Abstract
The aim of the study is to utilize electrical impedance tomography (EIT) to guide positive end-expiratory pressure (PEEP) and to optimize oxygenation in patients undergoing laparoscopic abdominal surgery.
Fifty patients were randomly assigned to the control (C) group and the EIT (E) group (n = 25 each). We set the fraction of inspired oxygen (FiO2) at 0.30. The PEEP was titrated and increased in a 2-cm H2O stepwise manner, from 6 to 14 cm H2O. Hemodynamic variables, respiratory mechanics, EIT images, analysis of blood gas, and regional cerebral oxygen saturation were recorded. The postoperative pulmonary complications within the first 5 days were also observed.
We chose 10 cm H2O and 8 cm H2O as the “ideal” PEEP for the C and the E groups, respectively. EIT-guided PEEP titration led to a more dorsal shift of ventilation. The PaO2/FiO2 ratio in the E group was superior to that in the C group in the pneumoperitoneum period, though the difference was not significant (330 ± 10 vs 305.56 ± 4 mm Hg; P = 0.09). The C group patients experienced 8.7% postoperative pulmonary complications versus 5.3% among the E group patients (relative risk 1.27, 95% confidence interval 0.31–5.3, P = 0.75).
Electrical impedance tomography represents a new promising technique that could enable anesthesiologists to assess regional ventilation of the lungs and optimize global oxygenation for patients undergoing laparoscopic abdominal surgery.
INTRODUCTION
After the induction of general anesthesia and mechanical ventilation, the alveoli in the dependent lung regions may collapse.1,2 During laparoscopic abdominal surgery, pulmonary ventilation is further impaired by the application of pneumoperitoneum.3 The recruitment maneuvers and positive end-expiratory pressure (PEEP) might lower the risk of atelectasis,4,5 but high-level PEEP could also lead to overdistension in the nondependent areas. Current strategies to provide lung-protective ventilation rely on avoiding conditions that may cause lung injury, such as lung overdistension and the repeated opening and collapse of atelectasis. Based on the results of the Acute Respiratory Distress Syndrome Network (ARDSNet) trial,6 many anesthesiologists have gradually preferred to use low tidal volume (TV) ventilation intraoperatively. Further studies have demonstrated that mechanical ventilation, low TV ventilation combined with PEEP, might be beneficial not only in injured lungs but in healthy lungs as well.7,8 Although PEEP is widely used in clinical practice, it remains under debate as to how to individually titrate the adequate PEEP level for patients undergoing laparoscopic abdominal surgery.
Electrical impedance tomography (EIT), a noninvasive, functional imaging technology, can identify atelectatic, overdistended, and adequately recruited lung in different lung regions.9–11 Various studies have shown that EIT-guided PEEP titration proved to be a valuable method of optimizing PEEP in animals and humans.12–14 Cyclic variations in pulmonary air and blood content are the major determinants for the impedance, which allows EIT estimation of not only ventilation but also perfusion. Borges et al15 showed that, in both healthy and injured lung conditions, the distribution of pulmonary blood flow as assessed by EIT agreed well with that obtained by computerized tomography. However, it remains unclear whether EIT-guided PEEP titration could improve ventilation and optimize the intraoperative global oxygenation for patients undergoing laparoscopic abdominal surgery in conditions of low fraction of inspiratory oxygen concentration (FiO2). The aim of this study was to assess the reliability of real-time EIT-derived parameters for intraoperative PEEP selection to provide adequate oxygenation while avoiding unnecessarily high FiO2.
METHODS
Study Population
This is a prospective randomized controlled trial, which has been registered at “http://www.chictr.org/cn/” (ChiCTR-IPR-14005305). Ethical approval was obtained from the ethical committee of Changzheng Hospital, the Second Military Medical University, Shanghai, China. The study was carried out in the Department of Anesthesiology at Changzheng Hospital, the Second Military Medical University, from October 2014 to March 2015. The inclusion criterion was individuals who were aged 18 years or older and scheduled for laparoscopic abdominal surgery (rectum and colon, performed by the same surgeons) under general anesthesia. The exclusion criteria were as follows: individuals older than 80 years; with severe cardiac or pulmonary comorbidities or another disorder that might have compromised the safety of the trial procedure; with a body mass index higher than 40 kg/m2; the presence of pregnancy, tracheostomy, facial, neck, or chest wall abnormalities; a history of abdominal aortic aneurysm surgery, chemotherapy, or immunosuppressive therapy within 3 months; and individuals unwilling or unable to give informed consent.
