Abstract
Dodecafluoropentane emulsion (DDFPe) nanodroplets are exceptional oxygen transporters and can protect ischemic brain in stroke models 24 h without reperfusion. Current stroke therapy usually fails to reach patients because of delays following stroke onset. We tested using DDFPe to extend the time window for tissue plasminogen activator (tPA). Longer treatment windows will allow more patients more complete stroke recovery. We test DDFPe to safely extend the time window for tPA thrombolysis to 9 h after stroke. With IACUC approval, randomized New Zealand white rabbits (3.4–4.7 kg, n=30) received angiography and 4-mm blood clot in the internal carotid artery for flow-directed middle cerebral artery occlusion. Seven failed and were discarded. Groups were IV tPA (n=11), DDFPe + tPA (n=7), and no therapy controls (n=5). DDFPe (0.3 ml/kg, 2 % emulsion) IV dosing began at 1 h and continued at 90 min intervals for 6 doses in one test group; the other received saline injections. Both got standard IV tPA (0.9 mg/kg) therapy starting 9 h post stroke. At 24 h, neurological assessment scores (NAS, 0–18) were determined. Following brain removal percent stroke volume (%SV) was measured. Outcomes were compared with Kruskal-Wallis analysis. For NAS, DDFPe + tPA was improved overall, p=0.0015, and vs. tPA alone, p=0.0052. For %SV, DDFPe + tPA was improved overall, p=0.0003 and vs. tPA alone, p=0.0018. NAS controls and tPA alone were not different but %SV was, p=0.0078. With delayed reperfusion, DDFPe + tPAwas more effective than tPA alone in preserving functioning brain after stroke. DDFPe significantly extends the time window for tPA therapy.
Keywords: Stroke, Animal model, Dodecafluoropentane emulsion (DDFPe), Tissue plasminogen activator (tPA)
Introduction
Delay is the most common factor in obstructing delivery of thrombolysis to acute stroke patients. Delays in recognition, delays in calling for help, delays in first responder arrival, triage, and transportation, and delays in door to needle time on arrival at the hospital all steadily accumulate. These delays routinely add up to more than the FDA-approved 3 h between stroke onset and intravenous tissue plasminogen activator (IV tPA) administration, and usually to more than the 4.5 h, the rest of the scientific world allows to start thrombolysis. Due to this and other contraindications, fewer than 5.2 % of acute stroke patients now actually qualify and receive IV tPA therapy [1].
Many clinical efforts have focused on accelerating tPA delivery, and some real progress has been made. Still, a neuroprotective technique to save brain until reperfusion can actually be accomplished remains an elusive goal. Only this year, the Field Administration of Stroke Therapy—Magnesium Phase 3 Clinical Trial (FAST-MAG) of acute strokes showed a 97 % success rate in delivering IV drugs to acute stroke patients by an emergency medical technician (EMT) in the first 2 h after stroke onset. Over 73 % received IV magnesium sulfate in the first “Golden Hour” when recovery is most achievable [2]. This field administration demonstrates success in rapid EMT arrival, rapid field diagnosis, and injection of IV drug, however, the presumptive neuroprotective drug failed to provide real neuroprotection. Applying this approach to all strokes is difficult, but it is estimated that 35 % of all acute stroke patients could be treated by pre-hospital therapy within 2 h of onset, and up to 25 % could be treated within 1 h of onset (personal communication, Jeffrey Saver, MD, UCLA). This corresponds to a projection of almost 200,000 patients receiving IV neuroprotection within the first hour and 275,000 within the first 2 h in the USA each year. Dodecafluoropentane emulsion (DDFPe), an oxygen-transporting perfluorocarbon given IV, provides neuroprotection equally well given at 1 or 2 or even 3-h delay after ischemic onset in rabbits [3, 4]. Even though human dose and timing is not yet defined and actual human application in strokes remains uncertain, it could potentially benefit a very large number of patients.
