Abstract
Amyotrophic lateral sclerosis (ALS) is a devastating progressive neurodegenerative disease with no effective treatment and death within 2 to 5 years after symptom onset. Here, we reported a case of ALS patient using modified Dihuang Yinzi (DHYZ), a classical traditional Chinese medicine (TCM) prescription, who has survived 12 years with significant improvement in bulbar paralysis.
A 41-year-old Chinese Han nationality woman was admitted to the hospital with complaints of weakened bilateral grip, slurred speech, stumbling, and muscle twitching for 3 years. The electromyography showed neurogenic injury in bilateral upper limbs and tongue. She was diagnosed with ALS according to the revised El escorial criteria. The patient was orally administrated with Riluzole 100 mg daily for 10 months and then stopped. Subsequently, she resorted to TCM. Based on the TCM theory, the patient was diagnosed with Yinfei syndrome because of kidney deficiency. DHYZ was chosen because it has the function of replenishing kidney essence to treat Yinfei syndrome. Up to now, she has been using modified DHYZ continuously for 12 years. The patient survived with ALS and did not require permanent continuous ventilator. In addition, the symptoms of choking on liquids are improved, and the utility of 30 mL water swallow test was improved with grade 2. The symptoms of muscle fibrillations of limbs are also reduced. However, muscle strength worsened slowly. The repeated electromyography showed motor conduction amplitude reducing gradually and velocity not changing more when compared with the initial electromyography.
Our findings suggested that DHYZ can be potentially used in ALS patients because of its multi-targeted neuroprotection and general safety, although ALS does not have a cure. In addition, we identified the area that is worthy of further study and DHYZ as a promising candidate for further clinical application and ALS trials. Rigorous randomized controlled trials are needed in the future.
INTRODUCTION
Amyotrophic lateral sclerosis (ALS) is a progressive neurodegenerative disorder characterized by relentless loss of motor neuron function.1 The selective degeneration of upper and lower motor neurons results in progressive weakness of the limb, bulbar, abdominal, and thoracic muscles. The reported annual prevalence and incidence rates of ALS were only 9.9 and 2.3 per 100,000 people per year respectively in Asian country, Japan.2 However, the importance of ALS should not be underestimated since there are more than one in 500 people who will die of the disease in the industrialized world.3 Without mechanical ventilation, most patients die within 2 to 5 years after symptom onset because of respiratory failure, although 5% to 10% of patients may live more than 10 years.4 Up to now, riluzole was the only drug approved by the Food and Drug Administration as neuroprotective treatment/disease-modifying treatment for ALS that has been shown to slightly slow the course of ALS.5 Oral administration of riluzole 100 mg daily for ALS patients is reasonably safe and prolongs a limited lengthening of median survival by ∼2 to 3 months after 18 months treatment.6 To our knowledge, other than riluzole, there are no other new treatment that can halt or reverse the progressive loss of neurons, leading to an increase of the ALS life expectancy. Given the fatalness of the illness and lack of effective neuroprotective treatment/disease-modifying agents, complementary and/or alternative medicine (CAM) is thus increasingly sought to treat ALS worldwide.7
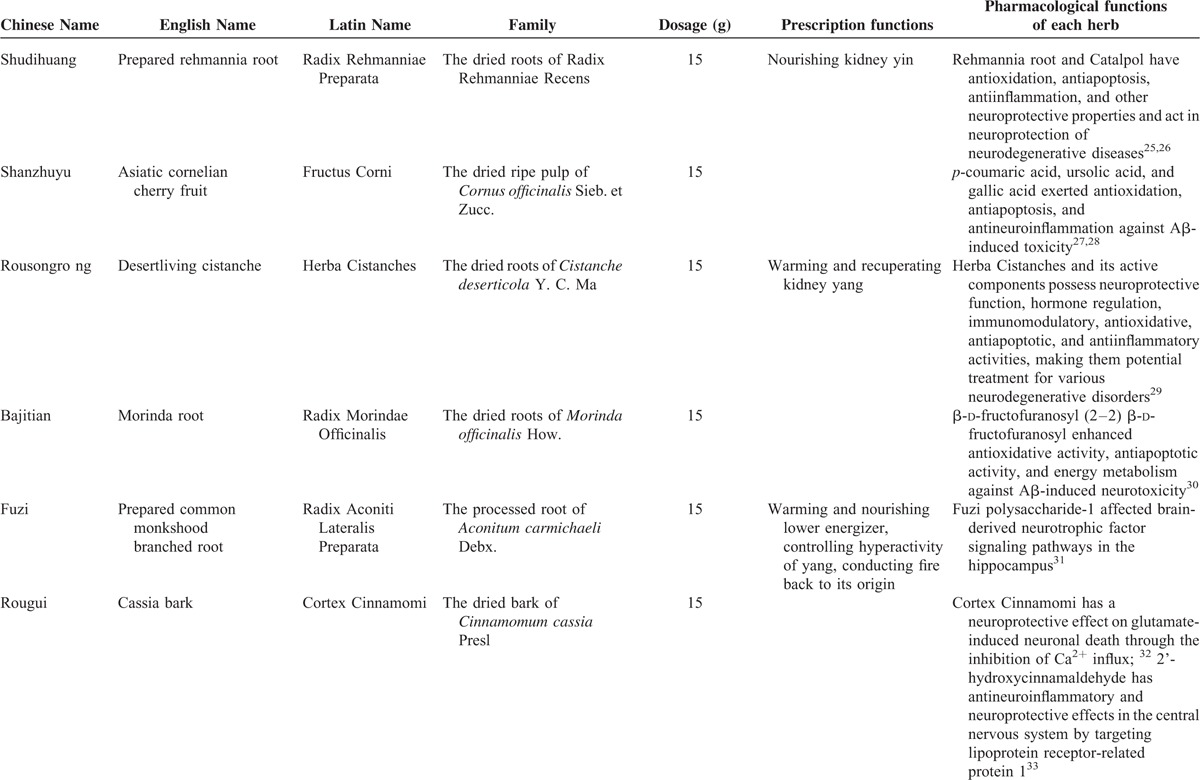
Traditional Chinese Medicine (TCM) is a main form of CAM originating in ancient China and has been practiced for a history of 3000 years. Accordingly, Chinese herbal medicines (CHMs) are frequently used in the treatment of ALS. Studies in vivo and in vitro showed that CHMs have a great potential for treatment of ALS, with neuroprotective function against excitatory amino acid toxicity, oxidative stress, calcium cytotoxicity, and neuroinflammation.8 During Song Dynasty (960–1279 AD), the TCM prescription book Shengji zonglu (Complete Record of Sacred Benevolence) compiled by Zhao Ji who was the eighth emperor of Song Dynasty of China in 1111 to 1117 AD. This book lists 20,000 TCM prescriptions and describes the causes, symptoms, and cures for different diseases. Dihuang Yinzi (DHYZ), a classical TCM prescription for neurological disorders Yinfei syndrome that centered on the symptoms of the speech and language disorders such as aphasis and logopathy (Yin syndrome) and disorders of motility such as motor paralysis and difficulty in walking (Fei syndrome), was first recorded in this book with the name Dihuang Yin. It is composed of 12 kinds of CHMs: (a) Radix Rehmanniae Preparata 15 g; prepared rehmannia root (Shudihuang), the dried roots of Radix Rehmanniae Recens; (b) Fructus Corni 15 g; asiatic cornelian cherry fruit (Shanzhuyu), the dried ripe pulp of Cornus officinalis Sieb. et Zucc.; (c) Herba Cistanches 15 g; desertliving cistanche (Rousongrong), the dried roots of Cistanche deserticola Y. C. Ma; (d) Radix Morindae Officinalis 15 g; morinda root (Bajitian), the dried roots of Morinda officinalis How.; (e) Radix Aconiti Lateralis Preparata 15 g; prepared common monkshood branched root (Fuzi), the processed root of Aconitum carmichaeli Debx.; (f) Cortex Cinnamomi 15 g; cassia bark (Rougui), the dried bark of Cinnamomum cassia Presl; (g) Herba Dendrobii 15 g; dendrobium (Shihu), the dried roots of Dendrobium loddigesli Rolfe. or Dendrobium fimbriatum Hook. var. oculatum Hook. or Dendrobium chrysanthum Wall. or Dendrobium officinale Kimra et Migo or Dendrobium nobile Lindl.; (h) Radix Ophiopogonis 15 g; dwarf lilyturf tuber (Maidong), the dried roots of Ophiopogon japonicus (Thunb.) Ker-Gawl.; (i) Fructus Schisandrae chinensis 15 g; Chinese magnoliavine fruit (Wuweizi), the dried ripe fruit of S chinensis (Turcz.) Baill.; (j) Rhizoma Acori Tatarinowii 15 g; grassleaf sweetflag rhizome (Shichangpu), the dried roots of Acorus tatarinowii Schott.; (k) Radix Polygalae 15 g; milkwort root (Yuanzhi), the dried roots of Polygala tenuifolia Willd. or Polygala sibirica L.; (l) Poria; Indian bread (Fuling) 15 g, the dried sclerotia of Poria cocos (Schw.) Wolf. (Table 1). In modern times, DHYZ is still used continuously and widely for treatment of neurological disorders such as stroke,9 Parkinson disease dementia,10 and spinal cord injury.11 Here, we reported a case of ALS patient using modified DHYZ who has survived 12 years with significant improvement in bulbar paralysis.
TABLE 1.
Overview of Dihuang Yinzi
CONSENT
Written informed consent was obtained from the patient before and after all procedures.
CASE PRSENTATION
Patient Information and Clinical Findings
On 31 July 2004, a 41-year-old Chinese Han nationality woman was admitted to the hospital with complaints of weakened bilateral grip, slurred speech, stumbling, and muscular twitchings for 3 years. The initial symptom, the intermittent weakness of right upper limb without numbness and pain, appeared in November 2001. The laryngological examination was normal. The magnetic resonance imaging (MRI) of head and cervical vertebrae were negative. After 6 months, uncontrollable twitching was observed in the right upper limb and the patient's speech became slurred. On 5 March 2002, the electromyography (EMG) done at the Huashan Hospital affiliated to Fudan University showed neurogenic injury in bilateral upper limbs and tongue (Table 2). Based on the revised El escorial criteria,12 clinically ALS was diagnosed. The patient was orally administrated with riluzole (50 mg, twice a day) for 10 months. She stopped the riluzole by herself because symptoms continuously deteriorated and could not bear the economic burden. On 31 July 2004, she was admitted to the hospital with weakness of limbs, dysarthria, dysphagia, and the clumsiness in daily activities. On examination, the four main vital signs, temperature, heart rate, blood pressure, and respiratory rate, were normal. The patient was alert with normal mental status. Cranial nerves examination showed the tongue muscle atrophy and tongue fasciculations. Motor system examination showed widespread muscle wasting and fasciculation of the bilateral thenar and interosseous muscles. Strength in the distal muscle of the upper bilateral limbs was of grade 2/5 to 3/5 and muscle strength in the lower bilateral limbs was of grade 3/5 to 4/5, graded on the Medical Research Council Muscle Strength Grading System.13 The sensory system was intact. Tendon reflexes were decreased with bilateral biceps, triceps, and brachioradialis. There were brisk reflexes of knee tendon and achilles tendon. The bilateral Hoffman signs were present, whereas the bilateral Babinski signs were not present. The utility of 30 mL water swallow test was grade 4. The laboratory blood test was generally normal. Her past drug history was not momentous except for the riluzole. The patient did not have the tobacco and alcohol consumption. She does not have a family history of ALS. TCM symptoms and signs were summarized as follows: sluggish speech, faint low voice, choke when drinking, weakness of limbs, muscle wasting of hands, muscular twitchings of upper limbs, pale facial complexion, soreness and weakness of waist and knees, excessive phlegm and saliva, constipation once every 3 days, pink tongue quality, tongue muscle atrophy and fibrillation, deep weak and thready pulse.
TABLE 1 (Continued).