Study Protocol
The patients were prospectively randomized into a control (C) group and an EIT (E) group (n = 25 each). Group assignment was concealed in a sealed envelope until the induction of anesthesia began. Briefly, the patients were ventilated with a volume-controlled mode. We set TVs at 6 mL/kg predicted body weight (PBW). The FiO2 was set at 0.30, to a target SpO2 of 95% or greater. We adjusted the respiratory rate to maintain the end-tidal partial pressure of carbon dioxide (ETCO2) between 35 and 45 mm Hg, with an I-to-E ratio of 1:2. The PEEP was increased in a 2-cm H2O stepwise manner, from 6 to 14 cm H2O, and was adjusted every 5 minutes. Periodic recruitment maneuvers, that is, sustained inflation of the lungs for 40 seconds to a peak inspiratory pressure (PIP) of 35 cm H2O,16 was done every 45 minutes. In the C group, “ideal” PEEP was selected based on the respiratory mechanics data, that is, when dynamic compliance reached the highest value, whereas in the E group, “ideal” PEEP was selected using the guidance of real-time EIT imaging, that is, when the dorsal regions (ROI3 and ROI4) reached the maximum and the ventral regions (ROI1 and ROI2) reached the minimum. Pneumoperitoneum was induced and maintained up to an intra-abdominal pressure (IAP) of 13 to 15 mm Hg.
Electrical Impedance Tomography Image Generation and Analysis
Sixteen equidistant electrodes were placed around the thorax (xiphoid process level), and a reference electrode was placed on the right thorax.17 We used this midthoracic level electrode placement strategy due to its good approximation of global lung behavior18 and due to its avoidance of imaging potential organ movements below the diaphragm.19 EIT images were acquired using the EIT monitor (PulmoVista 500, Draeger Medical, Germany). We used a software package (Draeger EIT Data Analysis Tool 6.1, Draeger, Germany) to quantitatively analyze the ventilation distributions. The lung region of interest (ROI) was then divided into 4 ROIs (ROI1, ROI2, ROI3, and ROI4; Figure 1) with equal height from ventral to dorsal.11
FIGURE 1.
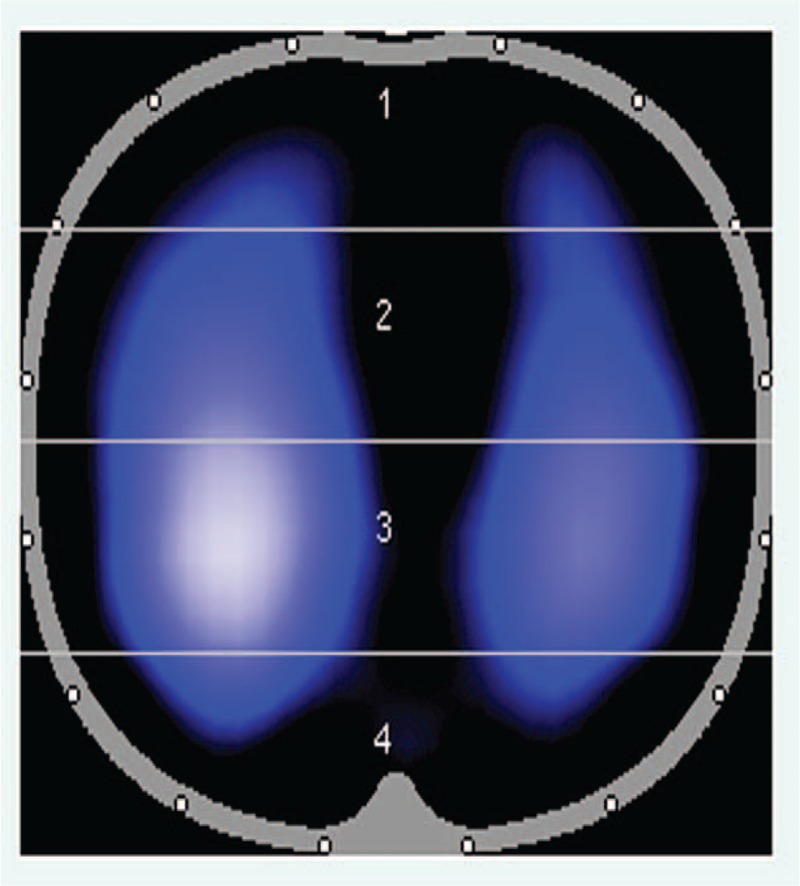
Lung regions of interest (ROIs) distribution for tidal ventilation.
Regional Tissue Oxygen Saturation
Regional tissue oxygen saturation was measured using the EQUANOXTM Model 7600 Cerebral/Somatic Oximetry System (Nonin Medical Inc., Plymouth, MN). Two sensors were placed above the supraorbital margin and along the left and right sites on the patient foreheads. The regional oxygen saturation (rSO2) is calculated and displayed as oxygen-saturated hemoglobin as a percentage of total hemoglobin.