In this study we tested DDFPe as a IV neuroprotective drug in a rabbit embolic ischemic stroke model which closely parallels the usual human clinical stroke situation. The IV DDFPe was started at 1 h after onset of embolic ischemia like the majority of patients in the FAST-MAG trial. Thrombolysis of the embolic clot was delayed until 9 h after embolization, three times the FDA-approved 3 h time limit. Then, at 9 h, thrombolysis was begun with standard IV tPA therapy. The results at 24 h were evaluated.
Methods
All procedures were approved of by the University’s Institutional Animal Care and Use Committee. This rabbit angiographic embolic stroke model is well proven in our laboratory with advantages of scale over smaller animals, especially for catheter delivery of clots. The published experience showing its reliability and consistency of reperfusion with IV tPA, even with the small number of animals in this study, is a strength [5.6].
Animals
Randomized New Zealand male or female rabbits 3.4 to 4.7 kg/bw (Harlan Labs) n=30 had a stroke induced with aged blood clot according to the rabbit embolic stroke procedure [5, 6]. Briefly, following sedation with intramuscular injection of ketamine of 30 mg/kg (Ketaset; Fort Dodge; Fort Dodge, IA) and xylazine of 5 mg/kg (AnaSed; Lloyd Laboratories; Shenandoah, IA) and 2 % isoflurane anesthesia maintenance (Novaplus; Hospira Inc.; Lake Forrest, IL); a modified 65-cm angled-tip 3 F catheter (SlipCath; Cook Inc.; Bloomington, IN) was placed in the femoral artery, and the internal carotid artery (ICA) selected for angiography and clot embolization.
Aged Clot Production
Aged clot was made using donor rabbit blood and allowed to clot at 37 °C for 6 h followed with 3 days at 4 °C in 1.5 mm glass tubing. The clot was then cut into 4 mm lengths for use [5, 6].
Angiography
A pre-stroke sub-selective magnified angiogram was made for comparison to post-embolization site identification of arterial occlusion using a C-arm DSA machine (OEC 9800; GE Healthcare; Salt Lake City, UT). Embolization with the 4-mm clot flushed into the ICA is flow directed into the middle cerebral artery (MCA) and/or the anterior cerebral artery (ACA) for standard infarcts. Animals with non-standard occlusions, usually posterior circulation occlusions or cerebrovascular spasm, were discarded and not included in the study. The catheter was then removed and the femoral artery ligated.
Treatment Groups
Before angiography, rabbits were randomized into one of three treatment groups: (i) control [CON] (saline only), (ii) 0.9 mg/kg tPA [tPA], or (iii) 0.3 ml/ kg DDFPe+0.9 mg/kg tPA [D + tPA]. Rabbits with combinations of MCA and/or ACA occlusions were placed in the recovery unit.
DDFPe
Animals received via ear vein IV treatment of saline or 0.3 ml/kg DDFPe beginning 1 h following embolization and additional doses at 90 min intervals (6 doses total). The administration of 0.3 ml/kg DDFPe was given using a slow push intravenous dose of a 2 % w/v DDFPe (NuvOx Pharma; Tucson, AZ). Following the last DDFPe dose at hour nine, tPA lytic therapy began.
tPA
For rabbits receiving tPA, the standard dose of IV tPA (Cathflo Activase; Genetech; South San Francisco, CA) was 0.9 mg/kg, given as a bolus of 10 % of the dose and the remainder of the doses at 5 min intervals over 1 h. The rabbit dose used here (0.9 mg/kg) corresponds to the classic paper of Zivin et al. [7] and was found to be efficacious in previous studies [6, 8].
Histopathology
At 24 h following embolization, the animals were evaluated for neurological assessment scores (NAS), and the brains were removed for quantification of core cerebral infarction stroke. The harvested brains were chilled in saline and sectioned coronally in 4.0 mm intervals using a brain mold (RBM-7000C; ASI Instruments Inc.; Warren, MI). Brain size and digitally captured images of stained coronal sections using a 1 % 2,3,5-triphenyltetrazolium chloride (TTC) were measured using ImageJ 1.48v (National Institutes of Health, USA) [3–6, 8]. Areas of core cerebral infarction were measured by a technician blinded to the treatment groups. Blinding of the TTC scoring was effected by randomizing the blinded image sets and having only one person doing a complete scoring of all these cases 2 months after the animal portion of the study. The percent of infarct was calculated as a percent of the whole brain. The incidence of hemorrhage and location were noted.
