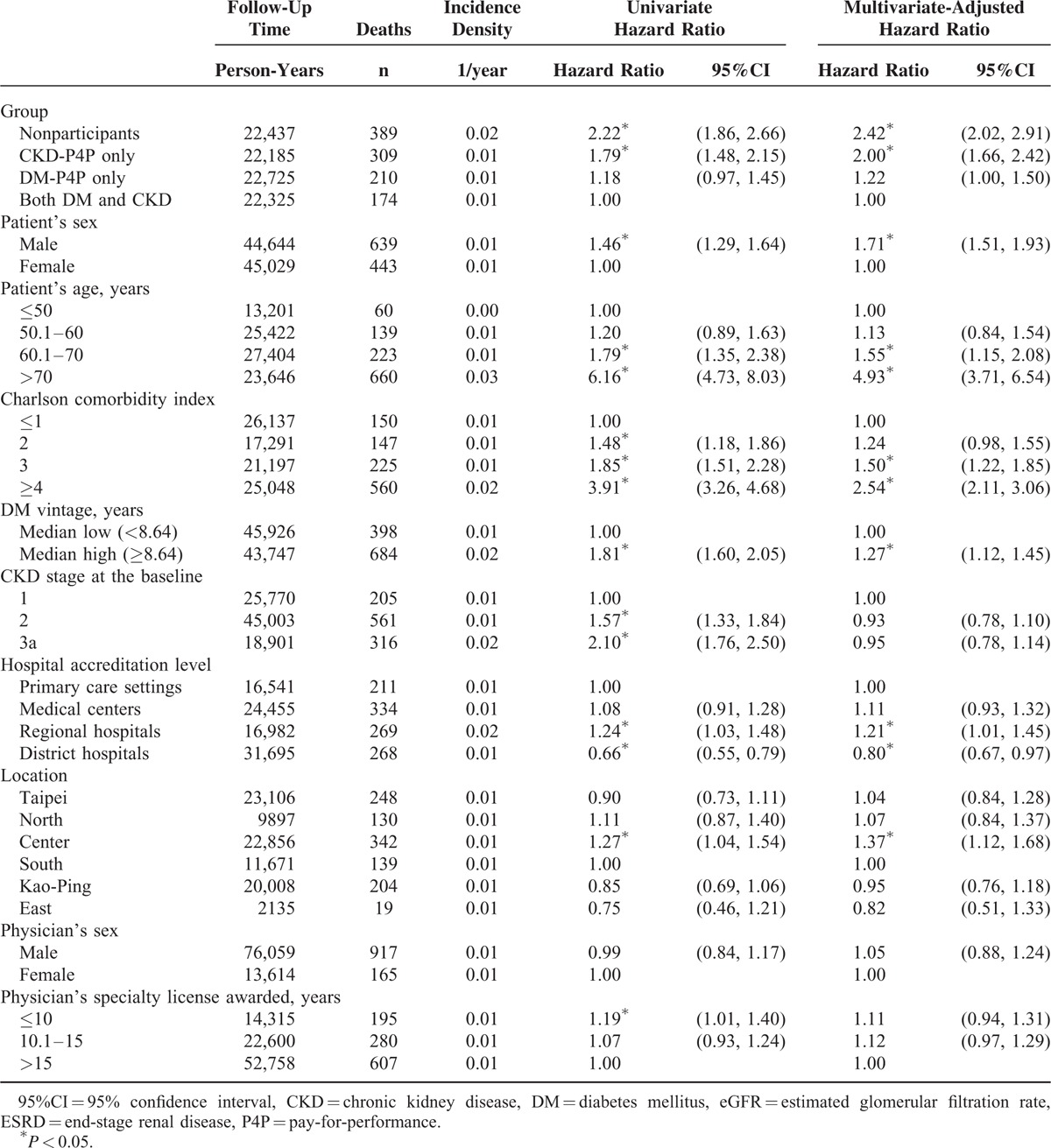
TABLE 3.
