Abstract
The aim of this study was to determine the antibiotic susceptibility profiles of bacteria in bile samples and to analyze the clinical relevance of the findings as only limited information about risk factors for elevated frequence of bacterial and fungal strains in routinely collected bile samples has been described so far.
A prospective cohort study at a tertiary care center was conducted. Seven hundred forty-four patients underwent 1401 endoscopic retrograde cholangiographies (ERCs) as indicated by liver transplantation (427/1401), primary sclerosing cholangitis (222/1401), choledocholithiasis only (153/1401), obstruction due to malignancy (366/1401), or other conditions (233/1401). Bile samples for microbiological analysis were obtained in all patients.
The 71.6% (823/1150) samples had a positive microbiological finding, and 57% (840/1491) of the bacterial isolates were gram-positive. The main species were Enterococcus spp (33%; 494/1491) and Escherichia coli (12%; 179/1491). Of the samples, 53.8% had enteric bacteria and 24.7% had Candida spp; both were associated with clinical and laboratory signs of cholangitis (C-reactive proteins 35.0 ± 50.1 vs 44.8 ± 57.6; 34.5 ± 51.2 vs 52.9 ± 59.7; P < 0.001), age, previous endoscopic intervention, and immunosuppression. Multi-resistant (MR) strains were found in 11.3% of all samples and were associated with clinical and laboratory signs of cholangitis, previous intervention, and immunocompromised status. In subgroup analysis, strain-specific antibiotic therapy based on bile sampling was achieved in 56.3% (89/158) of the patients. In cases with a positive bile culture and available blood culture, blood cultures were positive in 29% of cases (36/124), and 94% (34/36) of blood cultures had microbial species identical to the bile cultures.
Bactobilia and fungobilia can usually be detected by routine microbiological sampling, allowing optimized, strain-specific antibiotic treatment. Previous endoscopic intervention, clinical and laboratory signs of cholangitis, and age are independent risk factors. MR bacteria and fungi are an evolving problem in cholangitis, especially in immunocompromised patients.
INTRODUCTION
Biliary obstruction is often complicated by cholangitis, which can range from mild to life threatening. Endoscopic treatment to enable biliary drainage and the use of antibiotics are both mainstays of treatment for this condition.1–3 Although standard care for biliary obstruction includes antibiotic treatment, the basis for choosing an empiric antibiotic regimen has several limitations.
Data regarding the microbiological flora of the biliary tract are scarce and associated with methodological limitations such as sample collection during surgical or percutaneous interventional procedures. Such samples might not be representative of the risk profiles of patients undergoing only endoscopic drainage. Moreover, most studies were performed decades ago and, therefore, do not reflect recent developments in species and drug resistance.4,5 Although discovering the underlying strain is a general principle of anti-infective therapy, few clinical studies address the diagnostic and therapeutic value of routine biliary sampling during endoscopic retrograde cholangiographies (ERCs). Consistent with these limitations, current guidelines suggest that prospective studies are needed to obtain reliable results that can be used as a basis for further recommendations.1
There is no agreement on the optimum initial antibiotic regimen,6 and limited information is available regarding the antibiotic susceptibility profile of the pathogens isolated from bile samples.7,8 Therefore, the choice of antibiotics remains a challenge to the treating physician. Data on biliary tract candidiasis are emerging, but the clinical relevance and potential risk factors are still being debated.9,10
The aims of this prospective study of patients with biliary obstruction were to determine the incidence and spectrum of bactobilia, fungobilia, and drug resistance and to analyze the implications of these findings for therapeutic treatment decisions. We also tried to identify risk factors for stratisfication of optimized empirical treatment.