Overview of Dihuang Yinzi

Diagnostic Assessment
The patient is a middle-aged woman. With muscles atrophy and fasciculations, the onset symptom is insidiously developing asymmetric upper limb weakness and then bulbar muscle. The clinical features are accompanied with the pathological signs: overactive tendon reflexes and clonus. The sensory system was intact. EMG studies showed typically neurogenic abnormalities. Based on the revisited El escorial criteria, she was diagnosed with clinically definite ALS, depending on the clinical manifestations and consistent electrodiagnostic studies. The clinical signs of myasthenia and muscular atrophy belong to Fei syndrome in TCM. When the patient experienced bulbar paralysis with symptoms such as dysarthria and dysphagia, the clinical features were classified to Yin syndrome in TCM. The kidney meridian dominates feet, throat, and tongue according to meridian theory of TCM. Thus, the kidney deficiency leads to Yinfei syndrome affecting both bulbar muscles and limbs muscles. Following the TCM theory, the patient was diagnosed with Yinfei syndrome because of kidney deficiency.
Therapeutic Intervention
Once the patient was diagnosed with ALS firstly in 2002, she was started on riluzole (50 mg, twice a day) for 10 months. When she noted that the treatment cannot arrest the disease condition and she cannot bear the economic burden, the patient discontinued treatment because riluzole is not a cure for ALS. In that time, she resorted to the TCM. Modified DHYZ was chosen because it has the function of replenishing kidney essence to treat Yinfei syndrome. The compositions and dosage of modified DHYZ are as follows: (a) Radix Rehmanniae Preparata (Shudihuang) 15 g; (b) Fructus Corni (Shanzhuyu) 12 g; (c) Herba Cistanches (Rousongrong) 15 g; (d) Herba Epimedii 15 g; epimedium herb (Yinyanghuo) the dried above ground parts of Epimedium brevicornum Maxim., Epimedium sagittatum (Sieb. et Zucc.) Maxim., Epimedium pubescens Maxim., Epimedium wushanensis T. S. Ying and Epimedium koreanum Nakai; (e) Radix Aconiti Lateralis Preparata (Fuzi) 6 g; (f) Radix Ophiopogonis (Maidong) 15 g; (g) Rhizoma Anemarrhenae 15 g; common anemarrhena rhizome(Zhimu), the dried rhizome of Anemarrhena asphodeloides Bge.; (h) Rhizoma Acori Tatarinowii (Shichangpu) 6 g; (i) Radix Polygalae (Yuanzhi) 6 g; (j) Fructus Trichosanthis seed 30 g; snakegourd seed (Gualouren), the dried seed of Trichosanthes kirilowii Maxim. or Trichosanthes rosthorinii Harms.; (k) Scorpio 6 g; scorpion (Quanxie), the dried body of artificial breeding of Buthus martensii Karsch.; (l) Agkistrodon 9 g; long-nosed pit viper (Qishe), the dried body of artificial breeding of Agkistrodon acutus (Guenther). The prescription was prepared from the water decoction and oral for twice daily and the patient has been using it continuously for 12 years.
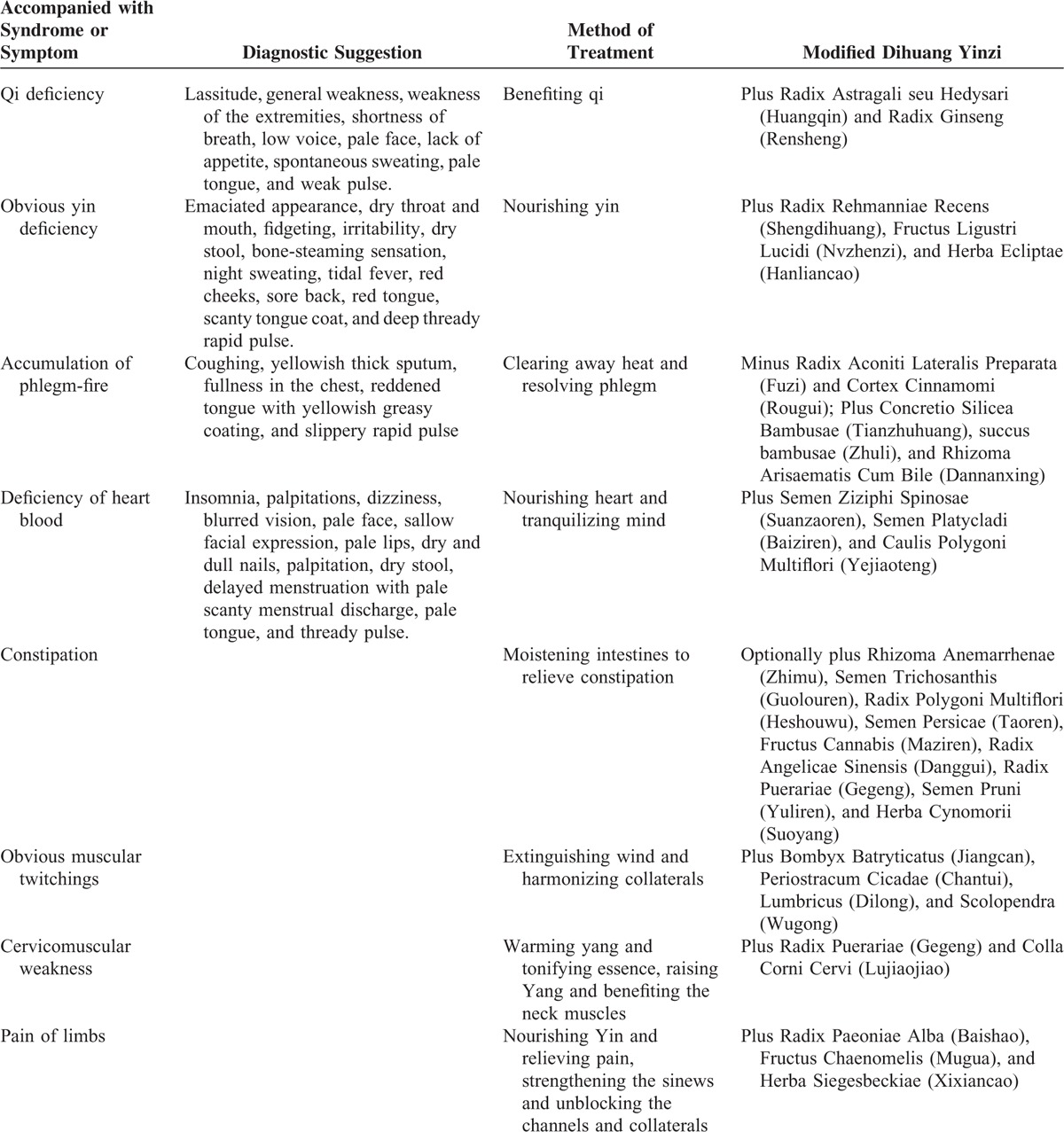