Data Acquisition
Hemodynamic variables [heart rate (HR), mean arterial pressure (MAP), cardiac output index (CI), and stroke volume variation (SVV)]; respiratory mechanics (plateau pressure [PP], PIP, and global respiratory system compliance); EIT images; and the analysis of the arterial, central venous blood, and regional cerebral oxygen saturation (SpO2, ScvO2, rSO2) were collected before the induction of anesthesia (T0), before the pneumoperitoneum/PEEP titration period (5 minutes after the induction of anesthesia and tracheal intubation, T1–T5), 5 minutes after the application of pneumoperitoneum (T6), 5 minutes after exsufflation of the pneumoperitoneum (T7), and 5 minutes after tracheal extubation (T8). Other data, such as the Qs/Qt and PaO2/FiO2, were calculated based on the raw data. PPCs within the first 5 days after surgery were recorded, and mainly included hypoxia (SpO2 < 90%), atelectasis, pneumonia, suspected pulmonary infection, and the development of ARDS.
Data Analysis and Statistics
The data in the text and figures are presented as the mean ± SE. Statistical analysis was performed using GraphPad Prism version 6.0 (GraphPad Software, San Diego). The data were tested for normal distribution with the Kolmogorov–Smirnov normality test. The comparisons between 2 groups or different time points were performed with the multiple t test or 2-way analysis of variance (ANOVA). The statistical tests were 2-tailed, and a P value less than 0.05 was accepted as statistically significant. We calculated the Kaplan–Meier estimates of survival curves and censored data used for Kaplan–Meier estimates when patients did not have PPCs during the study period.
RESULTS
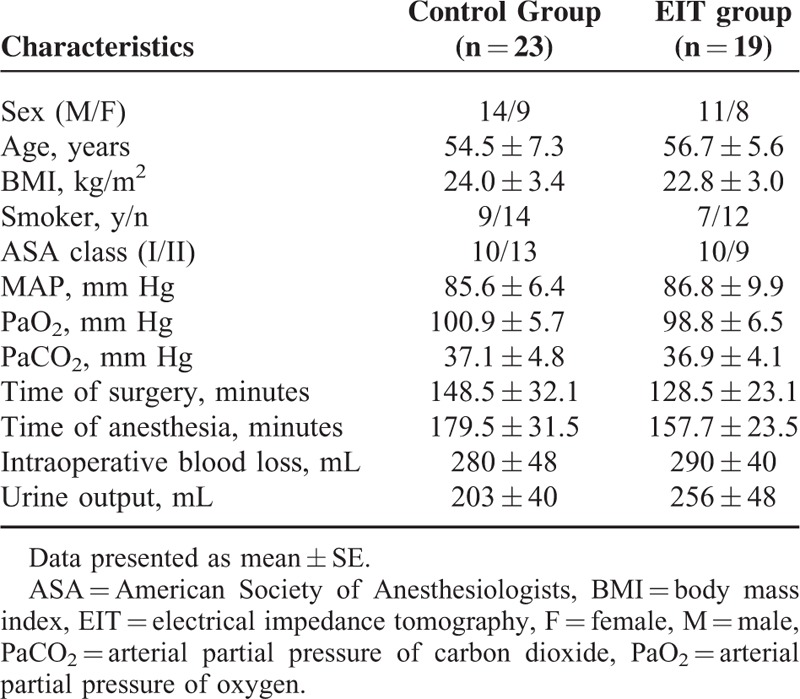
The time points for data sampling are presented in Figure 2. In the course of PEEP titration (T1–T5), 4 patients could not maintain a target SpO2 (≥95%), 2 patients underwent hypotension (MAP <50 mm Hg for more than 3 minutes requiring vasoactive drugs), and another 2 patients demonstrated a high peak pressure (>40 cm H2O). Thus, we included 42 out of 50 patients (23 in the C group and 19 in the E group). Details of the patient characteristics are presented in Table 1, and no significant difference existed between the 2 groups. The ARISCAT score (Risk for Postoperative Pulmonary Complications)20 was no higher than 44.
FIGURE 2.
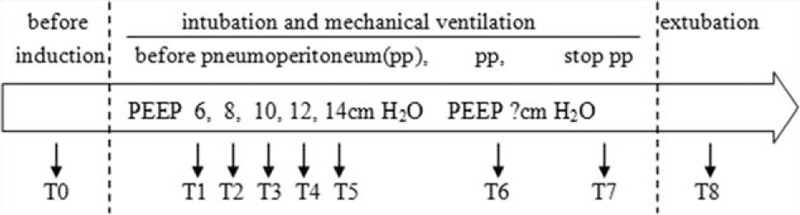
Timeline. Data collection was made after each intervention.
TABLE 1.