The Leading Causes of Death Among Total DM Nephropathy Patient Cohort (n = 63,923)
DISCUSSION
The aim of the present study was to examine the outcomes of diabetic patients, and later developed nephropathy, in participating P4P programs, including DM-P4P and early CKD-P4P, in a national compulsory health insurance system. This population-based cohort study followed 32,084 diabetic nephropathy patients at an early CKD stage and found a significantly better prognosis in all-cause mortality among matched participants in both P4P programs. This finding is consistent with studies that demonstrated a significantly lower risk of overall mortality in patients with diabetes or CKD who enrolled in a program.8,9,17,18 Although the effectiveness of a P4P intervention program was demonstrated by significant improvements on outcome measures (e.g., BP, HbA1C, LDL, creatinine, and eGFR) in previous studies,15 mixed findings were found due to a smaller sample size, data from different health care environments, short follow-up periods, and selection bias. The present study adds to the current knowledge using a design characterized by a nationwide; high coverage health insurance system; sufficient length of follow-up, starting from diabetes to early CKD; comprehensive data sources; and examinations of both P4P interventions using a dose–response approach.
The study has found that participants in both DM and early CKD P4P interventions had the lowest all-cause mortality. A dose–response relationship was found in the outcomes while considering the P4P intervention duration and combination as dose intensity according to both DM and CKD P4P, DM P4P only, CKD P4P only, and none. This study has shown a synergistic effect of both CKD and DM P4P interventions on the patients’ outcomes and demonstrated that an effect existed regardless of the length of early CKD intervention. Finding the lower risk of disease progression by early CKD P4P intervention and by previous DM P4P intervention is novel and consistent with previous studies that reported a higher likelihood of preventing adverse outcomes for patients enrolled in P4P programs than for patients who were not enrolled in a program.24,25
Previous articles have reported the underlying mechanisms – namely, health education, patient's medical seeking consistency, integrated information by continuity of care, and better physician-patient relationship – of such interventions to be effective.26–29 One of the prominent reasons for these underlying mechanisms is that physicians are willing to invest more time and effort to strive for predetermined benchmarks of quality care through financial incentives. A previous study has proposed that the effectiveness of P4P could be the consequence of a physician's adverse selection of patients.24 The present study has performed propensity score matching by sex, age, DM vintage, CCI, and CKD stage at the baseline, and adjusted the results by provider's factors to ensure the validity of the study. In addition to the multivariable adjustments, the stratified analysis has found that the strength of association between the study group and the outcome was reliable particularly on strata with high risk characteristics, including males, high age, high CKD stage, high disease comorbidities, and long-term DM disease course. Therefore, it is unlikely to have a significant effect due to different patient characteristics and selection bias on the results of this study.
Additionally, we noticed that the intervention duration of early CKD P4P among the participants was a median of 1.5 years. It was short compared to the DM P4P intervention in this study. However, the effect of an early CKD P4P intervention was unexpectedly significant and synergistically interactive with previous DM P4P intervention. Previous studies have documented that the intervention effect of the P4P program on reducing CKD progression could be found within a duration of 6 months to 2 years.17,18,21 An UK longitudinal study has also shown that the effect of the P4P program on CKD patients was found within 2 years after intervention.18 Our findings agree with these studies in terms of intervention duration but also reveal the interactive effect of previous DM P4P interventions. Other studies have proposed that BP control was the primary preventative strategy on the prognosis of patients with early CKD and ESRD.20 The stabilized control of BP as well as life-style complements within 2 years may be sufficient to affect patients’ prognoses.
The primary finding of this study is the long-term effects of diabetes P4P and its interactive effects with early CKD P4P on the prognosis of patients with diabetic nephropathy. The diabetes P4P program provides nutritional and self-management educational interventions while information integration and consistency of care were executed by the clinicians encouraged by the financial incentives of P4P programs.30 The contents of P4P intervention between diabetes and early CKD were complement to each other in the sense of disease progression and entity (Appendix 1). The incentive design of both P4P interventions is to ensure the process management, including health education, periodical biophysiological tests, patient's adherence, and more patients recruitment, but not on the patient's prognosis. Therefore, physicians are unlikely to exclude poor progression patients from the programs.
