Supplemental Digital Content is available in the text
Abstract
Nonthyroidal illness (NTI), often observed in critically ill patients, arises through diverse alterations in the hypothalamus-pituitary-thyroid (HPT) axis. However, the causal relationship between underlying disease and NTI diversity in critically ill patients is poorly understood.
The aim of this study was to examine NTI severity and adverse outcomes in critically ill patients with respect to their underlying disease(s).
The medical records of 616 patients admitted to the intensive care unit (ICU) between January 2009 and October 2014 were retrospectively reviewed. Patients with known diseases or taking medications that affect thyroid function were excluded. All-cause mortality (ACM) and length of stay (LOS) in the ICU were assessed as adverse outcomes.
The enrolled patients (n = 213) were divided into the following 4 groups according to the severity of NTI at the nadir of their thyroid function test (TFT): normal (n = 11, 5.2%), mild NTI (n = 113, 53.1%), moderate NTI (n = 78, 36.6%), and severe NTI (n = 11, 5.2%). There was no significant difference between the groups in terms of age and gender. NTI severity showed a significantly strong association with ACM (P < 0.0001) and a significant positive association with LOS in the ICU (P = 0.031). After adjusting for age, gender, and current medications affecting TFT, increasing NTI severity led to increased ACM (odds ratio = 3.101; 95% confidence interval = 1.711–5.618; P < 0.0001). Notably, the prevalence of moderate-to-severe NTI was markedly higher in patients with infectious disease than in those with noninfectious disease (P = 0.012). Consistent with this, serum C-reactive protein levels were higher in patients with moderate-to-severe NTI (P = 0.016).
NTI severity is associated with increased ACM, LOS, and underlying infectious disease. Future studies will focus on the biological and clinical implications of infectious disease on the HPT axis.
INTRODUCTION
Nonthyroidal illness (NTI) syndrome, also known as “sick euthyroid syndrome” or “low T3 syndrome,” can be described as variations in the results of thyroid function tests (TFTs) in the absence of hypothalamic-pituitary and thyroid gland dysfunction. The early adaptive stage of NTI usually presents as low triiodothyronine (T3) or thyroxine (T4) levels, without an increase in thyrotropin (TSH). In a clinical setting, NTI is frequently observed in patients with acute or chronic critical illnesses who require treatment in the intensive care unit (ICU).1–4 However, the pathophysiology of NTI is unclear.
During the acute phase of critical illness (e.g., postsurgery or severe physical stress), circulating levels of T3 fall rapidly. These changes are generated by reduced levels of thyroid hormone binding proteins such as thyroxine binding globulin and albumin; thyroid hormone is easily released from thyroid hormone binding proteins due to a reduction in their binding affinity, leading to incremental increases in the clearance of thyroid hormone.5 In addition, alterations in the peripheral conversion of T4, mainly due to decreased type-1 deiodinase (D1) activity and increased type-3 deiodinase (D3) activity, induce the generation of reverse (r)T3.6,7 Although all these changes reduce circulating T3 levels, plasma TSH concentrations usually remain normal.4
During a prolonged phase of critical illness, central suppression of the hypothalamus-pituitary-thyroid (HPT) axis occurs.8 As hypothalamic stimulation of the pituitary thyrotropes diminishes, the production and release of thyroid hormones is further reduced; this can resemble central hypothyroidism, characterized by low plasma levels of unbound T4 and/or low TSH concentrations. At this stage, pulsatile TSH secretion is almost abrogated.9 However, the mechanism underlying NTI progression from an adaptive response to a noncompensatory or maladaptive process that triggers hypothalamic suppression is poorly understood.3
Recent studies suggest that cytokines such as tumor necrosis factor-alpha (TNF-α), interferon-gamma (IFN-γ), interleukin (IL)-1, and IL-6 are potential mediators of NTI. These cytokines directly downregulate various components of the thyroid hormone synthesis pathway at the thyrocyte level10–16; however, no study has examined the role of cytokines in NTI progression in large human cohorts.
Because NTI in patients with a critical illness could be either adaptive or maladaptive,17 NTI during the acute phase of critical illness is beneficial; however, it may be deleterious during the prolonged phase. The latter interpretation stems from the observation that changes in plasma thyroid hormone levels are related to the risk of adverse outcomes.18–20 Here, we examined the correlation between NTI severity and adverse outcomes. In addition, we investigated NTI severity with respect to underlying disease.