METHODS
Patients and Endoscopic Interventions
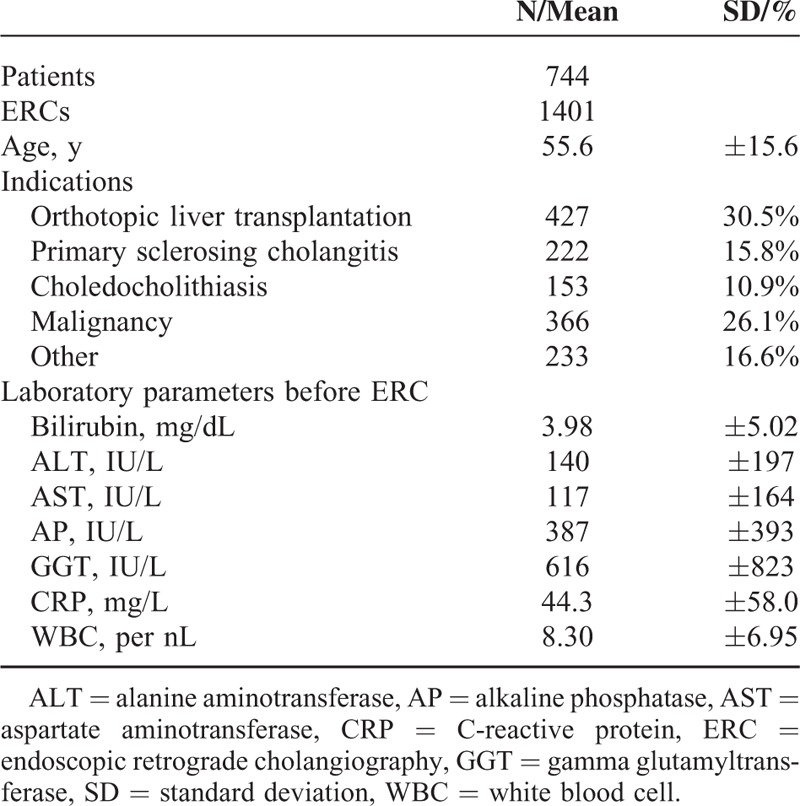
This prospective study was conducted at a tertiary care center, the University Hospital of Heidelberg, Germany, from December 2006 to January 2009. During the study period, 1519 ERC procedures were performed on 807. Of the 807 patients, 63 were excluded from the analysis because of a lack of agreement or a failure of the inclusion criteria. Thus, datasets from 1401 ERCs performed on 744 patients were available for further analysis. The main indications for ERC were cholestasis due to primary sclerosing cholangitis, common duct stones, malignant stenosis, and biliary complications after liver transplantation (Table 1). The ERCs were carried out using a therapeutic duodenoscope (TJF160R or TJF160VR, Olympus Corp., Tokyo, Japan), and the selective cannulation of the common bile duct was performed using a guide wire (Jagwire, 0.035 inch, Boston Scientific, Natick, MA or Visiglide 0.035 inch, Olympus Corp.) or a standard catheter for cases where there was a pre-existing sphincterotomy. All procedures were performed under conscious sedation using midazolam, propofol, and/or short-acting opiates. Many patients were on an antibiotic regimen before the initiation of ERC treatment because of suspected or proven cholangitis. The standard regimen in our institution is Mezlocillin i.v. or, in case of known allergy to penicillins, ciprofloxacin was used. Patients not receiving antibiotic treatment who needed intervention received peri-interventional antibiotic prophylaxis (mezlocillin or ciprofloxacin). Treatment of candida was fluconazole by mouth or i.v. The baseline characteristics of the patients are shown in Table 1.
TABLE 1.
Baseline Patients’ Characteristics
Bile Sampling and Microbiological Analysis
Bile sampling and microbiological analysis were performed as described previously.10 In brief, bile samples were obtained after selective intubation before any therapeutic procedure was performed. When bile could not be aspirated directly after cannulation, a small amount of sterile saline (2–4 mL) was applied and aspiration was reattempted. Aliquots of all biliary samples were placed in a sterile glass tube containing medium for anaerobic and aerobic bacterial cultures (BD BBL Port-A-Cul; Becton, Dickinson and Co., Sparks, MD). The material was delivered to the microbiology laboratory within 2 h of collection and cultured aerobically and anaerobically according to standard laboratory protocols. After endoscopic procedures all endoscopes were precleaned at the point of use with detergent solution. After brushing of endoscope channel, parts, connectors, and orifices, endoscopes were further reprocessed by automated high-level disinfection according to instructions of the manufacturers (Olympus EDT 3, Olympus Corp.; Korsolex Endo-cleaner© and Korsolex Endo-disinfectant©, Bode Chemie, Hamburg, Germany). All reprocessing steps as well as training of involved persons are in conformance with recommended guidelines.20 Cultures of endoscope surface, channel, and parts as well as worktops were performed routinely by our local department of microbiology. During the study period in none of the routinely performed cultures a growth of germs was observed. The analysis of susceptibility to antibiotics commonly used for treatment included results for all isolates for which routine antibiotic susceptibility tests were performed. For further analysis, we categorized the samples into groups according to the bacterial and fungal findings: sterile; low-grade pathogens, consisting mainly of alpha- or beta-hemolytic streptococci and coagulase-negative staphylococcus; enteric bacteria, including Escherichia coli, Enterococcus spp, Klebsiella spp, and other Enterobacteriaceae; Candida, including all Candida species; and multi-resistant (MR) bacteria, which included all samples containing at least 1 MR strain.
Study Design, Definitions, and Statistical Analysis
The study was designed to determine the incidence and spectrum of biliary infections and drug resistance and to assess the impact of these findings on therapeutic decisions. The study was also designed to identify potential risk factors for biliary infection.