Neurological Assessment Scores
Just before sacrifice at 24 h, the rabbits had a complete neurological assessment score (NAS) performed [9, 10]. This score of neurological function was expanded with additional behavior scores and ranged from 0 to 18 (Table 1). Blinding of the NAS scoring was not complete. However, all NAS values were a consensus reached by two independent observers after independent evaluations.
Table 1.
Neurological assessment score (NAS) application for post-stroke analysis
| Test | Clinical criteria | Score | Potential score |
|---|---|---|---|
| Behavior | Neck twist | 0-normal, 1-twisted neck | 1 |
| Behavior | Functional appearance | 0-normal, 1-fatigued, 2-very fatigued falls asleep during examination | 2 |
| Behavior | Ear sag | 0-normal, 2-lateral ear sags | 2 |
| Behavior | Ambulation | 0-normal, 1-ambulates with uneven gait, 2-unable to ambulate | 2 |
| Reflex | Righting reflex | 0-right in 1 s, 1-right in 5 s, 2-not right in 5 s | 2 |
| Reflex | Standing reflex | 0-upright on paws, 1-upright briefly, 2-unable to be upright | 2 |
| Stimuli reflex | Extension reflex (forepaws)a | 0-retract in 1 s, 1-retract in 5 s, 2-not retract in 5 s | 2 |
| Stimuli reflex | Extension reflex (hindpaws)a | 0-retract in 1 s, 1-retract in 5 s, 2-not retract in 5 s | 2 |
| Posture | Posture (contralateral push) | 0-normal resistance, 1-reduced resistance, 2-falls down | 2 |
| Death | Maximum score with death | 18 | |
| Total cumulative score possible | 18 | ||
The paws are gently pulled toward the body, the time to re-extend the paw is scored
Statistics
Median percent infarct volume and median NAS scores are reported for each group. The Kruskal-Wallis procedure, which is the nonparametric analog of one-way ANOVA, was used to compare percent infarct volume and NAS scores among the three treatment groups using an alpha-level of 0.05 to determine statistical significance. If this test was significant for a specific outcome (e.g., percent stroke volume or NAS), then pre-specified pairwise comparisons of CON vs. tPA and tPA vs. D + tPA were made using Bonferroni-adjusted alpha-levels of 0.025 to determine significance. The percentages of hemorrhage and of infarct observed in posterior brain tissue areas were compared among treatment groups using exact contingency table methods.
Results
Thirty rabbits were randomized and had angiography and embolization with 4-mm aged clot. Six had posterior circulation occlusions and 1 had a severe vasospasm of the ICA and were excluded. Rabbits fell into one of three treatment groups: (i) CON n=5, (ii) tPA n=11, or (iii) D + tPA n=7. Thus, 23 rabbits had successful occlusion of the MCA and/or the ACA as documented by angiography. These rabbits completed the treatment regimens with recorded percent stroke volumes and NAS scores and documentation of brain structures impacted by infarct.
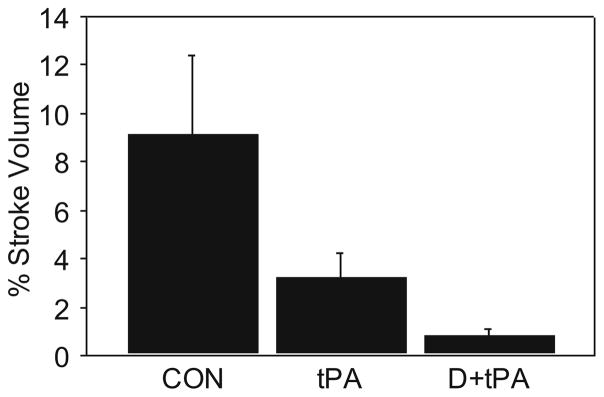
Overall, a three-group comparison using a Kruskal-Wallis analysis indicated significance for the percent stroke outcome variables at p=0.0003 and alpha of 0.05. The two group results were Bonferroni adjusted to test at alpha 0.025. Two group comparisons of %SV indicated that in the placebo CON, tPA, and D + tPA treatment groups, the median values were 5.61, 2.32, and 0.52 %, respectively (Figs. 1 and 2). The tPA group was significantly lower in median percent stroke volume than the placebo controls (p=0.0078 with alpha set at 0.025). The comparison of the D + tPA test group median percent stroke volume was significantly less than the tPA control group at p=0.0018, with alpha set at 0.025.