Baseline Characteristics of the Study Population
Dynamic compliance had the highest value (42.67 ± 3.48 mL/cm H2O in the C group and 43.33 ± 4.26 mL/cm H2O in the E group) at 10 cm H2O PEEP (Figure 3A, T1–T5). We chose 10 cm H2O as the “ideal” PEEP for the C group. The dorsal regions (ROI3 and ROI4) reached the maximum (43.55 ± 2.67 for ROI3 and 16.3 ± 0.81 for ROI4) and the ventral regions (ROI1 and ROI2) reached the minimum (9.20 ± 0.73 for ROI1 and 30.95 ± 1.74 for ROI2) at the PEEP level of 8 cm H2O (Figure 3B, T1–T5), which we chose as the “ideal” PEEP for the E group. In the pneumoperitoneum period, a decrease in compliance occurred in both groups (Figure 3A; T3 vs T6: 42.67 ± 3.48 vs 26.67 ± 3.18 mL/cm H2O in the C group; P = 0.0005; T2 vs T6: 40.67 ± 3.53 vs 28.67 ± 3.84 mL/cm H2O in the E group; P = 0.004), and a higher ventral shift of ventilation was observed even with the “ideal” PEEP (Figure 3B; T6 vs T2: 48.93 ± 2.68 vs 40.15 ± 2.05 for the ventral parts; P = 0.04; T6 vs T2: 50.63 ± 2.73 vs 59.85 ± 2.05 for the dorsal parts; P = 0.035). The PaO2/FiO2 ratio had the higher value (362.22 ± 19.28 mm Hg in the C group and 366.67 ± 13.88 mm Hg in the E group) at 8 cm H2O PEEP accompanied with the lower Qs/Qt (7.63 ± 0.99 in the C group and 7.23 ± 0.94 in the E group; Figure 3C and D, T1–T5). After pneumoperitoneum, a decrease in PaO2/FiO2 (305.56 ± 4.01 mm Hg in the C group vs 330 ± 10 mm Hg in the E group; P = 0.09) and an increase in Qs/Qt (11.77 ± 1.28 in the C group vs 10.37 ± 1.19 in the E group; P = 0.47) occurred (Figure 3C and D, T6). No significant differences were found in the other mechanical ventilation respiratory mechanics (PP, PIP), hemodynamic (HR, MAP, CI, SVV), or oxygenation parameters (SpO2, ScvO2, rSO2) between the groups (Figure 3E and F and Figure 4, T0–T8).
FIGURE 3.
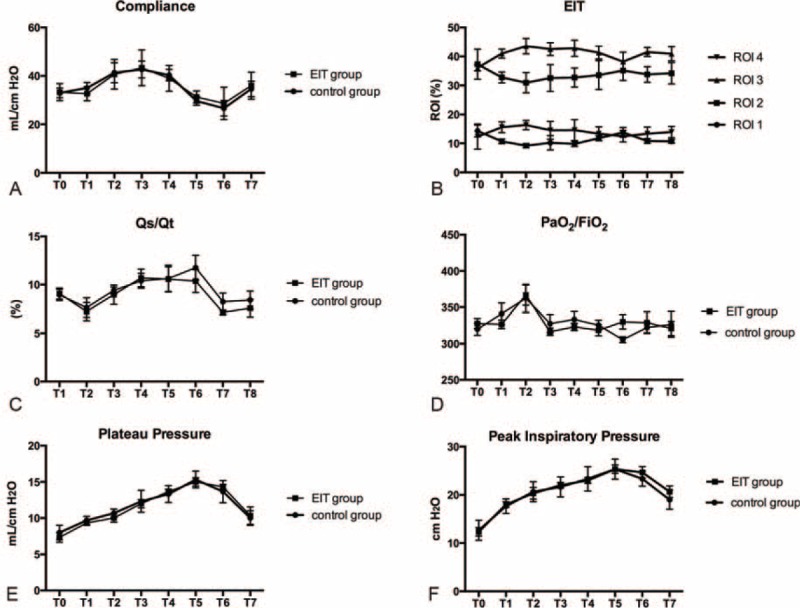
Respiratory and oxygenation parameters of the C group and the E group.
FIGURE 4.
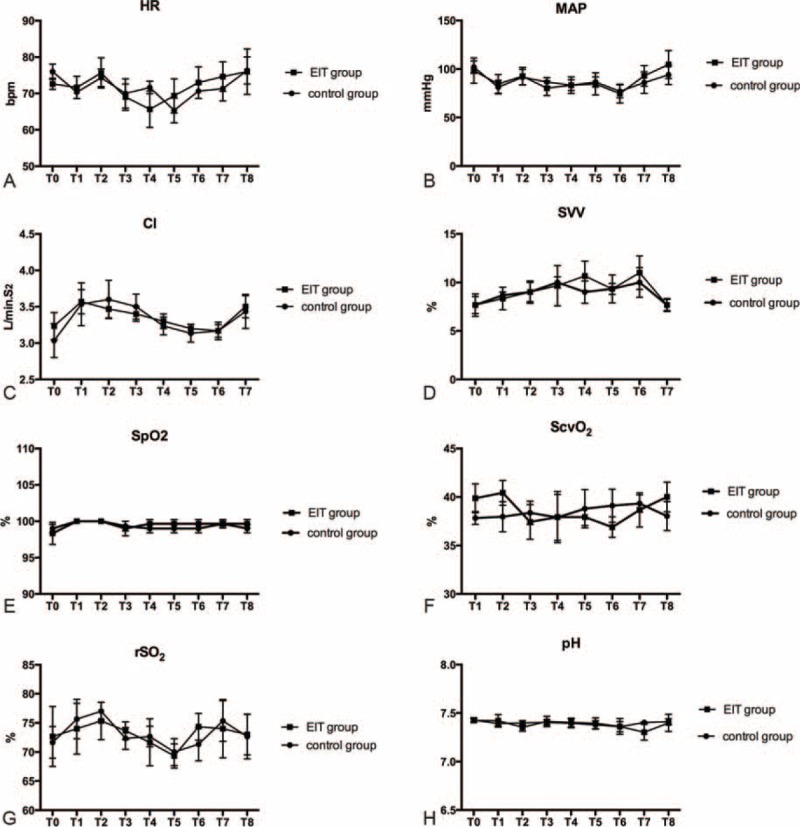
Hemodynamic and other physiological parameters of the C group and the E group.