METHODS
Study Population
The medical records of 616 patients admitted to the medical and surgical ICU at Severance Hospital, Yonsei College of Medicine (Seoul, South Korea) between January 2009 and October 2014 were retrospectively reviewed. All patients underwent a TFT (T3, fT4, TSH) while being treated in the ICU. Of the 616 patients, 403 were excluded under the following exclusion criteria: patients had subclinical or clinical thyrotoxicosis/hypothyroidism before admission to the ICU21; patients had pre-existing thyroid or pituitary-hypothalamic disease; patients had liver or renal disease, such as liver cirrhosis, nephrotic syndrome, and renal failure; patients had brain injury or surgery for brain tumor; or patients were taking levothyroxine, thyrostatic agents, or drugs that affect thyroid function (e.g., amiodarone, lithium, glucocorticoid, salicylates, furosemide, ferrous sulfate, phenytoin, carbamazepine, or phenobarbital). Finally, 213 patients, 202 with NTI (32.8%) and 11 with normal thyroid function (1.8%), were included in the study. The 202 NTI patients were divided into 3 groups according to NTI severity as previously proposed by a textbook22: mild NTI (n = 113), moderate NTI (n = 78), and severe NTI (n = 11) (Supplementary Table 1). The study was approved by the Institutional Review Board, which waived the requirement for informed consent because of the retrospective design of the study.
TFT, Biochemical Measurements, and Acute Inflammatory Indices
All blood samples were obtained after fasting patients for 12 hours. Serum-bound T3, free T4, and TSH were measured in a chemiluminescent microparticle immunoassay (Architect System; Abbott Ireland Diagnostic Division, Lisnamuck, Longford, Co. Longford, Ireland). The reference ranges were as follows: 0.58 to 1.59 ng/dL for bound T3, 0.70 to 1.48 ng/dL for free T4, and 0.35 to 4.94 μIU/mL for TSH. The most severe TFT results were analyzed. Serum total protein and albumin levels were measured using the Hitachi 7600 DDP analyzer (Hitachi High Technologies Co., Tokyo, Japan). Serum C-reactive protein (CRP) concentrations were measured using a nephelometric method (Beckman Coulter, Fullerton, CA). The erythrocyte sedimentation rate (ESR) was assessed using the Westergren method (Alifax, Padova, Italy). The reference ranges were as follows: 6 to 8 g/dL for total protein, 3.3 to 5.3 g/dL for albumin, 0 to 8 mg/L for CRP, and 0 to 20 mm/hour for ESR.
Diagnosis of Underlying Disease, Patient Outcome, and Drugs as Confounders
The main diagnoses upon admission to the ICU were recorded for analysis. In terms of patient outcome, all-cause mortality (ACM) and the length of stay (LOS) in the ICU were examined. Initiation of levothyroxine, hypothalamic-releasing factor, thyrostatic agent, dopamine, amiodarone, or steroid treatment in the ICU was also examined to determine whether NTI severity is an independent risk factor for a poor outcome.
Statistical Analysis
Continuous variables were compared using either the 2-tailed Student t test or analysis of variance (ANOVA) test, and categorical data were compared using either a 2-tailed χ2 test or Fisher's exact test. The relationship between NTI severity and ACM was analyzed using a multivariate logistic regression model adjusted for age, gender, and the use of drugs that can affect thyroid function. Pearson's correlation coefficient was used to examine the association between thyroid hormone levels and acute inflammatory indices. Statistical analyses were performed using Statistical Package for the Social Sciences (SPSS) software for Windows (Version 21.0; IBM Corp., NY) or GraphPad Prism (GraphPad Software, Inc., San Diego, CA). P value less than 0.05 was considered statistically significant.
RESULTS
Baseline Patient Characteristics
The baseline characteristics of the 213 patients with normal thyroid function or NTI are listed in Table 1. Of these, 11 had normal thyroid function and the other 202 had NTI, classed as mild (n = 113, 55.9%), moderate (n = 78, 38.6%), or severe (n = 11, 5.4%). There was no significant difference between the groups with respect to age and gender. As expected, NTI severity was closely related to T3, fT4, and TSH values (P < 0.0001). In addition, NTI severity also correlated with serum total protein (P < 0.0001) and albumin (P = 0.002) levels.
TABLE 1.
