Abstract
Although obesity has been identified as a risk factor for pancreatic cancer, the important question of whether obesity influences the prognosis of pancreatic cancer has not been explicated thoroughly. We therefore performed a meta-analysis to investigate the association between body mass index (BMI) and survival outcomes of patients with pancreatic cancer.
Studies that described the relationship between BMI and overall survival (OS) of pancreatic cancer were searched in PubMed, Embase, Ovid, and Cochrane Library Databases from the earliest available date to May 12, 2015. Hazard ratios (HRs) for OS in each BMI category from individual studies were extracted and pooled by a random-effect model. Dose–response meta-analysis was also performed to estimate summary HR and 95% confidence interval (CI) for every 5-unit increment. Publication bias was evaluated by Begg funnel plot and Egger linear regression test.
Ten relevant studies involving 6801 patients were finally included in the meta-analysis. Results showed that obesity in adulthood significantly shortened OS of pancreatic cancer patients (HR: 1.29, 95% CI: 1.17–1.41), whereas obesity at diagnosis was not associated with any increased risk of death (HR: 1.10, 95% CI: 0.78–1.42). For every 5-kg/m2 increment in adult BMI, the summary HR was 1.11 (95% CI: 1.05–1.18) for death risk of pancreatic cancer. However, no dose–response relationship was found in the BMI at diagnosis. Egger regression test and Begg funnel plot both revealed no obvious risk of publication bias.
In conclusion, increased adult BMI is associated with increased risk of death for pancreatic cancer patients, which suggested that obesity in adulthood may be an important prognostic factor that indicates an abbreviated survival from pancreatic cancer. More studies are needed to validate this finding, and the mechanism behind the observation should be evaluated in further studies.
INTRODUCTION
Pancreatic cancer is a devastating malignancy with 1-year survival rate of <18%.1 As the fourth leading cause of cancer-related death for both men and women in the United States,2 tumors progress rapidly with few specific symptoms and are thus at an advanced stage at diagnosis in most patients. Smoking, diabetes, and obesity are established risk factors for pancreatic cancer.3–5 Prevalence of overweight and obesity has rapidly increased during the last 2 decades. Obesity and overweight are commonly measured by body mass index (BMI), which is defined as weight in kilograms divided by height in meters squared. Obesity is defined by the World Health Organization (WHO) as BMI ≥30.0 kg/m2, and overweight is defined as BMI of 25.0 to 29.9 kg/m2. A most recent meta-analysis of 23 studies with 9504 cases suggests that higher BMI is a risk factor for pancreatic cancer with a relative risk (RR) of 1.10 (95% confidence interval [CI]: 1.07–1.14) for every 5-unit increment.6 However, prognostic effect of BMI still remains unclear for patients with established diagnosis of pancreatic cancer. Furthermore, results of published studies focusing on this topic are inconsistent. To explicate the question, we performed the meta-analysis to identify the relationship between BMI and OS in pancreatic cancer.
MATERIALS AND METHODS
Search Strategy
Two reviewers (Y-QS, JY) performed the search independently in PubMed database from its earliest available date to May 12, 2015. The following key words were used: pancreatic, pancreas, cancer, adenocarcinoma, tumor, neoplasm, mortality, survival, BMI, and body mass index. Boolean logic words (AND and OR) were jointly used to combine the key words. The EMBASE, Ovid, and Cochrane Library Databases were further searched for additional relevant articles. Full article was investigated if 1 of the 2 reviewers considered it potentially relevant. References of the relevant articles were further screened for earlier original studies. Disagreements were solved by group discussion (C-FX, Y-QS, JY, PD, J-QS).
Inclusion Criteria
Studies were considered eligible if they satisfied all the following items: research objects of individual study were patients diagnosed as having pancreatic cancer histologically or pathologically; relationship between BMI and OS was investigated; BMI was categorized as underweight (<18.5 kg/m2), normal weight (18.5–24.9 kg/m2), overweight (25.0–29.9 kg/m2), and obese (≥30.0 kg/m2) in each study; HR and 95% CI were available for each BMI category (normal weight group was defined as reference, HR = 1) in individual study. Only full texts written in English were included.
Data Collection
We extracted the following information from each included article: author, year of publication, country, recruitment period, age, sample size, median OS, and HR with 95% CI for each BMI category. Authors were contacted for important missing information. We used multivariate Cox proportional HRs for the quantitative analysis. If multivariate HRs were not available and the corresponding authors did not respond to our request, then the univariate HRs were used instead. Data extraction was accomplished by 4 authors (Y-QS, JY, TX, X-HZ).
