Abstract
At present, we do not know the exact prevalence of Barrett esophagus (BE) developing later in patients without BE in their first endoscopic screening. The purpose of this study was to determine the prevalence of BE on the second endoscopic examination of patients who had no BE in their first endoscopic examination.
The data of the patients older than 18 years who had undergone upper gastrointestinal system endoscopy more than once at the endoscopy unit of our clinic during the last 6 years were retrospectively analyzed.
During the last 6 years, 44,936 patients had undergone at least one endoscopic examination. Among these patients, 2701 patients who had more than one endoscopic screening were included in the study. Of the patients, 1276 (47.3%) were females and 1425 (52.7%) were males, with an average age of 54.9 (18–94) years. BE was diagnosed in 18 (0.66%) of the patients who had no BE in the initial endoscopic examination. The patients with BE had reflux symptoms in their medical history and in both endoscopies, they revealed a higher prevalence of lower esophageal sphincter laxity, hiatal hernia, and reflux esophagitis when compared to patients without BE (P < 0.001).
Our study showed that in patients receiving no diagnosis of BE on their first endoscopic examination performed for any reason, the prevalence of BE on their second endoscopy within 6 years was very low (0.66%).
INTRODUCTION
Barrett esophagus (BE) has been accepted as a complication of chronic gastroesophageal reflux disease (GERD). It is characterized by replacement of the normal stratified squamous epithelium lining of the distal esophagus by columnar epithelium with intestinal metaplasia. In BE gastroesophageal junction is found to be migrated down adjacent to gastric folds. BE is strongly associated with esophageal adenocarcinoma (EAC).1 In prior studies, patients with BE have been reported to have increased risk of EAC up to 30- to 60-fold when compared to normal population.2,3 However recent studies showed that this risk has decreased down to 11- to 30-fold.1,4
Chronic GERD has been accepted to be one of the most important risk factors for BE and EAC.5 The other risk factors described for BE are age over 50, male gender, white race, hiatal hernia (HH), increased body-mass index, metabolic syndrome, intraabdominal distribution of body fat, increased insulin resistance, increased serum leptin level, and low adiponectin levels.6,7
At present, we do not know the exact prevalence of BE diagnosed in the second endoscopy in patients without BE on their first endoscopic screening. The purpose of this study was to determine the prevalence of BE on the second endoscopic examination of patients who had no BE on their first endoscopic examination.
MATERIALS AND METHODS
The data of the patients older than 18 years who had undergone more than one upper gastrointestinal system endoscopy for different reasons at our endoscopy unit between April 15, 2007 and April 27, 2013 were retrospectively analyzed. Patients who were diagnosed to have dysplasia and/or cancer in the upper gastrointestinal tract on their first endoscopic examination were excluded from the study. The prevalence of BE on the second endoscopic examination of patients who had no BE on their first endoscopic examination was determined. The patients’ demographic data, endoscopy indications (such as reflux-like, ulcus-like dyspepsia-like), endoscopic findings [such as lower esophageal sphincter (LES) laxity, HH, reflux esophagitis], and the histological findings were noted, and their effects on the prevalence of BE determined on the second endoscopy were assessed.
The following predetermined criteria were used for diagnosing BE and definition of endoscopic findings. The endoscopic diagnosis of BE was confirmed by histopathological examination.8 Esophageal biopsies were obtained from 4 quadrants of every 2 cm Barrett mucosa. Also, endoscopists took biopsies both from the circular changes of the Barrett nucosa and Barrett islands. BE was labeled as long segment BE (when the metaplastic epithelium reaches ≥3 cm above the cardioesophageal junction) or short-segment BE (when the metaplastic epithelium settles <3 cm above the cardioesophageal junction). The length of BE was reported routinely.