During the 12 years, DHYZ has been modified according to the mainly accompanied syndrome or symptoms as follows (Table 3): (1) Qi deficiency: plus Radix Astragali seu Hedysari, milkvetch root (Huangqin), the dried roots of Astragalus membranaceus (Fisch.) Bge. var. Mongolicus (Bge.) Hsiao or A membranaceus (Fisch.) Bge.; and Radix Ginseng, ginseng (Rensheng), the dried roots of Panax ginseng C. A. Mey.; (2) obvious yin deficiency: plus Radix Rehmanniae Recens, unprocessed rehmannia root (Shengdihuang), the dried roots of Rehmannia glutinosa Libosch.; Fructus Ligustri Lucidi, glossy privet fruit (Nvzhenzi), the dried ripe fruit of Ligustrum lucidum Ait.; and Herba Ecliptae, yerbadetajo herb (Hanliancao), the dried stems of Eclipta prostrata L.; (3) accumulation of phlegm-fire: minus Radix Aconiti Lateralis Preparata (Fuzi) and Cortex Cinnamomi (Rougui); Plus Concretio Silicea Bambusae, tabasheer (Tianzhuhuang), the saps from Bambusa textilis McClure or Schizostachyum chinense Rendle, succus bambusae; fresh bamboo sap (Zhuli), the saps from Bambusa tuldoides Munro or Sinocalamus beecheyanus (Munro) McClure var. pubescens P. F. Li or Phyllostachys nigra (Lodd.) Munro var. henonis (Mitf.) Stapf ex Rendle; and Rhizoma Arisaematis Cum Bile; bile arisaema (Dannanxing), the dried roots of Arisaema erubescens (Wall.) Schott or Arisaema heterophyllum Bl. or Arisaema amurense Maxim.; (4) deficiency of heart blood: plus Semen Ziziphi Spinosae, spine date seed (Suanzaoren), the dried ripe seeds of Ziziphus jujuba Mill. var. spinosa (Bunge) Hu ex H. F. Chou; Semen Platycladi, Chinese arborvitae kernel (Baiziren), the dried ripe seeds of Platycladus orientalis (L.) Franco; and Caulis Polygoni Multiflori, tuber fleeceflower stem (Yejiaoteng), the dried stems of Polygonum multiflorum Thunb.; (5) constipation: optionally plus Rhizoma Anemarrhenae (Zhimu); Semen Trichosanthis (Guolouren); Radix Polygoni Multiflori, fleeceflower root (Heshouwu), the dried roots of P multiflorum Thunb.; Semen Persicae, peach seed (Taoren), the dried seeds of Amygdalus persica L. or Amygdalus davidiana (Carr.) C. de Vos ex Henry; Fructus Cannabis, hemp seed (Maziren), the dried ripe fruit of Cannabis sativa L.; Radix Angelicae sinensis, Chinese angelica (Danggui), the dried roots of A sinensis (Oliv.) Diels; Radix Puerariae, kudzuvine root (Gegeng), the dried roots of Pueraria lobata (Willd.) Ohwi or Pueraria thomsonii Benth; Semen Pruni, Chinese dwarf cherry seed (Yuliren), the dried ripe seeds of Cerasus humilis (Bge.) Sok. or Cerasus japonica (Thunb.) Lois. or Amygdalus pedunculata Pall.; and Herba Cynomorii, songaria cynomorium herb (Suoyang), the dried stems of Cynomorium songaricum Rupr.; (6) obvious muscular twitchings: plus Bombyx Batryticatus, stiff silkworm (Jiangcan), the dried body of Bombyx mori Linnaeus.; Periostracum Cicadae, cicada slough (Chantui), the dried shell of Cryptotympana pustulata Fabricius; Lumbricus, earthworm (Dilong), the dried body of Pheretima aspergillum (E. Perrier) or Pheretima vulgaris Chen or Pheretima guillelmi (Michaelsen) or Pheretima pectinifera Michaelsen; Scolopendra, centipede (Wugong), the dried body of Scolopendra subspinipes mutilans L. Koch; (7) cervicomuscular weakness: plus Radix Puerariae (Gegeng); and Colla Corni Cervi, deerhorn glue (Lujiaojiao), the glue of horn of Cervus nippon Temminck or Cervus elaphus L.; (8) pain of limbs: plus Radix Paeoniae Alba, white peony root (Baishao), the dried stems of paeonia lactiflora pall.; Fructus Chaenomelis, common floweringqince fruit (Mugua), the dried ripe fruit of Chaenomeles speciosa (Sweet) Nakai; and Herba Siegesbeckiae, siegesbeckia herb (Xixiancao), the dried stems of Siegesbeckia orientalis L. or Siegesbeckia pubescens Makino or Siegesbeckia glabrescens Makino.
TABLE 2.