Baseline Characteristics of the Study Population
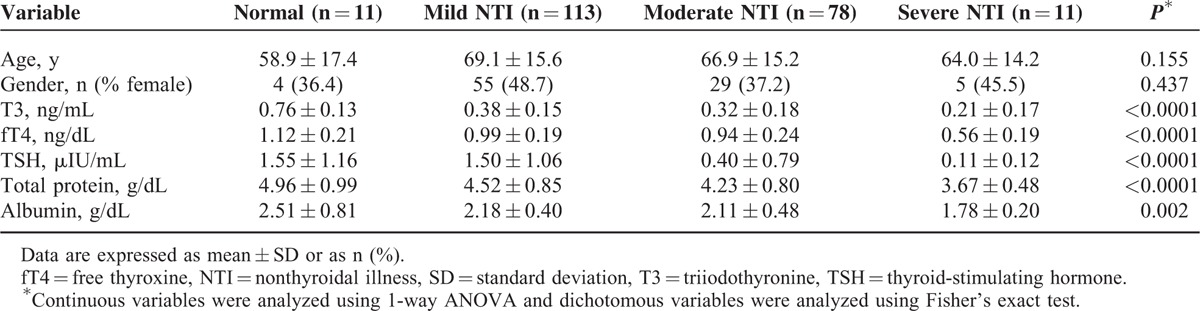
Association Between NTI Severity and Patient Outcome
We next examined the relationship between NTI severity and ACM and LOS. NTI severity was strongly associated with ACM (P < 0.0001; Figure 1A). In particular, ACM for patients with moderate or severe NTI was significantly higher than that for patients with mild NTI (P = 0.001; Figure 1A). LOS in the ICU was also related to NTI severity (P = 0.031; Figure 1B). To determine whether NTI is an independent risk factor for ACM, we used a multivariate logistic regression model adjusted for age, gender, and use of drugs that can affect thyroid function (such as dopamine, amiodarone, and steroids). Patients were split into 2 groups: Group I contained patients with normal or mild NTI, and Group II contained patients with moderate or severe NTI. According to model 1 (adjusted for age and gender), the risk of ACM was significantly higher in Group II [odds ratio (OR) = 3.519; 95% confidence interval (95% CI) = 1.975–6.271; P < 0.0001; Table 2]. According to model 2 (adjusted for age, gender, and the use of drugs that can affect thyroid function, prescribed after ICU admission), the risk of ACM was again higher for Group II (OR = 3.101; 95% CI = 1.711–5.618; P < 0.0001; Table 2).
FIGURE 1.
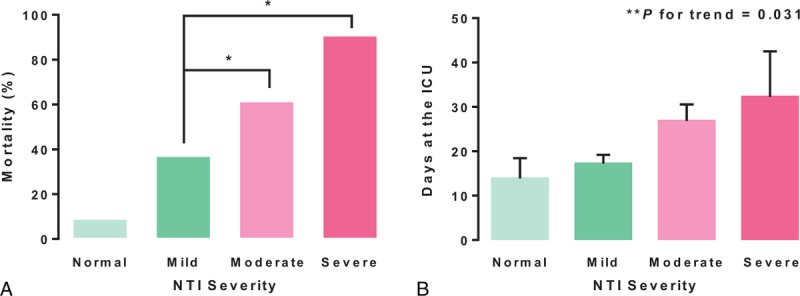
Relationship between NTI severity and patient outcome. (A) The severity of NTI was significantly associated with an increase in all-cause mortality (ACM). (B) The length of stay (LOS) in the ICU tended to be longer in the moderate-to-severe NTI group. The relationship between NTI severity and ACM and LOS was examined using the Chi-square test/Fisher's exact test and 1-way ANOVA, respectively. Error bars represent the standard error. ICU = intensive care unit; NTI = nonthyroidal illness. ∗P = 0.001. ∗∗P for trend was determined between the four groups.
TABLE 2.
Relationship Between NTI Severity and All-Cause Mortality After Adjusting for Confounders
NTI Severity and Infection
Because we observed a positive correlation between NTI severity and ACM and LOS, we next investigated the relationship between NTI and the underlying diseases that caused patients to be admitted to the ICU. NTI was more severe in patients with infectious diseases such as pneumonia and infections of nonlung origin (Table 3). To examine the effect of infection on NTI severity, we sorted the study patients into 2 groups: those with infectious disease and those with noninfectious disease (n = 75 and n = 138, respectively). There was a significant difference in CRP levels between those with infectious disease and those with noninfectious disease, indicating that the sorting was conducted appropriately (Supplementary Table 2). The percentage of patients with moderate or severe NTI was significantly higher in the infectious disease group (P = 0.012); in particular, severe NTI was only observed in the infectious disease group (Figure 2).
TABLE 3.