The severity of esophagitis was determined according to the Los Angeles Classification.9 On the endoscopic examination, a sliding type of HH was determined if the distance between the gastroesophageal junction and the hiatal clamp was longer than 2 cm. The value of 2 or higher in the Hill Classification was accepted as LES laxity.10
Endoscopic procedure in our clinic was performed by gastroenterology specialists with practice of endoscopy for long years and fellows accompanied by them. The standard endoscopy equipment (GIF-H260; Olympus, Tokyo, Japan, and EG-530-WR; Fuji Film Cooperation, Tokyo, Japan) were used. The patients’ data were obtained from the AviCenna Hospital Information Management System (Datasel Information Systems, Ankara, Turkey), which supports the internationally accepted standards (ICD-10, SNOMED, ATC, GMDN, etc.). The study was confirmed by the hospital's ethics council.
Statistical Analysis
The SPSS 15.0 software package (SPSS, Inc., Chicago, IL) was used for the statistical analysis. The Chi-square test and the Fisher exact test were performed for the analyses. A P-value of <0.05 was accepted as significant.
RESULTS
During the study period, 44,936 patients had undergone at least 1 endoscopic examination. Out of these patients, 2701 patients who had undergone more than 1 endoscopic examination and who were consistent with the study protocol were included in the study. Of the patients, 1276 (47.3%) were female and 1425 (52.7%) were male, with an average age of 54.9 (18–94) years. The average interval between the 2 endoscopies was 24 (1–78) months.
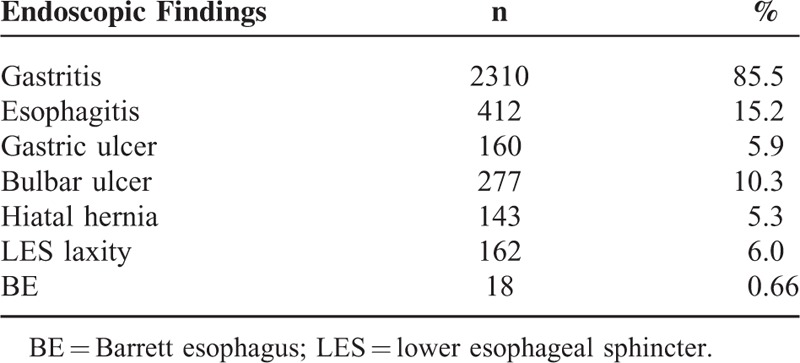
The most frequent indications for the second endoscopic examination were dyspepsia-like, ulcus-like, and reflux-like complaints (46.7%, 20.2%, and 14.6%, respectively) (Table 1). The results of the second endoscopic examination are shown in Table 2. The most common findings on the second examination, in decreasing order, were gastritis (85.5%), reflux esophagitis (15.2%), and bulbar ulcer (10.3%). Based on endoscopy, BE was suspected in 29 (1.07%) patients, and 18 (0.66%) of these had intestinal metaplasia confirmed by histopathology. Of the 18 patients, 15 (83%) had short-segment BE and 3 (17%) had long-segment BE. The length of metaplasia among patients with short-segment BE was reported to be <1 cm in 4 patients, 1 to 2 cm in 9 patients, and 2 to 3 cm in 5 patients. The biopsies revealed no dysplasia or malignancy in patients with a BE. Among patients with the diagnosis of BE, the average interval between the 2 endoscopic examinations was 20 (2–61) months.
TABLE 1.
Indications of the Second Endoscopy
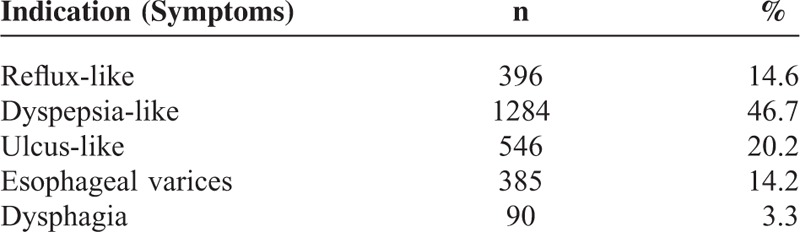
TABLE 2.