One of the primary goals of this study was to examine the intervention effects of P4P programs on all-cause mortality and major causes of death. To the best of our knowledge, this is the 1st study demonstrating universal effects of P4Ps on all-cause and major causes among the diabetic nephropathy patients in a nationwide longitudinal study cohort. Our findings suggest a universal effect on all causes of death by P4P programs and are plausible to the pathogenesis of diabetes. The key process indicators control as required by P4Ps is important to explain the results (Appendix 1). A diabetic patient's complications can be classified into microangiopathy and macroangiopathy.31 The risk of developing diabetic microvascular complications seems to be directly related to the degree of glucose control.32 However, the etiology of diabetic macrovascular complications are multifactorial, such as hypertension, dyslipidemia, obesity, smoking, sedentary lifestyle, or family history. The initial presentation of diabetic nephropathy is microalbuminuria, and the risks for developing nephropathy are escalated by increasing the duration of diabetes, poor sugar control (untargeted A1C level), and poorly controlled hypertension. Hypertension is associated with accelerated atherosclerosis and the progression of CKD,33 and elevated BP levels may increase the risk of a renal event in diabetic patients.34 The primary intervention required for both pathways was diet control; however, medical examinations for the clinician's medication reference were also important but different in each pathway. The administration of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers are the 2 dominate BP-lowering therapies in both types of diabetes and have demonstrated a significant renal benefit in patients with nephropathy.35 The benefit of good BP control using P4P interventions on renal outcomes and major cardiovascular events had been previously shown.36,37 A lower A1C level, the most important biochemical test for diabetes, can help to reduce nephropathy by 53% to 86% using intensive therapy from the Diabetes Control Complications Trial.38 The important examination for CKD patient care is renal function changes in which periodically measuring urine microalbumin and serum creatinine levels is important for evaluating the status of CKD. Patients will receive more care and health promotion from routine chronic complication evaluations and management under P4P programs.
This study was one of the first to examine a large nationwide diabetic nephropathy population and to assess the quality of care of diabetes and early CKD P4P programs with long-term follow-up. Although, the study has several limitations that merit caution. First, patients who may have higher education and better medical compliance or doctor–patient interaction could have an effect. However, it is difficult to control these situations. Second, the NHI claim database lacks detailed biophysiological measures; thus, this study was unable to investigate the intermediate measures, such as the levels of HbA1c, BP, and other CKD-specific examinations. Third, several patients in both interventions may not have had sufficient time of interventions, which is likely to cause an underestimate of the P4P intervention effects. Fifth, the declination of renal function was higher in this population (8.38% of the diabetic nephropathy patients developed late CKD or ESRD within 2 years of follow-up) as opposed to that of Western population. The generalizability of the findings to other populations is suggested to be cautious. Finally, residual effects of the confounding variables may still exist under multivariable models. As such, patient's medical seeking behavior and adherence to providers were not able to be examined in this national health claim database.
In conclusion, this study showed that either diabetes or early CKD P4P programs could significantly decrease the mortality rates and the adverse manifestations for patients with diabetic nephropathy. There is evidence to support the intervention effects by P4P programs, which motivate physicians to strive to improve the quality of care. The complement effects derived from both P4P interventions may suggest that a more comprehensive, integrated, and continuous program for patient care starting from the early course of the disease is warranted in future care.
Supplementary Material
Acknowledgments
This study was partly supported by grants NSC102–2410-H-182–010-MY2, NSC103–2410-H-161–001, EMRPD 1D0911, EMRPD 1E1691, EMRPD 1F0301, CMRPD390041, CMRPD390042, and CMRPD390043.
Footnotes
Abbreviations: BP = blood pressure, CCI = Charlson comorbidity index, CKD = chronic kidney disease, DM = diabetes mellitus, eGFR = estimated glomerular filtration rate, ESRD = end-stage renal disease, NHI = National Health Insurance, P4P = pay-for-performance.
P-JL, T-YL, and T-CW contributed equally to this paper.