NTI Severity According to Underlying Disease
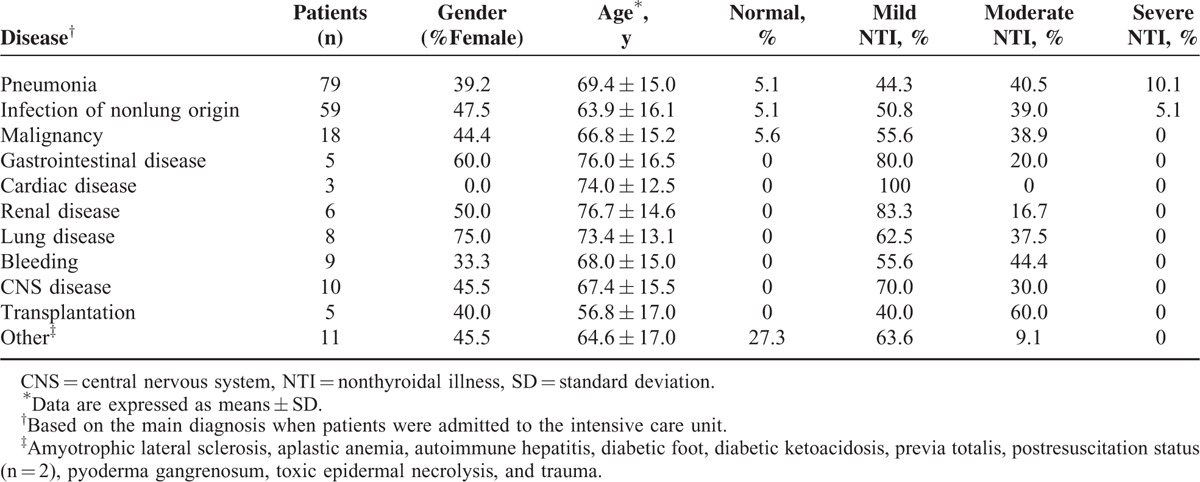
FIGURE 2.
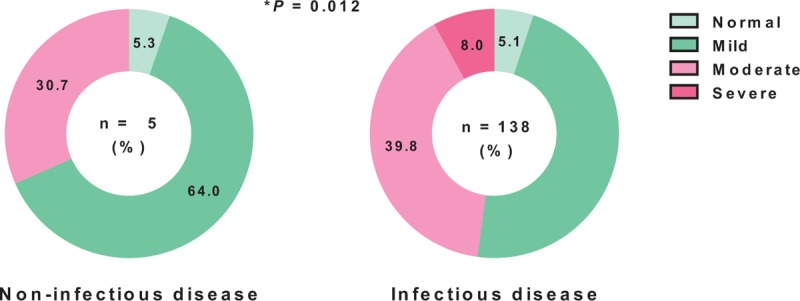
NTI severity in patients with infectious disease and noninfectious disease. NTI was significantly more severe in the patients with infectious disease than in those with noninfectious disease. Data were examined using Fisher's exact test. NTI = nonthyroidal illness.
Association Between NTI Severity and Acute Inflammatory Indices
To verify the relationship between NTI severity and infectious disease, we examined the relationship between NTI severity and acute inflammatory indices such as CRP levels and the ESR. CRP levels tended to increase according to NTI severity (P = 0.045; Figure 3A, upper panel). In addition, CRP levels were significantly higher in NTI group II than in NTI group I (P = 0.016; Figure 3A, lower panel). By contrast, there was no significant difference in ESR levels between the 2 groups. CRP levels in the infectious disease group also tended to increase with NTI severity, although the difference was not statistically significant (Supplementary Figure 1). Finally, we performed bivariate correlation analysis to explore the correlation between thyroid hormone and CRP levels, and found that T3 levels negatively correlated with CRP levels (r = −0.199; P = 0.004; Figure 3B).
FIGURE 3.
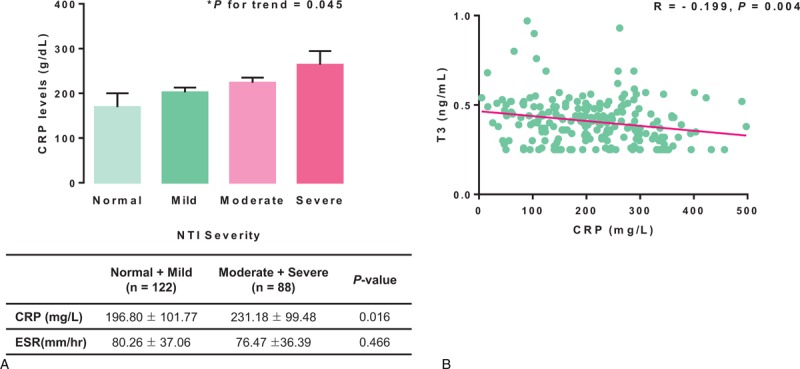
Relationship between NTI severity and acute inflammatory indices. (A) The relationship between NTI severity and acute inflammatory indices in the entire cohort. CRP levels increased significantly with the severity of NTI. When CRP levels in patients with moderate and severe NTI were compared with those in patients with normal thyroid function or those with mild NTI, CRP levels were significantly higher in the former. However, there were no significant differences in ESR levels between the groups. (B) Correlation between T3 and CRP levels. T3 levels were inversely correlated with CRP levels (r = −0.199, P = 0.004). ∗P for trend was determined by 1-way ANOVA. A 2-sample t test was conducted to compare acute inflammatory indices in the moderate and severe NTI group with those in the normal and mild NTI group. Pearson's correlation coefficient was used for bivariate correlation analysis. Error bars represent the standard error. Data in the tables are expressed as means ± SD. CRP = C-reactive protein; ESR = erythrocyte sedimentation rate; NTI = nonthyroidal illness; SD = standard deviation; T3 = triiodothyronine.