Results of the Second Endoscopic Examination
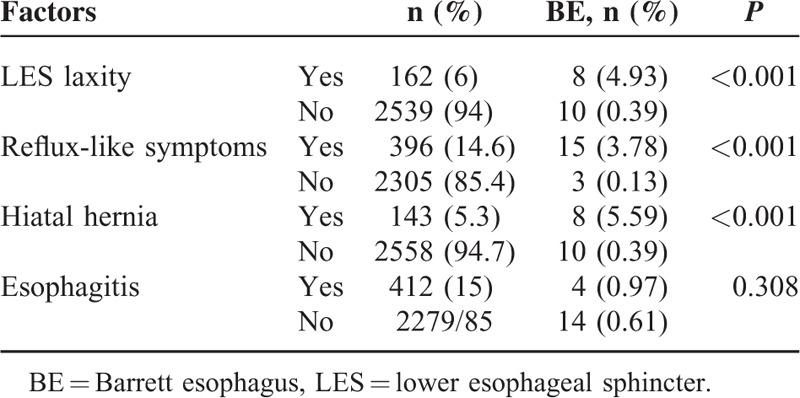
The factors effecting the occurrence of BE on the first and second endoscopic evaluation are shown in Tables 3 and 4, respectively. The prevalence of BE was found to be higher in patients with LES laxity on the first and second endoscopic examinations (P < 0.001 and P < 0.001, respectively). Similarly, the prevalence of BE was higher in patients with reflux symptoms on the first and second endoscopic examinations than in patients without these symptoms on both examinations (P < 0.001 and P < 0.001, respectively). Furthermore, the prevalence of BE was higher in patients with HH diagnosed on the first and second endoscopic examinations than in patients without HH in none of the examinations (P < 0.001 and P < 0.001, respectively). The prevalence of BE was found to be higher in patients with reflux esophagitis on the first endoscopic examination than in those without reflux esophagitis (P < 0.001). However patients with and without reflux esophagitis did not show any significant difference by means of BE according to the second endoscopic evaluation (P = 0.308). In addition, BE prevalence was found to have no association with age, gender, or the interval between the 2 endoscopies (P = 0.829, P = 0.230, and P = 0.449, respectively).
TABLE 3.
Factors Related to the Barrett Esophagus at the Initial Endoscopic Examination
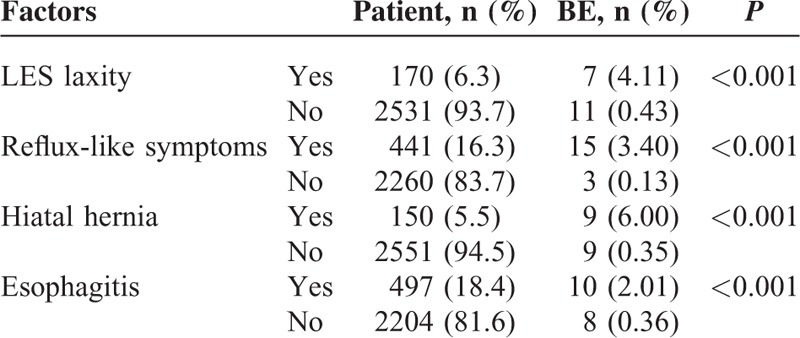
TABLE 4.
Factors Related to Barrett Esophagus at the Second Endoscopy
DISCUSSION
In our study we observed that for patients without a diagnosis of BE on their first endoscopic examination performed for any reason, the probability of having a BE on their second endoscopy within 6 years was very low (0.66%). Depending on this finding we do not recommend a second endoscopic evaluation among patients without any sign of BE in the initial endoscopy.
Prevalence of EAC increases with existence of BE. This increase had been reported to be high in prior series (0.48–0.58% per year),2,3 but recent studies reported firmer increase in prevalence (0.12–0.27% per year).1,11,12 Furthermore, longer BE length has been consistently observed to have a higher rate of progression. Three meta-analysis have reported a higher incidence of progression to EAC in patients diagnosed with long (>3 cm) when compared to short (<3 cm) segment nondysplastic BE.13,14 In addition, 3 multicentre studies consistently showed the relative risk of length (per centimeter) on progression to EAC on multivariant analysis as 1.1 to 1.21.15–17 According to these findings, the dominancy of patients with short-segment BE in our series is supporting our recommendation against performing a second endoscopic evaluation.