Laboratory values for white blood cell (WBC), C-reactive protein (CRP), AP, GGT, AST, ALT, and bilirubin were determined, and clinical signs of cholangitis, including fever, right upper quadrant pain, and jaundice were recorded before the ERCs were performed. These data are in line with published criteria for acute cholangitis.1 Previous endoscopic intervention, laboratory parameters at baseline, clinical signs of cholangitis, and patient age were defined as potential risk factors for biliary infection. Immunosuppression was also analyzed as a risk factor, and patients with malignancy, end-stage renal or end-stage liver disease as well as patients receiving immunosuppressive medical treatment were considered to be immunosuppressed. The parameters were compared initially in a univariate analysis, and a regression analysis was performed to assess the prognostic values of the identified risk factors. Changes in antibiotic treatment regimens as a consequence of bile analysis and longer hospital stay were identified by reviewing each patient's medical chart.
Continuous data were compared using the nonparametric Mann–Whitney U test. Frequency differences were compared using the chi-squared test or Fisher exact test if frequencies were below 5. Differences were considered significant when P < 0.05. The covariates that showed a high level of significance in the univariate analysis were analyzed further in a multivariate analysis using the binary logistic regression method. Statistical analyses were performed using PASW Statistics 17.0 (SPSS, Inc., Chicago, IL).
Informed Consent
Written informed consent was obtained from each patient, and the study protocol conformed to the ethical guidelines of the Declaration of Helsinki in its current version, as reflected in a priori approval by the institution's human research review committee. The study was approved by the local ethics committee of Heidelberg University.
RESULTS
Detection Rates of Bacteria and Fungi in Bile
Of the 1401 ERC examinations included in this prospective study, bile cultures were not performed in 251 cases because of technical or organizational failure. Thus, 1491 bacterial isolates were identified from a total of 1150 bile cultures. In total, 840/1491 (57%) of all isolates were gram-positive and 651/1491 (43%) were gram-negative (Table 2). A total of 350/1150 (30%) bile samples showed only 1 bacterial species, 265/1150 (23%) showed 2 different species, and 181/1150 (16%) showed 3 or more bacterial isolates.
TABLE 2.
Microbiological Isolates From Bile Specimens
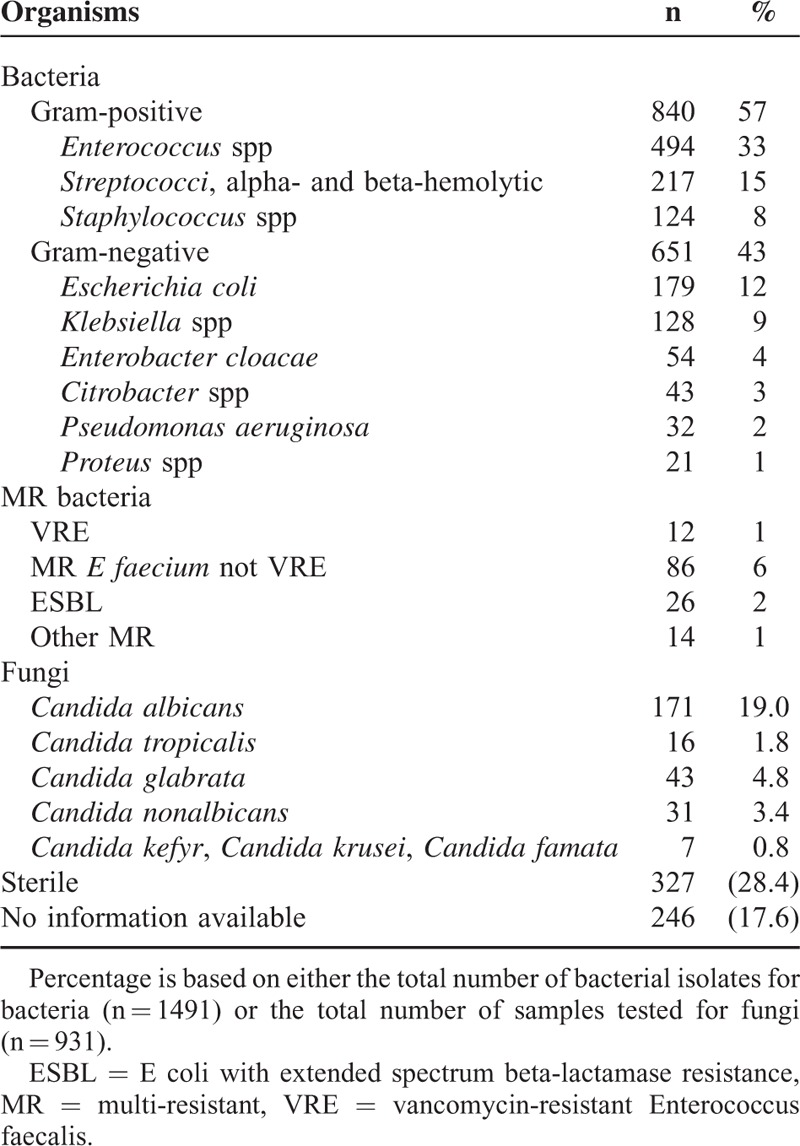
Enterococcus species were predominant (494/1150 samples; 33%). E coli and Klebsiella spp were detected in 12% (179/1150) and 9% (128/1150) of the samples, respectively. A total of 840 gram-positive bacteria could be identified. Alpha- and beta-hemolytic streptococci were detected in 15% of the samples and coagulase-negative staphylococci in 8%. The gram-negative species were Enterobacter cloacae (4%), Citrobacter spp (3%), Pseudomonas aeruginosa (2%), and Proteus spp (1%). In total, 651 gram-negative isolates could be detected. MR bacteria included the following: 1% vancomycin-resistant Enterococcus faecalis (VRE), 2% ESBL (E coli with extended spectrum beta-lactamase resistance), and 6% Enterococcus faecium strains, which were only sensitive to vancomycin, linezolid, or other reserve antibiotics (Tables 2 and 3).