Quality Assessment
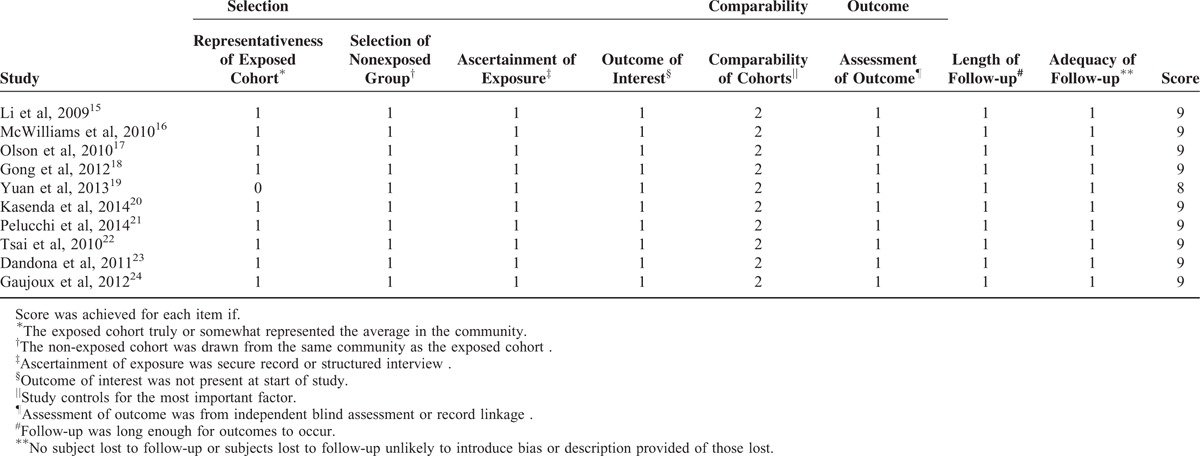
The Newcastle Ottawa scale (NOS), which was recommended by the Cochrane Non-Randomized Studies Methods Working Group, was used in this meta-analysis for quality assessment. NOS was designed to assess the quality of observational studies. It assessed study quality by 3 classifications, namely, selection, comparability, and outcome with a total score of nine stars. Among the 9 stars, 4 stars represented for the appropriate selection of exposure and nonexposure cohort participants, 2 stars represented for the comparability of cohort, and the last 3 stars described the assessment of outcome and follow-up. Studies that scored ≥5 of the 9 stars were considered to be of high quality.
Statistical Methods
We performed analysis using a random-effect model in case that there was significant heterogeneity. We also performed sensitivity analysis to assess whether the summary estimates are robust to inclusion of studies. One study was removed every time, and the rest were analyzed to evaluate whether the results could have been affected significantly by a single study. Heterogeneity was assessed by value of I2. Publication bias was evaluated by the use of Begg funnel plot and Egger linear regression test. A pooled HR >1 suggested that underweight, overweight, or obesity predicted an unfavorable prognosis for pancreatic cancer patients. Oppositely, a pooled HR <1 suggested a favorable prognosis for those patients. It was regarded as statistically significant if the 95% CI of HR did not overlap 1. All P values were 2-sided. P < 0.05 was regarded as statistically significant. All analyses were performed using the Stata version 12.0 software (StataCorp, College Station, TX, http://www.stata.com). Given that our study was a review of previous published studies, ethical approval or patient consent was not required.
RESULT
Study Characteristics
The search strategy identified 212 articles, of which 10 met the inclusion criteria and were finally included (Figure 1). No additional relevant articles were yielded in EMBASE, Ovid, and Cochrane Library Databases. Eight studies were excluded because of the absence of HR7,8 or different reference groups of BMI.9–14 All the 10 included studies were cohort analysis containing a total of 6801 patients recruited between 1982 and 2010, with a sample size ranging from 314 to 1861. Among all the studies, 5 studies explicated the impact of adult BMI on pancreatic cancer survival,15–19 for which OS was calculated from the date of pathological diagnosis to the date of death or last follow-up visit, whereas the remaining 5 focused on BMI at diagnosis20,21 or before operation.22–24 For the 5 studies, OS was calculated from the date of diagnosis or surgery.20–24 According to the different points of BMI, we renamed the 2 groups as adult BMI group and BMI at diagnosis group, respectively. In the former group, usual adult weight and height available from questionnaires were used for calculating BMI. In the latter group, BMI was calculated by weight and height at diagnosis or measured by physical examination before operation. Characteristics and important information of relevant studies are listed in Table 1. Quality assessment of studies is shown in Table 2.
FIGURE 1.
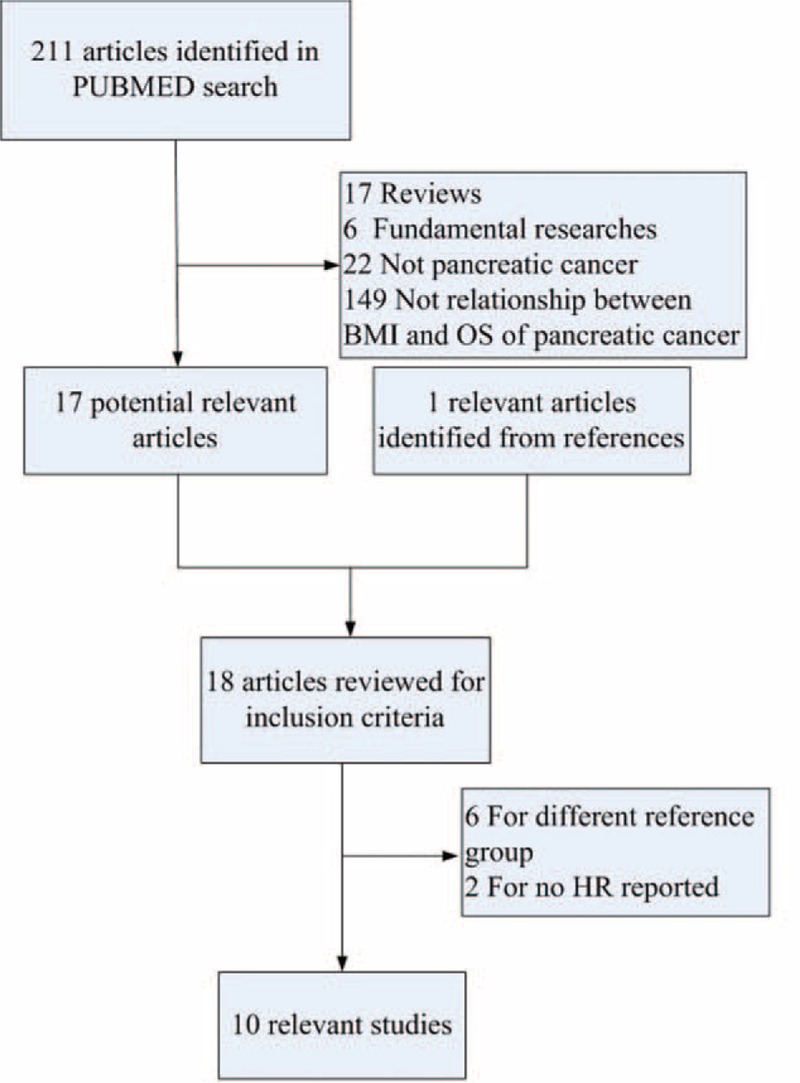
Literature screening process.