The Electromyography Examination
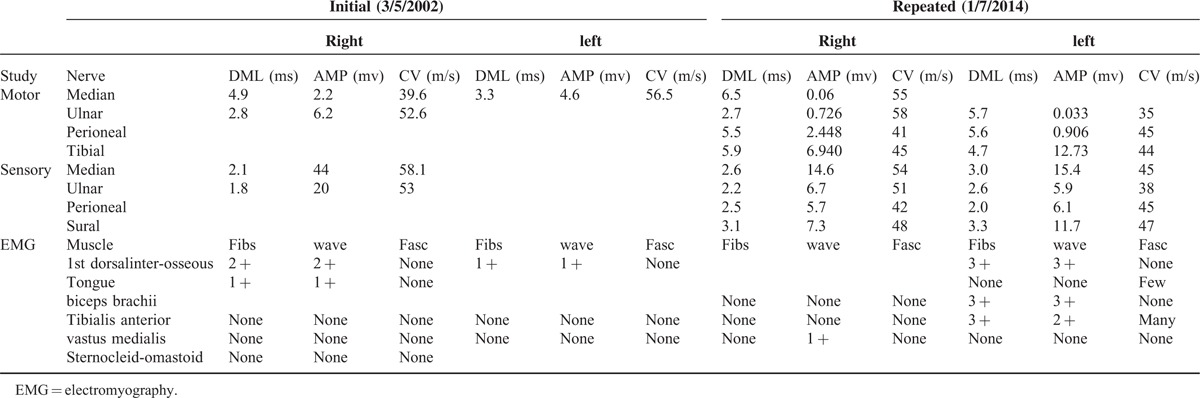
TABLE 3.
Accompanied with Syndrome or Symptom and Corresponding Modified Dihuang Yinzi for Amyotrophic Lateral Sclerosis
Follow-up and Outcomes
After 12-year treatment and follow-up, no obvious adverse event occurred during the treatment period. In addition, the patient still survived with ALS and does not require permanent continuous ventilator till today (Figure 1). The symptoms of choking on liquids are improved, and the utility of 30 mL water swallow test was improved with grade 2. The symptoms of muscle twitching of limbs were also reduced. However, muscle strength worsened slowly as follows: tongue muscle atrophy and tongue fasciculations, the slurring of speech, difficulty in communication and use of facial expression, difficulty in activity with both hands and in neck-lifting, muscle wasting of limbs, and presenting with claw hand. Strength in the distal and proximal muscle of the upper bilateral limbs was of grade 0/5 and 1/5 to 2/5 respectively, and muscle strength in the lower bilateral limbs was of grade 2/5, graded on the Medical Research Council Muscle Strength Grading System. At last, we will follow-up the patient continuously. On 7 January 2014, the repeated EMG showed motor conduction amplitude reducing gradually and velocity not changing more when compared with the initial EMG (Table 2).
FIGURE 1.
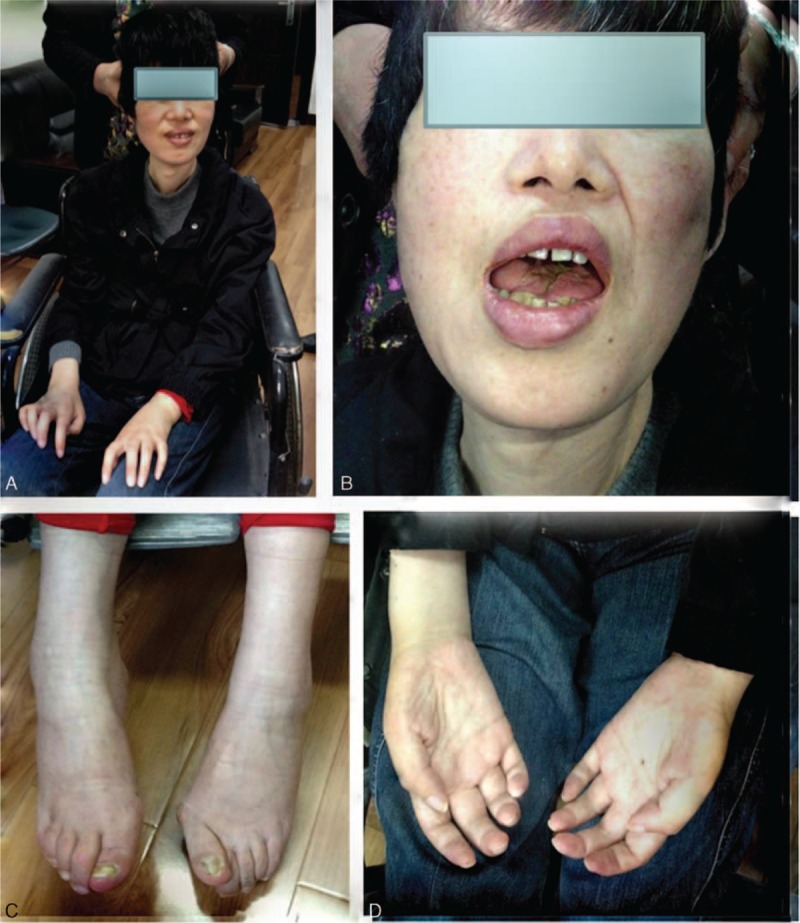
A case of amyotrophic lateral sclerosis patient was treated by using a classical Chinese herbal prescription Dihuang Yinzi, who has survived 12 years with significant improvement in bulbar paralysis and don’t require permanent continuous ventilator till now. (A) The patient's appearance; (B) The patient's tongue muscle atrophy; (C) The patient's feet; (D) The patient's palms.
DISCUSSION
This is a long-term follow-up study on a case with ALS treated by TCM prescription. A middle-aged woman was diagnosed with ALS based on the revised El escorial criteria. After consuming orally administrated riluzole 100 mg daily for 10 months, she stopped this drug and then started DHYZ that she has been using for 12 years. The main findings were that DHYZ therapy for ALS may potentially improve bulbar paralysis, delay use of ventilator support, and prolong survival time; there were fewer adverse effects.
ALS is an adult-onset fatal neurodegenerative disorder affecting motor neurons. Approximately 90% of ALS cases are sporadic, but the remaining 10% of the cases are familial.1 The mean age of onset is 58 to 63 years in sporadic and 43 to 52 years in familial cases of ALS.14 Only 5% of the cases of ALS have an onset <30 years of age.15 An increased risk in the sex incidence ratio was male:female = 1.5:1.2 In the present case, a female suffering from ALS was of age 38 years in sporadic who presented with limb onset and subsequently bulbar symptoms.