This study was partly supported by grants NSC102–2410-H-182–010-MY2, NSC103–2410-H-161–001, EMRPD 1D0911, EMRPD 1E1691, EMRPD 1F0301, CMRPD390041, CMRPD390042, and CMRPD390043.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Centers for Disease Control and Prevention. National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States, 2014. Atlanta, GA: US Department of Health and Human Services; 2014. [Google Scholar]
- 2.Fowler MJ. Microvascular and macrovascular complications of diabetes. Clin Diabetes 2008; 26:77–82. [Google Scholar]
- 3.Yang WC, Hwang SJ. Incidence, prevalence and mortality trends of dialysis end-stage renal disease in Taiwan from 1990 to 2001: the impact of national health insurance. Nephrol Dial Transplant 2008; 23:3977–3982. [DOI] [PubMed] [Google Scholar]
- 4.Arnal LL, Gutiérrez BC, Izquierdo MC, et al. Prevalence of chronic kidney disease in patients with type 2 diabetes mellitus treated in primary care. Nefrologia 2010; 30:552–556. [DOI] [PubMed] [Google Scholar]
- 5.Kinchen KS, Sadler J, Fink N, et al. The timing of specialist evaluation in chronic kidney disease and mortality. Ann Intern Med 2002; 137:479–486. [DOI] [PubMed] [Google Scholar]
- 6.United States Renal Data System. 2014 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2014. [Google Scholar]
- 7.De Bruin SR, Baan CA, Struijs JN. Pay-for-performance in disease management: a systematic review of the literature. BMC Health Serv Res 2011; 11:272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eijkenaar F, Emmert M, Scheppach M, et al. Effects of pay for performance in health care: a systematic review of systematic reviews. Health Policy 2013; 110:115–130. [DOI] [PubMed] [Google Scholar]
- 9.Huang J, Yin S, Lin Y, et al. Impact of pay-for-performance on management of diabetes: a systematic review. J Evid Based Med 2013; 6:173–184. [DOI] [PubMed] [Google Scholar]
- 10.Van Herck P, De Smedt D, Annemans L, et al. Systematic review: effects, design choices, and context of pay-for-performance in health care. BMC Health Serv Res 2010; 10:247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levin-Scherz J, DeVita N, Timbie J. Impact of Pay-for-Performance Contracts and Network Registry on Diabetes and Asthma HEDIS (R)Measures in an Integrated Delivery Network. Med Care Res Rev 2006; 63 (1 suppl):14S–28S. [DOI] [PubMed] [Google Scholar]
- 12.Millett C, Netuveli G, Saxena S, et al. Impact of pay for performance on ethnic disparities in intermediate outcomes for diabetes: a longitudinal study. Diabetes Care 2009; 32:404–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith AL. Merging P4P and disease management: how do you know which one is working? J Manag Care Pharm 2007; 13:S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.James W, Snyder JM, Griego J, et al. Quality improvement and cost reduction realized by a purchaser through diabetes disease management. Dis Manag 2003; 6:233–241. [DOI] [PubMed] [Google Scholar]
- 15.Beaulieu ND, Horrigan DR. Putting smart money to work for quality improvement. Health Serv Res 2005; 40:1318–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cupples ME, Byrne MC, Smith SM, et al. Secondary prevention of cardiovascular disease in different primary healthcare systems with and without pay-for-performance. Heart 2008; 94:1594–1600. [DOI] [PubMed] [Google Scholar]
- 17.Howard K, White S, Salkeld G, et al. Cost-effectiveness of screening and optimal management for diabetes, hypertension, and chronic kidney disease: a modeled analysis. Value Health 2010; 13:196–208. [DOI] [PubMed] [Google Scholar]
- 18.Karunaratne K, Stevens P, Irving J, et al. The impact of pay for performance on the control of blood pressure in people with chronic kidney disease stage 3-5. Nephrol Dial Transplant 2013; 28:2107–2116. [DOI] [PubMed] [Google Scholar]