TABLE 1.
Characteristics of Relevant Studies
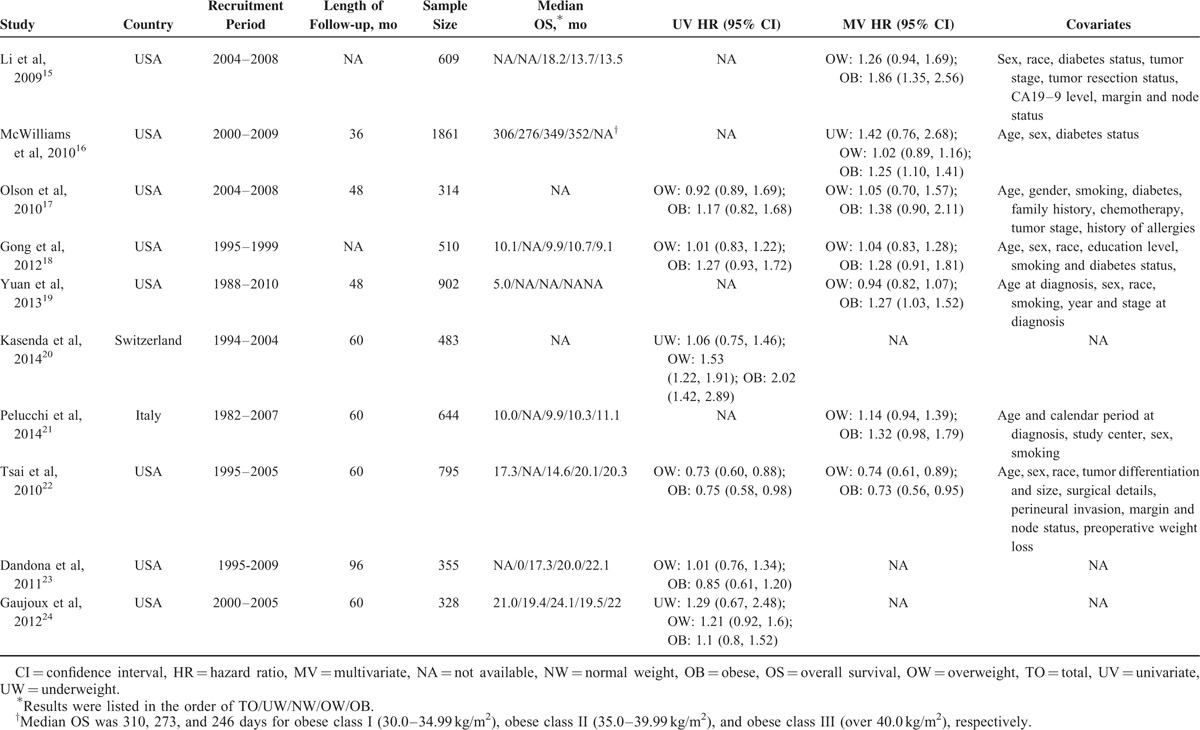
TABLE 2.
Assessment of Study Quality
Meta-analysis
Overweight or Obesity Versus Normal BMI
Meta-analysis was performed in the adult BMI group and BMI at diagnosis group in a random-effect model. To determine the relationship between adult BMI and OS, meta-analysis was conducted for subgroups of overweight and obesity, respectively. Results informed that adult overweight was not statistically significantly associated with OS (HR: 1.00, 95% CI: 0.92–1.08, P = 0.560, I2 = 0.0%, Figure 2), whereas OS was significantly reduced in obesity subgroup (HR: 1.29, 95% CI: 1.17–1.41), with no obvious heterogeneity (P = 0.437, I2 = 0.0%, Figure 2). Compared with normal BMI, patients with BMI ≥25.0 kg/m2 survived obviously shorter with a 15% higher risk of death (HR: 1.15; 95% CI: 1.03–1.27, P = 0.010, I2 = 58.2%, Figure 2). Meta-analysis was performed to determine the impact of BMI at diagnosis. Results suggested that overweight and obesity both failed to result in an altered risk of death (HR: 1.10; 95% CI: 0.83–1.38 and HR: 1.10; 95% CI: 0.78–1.42, respectively, Figure 3).
FIGURE 2.