TABLE 3.
Microbiological Spectrum of Bile Samples
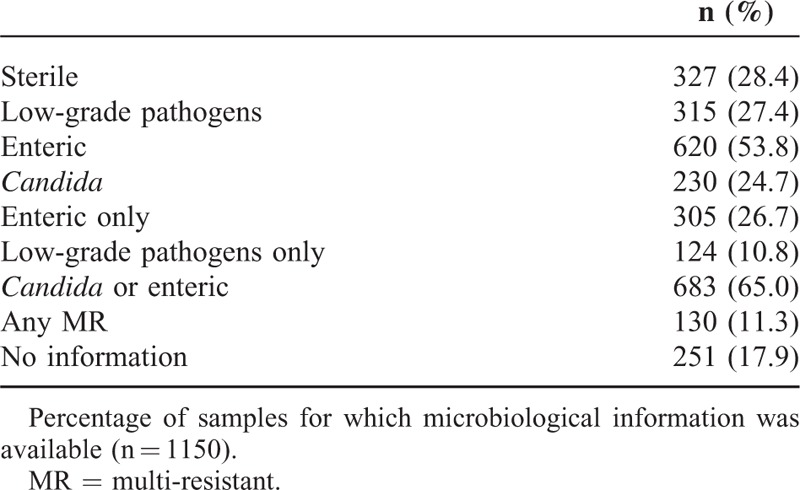
Only 28.3% of all samples were sterile. In 27.4%, low-grade pathogens were detected, and in 10.8% of the samples, only low-grade pathogens were detected. In 65% of all samples with complete microbiological information, enteric bacteria and/or Candida were detected.
We also analyzed the bile samples for fungi. A total of 19% showed Candida albicans; however, other Candida species were detected at low rates (Tables 2 and 3). Some Candida species, including C glabrata and C tropicalis, were detected at low frequencies, while still other fungal organisms, such as Aspergillus fumigatus, were be detected.
Summarizing the results of analyses resulted in the following: 327/1150 (28.4%) sterile bile cultures; 315/1150 (27.4%) samples with low-grade pathogens; 124/1150 (10.8%) with low-grade pathogens only; 620/1150 (53.8%) with enteric bacteria; 305/1150 (26.7%) with enteric bacteria only; 230/930 (24.7%) samples with Candida; and 683/1050 (65.0%) with enteric bacteria and/or Candida. At least 1 MR strain was detected in 130 (11.3%) of the samples. These data are summarized in Table 3.
Risk Factors for Biliary Infection According to Uni- and Multivariate Analyses
Bactobilia by enteric bacteria (35.0 ± 50.1 vs 44.8 ± 57.6; P < 0.001), detection of Candida (34.5 ± 51.2 vs 52.9 ± 59.7; P < 0.001), and detection of enteric bactobilia and/or fungi (28.7 ± 45.0 vs 46.1 ± 57.6; P < 0.001) were associated with increased serum levels of CRP. Detection of low-grade pathogens or low-grade pathogens only was not associated with elevated CRP levels (Table 4). WBC was not associated with the presence of bacteria or fungi. The clinical signs of cholangitis were recorded before ERC treatment and were much more frequent in the groups with enteric bactobilia, fungobilia, and enteric bacteria and/or Candida (P < 0.001 for all comparisons). There was no correlation with either of the low-grade pathogen groups (Table 4). As with the clinical signs of cholangitis and CRP, patient age and previous endoscopic intervention were associated with the detection of enteric bacteria or Candida (Table 5).
TABLE 4.