Reendoscopy for BE screening among patients with normal findings in their prior endoscopic evaluation is not recommended according to the guidelines of American Society of Gastrointestinal Endoscopy (ASGE), depending on the results of the study reported by Rodriguez et al.18 The results of 24,406 patients with 2 endoscopic evaluations within a period of 5 years were assessed in the study conducted by Rodriguez et al.18 They have found the prevalence of suspicious BE as 2.3% on the second endoscopy of patients who did not have BE on their first endoscopy. The main finding in our study supports the recommendation of the ASGE directory. In our study, BE was diagnosed on the second endoscopy in 18 (0.66%) patients who had no BE on their first endoscopic examination. This result shows that patients without a BE diagnosed on their first endoscopy performed for any reason are apt to develop BE very rarely, as determined on their second endoscopy.
The prevalence of BE determined on the second endoscopy in our study was lower than the results found by Rodriguez et al.18 We think that our low prevalence may be associated with geographical aspect of Turkey, settled on the Asian continent, because former studies have reported that the prevalence of BE in patients with GERD in routine endoscopic examinations in western countries is 10% to 20%, whereas the prevalence is 0.2% to 5% in Asia.19,20 The prevalence rates reported form Turkey are markedly lower than those determined in western countries. In their prospective study, Odemiş et al21 have reported a BE prevalence of 1.2% in Turkish patients undergoing endoscopy for any reason. In a retrospective Turkish study on 18,766 patients undergoing endoscopy for various reasons, the prevalence of BE has been reported as 0.4%.22 Again in a Turkish study by Bayrakçi et al23 BE has been diagnosed in 3 (2%) of 160 patients undergoing upper gastrointestinal system endoscopy because of pyrosis or regurgitation at least once a week.
Although the prevalence of BE in GERD patients is high, the prevalence of EAC is relatively low. For this reason, the question of whether endoscopic screening for BE is life-saving and cost-effective or not is a controversial issue. Another potential problem is that it is not known whether BE develops or not after the first BE-negative endoscopy, because it is not known when BE develops exactly. Furthermore, it is not yet clear whether endoscopy is the perfect screening method for BE or not. A related study has reported 82% as the sensitivity of endoscopy for BE, stating that the rest of the patients had probably been misdiagnosed.24 In addition, the endoscopic diagnosis of BE has an unacceptably low interobserver reliability for very short-segment (<1 cm) BE.25 Due to these controversies, we named the diagnosis of BE only for patients with columnar metaplasia in their histopathologic examination.
Allison and Johnstone26 were the first who have shown the mucosal changes in GERD patients in 1953. The prevalence of BE is 0.5% to 1.3% in patients undergoing endoscopy for various reasons, 5% to 15% in patients with GERD, and this rate can increase up to 50% in patients with esophageal peptic stricture.22,27 BE is generally caused by oxidative damage and inflammation due to contact of the distal esophageal mucosa with the gastric contents in GERD. Exposure to acid enhances proliferation and survival, and decreases apoptosis by activating the mitogen-activated protein kinase pathways.28 This mucosa, which is modified by intestinal metaplasia, is more resistant to the chronic damage by gastric acidity than squamous epithelium lining the normal esophageal surface.29 This adaptive mechanism also brings along the risk for cancer. Disorders such as GERD, HH, LES laxity, delayed esophageal acid clearance, and duodeno-gastro-esophageal reflux predispose to the development of BE.30,31
Rodriguez et al18 have found a higher prevalence of suspicious BE in patients with reflux symptoms than in those without these symptoms on the second endoscopy of patients who had been found to be BE-negative on the first endoscopy. Similarly, in our study, we also observed a higher prevalence of BE in patients with reflux symptoms than in patients without reflux symptoms on the second endoscopy of patients who had been found to be BE-negative on their first endoscopy (3.8% and 0.13%, respectively).