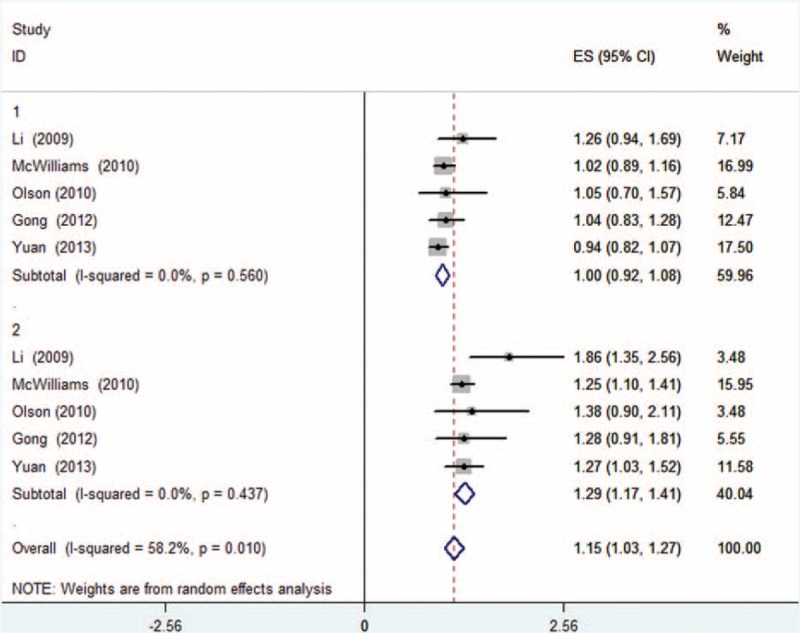
Forest plot showed the association between adult overweight/obesity and overall survival (OS) for pancreatic cancer. Random-effect model was used. For each study, the estimates of hazard ratio (HR) and 95% confidence interval (CI) were plotted with a box and a horizontal line. Closed diamond indicates pooled HR and 95% CI (1: overweight, 2: obesity).
FIGURE 3.
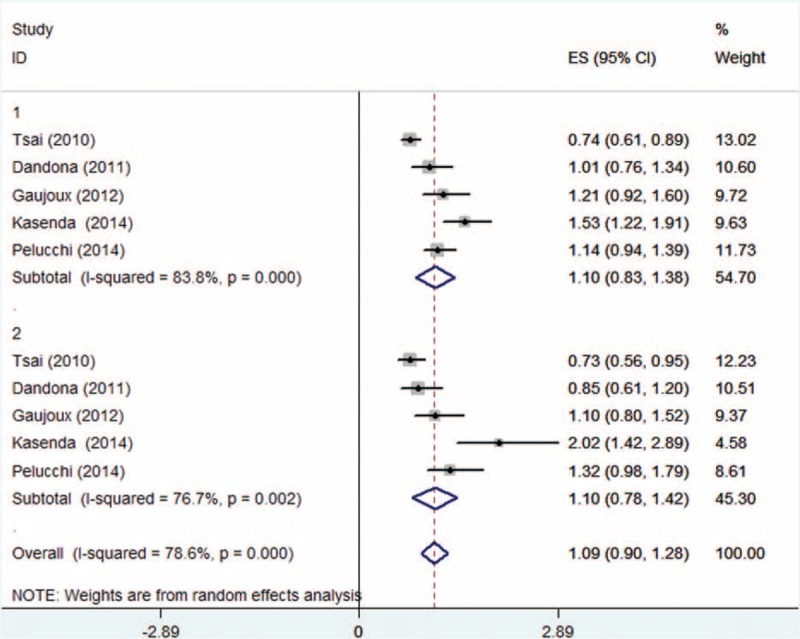
Forest plot showed the association between overweight/obesity at diagnosis and overall survival (OS) for pancreatic cancer. Random-effect model was used (1: overweight, 2: obesity).
Dose–response Meta-analysis
Dose–response meta-analysis was also performed for each group. Analysis from the 5 cohort studies about the relationship between adult BMI and overall survival (OS) showed an increased death risk of 1.11 (95% CI: 1.05–1.18) for every 5-kg/m2 increase in BMI (Figure 4). As shown in Figure 5, BMI at diagnosis showed no significant dose–response relationship to survival outcome of pancreatic cancer patients with an HR of 1.07 (95% CI: 0.85–1.30, I2 = 16.6%, P = 0.309). In consideration to the significant heterogeneity (Figure 5), we performed a subgroup analysis by excluding those studies20,23,24 not providing multivariate HR. However, no significant relationship was observed with an HR of 0.97 (95% CI: 0.58–1.35). Studies of Kasenda et al and Pelucchi et al were separated and analyzed as a subgroup, as the rest22–24 all focused on relationship between BMI and OS of pancreatic cancer patients who underwent operation. Results also suggested no significant relationship between BMI and survival for each subgroup (HR: 0.91, 95% CI: 0.74–1.08 and HR: 1.35, 95% CI: 0.97–1.73, respectively), which is shown in Figure 6.
FIGURE 4.
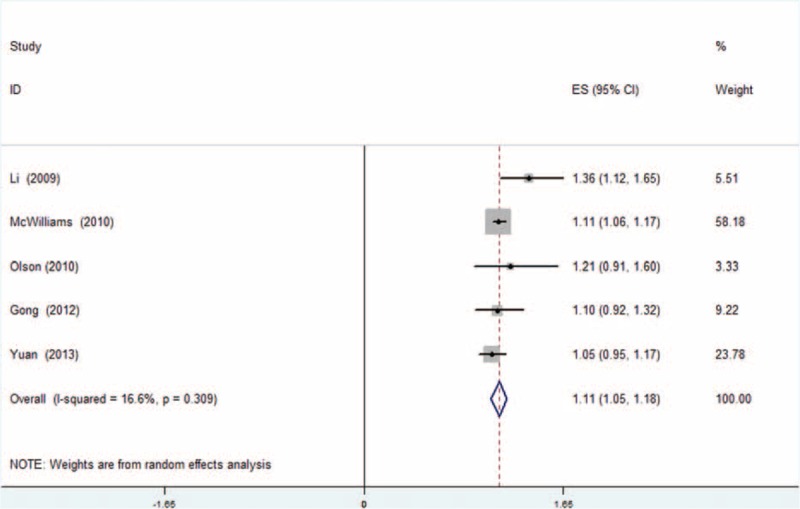
Dose–response meta-analysis for adult BMI group. Random-effect model was used. For each study, the estimates of dose-associated hazard ratio (HR) and 95% confidence interval (CI) were plotted with a box and a horizontal line. Closed diamond indicates pooled HR and 95% CI.