Postoperative pulmonary complications within the first 5 days after surgery were recorded in 2 of 23 (8.7%) patients in the C group versus 1 of 19 (5.3%) individuals in the E group (relative risk 1.27, 95% confidence interval [CI] 0.31–5.3, P = 0.75; Table 2, Figure 5). Hypoxia and atelectasis were the only observed PPCs (Table 2).
TABLE 2.
Postoperative Pulmonary Complications
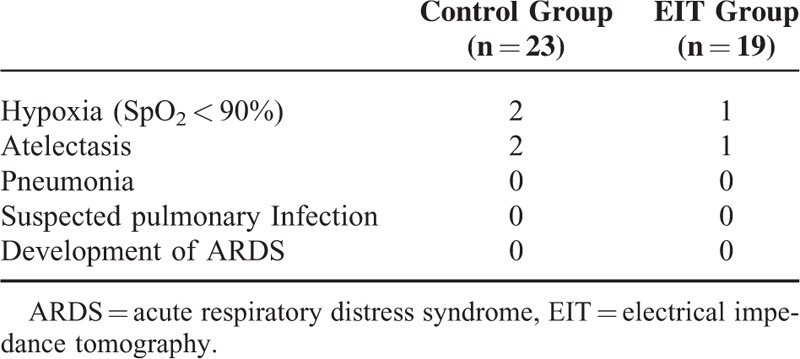
FIGURE 5.
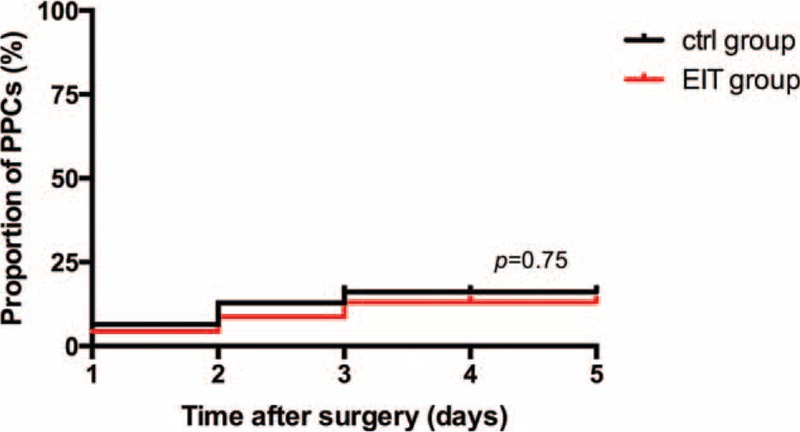
Kaplan–Meier curve showing the probability of PPCs by postoperative day 5. PPCs = postoperative pulmonary complications.
DISCUSSION
The main findings of this study are as follows: EIT-guided mechanical ventilation resulted in better ventilation distribution and oxygenation in patients undergoing laparoscopic abdominal surgery; and a FiO2 of 0.3 was sufficient to maintain the intraoperative balance between oxygen supply and consumption.