Clinical and Laboratory Signs of Cholangitis
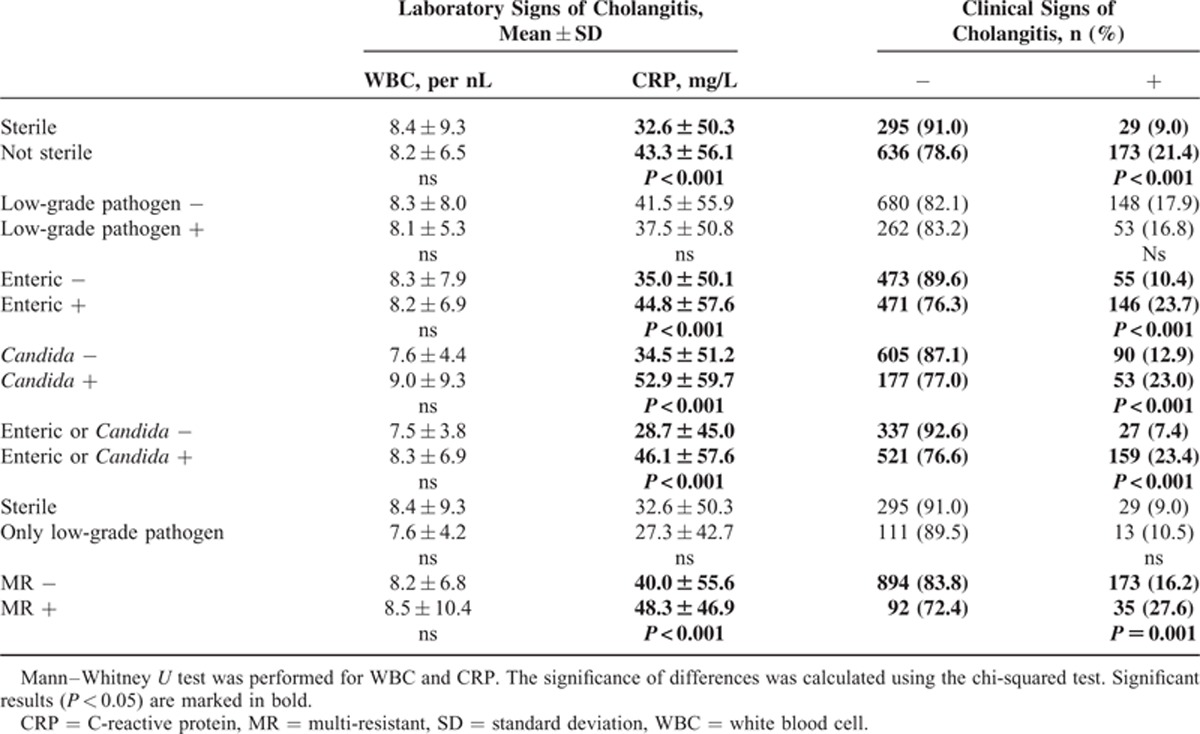
TABLE 5.
Patient Age, Liver Function Tests, and Previous Endoscopic Intervention Associated With Detection of Bacteria and Fungi
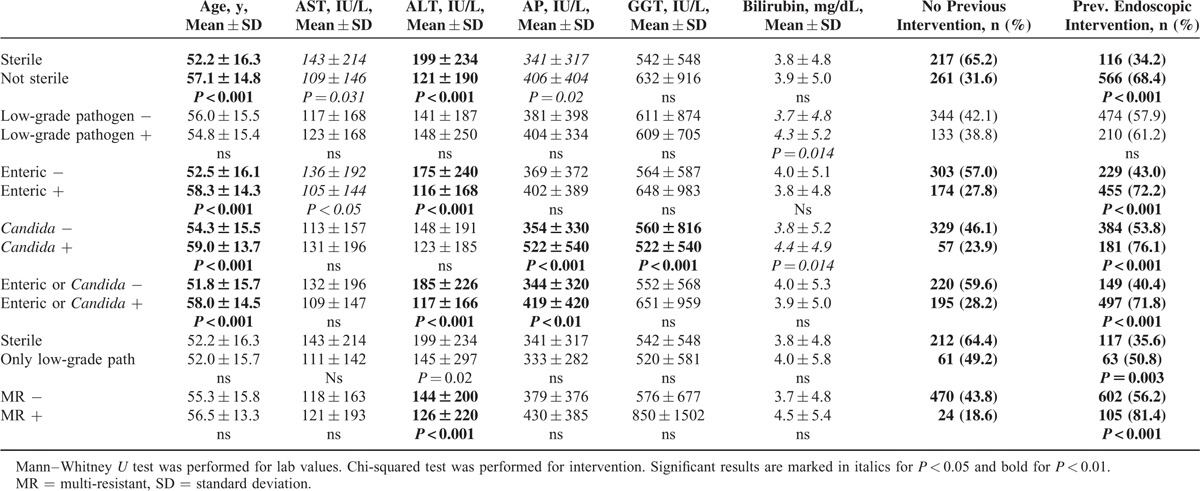
A total of 405/1337 (30.3%) samples were acquired from immunocompetent patients, and 932/1337 (69.7%) were acquired from patients suffering from either drug-induced immunosuppression (495/1337; 37.0%) or immunosuppression due to underlying disease (437/1337; 32.7%). The incidence of enteric bacteria and Candida in bile was significantly higher in patients defined as immunocompromised (Figure 1).