FIGURE 5.
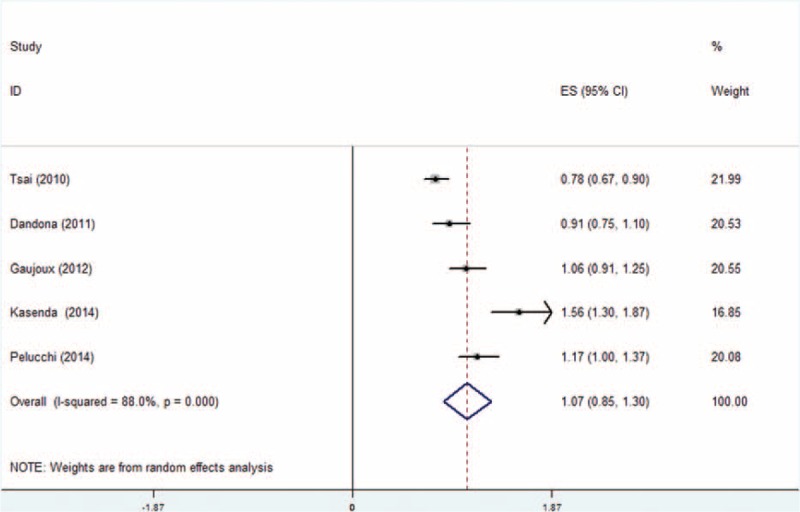
Dose–response meta-analysis for body mass index (BMI) at diagnosis group. Random-effect model was used. For each study, the estimates of dose-associated hazard ratio (HR) and 95% confidence interval (CI) were plotted with a box and a horizontal line. Closed diamond indicates pooled HR and 95% CI.
FIGURE 6.
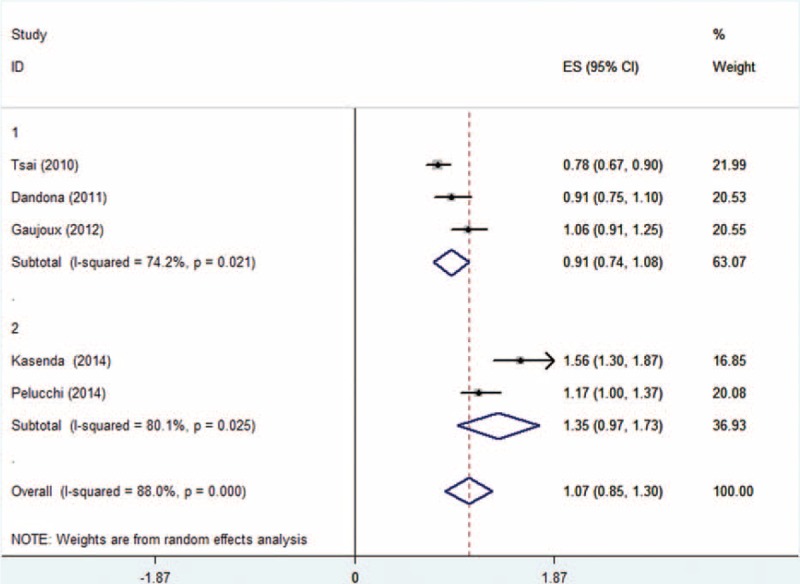
Dose–response meta-analysis for subgroups of BMI at diagnosis group studies.(1: preoperative BMI, 2:BMI at diagnosis).
Sensitivity Analysis
In sensitivity analysis, one study was removed at a time and the rest were analyzed. The pooled HRs ranged from 1.10 to 1.14 for adult BMI group and from 0.97 to 1.15 for BMI at diagnosis group. Results of sensitivity analysis are detailed in Table 3. For the adult BMI group, the pooled estimates were robust and not influenced by a single study. However, for the BMI at diagnosis group, HRs ranged from 0.97 to 1.15 with 95% CIs all overlapping 1. This finding indicated that no statistically significant relationship was observed between BMI at diagnosis and OS.
TABLE 3.
Sensitivity Analysis

Publication Bias
Publication bias was assessed by use of Egger regression test and Begg funnel plot. Begg funnel plot for both groups revealed no obvious publication bias (Figures 7A and 8A). Further confirmation by use of Egger regression test also failed to find evidence of publication bias in both former group (t = 0.83, P = 0.465) and latter group (t = 1.13, P = 0.341, Figure 7B, 8B).
FIGURE 7.