DISCUSSION
This cohort study examined the association between NTI severity and patient outcome (ACM and LOS in the ICU). In addition, to understand the relationship between NTI severity and underlying disease, we also examined the etiology underlying NTI in our study patients. NTI severity was associated with an increased risk of an adverse outcome; the correlation remained statistically significant even after adjusting for iatrogenic drug interference. NTI was more severe in the group with infectious disease than in the group with noninfectious disease; moreover, CRP levels were significantly higher in those with more severe NTI. These findings suggest that NTI severity is an independent risk factor for an adverse outcome for critically ill patients, and that infectious diseases play a role in the development of moderate-to-severe NTI. To the best of our knowledge, this is the first population-based cohort study to examine patient outcomes with respect to NTI severity, and the association between NTI severity and infection in critically ill patients.
NTI is an adaptive response that occurs during the acute phase of a critical illness; however, it can be deleterious during a prolonged critical illness. As mentioned previously, during the acute phase of a critical illness, circulating T3 levels fall rapidly, accompanied by an acute rise in rT3. These changes mainly result from changes in the circulation and peripheral organs such as reduced thyroid hormone binding and increased inactivation of thyroid hormones by type-3 deiodinases. Meanwhile, during the prolonged phase, central suppression of the HPT axis occurs. Here, we found that the more severe the NTI, the lower the total protein and albumin levels. Moreover, serum T3 levels were positively correlated with plasma protein and albumin levels (Supplementary Figure 2). These results indicate that reduced thyroid hormone binding (due to reduced protein and albumin levels) plays a role in the development of NTI; this condition is probably persistent, even in cases of moderate or severe NTI involving hypothalamic suppression.
A few studies exploring the relationship between NTI and outcome in critically ill patients have been published.18,23–28 Two of these show that reduced fT4 levels are associated with mortality independently of TSH levels,25,27 whereas others found that T3 is a major predictor of survival.26,28 However, all of these studies were conducted on small numbers of selected patients. Here, we assessed thyroid function in 616 patients admitted to the ICU, and examined the association between NTI and outcomes in a final group of 202 patients with NTI; this is one of the largest cohorts reported to date. To minimize the effects of confounding factors, we excluded patients with underlying liver or renal diseases because these diseases can affect thyroid function directly by decreasing thyroid hormone binding capacity or changing clearance, which can make the severity of NTI worse than those caused by event-related changes. Several studies show that NTI is associated with patient outcome in specific clinical settings.19,20,29–32 For example, Tognini et al reported that T3 levels are associated with short-term survival in a hospitalized older population,20 and Meuwese et al reported that fT3 levels were negatively associated with vascular calcification in patients with end-stage renal disease.30 However, these results are not generalizable to the association between NTI and critical illness. Furthermore, few studies have examined patient outcome according to NTI severity. Plikat et al reported that patients with low fT3 and fT4 levels had a higher mortality than patients with normal thyroid function and low fT3 levels. However, additional reductions in TSH levels, that is, severe NTI indicating central suppression of the HPT axis, did not affect patient outcome, including mortality.2 Here, we found that mortality was higher for patients with severe NTI (90.9%) than for those with mild or moderate NTI (37.2% and 61.5%, respectively), indicating that NTI severity is an important risk factor for an adverse outcome in critically ill patients.
Inflammatory cytokines are linked to the development of NTI. According to several studies, IL-1α and IL-1β inhibit both 125I uptake and thyroid hormone secretion by human-cultured thyrocytes in the presence of TSH.33 IL-6 also inhibits TSH-induced increases in thyroid peroxidase mRNA expression and T3 release by thyrocytes obtained from patients with Graves’ disease.14 Furthermore, TNF-α and IFN-γ directly downregulate several components of the thyroid hormone synthesis pathway at the thyrocyte level.11,34,35 Also, thyroid hormone levels in an animal model fell after the administration of inflammatory cytokines.36 Accordingly, a few human studies describe a negative relationship between IL-6 and fT3 levels.37–39 Consistent with these previous reports, we found that NTI is more severe in patients with infectious disease than in those with noninfectious disease, and that NTI severity is significantly associated with higher CRP levels, which are directly related to IL-6 levels.20 Moreover, T3 levels inversely correlated with CRP levels. However, this association lost significance when analyzing patients with infectious disease, although the trend was maintained. This might be because patients with infectious disease have worse liver function than patients with other diseases (possibly due to sepsis), which affects CRP levels.40 Overall, the present study supports the hypothesis that inflammatory cytokines, along with binding proteins and deiodinases, play a pivotal role in the development of NTI as well as in the crosstalk between NTI and infection.