The presence of erosive esophagitis in endoscopic examination complicates the diagnosis of BE by masking it. In a study on 172 patients with reflux symptoms and erosive esophagitis, but without a BE on their first endoscopic examination, following treatment of esophagitis with acid suppression, only 12% of the patients were found to develop BE.32 Likewise, Rodriguez et al18 have found suspicious BE in about 12% of patients undergoing a second endoscopic examination, who were erosive esophagitis-positive on their first endoscopy. The probability of missing BE in endoscopic imaging has increased in parallel with the increase in severity of esophagitis.18 Our study were in accord with these studies. The prevalence of BE was higher in patients with reflux esophagitis on their first endoscopy than in patients without esophagitis (2% and 0.36, respectively). For this reason, parallel to the former 2 studies,18,32 we also recommend that patients with erosive esophagitis determined on the first endoscopy should undergo a control endoscopy later in order to exclude the presence of BE.
BE is closely related with HH. HH is found in 72% to 96% of patients with BE.27,33 Rodriguez et al18 have found a higher prevalence of HH in patients with diagnosis of BE on their second endoscopy than in patients undergoing their first endoscopy. Similarly in our study, the prevalence of BE in patients with HH was higher on the second endoscopy than on the first one (5.6% and 0.4%, respectively). Our study and the former study show a close relation between BE and HH.18
One of the important risk factors for BE is LES laxity. However, the effect of LES laxity on the prevalence of BE determined in following endoscopy of patients who were found to be BE-negative in their first endoscopy is unknown. In our study, the BE prevalence on the second endoscopy of patients who had formerly been BE-negative was higher in those with LES laxity than in those without (4.9% and 0.39%, respectively). Consequently, we think that LES laxity is a risk factor for development of BE.
Although BE is seen in advanced age, the age interval of its development is not exactly known. For this reason, endoscopy performed at very early ages may not reveal BE, and endoscopy at very advanced ages may reveal a tumor. Rodriguez et al18 have reported that the prevalence of BE is higher in patients undergoing their first and second endoscopy within 1 year than in those undergoing their second endoscopy 1 year after their first endoscopic examination. In our study, we found no significant difference in terms of BE prevalence between these groups of patients mentioned above (0.7% and 0.63%, respectively). Since the interval between the first and second examinations was relatively short, it is difficult to comment on the appropriate timing of the second endoscopic examination.
The prevalence of BE increases with age and reaches its peak between 5th and 6th decades of life.34,35 However, in our study, there was no significant difference in the prevalence of BE determined on the second endoscopic examination between patients under and over 50 (0.41% and 0.8%, respectively) years of age. Rodriguez et al18 have reported that suspicious BE on the second endoscopy was seen most frequently in the 50 to 59 age group. In our study, we found that the rates of BE in female and male patients on the second endoscopy were similar (0.62% and 0.7%, respectively). But, in our study, due to the less number of patients with BE, it would be hard to comment on the effects of age and gender on the necessity of a second endoscopic evaluation.
This study had some limitations. This was a retrospective study. All of the procedures have not been performed by the same endoscopists and it was possible for endoscopists to have a misdiagnosis on BE. As the squamocolumnar junction was displaced proximally in BE, BE can be missed, and if the endoscopist insufflates too much air, the gastric folds may be flattened and BE may be missed. Thus in our study 11 patients with a suspect of BE in endoscopy did not show columnar metaplasia in histological examination. In addition, because of the retrospective nature of the study we could not get information about the medications of the patients therefore cannot the rule out the effect of proton pump inhibitor use on the results. However, diagnosing BE not only according to endoscopic appearance but also with histological examination, the marked experience of the endoscopists performing the procedures and the habit of careful evaluation of esophagogastric junction by the endoscopists can overcome these theoretical deficiencies. The high number of patients beside the ones with tumor and BE in the initial examination, and assessing the risk factors concerning this group of patients were the strong sides of the study.
In conclusion, we have assessed that the development of BE was observed very rarely (0.66%) in the second endoscopy after a normal initial examination. According to this finding we do not think that a second endoscopic evaluation is necessary for BE screening among patients with a normal initial endoscopy.