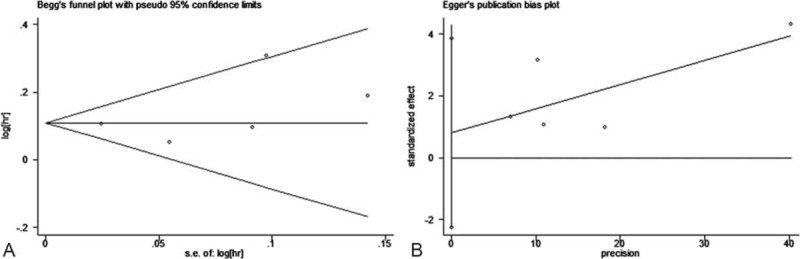
Begg funnel plot and Egger linear regression test for adult body mass index (BMI) group studies. (A: Begg funnel plot, B: Egger linear regression).
FIGURE 8.
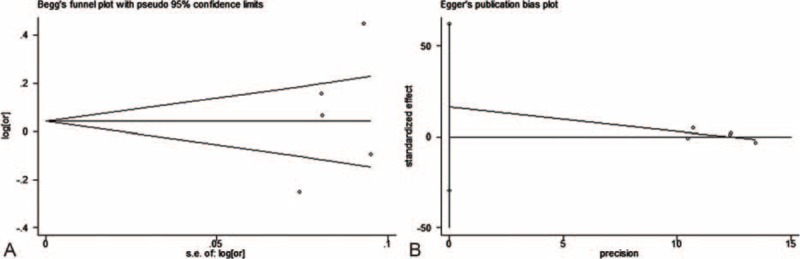
Begg funnel plot and Egger linear regression test for body mass index (BMI) at diagnosis group studies.(A: Begg funnel plot, B: Egger linear regression).
DISCUSSION
Overweight/obesity and its related morbidities are a growing health problem claiming 2.8 million lives annually according to the WHO 2010 global report.25 Obesity has been found to be associated with a wide array of morbidities, including cardiovascular disease, chronic kidney disease, sleeping disorder, type 2 diabetes, and several forms of cancer. Relationship between obesity and risk of pancreatic cancer has been discussed for decades. Four meta-analyses6,26–28 and 3 pooled analyses29–31 have attempted to explicate the question, evaluating the risk either in dose–response relationship or according to BMI categories. A recent review summarized that obese individuals have an RR ranging from 1.19 to 1.47, when compared with those of normal weight, regardless of diabetes or smoking status.32
Obesity is considered to be an adverse prognostic factor in malignancies, such as breast33 and colon cancers34. Prognostic influence of obesity on pancreatic cancer has also been searched in recent years. However, the role of obesity as a prognostic factor of pancreatic cancer is still unclear. Thus, we conducted the meta-analysis to identify the prognostic role of BMI on survival from pancreatic cancer. Results indicated that obesity in adulthood was associated with a significantly worse survival outcome, suggesting that obesity may be a potential important prognostic factor for pancreatic cancer. In addition, compared with normal BMI, increased adult BMI was related to higher risk of death with 11% percentage for every 5-unit increment.
No statistically significant relationship between preoperative obesity and survival is shown in Figure 6. Although not included in the analysis, many studies made effort on this topic. A study reported that patients with more intra-abdominal fat demonstrated worse OS (individuals in the second quartile showed a 4-fold increase in likelihood of death relative to the lowest quartile), though in a nonlinear fashion.11 A recent study reported that obesity did not impact operative complexity or length of stay, but resulted in a shortened survival for patients with pancreatic cancer undergoing pancreatoduodenectomy.7 Fleming et al13 summarized that obese patients with a BMI of >35 kg/m2 are more likely to have decreased survival after surgical resection, whereas Benns et al12 found no significant difference in OS among patients with pancreatic cancer undergoing various types of operations (obese patients had an OS of 19.8 months compared with nonobese patients who had an OS of 23.5 months, P = 0.46). Prognostic effect of preoperative obesity on survival after operation needed further investigation.
To our knowledge, this study is the first meta-analysis evaluating the effect of BMI on survival from pancreatic cancer. However, limitations of our study should be addressed. First, several relevant studies were not primarily designed to monitor the effect of BMI on survival. Pelucchi et al21 considered data from 2 previous hospital-based case-control studies on risk factors for pancreatic cancer. Cases in analysis of Olson et al17 came from an ongoing hospital-based case-control study and Familial Pancreatic Cancer Registry at Memorial Sloan-Kettering Cancer Center. Second, credible conclusion established on more studies was necessary because of the relative small sample size of the current meta-analysis. Third, all included studies were from the United States or European developed countries. Compared with developing countries, techniques, devices, therapies, and patient population were vastly different in other countries. Collection of information from developed countries may help in investigating the topic more comprehensively and thoroughly. Cancer stage and treatments, such as surgery and chemotherapy, were important confounding factors for OS, and not all the studies provide the information.
Weight loss, which could influence the results of the included studies, was not considered. As we know, loss of weight was usual for pancreatic cancer patients either before or after diagnosis. Dalal et al35 suggested that obese patients experienced higher losses in weight, skeletal muscle, and visceral adipose tissue, which may contribute to poorer survival in pancreatic cancer patients. Choi et al9 also suggested that weight loss at diagnosis and during first-line chemotherapy was associated with shortened OS (HR: 1.300; P = 0.012 and HR: 1.367; P = 0.010, respectively). Furthermore, limited information was available on the possible joint effect of diabetes and obesity on survival. Diabetes, which often coexists with obesity, has been found to be associated with increased risk of pancreatic cancer, but little is known about its influence on survival. HR was adjusted for diabetes status in 4 studies only,15–18 and Gong et al18 suggested a poorer survival of obese diabetics as compared with obese patients without diabetes. Joint effects of obesity and diabetes on survival from pancreatic cancer require further identification.