ALS is primarily a clinically diagnosed disease because of the lack of an established biological marker. Diagnosis of ALS is usually straightforward according to the progressive, generalized symptoms in the limb and bulbar regions.16 This can result in a delay in diagnosis because ALS inclines to be focal in onset presented with few clinical signs in the early disease course. The mean lag time between the onset of symptoms of ALS and confirmation of the diagnosis is 10 to 18 months.17 In 1994, the El Escorial criteria for diagnosing ALS clinically were published by a subcommittee on ALS of the world federation of neurology and the revisited criteria were revised in 1999, which included the clinical neurophysiolgical measurements as diagnostic tools to exclude differential diagnosis.12 In 2006, the Awaji criteria18 placed equal emphasis on both electromyographic evidence of degeneration and clinical abnormalities, thereby potentially enabling an earlier secure diagnosis of ALS, and this criterion has successfully increased in sensitivity to detect patients with ALS but additionally showed that this is achieved without increasing the number of false-positives.19 In 2015, an updated version of El Escorial criteria for the diagnosis of ALS was published with the purpose of both clinical practice and clinical trial.20 The new diagnostic criteria of ALS require at least one of the following: (1) progressive upper and lower motor neuron deficits in at least one limb or region of the human body; that is,that is meeting the revised El Escorial criteria for possible ALS; (2) lower motor neuron deficits as defined by clinical examination (one region) and/or by EMG in two body regions (defined as bulbar, cervical, thoracic, and lumbosacral). The EMG findings consist of neurogenic potentials and fibrillation potentials and/or sharp waves. In the present study, the patient was diagnosed with ALS according to revised El Escorial criteria 2000 and confirmed both clinical and electrophysiological evidences during long-term follow-up. Electrophysiological evaluation is important for the diagnosis of ALS. As for this patient, repeated investigations were required and the diagnosis can be confirmed with disease progression over time. In the initial EMG, the damages of different extents occurred in motor nerves which were controlled by neurogenic changed muscle of the patient. The patient's sensory nerve conduction was normal. Motor conduction velocity in the upper and lower limbs was almost normal despite the amplitude decreased in the initial and repeated examination, because the primary abnormality of the peripheral nerve was axonal loss, rather than demyelination. It is critical that tongue innervated by a cranial nerve demonstrated evidence of acute reinnervation confirmed the diagnosis for the positive changes of resting potential, for example, fibs and wave. From the repeated EMG, we can find motor conduction amplitude reducing gradually and velocity not changing more when compared with the initial EMG.
Despite advances in the treatments and interventions, there are no medications that stop or reverse the progressive loss of motor neurons of ALS because of uncertainty on the pathogenic mechanisms underlying degeneration of motor neuron. Riluzole remains the only available neuroprotective/disease-modifying drug for ALS, with only marginal effects on disease survival.5 Although still incurable, ALS is not untreatable. Over the past two decades, remarkable progression in integrative and aggressive supportive care has altered the quality of life of ALS patient. In addition, emphasis has been made in therapies that may even improve the disease course of ALS. Presently, ALS is considered as a complex disease with broad pathophysiological framework and numerous theories, including oxidative stress, glutamate and neuronal cytotoxicity, protein aggregation, mitochondrial impairment, cytoskeletal disruption, inflammation, apoptotic cell death, and altered regulation of gene expression.21 Thus, combined therapies that focus on more than one pathogenic pathway may slow disease progression in multiple targets/organs interactions. Impressively, the key to TCM prescription is to choose a combination of CHMs guided the combinatorial principle of Sovereign-Minister-Assistant-Envoy according to the patient's syndrome in order to regain the balance state of body functions.22 Over the past decades, many experimental and clinical studies demonstrated TCM prescriptions; herbal components may have multiple targets and exert neuroprotection or treatment of ALS.8,23 Pharmacological studies indicated that DHYZ exerts neuroprotective function. For example, DHYZ possesses neuroprotective and antidementia properties through preventing the loss of neural cells and synapses in rats of ischemic brain injury.24 Many studies have demonstrated that each ingredient or its active components of DHYZ exerted potential neuroprotective functions (Table 1). Rehmannia root and Catalpol have antioxidation, antiapoptosis, antiinflammation, and other neuroprotective properties and act in neuroprotection of neurodegenerative diseases.25,26p-coumaric acid, ursolic acid, and gallic acid from Corni fructus exerted antioxidation, antiapoptosis, and antineuroinflammation against Aβ-induced toxicity.27,28 Herba Cistanches and its active components possess neuroprotective function, hormone regulation, immunomodulatory, antioxidative, antiapoptotic, and antiinflammatory activities, making them potential treatment for various neurodegenerative disorders.29 β- d-fructofuranosyl (2–2) β-d-fructofuranosyl from Radix Morindae Officinalis enhanced antioxidative activity, antiapoptotic activity, and energy metabolism against Aβ-induced neurotoxicity.30 Fuzi polysaccharide-1 affected brain-derived neurotrophic factor-signaling pathways in the hippocampus.31 Cortex Cinnamomi has a neuroprotective effect on glutamate-induced neuronal death through the inhibition of Ca2+ influx;32 2′-hydroxycinnamaldehyde has antineuroinflammatory and neuroprotective effects in the central nervous system by targeting lipoprotein receptor-related protein 1.33 An active compound from Dendrobium and Chrysotoxine exhibit antioxidant, antiapoptosis, and neuroprotective activities against neurotoxion-induced cell death.34,35O japonicus displays antiinflammatory, antioxidative, and immunomodulative activities.36 Schisantherin A, schisandrin B, and schisandrin C from Fructus Schisandrae Chinensis attenuate abnormal oxidative stress and modulate the apoptotic signal pathway, exhibiting their neuroprotective function on neurodegenerative diseases.37,38 β-asarone and eugenol, components of Rhizoma Acori Tatarinowii, have neuroprotective function cells through inhibiting apoptosis against Aβ- induced neurotoxicity.39 Polygalae radix has neuroprotective effects against toxin-induced neuronal death through its antioxidant and antiapoptotic activities;40 its ethanol extracts as a novel autophagy inducer modulate neurodegenerative disorders via reducing toxicity and clearing of mutant proteins in the cellular level.41P cocos water extract has neuroprotective action against Aβ-induced neuronal injury through suppressing the oxidative stress and the apoptosis.42
In conclusion, we reinforce that TCM prescription, especially DHYZ, can be potentially used in ALS patients because of its multitargeted neuroprotection and general safety, although ALS has not a cure. In addition, we identified the area that is worthy of further study and DHYZ as a promising candidate for further clinical application and ALS trials. Further rigorous randomized controlled trials are needed.