There is much controversy surrounding the treatment of patients with NTI. Previous studies used animal models to examine the effect of treatment with thyroid hormones (T3 and T4) compared with treatment with releasing factors such as thyrotropin-releasing hormone (TRH) or growth hormone-releasing hormone (GHRH).41–43 These studies demonstrate that during a prolonged critical illness, treatment with T3 or T4 does not increase plasma thyroid hormone levels, whereas infusion of TRH increases plasma T3 and T4 levels. Furthermore, as the main cause aggravating NTI during a prolonged phase of critical illness is central suppression of the HPT axis rather than thyroidal capacity or changes in peripheral metabolism, treatment with hypothalamic-releasing factors such as TRH or GHRH may be better and more rational than treatment with thyroid hormones. However, few studies have investigated the effect of treatment with hypothalamic-releasing factors on patient outcomes in adequately powered randomized controlled trial. With regard to treatment of NTI during an acute phase of critical illness, underlying diseases have been treated without thyroid hormone replacement therapy, as NTI is an adaptive response to reduce energy expenditure by lowering T3 levels.3 According to our current treatment policy, which is based on treatments reported in the relevant literature, we commonly monitor the thyroid function of patients with NTI but without performing thyroid hormone supplementation or treatments with hypothalamic-releasing factors, while maintaining treatment for the underlying disease. The present study showed that NTI was more severe in patients with infectious disease, and that patient outcomes were worse for patients with moderate or severe NTI. Therefore, future studies should enroll patients with infectious disease who have moderate-to-severe NTI and examine the effect of treatment with hypothalamic-releasing factors on patient outcomes.
One limitation of the present study is its retrospective and observational design. This may lead to selection bias and the presence of confounding factors, although iatrogenic drug interference was excluded.
In conclusion, the severity of NTI is associated with adverse outcomes for critically ill patients, even after excluding confounding factors. Also, NTI is more severe in patients with infectious disease, possibly due to the presence of pro-inflammatory cytokines. Future studies should focus on the biological and clinical implications of infection with respect to the HPT axis. Furthermore, the outcomes of patients with infectious disease and moderate-to-severe NTI should be examined after treatment with hypothalamic-releasing factors.
Supplementary Material
Footnotes
Abbreviations: ACM = all-cause mortality, CRP = C-reactive protein, D1 = type 1 deiodinase, D3 = type 3 deiodinase, ESR = erythrocyte sedimentation rate, fT3 = free triiodothyronine, fT4 = free thyroxine, GHRH = growth hormone-releasing hormone, HPT = hypothalamus-pituitary-thyroid axis, ICU = intensive care unit, IFN-γ = interferon-gamma, IL-1 = interleukin-1, IL-6 = interleukin-6, LOS = length of stay, NTI = non-thyroidal illness, rT3 = reverse triiodothyronine, T3 = triiodothyronine, T4 = thyroxine, TFT = thyroid function test, TNF-α = tumor necrosis factor-alpha, TRH = thyrotropin-releasing hormone, TSH = thyrotropin or thyroid-stimulating hormone.
This study was supported by National Research Foundation of Korea (NRF) grants funded by the Korean government (MEST) (2014R1A1A2059343).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Boonen E, Van den Berghe G. Endocrine responses to critical illness: novel insights and therapeutic implications. J Clin Endocrinol Metab 2014; 99:1569–1582. [DOI] [PubMed] [Google Scholar]
- 2.Plikat K, Langgartner J, Buettner R, et al. Frequency and outcome of patients with nonthyroidal illness syndrome in a medical intensive care unit. Metabolism 2007; 56:239–244. [DOI] [PubMed] [Google Scholar]
- 3.Van den Berghe G. Non-thyroidal illness in the ICU: a syndrome with different faces. Thyroid 2014; 24:1456–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van den Berghe GH. Acute and prolonged critical illness are two distinct neuroendocrine paradigms. Verh K Acad Geneeskd Belg 1998; 60:487–518. [PubMed] [Google Scholar]