Mechanisms behind the observation that obesity in adulthood is associated with decreased post-diagnostic survival have not been clarified thoroughly. As pancreatic cancer progresses rapidly, the chronic comorbidity associated with obesity, such as cardiovascular disease, can hardly explain the reduced survival. Increased insulin resistance may be the pathogenic mechanism between obesity and reduced OS in patients with pancreatic cancer.36 In obese individuals, the amount of glycerol, hormones, nonesterified fatty acids, cytokines, proinflammatory markers, and other substances that are involved in the development of insulin resistance is increased.37 To maintain normal blood sugar, islet β-cells secrete insulin compensatorily. In a status of hypersinulinemia, increased circulating level of insulin-like growth factor-1 induces proliferation and inhibits apoptosis of pancreatic cancer cells, thus contributing to tumorigenesis. In addition, DNA damage pathways, adipokines such as leptin,38 and proinflammatory environment induced by obesity39 may also contribute to tumorigenesis, angiogenesis, and metastasis and then resulted in a worse survival outcome. However, BMI at the time of diagnosis or operation showed no obvious effect on survival outcome. Overweight or obese patients possibly experienced less loss of weight and may be more tolerable to tumor burden, chemotherapy, and operation.
For patients undergoing pancreaticoduodenectomy, which is a major surgical procedure involving resection of the duodenum, the pancreatic head, uncinate process, and the distal common bile duct, complications, such as fistula, intra-abdominal hemorrhage and infection, and gastrointestinal bleeding, were usual and may also contribute to death. Patients undergoing surgery experience different degrees of weight loss and malnutrition, which increases the risk of postoperative complications and mortality. Possibility of intraoperative hemorrhage, incision infection, postoperative lower extremity deep venous thrombosis, pulmonary embolism, and metabolic disorder is relatively higher in obese patients. Whether obesity is related to survival after operation for patients suffering from pancreatic cancer needs further research.
In conclusion, obesity in adulthood is related to an abbreviated survival from pancreatic cancer. Higher adult BMI is also associated with a worse survival outcome in a dose–response relationship. However, BMI at diagnosis has not shown any clear relationship to survival outcome of pancreatic cancer. More studies are needed to validate this finding and the mechanism behind the observation should be evaluated in further studies.
Footnotes
Abbreviations: BMI = body mass index, CI = confidence interval, HR = hazard ratio, NOS = Newcastle Ottawa scale, OS = overall survival.
Y-QS and JY contributed equally in the meta-analysis (co-first authors and PD: co-second author).
REFERENCES
- 1.Hidalgo M, Cascinu S, Kleeff J, et al. Addressing the challenges of pancreatic cancer: future directions for improving outcomes. Pancreatology 2015; 15:8–18. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015; 65:5–29. [DOI] [PubMed] [Google Scholar]
- 3.Zou L, Zhong R, Shen N, et al. Non-linear dose-response relationship between cigarette smoking and pancreatic cancer risk: evidence from a meta-analysis of 42 observational studies. Eur J Cancer 2014; 50:193–203. [DOI] [PubMed] [Google Scholar]
- 4.Elena JW, Steplowski E, Yu K, et al. Diabetes and risk of pancreatic cancer: a pooled analysis from the pancreatic cancer cohort consortium. Cancer Causes Control 2013; 24:13–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dobbins M, Decorby K, Choi BC. The association between obesity and cancer risk: a meta-analysis of observational studies from 1985 to 2011. ISRN Prev Med 2013; 2013:680536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aune D, Greenwood DC, Chan DS, et al. Body mass index, abdominal fatness and pancreatic cancer risk: a systematic review and non-linear dose-response meta-analysis of prospective studies. Ann Oncol 2012; 23:843–852. [DOI] [PubMed] [Google Scholar]
- 7.Mathur A, Luberice K, Paul H, et al. Increasing body mass index portends abbreviated survival following pancreatoduodenectomy for pancreatic adenocarcinoma. Am J Surg 2015; 209:969–973. [DOI] [PubMed] [Google Scholar]
- 8.Del CM, Rangelova E, Ansorge C, et al. Impact of body mass index for patients undergoing pancreaticoduodenectomy. World J Gastrointest Pathophysiol 2013; 4:37–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi Y, Kim TY, Lee KH, et al. The impact of body mass index dynamics on survival of patients with advanced pancreatic cancer receiving chemotherapy. J Pain Symptom Manage 2014; 48:13–25. [DOI] [PubMed] [Google Scholar]
- 10.Hartwig W, Hackert T, Hinz U, et al. Pancreatic cancer surgery in the new millennium: better prediction of outcome. Ann Surg 2011; 254:311–319. [DOI] [PubMed] [Google Scholar]
- 11.Balentine CJ, Enriquez J, Fisher W, et al. Intra-abdominal fat predicts survival in pancreatic cancer. J Gastrointest Surg 2010; 14:1832–1837. [DOI] [PubMed] [Google Scholar]
- 12.Benns M, Woodall C, Scoggins C, et al. The impact of obesity on outcomes following pancreatectomy for malignancy. Ann Surg Oncol 2009; 16:2565–2569. [DOI] [PubMed] [Google Scholar]