Acknowledgments
The authors specially thank Professor C-LQ as a chief physician who treated this patient suffering from amyotrophic lateral sclerosis.
Footnotes
Abbreviations: ALS = amyotrophic lateral sclerosis, CAM = complementary and/or alternative medicine, CHM = Chinese herbal medicines, DHYZ = Dihuang Yinzi, EMG = electromyography, MRI = magnetic resonance imaging, TCM = traditional Chinese medicine.
Funding: this project was supported by the grant of national project on inheritance workshop of famous TCM experts (GZYYRJF, 2012149, C.-l. Q.); the Young and Middle-Aged University Discipline Leaders of Zhejiang Province, China (2013277, G.-q. Z.); Zhejiang Provincial Program for the Cultivation of High-level Health talents (2015, G.-q. Z.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors have no conflicts of interest to disclose.
This is an open access article distributed under the Creative Commons Attribution License 4.0, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
REFERENCES
- 1.Poppe L, Rué L, Robberecht W, et al. Translating biological findings into new treatment strategies for amyotrophic lateral sclerosis (ALS). Exp Neurol 2014; 262 (Pt B):138–151. [DOI] [PubMed] [Google Scholar]
- 2.Doi Y, Atsuta N, Sobue G, et al. Prevalence and incidence of amyotrophic lateral sclerosis in Japan. J Epidemiol 2014; 24:494–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ludolph AC, Bendotti C, Blaugrund E, et al. Guidelines for preclinical animal research in ALS/MND: a consensus meeting. Amyotroph Lateral Scler 2010; 11:38–45. [DOI] [PubMed] [Google Scholar]
- 4.Forsgren L, Almay BG, Holmgren G, et al. Epidemiology of motor neuron disease in northern Sweden. Acta Neurol Scand 1983; 68:20–29. [DOI] [PubMed] [Google Scholar]
- 5.Andersen PM, Abrahams S, Borasio GD, et al. EFNS Task Force on Diagnosis and Management of Amyotrophic Lateral Sclerosis. EFNS guidelines on the clinical management of amyotrophic lateral sclerosis (MALS)—revised report of an EFNS task force. Eur J Neurol 2012; 19:360–375. [DOI] [PubMed] [Google Scholar]
- 6.Miller RG, Mitchell JD, Moore DH. Riluzole for amyotrophic lateral sclerosis (ALS)/motor neuron disease (MND). Cochrane Database Syst Rev 2012; 3:CD001447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bedlack RS, Joyce N, Carter GT, et al. Complementary and alternative therapies in amyotrophic lateral sclerosis. Neurol Clin 2015; 33:909–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang X, Hong YL, Xu DS, et al. A review of experimental research on herbal compounds in amyotrophic lateral sclerosis. Phytother Res 2014; 28:9–21. [DOI] [PubMed] [Google Scholar]
- 9.Yu M, Sun ZJ, Li LT, et al. The beneficial effects of the herbal medicine Di-Huang-Yin-Zi (DHYZ) on patients with ischemic stroke: a randomized, placebo controlled clinical study. Complement Ther Med 2015; 23:591–597. [DOI] [PubMed] [Google Scholar]
- 10.Gu C, Shen T, An HM, et al. Combined therapy of Di-Huang-Yi-Zhi with Donepezil in patients with Parkinson's disease dementia. Neurosci Lett 2015; 606:13–17. [DOI] [PubMed] [Google Scholar]
- 11.Li YL, Li LT, Yu M, et al. Beneficial effects of the herbal medicine Di Huang Yin Zi in patients with spinal cord injury: a randomized, placebo-controlled clinical study. J Int Med Res 2012; 40:1715–1724. [DOI] [PubMed] [Google Scholar]
- 12.Brooks BR, Miller RG, Swash M, et al. World Federation of Neurology Research Group on Motor Neuron Diseases. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord 2000; 1:293–299. [DOI] [PubMed] [Google Scholar]
- 13.James MA. Use of the Medical Research Council muscle strength grading system in the upper extremity. J Hand Surg Am 2007; 32:154–156. [DOI] [PubMed] [Google Scholar]
- 14.Haverkamp LJ, Appel V, Appel SH. Natural history of amyotrophic lateral sclerosis in a database population. Validation of a scoring system and a model for survival prediction. Brain 1995; 118:707–719. [DOI] [PubMed] [Google Scholar]
- 15.Logroscino G, Traynor BJ, Hardiman O, et al. Descriptive epidemiology of amyotrophic lateral sclerosis: new evidence and unsolved issues. J Neurol Neurosurg Psychiatry 2008; 79:6–11. [DOI] [PubMed] [Google Scholar]
- 16.Li TM, Day SJ, Alberman E, et al. Differential diagnosis of motoneurone disease from other neurological conditions. Lancet 1986; 2:731–733. [PubMed] [Google Scholar]
- 17.Chio A, Mora G, Calvo A, et al. Epidemiology of ALS in Italy: a 10-year prospective population-based study. Neurology 2009; 72:725–731. [DOI] [PubMed] [Google Scholar]
- 18.de Carvalho M, Swash M. Awaji diagnostic algorithm increases sensitivity of El Escorial criteria for ALS diagnosis. Amyotroph Lateral Scler 2009; 10:53–57. [DOI] [PubMed] [Google Scholar]
- 19.Douglass CP, Kandler RH, Shaw PJ, et al. An evaluation of neurophysiological criteria used in the diagnosis of motor neuron disease. J Neurol Neurosurg Psychiatry 2010; 81:646–649. [DOI] [PubMed] [Google Scholar]