- 5.Docter R, van Toor H, Krenning EP, et al. Free thyroxine assessed with three assays in sera of patients with nonthyroidal illness and of subjects with abnormal concentrations of thyroxine-binding proteins. Clin Chem 1993; 39:1668–1674. [PubMed] [Google Scholar]
- 6.Peeters RP, Wouters PJ, Kaptein E, et al. Reduced activation and increased inactivation of thyroid hormone in tissues of critically ill patients. J Clin Endocrinol Metab 2003; 88:3202–3211. [DOI] [PubMed] [Google Scholar]
- 7.Rodriguez-Perez A, Palos-Paz F, Kaptein E, et al. Identification of molecular mechanisms related to nonthyroidal illness syndrome in skeletal muscle and adipose tissue from patients with septic shock. Clin Endocrinol (Oxf) 2008; 68:821–827. [DOI] [PubMed] [Google Scholar]
- 8.Mebis L, Van den Berghe G. Thyroid axis function and dysfunction in critical illness. Best Pract Res Clin Endocrinol Metab 2011; 25:745–757. [DOI] [PubMed] [Google Scholar]
- 9.Van den Berghe G, de Zegher F, Veldhuis JD, et al. Thyrotrophin and prolactin release in prolonged critical illness: dynamics of spontaneous secretion and effects of growth hormone-secretagogues. Clin Endocrinol (Oxf) 1997; 47:599–612. [DOI] [PubMed] [Google Scholar]
- 10.Bartalena L, Bogazzi F, Brogioni S, et al. Role of cytokines in the pathogenesis of the euthyroid sick syndrome. Eur J Endocrinol 1998; 138:603–614. [DOI] [PubMed] [Google Scholar]
- 11.Rasmussen AK, Kayser L, Feldt-Rasmussen U, et al. Influence of tumour necrosis factor-alpha, tumour necrosis factor-beta and interferon-gamma, separately and added together with interleukin-1 beta, on the function of cultured human thyroid cells. J Endocrinol 1994; 143:359–365. [DOI] [PubMed] [Google Scholar]
- 12.Yamashita S, Kimura H, Ashizawa K, et al. Interleukin-1 inhibits thyrotrophin-induced human thyroglobulin gene expression. J Endocrinol 1989; 122:177–183. [DOI] [PubMed] [Google Scholar]
- 13.Nolte A, Bechtner G, Rafferzeder M, et al. Interleukin-1 beta (IL-1 beta) binds to intact porcine thyroid follicles, decreases iodide uptake but has no effect on cAMP formation or proliferation. Horm Metab Res 1994; 26:413–418. [DOI] [PubMed] [Google Scholar]
- 14.Tominaga T, Yamashita S, Nagayama Y, et al. Interleukin 6 inhibits human thyroid peroxidase gene expression. Acta Endocrinol (Copenh) 1991; 124:290–294. [DOI] [PubMed] [Google Scholar]
- 15.Ajjan RA, Watson PF, Findlay C, et al. The sodium iodide symporter gene and its regulation by cytokines found in autoimmunity. J Endocrinol 1998; 158:351–358. [DOI] [PubMed] [Google Scholar]
- 16.Tang KT, Braverman LE, DeVito WJ. Tumor necrosis factor-alpha and interferon-gamma modulate gene expression of type I 5’-deiodinase, thyroid peroxidase, and thyroglobulin in FRTL-5 rat thyroid cells. Endocrinology 1995; 136:881–888. [DOI] [PubMed] [Google Scholar]
- 17.De Groot LJ. Dangerous dogmas in medicine: the nonthyroidal illness syndrome. J Clin Endocrinol Metab 1999; 84:151–164. [DOI] [PubMed] [Google Scholar]
- 18.Peeters RP, Wouters PJ, van Toor H, et al. Serum 3,3′,5′-triiodothyronine (rT3) and 3,5,3′-triiodothyronine/rT3 are prognostic markers in critically ill patients and are associated with postmortem tissue deiodinase activities. J Clin Endocrinol Metab 2005; 90:4559–4565. [DOI] [PubMed] [Google Scholar]
- 19.Bello G, Pennisi MA, Montini L, et al. Nonthyroidal illness syndrome and prolonged mechanical ventilation in patients admitted to the ICU. Chest 2009; 135:1448–1454. [DOI] [PubMed] [Google Scholar]
- 20.Tognini S, Marchini F, Dardano A, et al. Non-thyroidal illness syndrome and short-term survival in a hospitalised older population. Age Ageing 2010; 39:46–50. [DOI] [PubMed] [Google Scholar]
- 21.Kim YA, Park YJ. Prevalence and risk factors of subclinical thyroid disease. Endocrinol Metab 2014; 29:20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Melmed S, Polonsky KS, Larsen PR, et al. Williams Textbook of Endocrinology. Elsevier Health Sciences. 2011. [Google Scholar]
- 23.Leon-Sanz M, Lorente JA, Larrodera L, et al. Pituitary-thyroid function in patients with septic shock and its relation with outcome. Eur J Med Res 1997; 2:477–482. [PubMed] [Google Scholar]
- 24.Silberman H, Eisenberg D, Ryan J, et al. The relation of thyroid indices in the critically ill patient to prognosis and nutritional factors. Surg Gynecol Obstet 1988; 166:223–228. [PubMed] [Google Scholar]