- 13.Fleming JB, Gonzalez RJ, Petzel MQ, et al. Influence of obesity on cancer-related outcomes after pancreatectomy to treat pancreatic adenocarcinoma. Arch Surg 2009; 144:216–221. [DOI] [PubMed] [Google Scholar]
- 14.Park SM, Lim MK, Shin SA, et al. Impact of prediagnosis smoking, alcohol, obesity, and insulin resistance on survival in male cancer patients: National Health Insurance Corporation Study. J Clin Oncol 2006; 24:5017–5024. [DOI] [PubMed] [Google Scholar]
- 15.Li D, Morris JS, Liu J, et al. Body mass index and risk, age of onset, and survival in patients with pancreatic cancer. JAMA 2009; 301:2553–2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McWilliams RR, Matsumoto ME, Burch PA, et al. Obesity adversely affects survival in pancreatic cancer patients. Cancer Am Cancer Soc 2010; 116:5054–5062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Olson SH, Chou JF, Ludwig E, et al. Allergies, obesity, other risk factors and survival from pancreatic cancer. Int J Cancer 2010; 127:2412–2419. [DOI] [PubMed] [Google Scholar]
- 18.Gong Z, Holly EA, Bracci PM. Obesity and survival in population-based patients with pancreatic cancer in the San Francisco Bay Area. Cancer Causes Control 2012; 23:1929–1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yuan C, Bao Y, Wu C, et al. Prediagnostic body mass index and pancreatic cancer survival. J Clin Oncol 2013; 31:4229–4234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kasenda B, Bass A, Koeberle D, et al. Survival in overweight patients with advanced pancreatic carcinoma: a multicentre cohort study. BMC Cancer 2014; 14:728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pelucchi C, Galeone C, Polesel J, et al. Smoking and body mass index and survival in pancreatic cancer patients. Pancreas 2014; 43:47–52. [DOI] [PubMed] [Google Scholar]
- 22.Tsai S, Choti MA, Assumpcao L, et al. Impact of obesity on perioperative outcomes and survival following pancreaticoduodenectomy for pancreatic cancer: a large single-institution study. J Gastrointest Surg 2010; 14:1143–1150. [DOI] [PubMed] [Google Scholar]
- 23.Dandona M, Linehan D, Hawkins W, et al. Influence of obesity and other risk factors on survival outcomes in patients undergoing pancreaticoduodenectomy for pancreatic cancer. Pancreas 2011; 40:931–937. [DOI] [PubMed] [Google Scholar]
- 24.Gaujoux S, Torres J, Olson S, et al. Impact of obesity and body fat distribution on survival after pancreaticoduodenectomy for pancreatic adenocarcinoma. Ann Surg Oncol 2012; 19:2908–2916. [DOI] [PubMed] [Google Scholar]
- 25.Esteghamati A, Mazaheri T, Vahidi RM, et al. Complementary and alternative medicine for the treatment of obesity: a critical review. Int J Endocrinol Metab 2015; 13:e19678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berrington DGA, Sweetland S, Spencer E. A meta-analysis of obesity and the risk of pancreatic cancer. Br J Cancer 2003; 89:519–523.2003-08-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Larsson SC, Orsini N, Wolk A. Body mass index and pancreatic cancer risk: a meta-analysis of prospective studies. Int J Cancer 2007; 120:1993–1998. [DOI] [PubMed] [Google Scholar]
- 28.Renehan AG, Tyson M, Egger M, et al. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet 2008; 371:569–578. [DOI] [PubMed] [Google Scholar]
- 29.Arslan AA, Helzlsouer KJ, Kooperberg C, et al. Anthropometric measures, body mass index, and pancreatic cancer: a pooled analysis from the Pancreatic Cancer Cohort Consortium (PanScan). Arch Intern Med 2010; 170:791–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiao L, Berrington DGA, Hartge P, et al. Body mass index, effect modifiers, and risk of pancreatic cancer: a pooled study of seven prospective cohorts. Cancer Causes Control 2010; 21:1305–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Genkinger JM, Spiegelman D, Anderson KE, et al. A pooled analysis of 14 cohort studies of anthropometric factors and pancreatic cancer risk. Int J Cancer 2011; 129:1708–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Preziosi G, Oben JA, Fusai G. Obesity and pancreatic cancer. Surg Oncol 2014; 23:61–71. [DOI] [PubMed] [Google Scholar]
- 33.Dawood S, Broglio K, Gonzalez-Angulo AM, et al. Prognostic value of body mass index in locally advanced breast cancer. Clin Cancer Res 2008; 14:1718–1725. [DOI] [PubMed] [Google Scholar]
- 34.Siegel EM, Ulrich CM, Poole EM, et al. The effects of obesity and obesity-related conditions on colorectal cancer prognosis. Cancer Control 2010; 17:52–57. [DOI] [PubMed] [Google Scholar]
- 35.Dalal S, Hui D, Bidaut L, et al. Relationships among body mass index, longitudinal body composition alterations, and survival in patients with locally advanced pancreatic cancer receiving chemoradiation: a pilot study. J Pain Symptom Manage 2012; 44:181–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim HG, Han J. [Obesity and pancreatic diseases]. Korean J Gastroenterol 2012; 59:35–39. [DOI] [PubMed] [Google Scholar]
- 37.Al-Goblan AS, Al-Alfi MA, Khan MZ. Mechanism linking diabetes mellitus and obesity. Diabetes Metab Syndr Obes 2014; 7:587–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.White PB, True EM, Ziegler KM, et al. Insulin, leptin, and tumoral adipocytes promote murine pancreatic cancer growth. J Gastrointest Surg 2010; 14:1888–1893.1893-1894. [DOI] [PubMed] [Google Scholar]
- 39.Aggarwal BB, Shishodia S, Sandur SK, et al. Inflammation and cancer: how hot is the link? Biochem Pharmacol 2006; 72:1605–1621. [DOI] [PubMed] [Google Scholar]