- 20.Ludolph A, Drory V, Hardiman O, et al. WFN Research Group on ALS/MND. A revision of the El Escorial criteria—2015. Amyotroph Lateral Scler Frontotemporal Degener 2015; 16:291–292. [DOI] [PubMed] [Google Scholar]
- 21.Goodall EF, Morrison KE. Amyotrophic lateral sclerosis (motor neuron disease): proposed mechanisms and pathways to treatment. Expert Rev Mol Med 2006; 8:1–22. [DOI] [PubMed] [Google Scholar]
- 22.Qiu J. Traditional medicine: a culture in the balance. Nature 2007; 448:126–128. [DOI] [PubMed] [Google Scholar]
- 23.Nabavi SF, Daglia M, D’Antona G, et al. Natural compounds used as therapies targeting to amyotrophic lateral sclerosis. Curr Pharm Biotechnol 2015; 16:211–218. [DOI] [PubMed] [Google Scholar]
- 24.Hu R, Yin CL, Wu N, et al. Traditional Chinese herb Dihuang Yinzi (DY) plays neuroprotective and anti-dementia role in rats of ischemic brain injury. J Ethnopharmacol 2009; 121:444–450. [DOI] [PubMed] [Google Scholar]
- 25.Jiang B, Shen RF, Bi J, et al. Catalpol: a potential therapeutic for neurodegenerative diseases. Curr Med Chem 2015; 22:1278–1291. [DOI] [PubMed] [Google Scholar]
- 26.Lee B, Shim I, Lee H, et al. Rehmannia glutinosa ameliorates scopolamine-induced learning and memory impairment in rats. J Microbiol Biotechnol 2011; 21:874–883. [DOI] [PubMed] [Google Scholar]
- 27.Yoon JH, Youn K, Ho CT, et al. p-Coumaric acid and ursolic acid from Corni fructus attenuated β-amyloid(25-35)-induced toxicity through regulation of the NF-κB signaling pathway in PC12 cells. J Agric Food Chem 2014; 62:4911–4916. [DOI] [PubMed] [Google Scholar]
- 28.Hong SY, Jeong WS, Jun M. Protective effects of the key compounds isolated from Corni fructus against β-amyloid-induced neurotoxicity in PC12 cells. Molecules 2012; 17:10831–10845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang T, Zhang X, Xie W, et al. Desert ginseng: a review. Am J Chin Med 2012; 40:1123–1141. [DOI] [PubMed] [Google Scholar]
- 30.Chen DL, Zhang P, Lin L, et al. Protective effects of bajijiasu in a rat model of Aβ-induced neurotoxicity. J Ethnopharmacol 2014; 154:206–217. [DOI] [PubMed] [Google Scholar]
- 31.Yan HC, Qu HD, Sun LR, et al. Fuzi polysaccharide-1 produces antidepressant-like effects in mice. Int J Neuropsychopharmacol 2010; 13:623–633. [DOI] [PubMed] [Google Scholar]
- 32.Shimada Y, Goto H, Kogure T, et al. Extract prepared from the bark of Cinnamomum cassia Blume prevents glutamate-induced neuronal death in cultured cerebellar granule cells. Phytother Res 2000; 14:466–468. [DOI] [PubMed] [Google Scholar]
- 33.Hwang H, Jeon H, Ock J, et al. 2′Hydroxycinnamaldehyde targets low-density lipoprotein receptor-related protein-1 to inhibit lipopolysaccharide-induced microglial activation. J Neuroimmunol 2011; 230:52–64. [DOI] [PubMed] [Google Scholar]
- 34.Yoon MY, Hwang JH, Park JH, et al. Neuroprotective effects of SG-168 against oxidative stress-induced apoptosis in PC12 cells. J Med Food 2011; 14:120–127. [DOI] [PubMed] [Google Scholar]
- 35.Song JX, Shaw PC, Wong NS, et al. Chrysotoxine, a novel bibenzyl compound selectively antagonizes MPP + , but not rotenone, neurotoxicity in dopaminergic SH-SY5Y cells. Neurosci Lett 2012; 521:76–81. [DOI] [PubMed] [Google Scholar]
- 36.Chen MH, Chen XJ, Wang M, et al. Ophiopogon japonicus—A phytochemical, ethnomedicinal and pharmacological review. J Ethnopharmacol 2016; 181:193–213. [DOI] [PubMed] [Google Scholar]
- 37.Zhang LQ, Sa F, Chong CM, et al. Schisantherin A protects against 6-OHDA-induced dopaminergic neuron damage in zebrafish and cytotoxicity in SH-SY5Y cells through the ROS/NO and AKT/GSK3β pathways. J Ethnopharmacol 2015; 170:8–15. [DOI] [PubMed] [Google Scholar]
- 38.Song JX, Lin X, Wong RN, et al. Protective effects of dibenzocyclooctadiene lignans from Schisandra chinensis against beta-amyloid and homocysteine neurotoxicity in PC12 cells. Phytother Res 2011; 25:435–443. [DOI] [PubMed] [Google Scholar]
- 39.Liang ZH, Cheng XH, Ruan ZG, et al. Protective effects of components of the Chinese herb grassleaf sweetflag rhizome on PC12 cells incubated with amyloid-beta42. Neural Regen Res 2015; 10:1292–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Choi JG, Kim HG, Kim MC, et al. Polygalae radix inhibits toxin-induced neuronal death in the Parkinson's disease models. J Ethnopharmacol 2011; 134:414–421. [DOI] [PubMed] [Google Scholar]
- 41.Wu AG, Wong VK, Xu SW, et al. Onjisaponin B derived from Radix Polygalae enhances autophagy and accelerates the degradation of mutant α-synuclein and huntingtin in PC-12 cells. Int J Mol Sci 2013; 14:22618–22641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Park YH, Son IH, Kim B, et al. Poria cocos water extract (PCW) protects PC12 neuronal cells from beta-amyloid-induced cell death through antioxidant and antiapoptotic functions. Pharmazie 2009; 64:760–764. [PubMed] [Google Scholar]