After tracheal intubation and mechanical ventilation, a higher amount of ventilation in the ventral regions of the lungs was noticed, which would cause a mismatch between ventilation and perfusion. Several studies have been published in which EIT was used to assess mechanical ventilation in intubated patients.21–23 Bikker et al21 performed PEEP decrementation at 4 PEEP levels (15 to 10 to 5 to 0 cm H2O) in ICU patients. They found tidal impedance increased only in the nondependent parts in the patients without lung disorders after decreasing the PEEP from 15 to 10 cm H2O. Another study published by the same author demonstrated that the recruitment and excessive distension of alveoli in response to variable PEEPs could be observed in the higher position on the thorax.22 In our study, EIT electrodes were placed around the thorax at the level of the xiphoid process, due to its good approximation of global lung behavior18 and due to its avoidance of imaging potential organ movements below the diaphragm.19 EIT was also used to assess the modifying effects of a recruitment maneuver and PEEP on lung ventilation in patients undergoing general anesthesia for laparoscopy.23 A PEEP of 10 cm H2O proved sufficient for reversing the ventilation distribution changes resulting from general anesthesia, yet was too low to prevent the adverse effects of pneumoperitoneum.23 The concomitant application of PEEP and recruitment maneuvers are inseparable parts of a lung-protective mechanical ventilation strategy in patients undergoing abdominal surgery.24 Our data revealed that the recruitment maneuver accompanied with PEEP was able to prevent the ventral shift of ventilation in most laparoscopic surgical patients. Furthermore, the PEEP titration intervals were small (2 cm H2O), which was more accurate and might help identify slight changes. Previous studies have provided options for “ideal” PEEP scanning, such as global dynamic compliance25 and intratidal compliance–volume curves.26 Here, we employed the global dynamic compliance and regional EIT to guide intraoperative PEEP selection. In the course of PEEP titration, oxygenation in the PEEP-ventilated patients improved in some specific levels. A higher PaO2/FiO2 ratio could be observed at the PEEP level of 8 cm H2O, which should be associated with more well-distributed ventilation. In the pneumoperitoneum period, the PaO2/FiO2 ratio in the E group was better than that in the C group, although no significant difference was ascertained in both groups. It seemed that EIT was superior to compliance in scanning the “ideal” PEEP, even if the difference about this “ideal” value was slight (8 cm H2O PEEP for the E group vs 10 cm H2O PEEP for the C group). Our results showed that the 8-cm H2O PEEP could better improve ventilation (maximizing the recruitment of dependent lungs and minimizing the overdistension of nondependent lungs) and oxygenation (avoiding impairing PaO2/FiO2 and increasing the shunt upon the initiation of pneumoperitoneum) in patients undergoing laparoscopic surgery, without significantly interfering with other respiratory or hemodynamic parameters. However, pneumoperitoneum had a considerate influence on the change of ventilation distributions, and the 8 or 10-cm H2O PEEP was not sufficient to prevent a secondary ventral shift in all patients.
There have been conflicting findings regarding the role of high intraoperative FiO2 and perioperative outcomes,27,28 mainly the potentially beneficial (decreases in the risk of surgical site infection/SSI or postoperative nausea and vomit/PONV) and harmful (increases in the risk of pulmonary complications, such as atelectasis, deterioration of gas exchange) effects on surgical patients. Arterial hyperoxia has also been shown to induce vasoconstriction and reduce cardiac output, which may impair blood flow to the at risk organs.29–31 In addition, hyperoxia facilitates a complex proinflammatory response and has been associated with cell injury by reactive oxygen species.32,33 In this clinical trial, we set FiO2 as 0.3, which was adequate to maintain the intraoperative oxygen supply and consumption balance, with no signs of decreased SpO2, ScvO2, or rSO2. Meanwhile, no SSI, severe PONV, or other cardiovascular events were reported. A recent systemic review suggested that arterial hyperoxia was associated with poor hospital outcomes.34 Although the authors noted that these harmful effects depended on the degree of hyperoxia and were more prominent in certain subgroups, a high intraoperative FiO2 might be considered unnecessary and even disadvantageous. Additionally, it is necessary to realize that not only the lung mechanics but also the hemodynamic effect of PEEP may influence the outcome of oxygenation. Thus, we should always consider cardiorespiratory physiology to be the principal determinant for FiO2. Based on our results, a FiO2 of 0.3 was appropriated for most American Society of Anesthesiologists class I to II patients while avoiding the potential deleterious effects of both hyperoxia and hypoxia. More convincing data are needed to provide tailored oxygen targets for clinicians.
As we know, PPCs account for a substantial proportion of risks related to major abdominal surgery and intraoperative mechanical ventilation strategy, which may impair patient recovery and increase hospital costs.24 Generally, most patients can be considered for laparoscopic surgery if they could tolerate general anesthesia. However, caution must be observed in the choice of laparoscopic surgery because pneumoperitoneum may influence the pulmonary capacity or reserve for patients with severe pulmonary disease. Thus, we evaluated a sample of patients and found that they were not at high risk (the ARISCAT score no higher than 44). Still, we observed some PPCs, that is, hypoxia (SpO2 < 90%) and atelectasis, within the first 5 days after surgery. The notable finding of the current study was a considerable decrease in the development of PPCs. This was most likely due to the strict patient selection, which excluded patients with many comorbidities and the intraoperative protective lung ventilation strategy. The low incidence of serious PPCs in both groups can be associated with rapid postoperative rehabilitation and decreased perioperative morbidity and mortality in patients undergoing laparoscopic abdominal surgery.
Our study has some methodological limitations, including the small sample size and short-term postoperative follow-up, which might not identify difference in the outcomes of both groups. Future studies should improve upon these methodological flaws. With the increasing availability of EIT to our surgical patients and its potential to improve outcomes (possibly in conjunction with intraoperative lung-protective ventilation strategies), large-sample, high-quality research is needed to evaluate the utility of real-time EIT in guiding PEEP titration after major laparoscopic abdominal surgery. This technique may offer anesthesiologists prospects for establishing a better, direct assessment of the patient–ventilator interaction and a more protective mechanical ventilation protocol.