- 25.Slag MF, Morley JE, Elson MK, et al. Hypothyroxinemia in critically ill patients as a predictor of high mortality. JAMA 1981; 245:43–45. [PubMed] [Google Scholar]
- 26.Maldonado LS, Murata GH, Hershman JM, et al. Do thyroid function tests independently predict survival in the critically ill? Thyroid 1992; 2:119–123. [DOI] [PubMed] [Google Scholar]
- 27.Van den Berghe G, Baxter RC, Weekers F, et al. The combined administration of GH-releasing peptide-2 (GHRP-2), TRH and GnRH to men with prolonged critical illness evokes superior endocrine and metabolic effects compared to treatment with GHRP-2 alone. Clin Endocrinol (Oxf) 2002; 56:655–669. [DOI] [PubMed] [Google Scholar]
- 28.Loh KC, Eng PC. Prevalence and prognostic relevance of sick euthyroid syndrome in a medical intensive care unit. Ann Acad Med Singapore 1995; 24:802–806. [PubMed] [Google Scholar]
- 29.Anastasiou O, Sydor S, Sowa JP, et al. Higher thyroid-stimulating hormone, triiodothyronine and thyroxine values are associated with better outcome in acute liver failure. PLoS One 2015; 10:e0132189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meuwese CL, Olauson H, Qureshi AR, et al. Associations between thyroid hormones, calcification inhibitor levels and vascular calcification in end-stage renal disease. PLoS One 2015; 10:e0132353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Han G, Ren J, Liu S, et al. Nonthyroidal illness syndrome in enterocutaneous fistulas. Am J Surg 2013; 206:386–392. [DOI] [PubMed] [Google Scholar]
- 32.Yasar Z, Kirakli C, Cimen P, et al. Is non-thyroidal illness syndrome a predictor for prolonged weaning in intubated chronic obstructive pulmonary disease patients? Int J Clin Exp Med 2015; 8:10114–10121. [PMC free article] [PubMed] [Google Scholar]
- 33.Sato K, Satoh T, Shizume K, et al. Inhibition of 125I organification and thyroid hormone release by interleukin-1, tumor necrosis factor-alpha, and interferon-gamma in human thyrocytes in suspension culture. J Clin Endocrinol Metab 1990; 70:1735–1743. [DOI] [PubMed] [Google Scholar]
- 34.Nagayama Y, Izumi M, Ashizawa K, et al. Inhibitory effect of interferon-gamma on the response of human thyrocytes to thyrotropin (TSH) stimulation: relationship between the response to TSH and the expression of DR antigen. J Clin Endocrinol Metab 1987; 64:949–953. [DOI] [PubMed] [Google Scholar]
- 35.Kung AW, Ma L, Lau KS. The role of interferon-gamma in lymphocytic thyroiditis: its functional and pathological effect on human thyrocytes in culture. Clin Exp Immunol 1992; 87:261–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Papanicolaou DA. Euthyroid Sick Syndrome and the role of cytokines. Rev Endocr Metab Disord 2000; 1:43–48. [DOI] [PubMed] [Google Scholar]
- 37.Murai H, Murakami S, Ishida K, et al. Elevated serum interleukin-6 and decreased thyroid hormone levels in postoperative patients and effects of IL-6 on thyroid cell function in vitro. Thyroid 1996; 6:601–606. [DOI] [PubMed] [Google Scholar]
- 38.Kimura T, Kanda T, Kotajima N, et al. Involvement of circulating interleukin-6 and its receptor in the development of euthyroid sick syndrome in patients with acute myocardial infarction. Eur J Endocrinol 2000; 143:179–184. [DOI] [PubMed] [Google Scholar]
- 39.Abo-Zenah HA, Shoeb SA, Sabry AA, et al. Relating circulating thyroid hormone concentrations to serum interleukins-6 and -10 in association with non-thyroidal illnesses including chronic renal insufficiency. BMC Endocr Disord 2008; 8:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest 2003; 111:1805–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Debaveye Y, Ellger B, Mebis L, et al. Tissue deiodinase activity during prolonged critical illness: effects of exogenous thyrotropin-releasing hormone and its combination with growth hormone-releasing peptide-2. Endocrinology 2005; 146:5604–5611. [DOI] [PubMed] [Google Scholar]
- 42.Debaveye Y, Ellger B, Mebis L, et al. Regulation of tissue iodothyronine deiodinase activity in a model of prolonged critical illness. Thyroid 2008; 18:551–560. [DOI] [PubMed] [Google Scholar]
- 43.Debaveye Y, Ellger B, Mebis L, et al. Effects of substitution and high-dose thyroid hormone therapy on deiodination, sulfoconjugation, and tissue thyroid hormone levels in prolonged critically ill rabbits. Endocrinology 2008; 149:4218–4228. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