FIGURE 1.
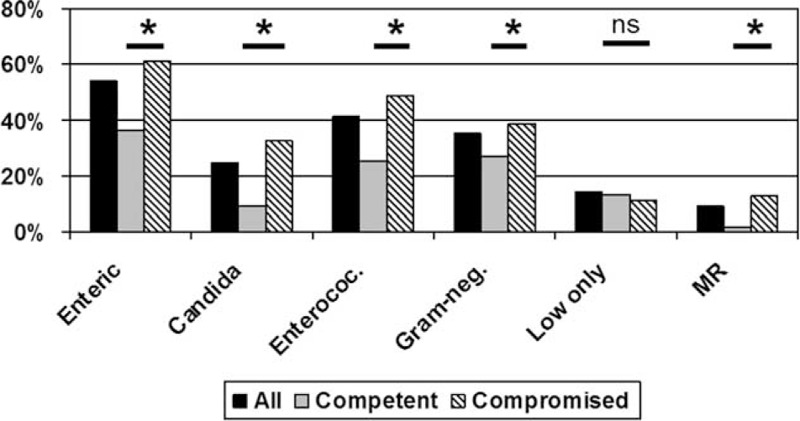
Detection rates for different groups of isolates. Results for all samples and for samples from immunocompetent and immunocompromised patients are shown. Candida = Candida spp, Enteric = enteric bacteria, enterococ = Enterococcus spp, Gram-neg. = gram-negative isolate, low only = only low-grade pathogens detected. ∗P < 0.001.
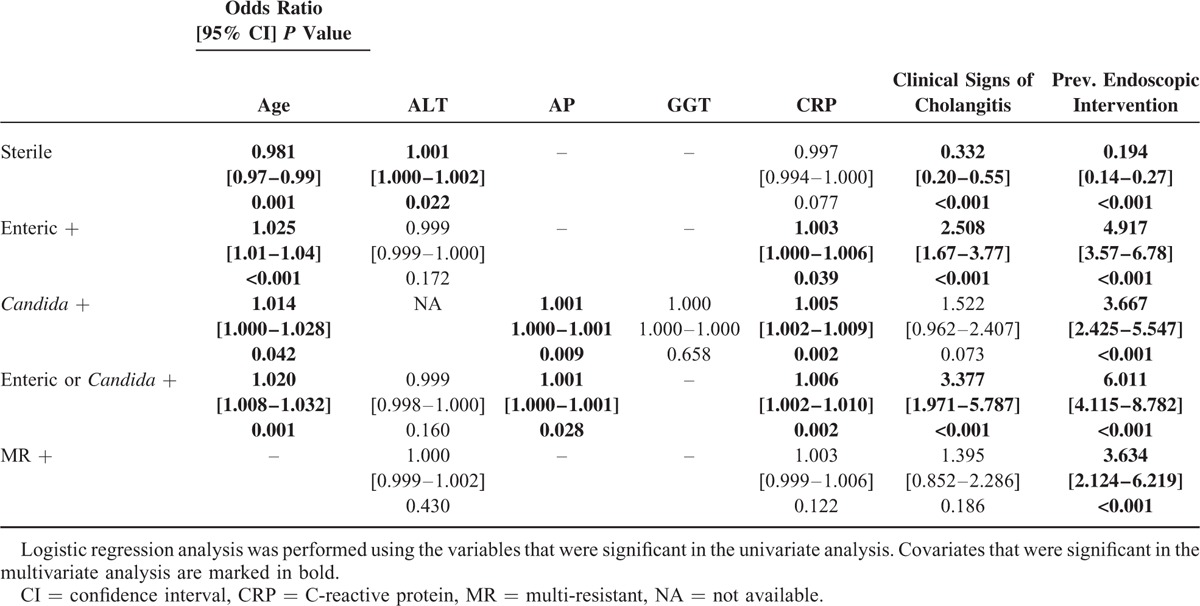
Age, laboratory and clinical signs of cholangitis, previous endoscopic intervention, and immunostatus were all analyzed using a logistic regression model. The covariates CRP, age, clinical signs of cholangitis, previous intervention, and immunostatus were identified as independent predictive factors for the presence of either enteric bacteria or Candida in bile (Table 6).
TABLE 6.