Fig. 1.
Percent stroke volume at 24 h post stroke. D + tPA was improved vs. controls and also vs. tPA alone, p=0.0003 overall; p=0.0018 for D+ tPA vs. tPA group; p=0.0078 for control vs. tPA
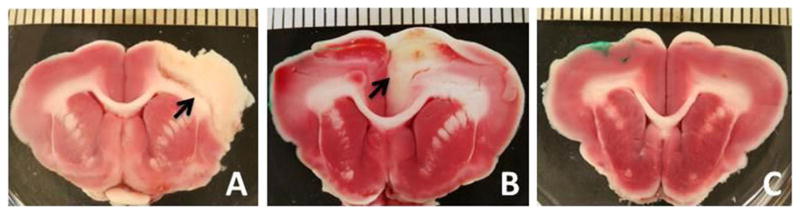
Fig. 2.
Representative TTC images from control, tPA (a), tPA alone (b), and D+ tPA (c). Arrows indicate stroke area on control and tPA alone. Scale bars=1 mm
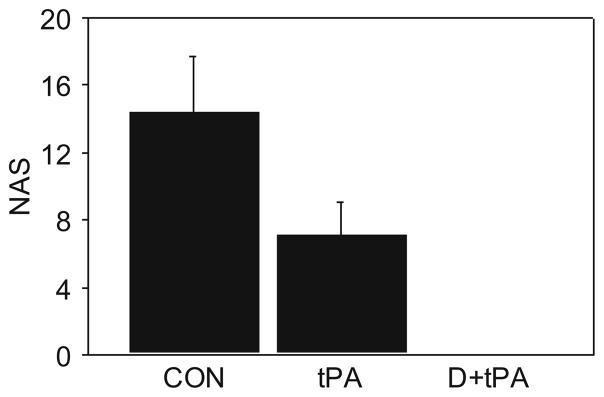
The neurological deficits were different in a Kruskal-Wallis analysis between treatment groups, at p=0.0015, with alpha at 0.05 (Fig. 3). In Bonferroni-adjusted two group comparisons of NAS, there was no difference between deficits in placebo controls (NAS 18) vs. tPA (NAS 9), p=0.051, when alpha was set at p=0.025. The addition of DDFPe with tPA lytic therapy (NAS 0) significantly decreased stroke neurological deficits compared to the tPA only group, p=0.0052, with alpha at 0.025.
Fig. 3.
Neurological assessment scores at 24 h. For NAS, results were significantly improved, p=0.0015 overall; p=0.0052 for D + tPA vs tPA; and non-significant p=0.051 (Bonferroni requires <0.025) for controls vs tPA alone (D+tPA=0)
The percent of positive hemorrhagic results between treatment groups was non-significant (Table 2) at an exact p value of 0.10. In the D + tPA group, the two identified with hemorrhage were also the only two that had infarct in tissues impacted from posterior cerebral arterial distribution. No angiographic evidence of posterior emboli was seen in these animals. Analysis of the placebo controls showed 80 % (4/5) were collected earlier than 24 h compared to tPA only when 18 % (2/11) was collected earlier and 0 % (0/6) when DDFPe was included as a treatment (Table 2). The early collections were due to severe symptoms in these animals that required early sacrifice to reduce pain and suffering.
Table 2.