Acknowledgments
We thank research assistant Yuqin Ke for the data collection, and technicians Mingyang Zhu and Jiabin Ren for the use of EIT.
Footnotes
Abbreviations: EIT = electrical impedance tomography, ETCO2 = end-tidal partial pressure of carbon dioxide, FiO2 = fraction of inspiratory oxygen concentration, IAP = intra-abdominal pressure, PBW = predicted body weight, PEEP = positive end-expiratory pressure, PONV = postoperative nausea and vomit, PPCs = postoperative pulmonary complications, ROI = region of interest, rSO2 = regional oxygen saturation, ScvO2 = central venous blood oxygen saturation, SSI = surgical site infection, TV = tidal volume.
This study was funded by Shanghai Municipal Science and Technology Commission medical guide project (15411966000), Shanghai Pujiang Program (15PJD003), the 12th Five-year Key Project Grant of the People's Liberation Army (BWS12J027), Natural Science Foundation of China (81100049, 81372103), and Shanghai Municipal Commission of Health and Family Planning project (201440039).
The authors report no conflicts of interest.
REFERENCES
- 1.Bendixen HH, Hedley-Whyte J, Laver MB. Impaired oxygenation in surgical patients during general anesthesia with controlled ventilation. A concept of atelectasis. N Engl J Med 1963; 269:991–996. [DOI] [PubMed] [Google Scholar]
- 2.Brismar B, Hedenstierna G, Lundquist H, et al. Pulmonary densities during anesthesia with muscular relaxation–a proposal of atelectasis. Anesthesiology 1985; 62:422–428. [DOI] [PubMed] [Google Scholar]
- 3.Andersson LE, Baath M, Thorne A, et al. Effect of carbon dioxide pneumoperitoneum on development of atelectasis during anesthesia, examined by spiral computed tomography. Anesthesiology 2005; 102:293–299. [DOI] [PubMed] [Google Scholar]
- 4.Rothen HU, Neumann P, Berglund JE, et al. Dynamics of re-expansion of atelectasis during general anaesthesia. Br J Anaesth 1999; 82:551–556. [DOI] [PubMed] [Google Scholar]
- 5.Tusman G, Bohm SH, Suarez-Sipmann F, et al. Alveolar recruitment improves ventilatory efficiency of the lungs during anesthesia. Can J Anaesth 2004; 51:723–727. [DOI] [PubMed] [Google Scholar]
- 6.Oba Y, Salzman GA. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury. N Engl J Med 2000; 343:813.[author reply 813-4]. [PubMed] [Google Scholar]
- 7.Choi G, Wolthuis EK, Bresser P, et al. Mechanical ventilation with lower tidal volumes and positive end-expiratory pressure prevents alveolar coagulation in patients without lung injury. Anesthesiology 2006; 105:689–695. [DOI] [PubMed] [Google Scholar]
- 8.Wolthuis EK, Choi G, Dessing MC, et al. Mechanical ventilation with lower tidal volumes and positive end-expiratory pressure prevents pulmonary inflammation in patients without preexisting lung injury. Anesthesiology 2008; 108:46–54. [DOI] [PubMed] [Google Scholar]
- 9.Costa EL, Borges JB, Melo A, et al. Bedside estimation of recruitable alveolar collapse and hyperdistension by electrical impedance tomography. Intensive Care Med 2009; 35:1132–1137. [DOI] [PubMed] [Google Scholar]
- 10.Gomez-Laberge C, Arnold JH, Wolf GK. A unified approach for EIT imaging of regional overdistension and atelectasis in acute lung injury. IEEE Trans Med Imaging 2012; 31:834–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Victorino JA, Borges JB, Okamoto VN, et al. Imbalances in regional lung ventilation: a validation study on electrical impedance tomography. Am J Respir Crit Care Med 2004; 169:791–800. [DOI] [PubMed] [Google Scholar]
- 12.Erlandsson K, Odenstedt H, Lundin S, et al. Positive end-expiratory pressure optimization using electric impedance tomography in morbidly obese patients during laparoscopic gastric bypass surgery. Acta Anaesthesiol Scand 2006; 50:833–839. [DOI] [PubMed] [Google Scholar]
- 13.Luepschen H, Meier T, Grossherr M, et al. Protective ventilation using electrical impedance tomography. Physiol Meas 2007; 28:S247–S260. [DOI] [PubMed] [Google Scholar]
- 14.Zhao Z, Steinmann D, Frerichs I, et al. PEEP titration guided by ventilation homogeneity: a feasibility study using electrical impedance tomography. Crit Care 2010; 14:R8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borges JB, Suarez-Sipmann F, Bohm SH, et al. Regional lung perfusion estimated by electrical impedance tomography in a piglet model of lung collapse. J Appl Physiol 19852012; 112:225–236. [DOI] [PubMed] [Google Scholar]