Multivariate Analysis of the Significant Covariates
Antimicrobial Resistance Profile of Bacterial Isolates
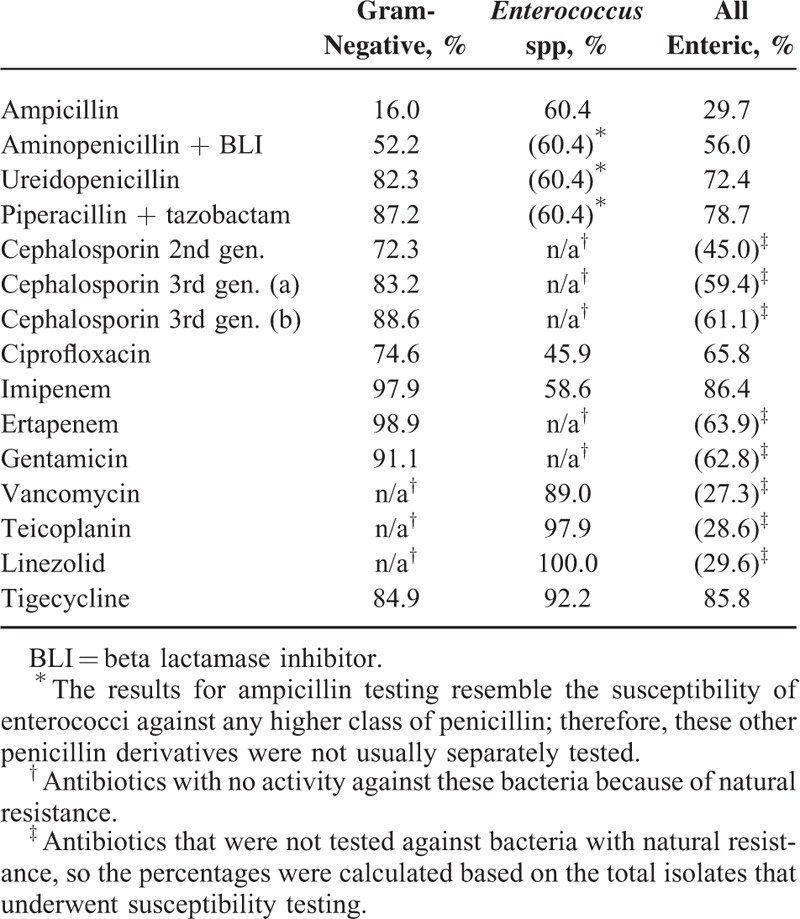
We analyzed 851 isolates for susceptibility to antibiotics. We found that 568/851 were gram-negative isolates, while 254/851 were gram-positive enteric bacteria that were almost completely Enterococcus spp (250/254). In total, 29/851 isolates of gram-positive nonenteric bacteria were excluded (Table 7). Only 16% of gram-negative isolates were susceptible to treatment with ampicillin, 52.2% were susceptible to treatment with aminopenicillin with a beta-lactamase inhibitor and 82.3% were susceptible to treatment with an ureidopenicillin; the latter was improved to 87.2% by the addition of tazobactam. Second- or third-generation (a and b) cephalosporins were effective in 72.3%, 83.2%, and 88.6% of the gram-negative isolates described above, respectively. Carbapenems and gentamycin showed the highest activity against gram-negative isolates. Regarding Enterococcus spp, penicillin derivatives showed activity against 60.4% of the isolates and imipenem showed activity against 58.6%. Ciprofloxacin, which was effective against 74.6% of the gram-negative species, was effective in only 45.9% of the enterococci. Vancomycin, teicoplanin, and linezolid were effective against 89.0%, 97.9%, and 100.0% of the enterococci isolates, respectively. Altogether, 84.9% of the gram-negative isolates and 92.2% of the Enterococcus spp were susceptible to tigecycline.
TABLE 7.
Susceptibility of the 818 Isolates to Antibiotics
Adaption of Antibiotic Therapy
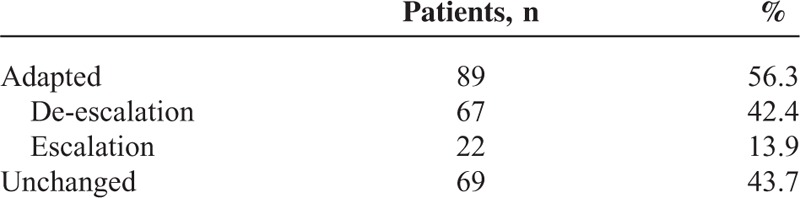
In addition to a bile culture, a blood culture was also drawn at the time of the ERC in 124/527 cases. Of the blood cultures, 71% were sterile and 29% showed a bacterial isolate. When a bacterial isolate was detected in the blood culture, the isolate was identical to the isolate in the bile culture in 94% cases (34/36). Enterococcus species were detected almost as frequently as E coli species in the blood cultures. We next analyzed the relevance of the bile sample microbiological profiles for antibiotic therapy in the subgroup of patients with choledocholithiasis and other indications for ERC (e.g., secondary sclerosing cholangitis and chronic pancreatitis). Complete microbiological and clinical information was available for 158 patients. In 67/158 patients (42.4%), the antibiotic regimen was changed to a more specific therapy as a result of the biliary microbiological analysis. In 22/158 patients (13.9%), the antibiotic regimen needed to be escalated because of bacterial resistance. In 69 patients (43.7%), the antibiotic therapy remained unchanged (Tables 8 and 9).