Results of DDFPe treatment prior to tPA delivery at 9 h
| Group | Sex | BW (kg) | Embolic site of occlusion | NAS | Time at sacrifice (h)a | Infarct volume (%) | Visible hemorrhage |
|---|---|---|---|---|---|---|---|
| Control | M | 3.6 | MCA/ACA | 18 | 12 | 21.89 | Yes |
| Control | M | 3.7 | MCA | 17 | 4 | 9.15 | No |
| Control | F | 3.4 | MCA | 18 | 7 | 5.61 | No |
| Control | F | 3.4 | MCA/ACA | 1 | 24 | 4.28 | No |
| Control | F | 4.2 | MCA | 18 | 9 | 4.5 | No |
| tPA 9 h | F | 4 | MCA | 0 | 24 | 2.1 | No |
| tPA 9 h | M | 4.5 | MCA/ACA | 0 | 24 | 1.06 | No |
| tPA 9 h | F | 4.4 | MCA | 2 | 24 | 2.13 | Yes |
| tPA 9 h | F | 4.5 | MCA | 2 | 24 | 1.91 | Yes |
| tPA 9 h | F | 4.2 | MCA | 9 | 24 | 2.32 | Yes |
| tPA 9 h | M | 4.2 | MCA | 9 | 24 | 2.46 | Yes |
| tPA 9 h | F | 4.7 | MCA | 0 | 24 | 2.67 | Yes |
| tPA 9 h | F | 4.4 | MCA | 9 | 24 | 3.23 | Yes |
| tPA 9 h | F | 4.4 | MCA | 15 | 9 | 2.33 | Yes |
| tPA 9 h | F | 4.4 | MCA | 14 | 5 | 1.76 | No |
| tPA 9 h | F | 3.9 | MCA | 18 | 12 | 13.16 | Yes |
| DDFPe + PA 9 h | M | 4.4 | MCA | 0 | 24 | 0.28 | No |
| DDFPe + PA 9 h | M | 4 | MCA | 0 | 24 | 0.52 | No |
| DDFPe + tPA 9 h | M | 4.5 | MCA | 0 | 24 | 0.99 | No |
| DDFPe + tPA 9 h | F | 4.2 | MCA | 0 | 24 | 0 | No |
| DDFPe + tPA 9 h | M | 4.4 | MCA | 0 | 24 | 1.92 | No |
| DDFPe + tPA 9 h | M | 3.9 | MCA/ACA | 0 | 24 | 0.28 | Yes |
| DDFPe + tPA 9 h | M | 3.8 | MCA/ACA | 0 | 24 | 1.62 | Yes |
Sacrifice was scheduled at 24 h unless early collections were due to severe symptoms
Discussion
The saying “Time is Brain” still holds true. Delay remains the biggest single enemy of successful ischemic stroke outcomes. After almost 20 years of massive effort striving toward prompt clinical tPA thrombolysis for ischemic stroke, we routinely fail to provide thrombolysis due to delays. They are real. They are common. They lurk everywhere.
The translation of animal study results into human therapies requires animal models that closely parallel the human situation and standard models may need changes to match clinical reality. After watching case after case of acute human stroke arrive too late for thrombolysis and reperfusion therapy by any method, it became painfully apparent to us that improved speed in transportation and clinical workup alone would not allow acute therapy to reach many patients. We needed something to “stop the clock” early in the process, in the field if possible, to preserve the ischemic brain in its “Golden Hour” when recovery is most possible, and allow the reperfusion techniques adequate time to be applied and take effect. This is not a new conclusion, just an old one that is again reconfirmed by current clinical experience. Therefore, to modify our proven embolic clot stroke model in rabbits to include the common delays that we experience clinically, we increased our previous delays of 1 to 3 h to a delay of 9 h following embolization for thrombolysis with tPA. Then we could test therapies in a meaningful model, one related to actual clinical delays.
This model addresses another facet of the problem, the effect of reperfusion using the clinical drug, IV tPA. Reperfusion is a double-edged sword which can be associated with severe side effects including brain swelling, tPA neurotoxicity, and hemorrhage in addition to the desired oxygenation and revitalization of ischemic penumbra. Several rat models have shown poor outcomes with reperfusion in strokes which further cloud the issue [11].
The nanodroplet perfluorocarbon, DDFPe, acting as a neuroprotectant in animals [3, 4, 12] has also been tested as an ultrasound contrast agent in more than 2200 humans in the past with only minor adverse events [13, 14]. Its effectiveness as an oxygen transport agent is well proven both in bench top studies and in multiple animal studies [12, 15]. The mechanism of action seems to primarily be in improved oxygen transport without need for red blood cell flow, but the details remain to be fully defined and are not addressed here. Basic pharmacokinetic characteristics have been reported in humans and rabbits [4, 16]. It is currently undergoing a human trial in brain tumor patients to augment tumor oxygenation and the effect of radiation on the tumors.