- 19.Kondo M, Yamagata K, Hoshi SL, et al. Cost-effectiveness of chronic kidney disease mass screening test in Japan. Clin Exp Nephrol 2012; 16:279–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Plantinga LC, Fink NE, Jaar BG, et al. Attainment of clinical performance targets and improvement in clinical outcomes and resource use in hemodialysis care: a prospective cohort study. BMC Health Serv Res 2007; 7:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stevens PE, de Lusignan S, Farmer CK, et al. Engaging primary care in CKD initiatives: the UK experience. Nephrol Dial Transplant 2012; 27 Suppl 3:iii5–11. [DOI] [PubMed] [Google Scholar]
- 22.Stock S, Drabik A, Buscher G, et al. German diabetes management programs improve quality of care and curb costs. Health Aff 2010; 29:2197–2205. [DOI] [PubMed] [Google Scholar]
- 23.Romano P, Romano L, Roos J, et al. Presentation adapting a clinical comorbidity index for use with ICD-9-CM administrative data: differing perspectives. J Clin Epidemiol 1993; 46:1075–1079. [DOI] [PubMed] [Google Scholar]
- 24.Chang RE, Lin SP, Aron DC. A pay-for-performance program in Taiwan improved care for some diabetes patients, but doctors may have excluded sicker ones. Health Aff 2012; 31:93–102. [DOI] [PubMed] [Google Scholar]
- 25.Cheng SH, Lee TT, Chen CC. A longitudinal examination of a pay-for-performance program for diabetes care: evidence from a natural experiment. Med Care 2012; 50:109–116. [DOI] [PubMed] [Google Scholar]
- 26.Alexander JA, Hearld LR, Mittler JN, et al. Patient-physician role relationships and patient activation among individuals with chronic illness. Health Ser Res 2012; 47:1201–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fairbrother G, Hanson KL, Friedman S, et al. The impact of physician bonuses, enhanced fees, and feedback on childhood immunization coverage rates. Am J Public Health 1999; 89:171–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liao PJ, Lin ZY, Huang JC, et al. The relationship between type 2 diabetic patients’ early medical care-seeking consistency to the same clinician and health care system and their clinical outcomes. Medicine (Baltimore) 2015; 94:e554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stewart MA. Effective physician-patient communication and health outcomes a review. Can Med Assoc J 1995; 152:1423–1433. [PMC free article] [PubMed] [Google Scholar]
- 30.Wu IW, Wang SY, Hsu KH, et al. Multidisciplinary predialysis education decreases the incidence of dialysis and reduces mortality–a controlled cohort study based on the NKF/DOQI guidelines. Nephrol Dial Transplant 2009; 24:3426–3433. [DOI] [PubMed] [Google Scholar]
- 31.Roderick P, Armitage A. Renal services for people with diabetes in the UK. Diabetic Med 2002; 19:56–60. [DOI] [PubMed] [Google Scholar]
- 32.Group DCaCTR. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993; 329:977–986. [DOI] [PubMed] [Google Scholar]
- 33.Bakris GL, Williams M, Dworkin L, et al. Preserving renal function in adults with hypertension and diabetes: a consensus approach. Am J Kidney Dis 2000; 36:646–661. [DOI] [PubMed] [Google Scholar]
- 34.Keane WF, Eknoyan G. Proteinuria, albuminuria, risk, assessment, detection, elimination (PARADE): a position paper of the National Kidney Foundation. Am J Kidney Dis 1999; 33:1004–1010. [DOI] [PubMed] [Google Scholar]
- 35.Wolf G, Ritz E. Combination therapy with ACE inhibitors and angiotensin II receptor blockers to halt progression of chronic renal disease: pathophysiology and indications. Kidney Int 2005; 67:799–812. [DOI] [PubMed] [Google Scholar]
- 36.Blood Pressure Lowering Treatment Trialists’ Collaboration. Effects of different blood pressure–lowering regimens on major cardiovascular events in individuals with and without diabetes mellitus: results of prospectively designed overviews of randomized trials. Arch Intern Med 2005; 165:1410–1419. [DOI] [PubMed] [Google Scholar]
- 37.Casas JP, Chua W, Loukogeorgakis S, et al. Effect of inhibitors of the renin-angiotensin system and other antihypertensive drugs on renal outcomes: systematic review and meta-analysis. Lancet 2005; 366:2026–2033. [DOI] [PubMed] [Google Scholar]
- 38.The Writing Team for the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. Effect of intensive therapy on the microvascular complications of type 1 diabetes mellitus. JAMA 2002; 287:2563–2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