Footnotes
Abbreviations: ASGE = American Society of Gastrointestinal Endoscopy, BE = Barrett esophagus, EAC = esophageal adenocarcinoma, GERD = gastroesophageal reflux disease, HH = hiatal hernia, LES = lower esophageal sphincter.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Hvid-Jensen F, Pedersen L, Drewes AM, et al. Incidence of adenocarcinoma among patients with Barrett's esophagus. N Engl J Med 2011; 365:1375–1383. [DOI] [PubMed] [Google Scholar]
- 2.Van der Veen AH, Dees J, Blankensteijn JD, et al. Adenocarcinoma in Barrett's oesophagus: an overrated risk. Gut 1989; 30:14–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drewitz DJ, Sampliner RE, Garewal HS. The incidence of adenocarcinoma in Barrett's esophagus: a prospective study of 170 patients followed 4.8 years. Am J Gastroenterol 1997; 92:212–215. [PubMed] [Google Scholar]
- 4.Solaymani-Dodaran M, Logan RF, West J, et al. Risk of oesophageal cancer in Barrett's oesophagus and gastro-oesophageal reflux. Gut 2004; 53:1070–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thrift AP, Kramer JR, Qureshi Z, et al. Age at onset of GERD symptoms predicts risk of Barrett's esophagus. Am J Gastroenterol 2013; 108:915–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Di Caro S, Cheung WH, Fini L, et al. Role of body composition and metabolic profile in Barrett's oesophagus and progression to cancer. Eur J Gastroenterol Hepatol 2016; 28:251–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thompson OM, Beresford SA, Kirk EA, et al. Serum leptin and adiponectin levels and risk of Barrett's esophagus and intestinal metaplasia of the gastroesophageal junction. Obesity (Silver Spring) 2010; 18:2204–2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharma P, McQuaid K, Dent J, et al. A critical review of the diagnosis and management of Barrett's esophagus: the AGA Chicago workshop. Gastroenterology 2004; 127:310–330. [DOI] [PubMed] [Google Scholar]
- 9.Lundell LR, Dent J, Bennet JR, et al. Endoscopic assessment of oesophagitis: clinical and functional correlated and further validation of the Los Angeles classification. Gut 1999; 45:172–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hill LD, Kozarek RA, Kraemer SJ, et al. The gastroesophageal flap valve: in vitro and in vivo observations. Gastrointest Endosc 1996; 44:541–547. [DOI] [PubMed] [Google Scholar]
- 11.Bhat S, Coleman HG, Yousef F, et al. Risk of malignant progression in Barrett's esophagus patients: results from a large population-based study. J Natl Cancer Inst 2011; 103:1049–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wani S, Falk G, Hall M, et al. Patients with nondysplastic Barrett's esophagus have low risks for developing dysplasia or esophageal adenocarcinoma. Clin Gastroenterol Hepatol 2011; 9:220–227. [DOI] [PubMed] [Google Scholar]
- 13.Desai TK, Krishnan K, Samala N, et al. The incidence of oesophageal adenocarcinoma in non-dysplastic Barrett's oesophagus: a meta-analysis. Gut 2012; 61:970–976. [DOI] [PubMed] [Google Scholar]
- 14.Thomas T, Abrams KR, De Caestecker JS, et al. Meta analysis: cancer risk in Barrett's oesophagus. Aliment Pharmacol Ther 2007; 26:1465–1477. [DOI] [PubMed] [Google Scholar]
- 15.Coleman HG, Bhat SK, Murray LJ, et al. Symptoms and endoscopic features at Barrett's esophagus diagnosis: implications for neoplastic progression risk. Am J Gastroenterol 2014; 109:527–534. [DOI] [PubMed] [Google Scholar]
- 16.Sikkema M, Looman CW, Steyerberg EW, et al. Predictors for neoplastic progression in patients with Barrett's esophagus: a prospective cohort study. Am J Gastroenterol 2011; 106:1231–1238. [DOI] [PubMed] [Google Scholar]
- 17.Rugge M, Zaninotto G, Parente P, et al. Barrett's esophagus and adenocarcinoma risk: the experience of the North-Eastern Italian Registry (EBRA). Ann Surg 2012; 256:788–794. [DOI] [PubMed] [Google Scholar]