Our earlier work has shown that rabbits can demonstrate about 80 % decrease in infarct size with DDFPe given IV starting as late as 3 h following embolization and, when starting at 1 h after embolization, the brain can maintain both neurological function and preserve viable brain for many hours with repeated doses of IV DDFPe. Dramatic neuroprotection has been demonstrated out to 24 h of permanent occlusion in some variations of the model. Similar permanent occlusion models in two strains of rats have also shown strong neuroprotective effects [9].
With its promising results, DDFPe became our primary candidate for testing in the long delay-to-lysis model and was compared to placebo + delayed tPA therapy.
At three times the official FDA acceptable delay and twice the acceptable delay outside the USA, DDFPe provided successful neuroprotection through 9 h of delay before standard IV tPA lytic therapy. With DDFPe, excellent and strongly significant improvements were shown in both %SV and NAS compared to the control group which received standard IV tPA therapy alone at 9 h. At 24 h the rabbits were fully recovered and evaluated for behavior and neurological deficits, free of any potential procedural anesthetic effects. This time also allowed definition of completely developed infarcts on TTC analysis. The excellent NAS scores and final appearance of the brains themselves discount concerns of possible brain swelling, toxicity, and delayed hemorrhage due to late reperfusion. The TTC stains confirm that pattern of neuroprotection with markedly reduced infarct size and without sign of edema or excessive bleeding.
Limitations
The study is limited by the small numbers tested. A much larger study would be required for confirmation and to assess uncommon side effects and complications. Still, the translation into humans is such a leap that more animal work may not need to be justified before human trials. Other limitations include more frequent fragmentation of the aged clot emboli in this series than in prior work. This provided extra fragments that not only occluded the anterior circulation but were flow directed to cause visible occlusions in the posterior circulation of several animals. This is by definition a non-standard occlusion and often causes such severe symptoms that the animal could not survive to scheduled sacrifice. These posterior occlusions were uniformly discarded when seen on angiography and contribute to the differences in final group numbers. However, there seems to have been some very small or late posterior occlusions seen on anatomic section which were not seen on angiography. All standard angiographic occlusions of the MCA and ACA were included in this analysis. Another limitation is that the effective rabbit window for tPA use is not fully defined by studying only one point at 9 h. Many more animals will be required to completely define that window for thrombolysis, but it seems that results at 9 h with D + tPA are clearly superior to only tPA.
Conclusion
Intravenous DDFPe can support brain function (NAS) and brain tissue (%SV) to the time of greatly delayed reperfusion in the rabbit embolic stroke model, basically stopping the clock on stroke progression. No significant adverse events were seen and rapid progress to testing in humans seems to be the next appropriate step. The long anticipated method to expand the “Golden Hour” and the time window for acute stroke therapy may finally be at hand.
Acknowledgments
Ms. Bridgette Engi, RLATG, is acknowledged for her important participation.
Funding This study was supported in part by the Hornick Foundation, University of Arkansas for Medical Sciences and the UAMS Foundation Fund for Cerebrovascular Research as well as the Translational Research Institute (TRI) (grant UL1TR000039) through the NIH National Center for Research Resources and National Center for Advancing Translational Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Compliance with Ethical Standards
Conflict of Interest A USPTC patent has been applied for using DDFPe in stroke therapy, by Drs. W.C. Culp and R.D. Skinner.