- 16.Hartland BL, Newell TJ, Damico N. Alveolar recruitment maneuvers: are your patients missing out? AANA J 2014; 82:307–314. [PubMed] [Google Scholar]
- 17.Gomez-Laberge C, Rettig JS, Smallwood CD, et al. Interaction of dependent and non-dependent regions of the acutely injured lung during a stepwise recruitment manoeuvre. Physiol Meas 2013; 34:163–177. [DOI] [PubMed] [Google Scholar]
- 18.Yang F, Patterson RP. The contribution of the lungs to thoracic impedance measurements: a simulation study based on a high resolution finite difference model. Physiol Meas 2007; 28:S153–S161. [DOI] [PubMed] [Google Scholar]
- 19.Adler A, Arnold JH, Bayford R, et al. GREIT: a unified approach to 2D linear EIT reconstruction of lung images. Physiol Meas 2009; 30:S35–55. [DOI] [PubMed] [Google Scholar]
- 20.Canet J, Gallart L, Gomar C, et al. Prediction of postoperative pulmonary complications in a population-based surgical cohort. Anesthesiology 2010; 113:1338–1350. [DOI] [PubMed] [Google Scholar]
- 21.Bikker IG, Leonhardt S, Reis Miranda D, et al. Bedside measurement of changes in lung impedance to monitor alveolar ventilation in dependent and non-dependent parts by electrical impedance tomography during a positive end-expiratory pressure trial in mechanically ventilated intensive care unit patients. Crit Care 2010; 14:R100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bikker IG, Preis C, Egal M, et al. Electrical impedance tomography measured at two thoracic levels can visualize the ventilation distribution changes at the bedside during a decremental positive end-expiratory lung pressure trial. Crit Care 2011; 15:R193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karsten J, Luepschen H, Grossherr M, et al. Effect of PEEP on regional ventilation during laparoscopic surgery monitored by electrical impedance tomography. Acta Anaesthesiol Scand 2011; 55:878–886. [DOI] [PubMed] [Google Scholar]
- 24.Futier E, Jaber S. Lung-protective ventilation in abdominal surgery. Curr Opin Crit Care 2014; 20:426–430. [DOI] [PubMed] [Google Scholar]
- 25.Suarez-Sipmann F, Bohm SH, Tusman G, et al. Use of dynamic compliance for open lung positive end-expiratory pressure titration in an experimental study. Crit Care Med 2007; 35:214–221. [DOI] [PubMed] [Google Scholar]
- 26.Mols G, Brandes I, Kessler V, et al. Volume-dependent compliance in ARDS: proposal of a new diagnostic concept. Intensive Care Med 1999; 25:1084–1091. [DOI] [PubMed] [Google Scholar]
- 27.Hovaguimian F, Lysakowski C, Elia N, et al. Effect of intraoperative high inspired oxygen fraction on surgical site infection, postoperative nausea and vomiting, and pulmonary function: systematic review and meta-analysis of randomized controlled trials. Anesthesiology 2013; 119:303–316. [DOI] [PubMed] [Google Scholar]
- 28.Pryor KO, Fahey TJ, 3rd, Lien CA, et al. Surgical site infection and the routine use of perioperative hyperoxia in a general surgical population: a randomized controlled trial. JAMA 2004; 291:79–87. [DOI] [PubMed] [Google Scholar]
- 29.Bak Z, Sjoberg F, Rousseau A, et al. Human cardiovascular dose-response to supplemental oxygen. Acta Physiol (Oxf) 2007; 191:15–24. [DOI] [PubMed] [Google Scholar]
- 30.Cornet AD, Kooter AJ, Peters MJ, et al. The potential harm of oxygen therapy in medical emergencies. Crit Care 2013; 17:313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Farquhar H, Weatherall M, Wijesinghe M, et al. Systematic review of studies of the effect of hyperoxia on coronary blood flow. Am Heart J 2009; 158:371–377. [DOI] [PubMed] [Google Scholar]
- 32.Altemeier WA, Sinclair SE. Hyperoxia in the intensive care unit: why more is not always better. Curr Opin Crit Care 2007; 13:73–78. [DOI] [PubMed] [Google Scholar]
- 33.Gore A, Muralidhar M, Espey MG, et al. Hyperoxia sensing: from molecular mechanisms to significance in disease. J Immunotoxicol 2010; 7:239–254. [DOI] [PubMed] [Google Scholar]
- 34.Helmerhorst HJ, Roos-Blom MJ, van Westerloo DJ, et al. Association between arterial hyperoxia and outcome in subsets of critical illness: a systematic review, metaanalysis, and meta-regression of cohort studies. Crit Care Med 2015; 43:1508–1519. [DOI] [PubMed] [Google Scholar]