- 18.Rodriguez S, Mattek N, Lieberman D, et al. Barrett's esophagus on repeat endoscopy: should we look more than once? Am J Gastroenterol 2008; 103:1892–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Modiano N, Gerson LB. Barrett's esophagus: incidence, etiology, pathophysiology, prevention and treatment. Ther Clin Risk Manag 2007; 3:1035–1145. [PMC free article] [PubMed] [Google Scholar]
- 20.Sollano JD, Wong SN, Andal-Gamutan T, et al. Erosive esophagitis in the Philippines: a comparison between two time periods. J Gastroenterol Hepatol 2007; 22:1650–1655. [DOI] [PubMed] [Google Scholar]
- 21.Odemiş B, Ciçek B, Zengin NI, et al. Barrett's esophagus and endoscopically assessed esophagogastric junction integrity in 1000 consecutive Turkish patients undergoing endoscopy: a prospective study. Dis Esophagus 2009; 22:649–655. [DOI] [PubMed] [Google Scholar]
- 22.Yilmaz N, Tuncer K, Tunçyürek M, et al. The prevalence of Barrett's esophagus and erosive esophagitis in a tertiary referral center in Turkey. Turk J Gastroenterol 2006; 17:79–83. [PubMed] [Google Scholar]
- 23.Bayrakçi B, Kasap E, Kitapçioğlu G, et al. Low prevalence of erosive esophagitis and Barrett esophagus in a tertiary referral center in Turkey. Turk J Gastroenterol 2008; 19:145–151. [PubMed] [Google Scholar]
- 24.Eloubeidi M, Provenzale D. Does this patient have Barrett's esophagus? The utility of predicting Barrett's esophagus at the index endoscopy. Am J Gastroenterol 1999; 94:937–943. [DOI] [PubMed] [Google Scholar]
- 25.Lee YC, Cook MB, Bhatia S, et al. Interobserver reliability in the endoscopic diagnosis and grading of Barrett's esophagus: an Asian multinational study. Endoscopy 2010; 42:699–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Allison PR, Johnstone AS. The oesophagus lined with gastric mucous membrane. Thorax 1953; 8:87–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zagari RM, Fuccio L, Wallander MA, et al. Gastro-oesophageal reflux symptoms, oesophagitis and Barrett's oesophagus in the general population: the Loiano-Monghidoro study. Gut 2008; 57:1354–1359. [DOI] [PubMed] [Google Scholar]
- 28.Souza RF, Shewmake K, Terada LS, et al. Acid exposure activates the mitogen-activated protein kinase pathways in Barrett's esophagus. Gastroenterology 2002; 122:299–307. [DOI] [PubMed] [Google Scholar]
- 29.Jovov B, Van Itallie CM, Shaheen NJ, et al. Claudin-18: a dominant tight junction protein in Barrett's esophagus and likely contributor to its acid resistance. Am J Physiol Gastrointest Liver Physiol 2007; 293:G1106–G1113. [DOI] [PubMed] [Google Scholar]
- 30.Nguyen TH, Thrift AP, Ramsey D, et al. Risk factors for Barrett's esophagus compared between African Americans and non-Hispanic Whites. Am J Gastroenterol 2014; 109:1870–1880. [DOI] [PubMed] [Google Scholar]
- 31.Lee TY, Lien HC, Chang CS, et al. Barrett's esophagus and severe reflux esophagitis share common pathophysiological characteristics among Chinese in Taiwan. Intern Med 2008; 47:1767–1773. [DOI] [PubMed] [Google Scholar]
- 32.Hanna S, Rastogi A, Weston AP, et al. Detection of Barrett's esophagus after endoscopic healing of erosive esophagitis. Am J Gastroenterol 2006; 101:1416–1420. [DOI] [PubMed] [Google Scholar]
- 33.Andrici J, Tio M, Cox MR, et al. Hiatal hernia and the risk of Barrett's esophagus. J Gastroenterol Hepatol 2013; 28:415–431. [DOI] [PubMed] [Google Scholar]
- 34.Rubenstein JH, Mattek N, Eisen G. Age- and sex-specific yield of Barrett's esophagus by endoscopy indication. Gastrointest Endosc 2010; 71:21–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rubenstein JH, Morgenstern H, Appelman H, et al. Prediction of Barrett's esophagus among men. Am J Gastroenterol 2013; 108:353–362. [DOI] [PMC free article] [PubMed] [Google Scholar]