References
- 1.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, et al. Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation. 2014;129:e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Savers JL. The field administration of stroke therapy—magnesium (FAST-MAG) Phase 3: Primary Results “Golden Hour” stroke trial. International Stroke Conference (ISC) 2014. Abstract 214 Presented; February 13, 2014.2014. [Google Scholar]
- 3.Culp WC, Woods SD, Skinner RD, Brown AT, Lowery JD, Johnson JLH, Unger EC, Hennings LJ, et al. Dodecafluoropentane emulsion decreases infarct volume in a rabbit ischemic stroke model. JVIR. 2012;23:116–121. doi: 10.1016/j.jvir.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woods SD, Skinner RD, Ricca AM, Brown AT, Lowery JD, Borrelli MJ, Lay JO, Culp WC. Progress in dodecafluoropentane emulsion as a neuroprotective agent in a rabbit stroke model. Mol Neurobiol. 2013;48:363–367. doi: 10.1007/s12035-013-8495-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Culp WC, Woods SD, Brown AT, Lowery JD, Hennings LJ, Skinner RD, Borrelli MJ, Roberson PK. Three variations in rabbit angiographic stroke models. J Neurosci Methods. 2012;212(2):322–328. doi: 10.1016/j.jneumeth.2012.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Culp WC, Flores R, Lowery JD, Brown AT, Hennings LJ, Roberson PK, Woods SD, Hatton JH, et al. Successful microbubble sonothrombolysis without tissue plasminogen activator in a rabbit model of acute ischemic stroke. Stroke. 2011;42:2280–2285. doi: 10.1161/STROKEAHA.110.607150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zivin JA, Fisher M, DeGirolami U, Hemenway CC, Stashak JA. Tissue plasminogen activator reduces neurological damage after cerebral embolism. Science. 1985;230:1289–1292. doi: 10.1126/science.3934754. [DOI] [PubMed] [Google Scholar]
- 8.Brown AT, Flores R, Hamilton E, Roberson PK, Borrelli MJ, Culp WC. Microbubbles improve sonothrombolysis in vitro and decrease hemorrhage in vivo in a rabbit stroke model. Investig Radiol. 2011;46(3):202–207. doi: 10.1097/RLI.0b013e318200757a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown AT, Arthur MC, Nix JS, Montgomery JA, Skinner RD, Roberson PK, Borrelli M, Culp WC. Dodecafluoropentane emulsion (DDFPe) decreases stroke size and improves neurological scores in a permanent occlusion rat stroke model. Open Neurol J. 2014;8(1):27–33. doi: 10.2174/1874205x01408010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown AT, Skinner RD, Flores R, Hennings L, Borrelli MJ, Lowery J, Culp W. Stroke location and brain function in an embolic rabbit stroke model. J Vasc Interv Radiol. 2010;21:903–909. doi: 10.1016/j.jvir.2010.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ren Y, Hashimoto M, Pulsinelli WA, Nowak TS., Jr Hypothermic protection in rat focal ischemia models: strain differences and relevance to “reperfusion injury”. J Cereb Blood Flow Metab. 2004;24:42–53. doi: 10.1097/01.WCB.0000095802.98378.91. [DOI] [PubMed] [Google Scholar]
- 12.Fitzgerald RT, Ou X, Nix JS, Arthur MC, Brown AT, Skinner RD, Borrelli MJ, Culp WC. Dodecafluoropentane emulsion delays and reduces MRI markers of infarction in a rat stroke model. Magn Reson Imaging, Magn Reson Imaging. 2015;33(2):236–239. doi: 10.1016/j.mri.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 13.The European Agency for the Evaluation of Medicinal Products Human Medicines Evaluation Unit. Jul 27, 1998. CPMP/1342/98. [Google Scholar]
- 14.The European Agency for the Evaluation of Medicinal Products Human Medicines Evaluation Unit. London: May 22, 2001. Doc. Ref: EMEA/37043/00. [Google Scholar]
- 15.Johnson JL, Dolezal MC, Kerschen A, Matsunaga TO, Unger EC. In vitro comparison of dodecafluoropentane (DDFP), perfluorodecalin (PFD), and perfluoroctylbromide (PFOB) in the facilitation of oxygen exchange. Artif Cells Blood Substit Immobil Biotechnol. 2009;37(4):156–162. doi: 10.1080/10731190903043192. [DOI] [PubMed] [Google Scholar]
- 16.Correas JM, Quay SC. EchoGen emulsion: a new ultrasound contrast agent based on phase shift colloids. Clin Radiol. 1996;51(S1):11–14. [PubMed] [Google Scholar]