TABLE 8.
Identity of Bacterial Isolates in Blood Cultures
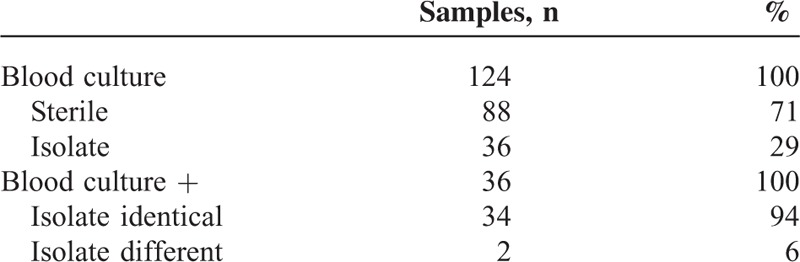
TABLE 9.
Adaptation of Antibiotic Therapy Based on Bile Sampling
DISCUSSION
Cholangitis is a serious complication of biliary obstruction that requires biliary drainage and antibiotic treatment.1–3 In this study, we determined the spectrum and antibiotic susceptibility profiles of bacteria in bile samples, identified risk factors for bactobilia and fungobilia, and validated the feasibility of bile cultures.
Evaluation of the spectrum of microbiological isolates, including fungi, in routine bile samples during ERCs helped us to establish a basis for empiric antibiotic treatment. Enterococcus spp accounted for 33% of all bacterial isolates, which is considerably higher than the detection rates of 22%, 19%, and 17% reported in previous studies of samples from ERCs.4,5,11 In light of the rising rates of fluoroquinolone-resistant enterococci, this must considered when choosing antibiotics and strongly supports routine bile sampling. The Tokyo guidelines for antimicrobial therapy in acute cholangitis, for example, recommend cephalosporins or fluoroquinolones for acute cholangitis.12 The recent US guidelines for intra-abdominal infection also recommend these antibiotics, but highlight the need for prospective studies to obtain more current data.13 By analyzing susceptibility profiles to antibiotics, we found a high frequency of resistance. Gram-negative strains were reasonably susceptible to ciprofloxacin and second-generation cephalosporins, and good and excellent susceptibility rates were found for broadband penicillins and carbapenems, respectively. However, the resistance rates for enterococci were very high. In our series, penicillin derivatives and carbapenems showed mediocre rates of susceptibility, and only small-spectrum antibiotics such as vancomycin were effective. Given the natural resistance of enterococci against cephalosporins, ertapenem and gentamicin, only ureidopenicillins with or without a beta-lactamase inhibitor, imipenem or tigecycline were effective in over 70% of the strains of enteric bacteria tested. The combination of ciprofloxacin and ampicillin was also effective in over 70% of isolates. This must be taken into account in future recommendations of empiric antibiotic treatment.
Our analysis identified pathogens considered low-grade in the context of cholangitis. Others have done this previously in samples from patients with primary sclerosing cholangitis.10,14 Our data suggest that detection of these isolates do not contribute to the pathology of this condition to the patient in the context of cholangitis/bactobilia. It is not clear whether this is because of colonization or merely due to sample contamination. We conclude that detecting low-grade pathogens in bile is not clinically relevant, in line with data from others regarding bacteremia after ERCs involving these pathogens.5,15,16 It is difficult to distinguish between colonization and significant infection. Signs of cholangitis were significantly more often associated with the detection of enteric bacteria, but there were still a substantial number of patients in which we found bacteria without signs of cholangitis. This is somewhat similar to data from patients with pancreatitis in which contaminated necrotizing pancreatitis was associated with worse clinical course.17 In addition, we identified risk factors for bactobilia and fungobilia that may be instrumental for risk stratification.
Fungal species are emerging as pathogens that are detected frequently in the setting of healthcare-associated biliary infection. In agreement with previous reports,4,9,18,19 we detected Candida species, especially C albicans in bile samples. However, the study by Lenz et al9 did not identify previous endoscopic intervention as a risk factor for fungobilia, even though the level of significance showed a trend toward association. In contrast, in our study endoscopic intervention was highly associated with bactobilia and fungobilia, perhaps because of the larger sample size. This might be important for patients for whom repeated endoscopic intervention is a mainstay of treatment.20
Many patients referred to a tertiary care center are immunocompromised because of chronic disease (e.g., end-stage renal disease or end-stage liver disease or malignancy) or due to pharmacological immunosuppression (e.g., patients who have undergone solid organ or bone marrow transplantation). Our data clearly show that isolates in these patients differ from those in patients who are immunocompetent. This was especially true for fungobilia, which was detected almost exclusively in samples from immunosuppressed patients. This change in the microbiological spectrum and the higher incidence of MR strains must be considered in the empiric treatment of these patients. These recommendations partly overlap current guidelines that emphasize different treatment for patients with healthcare-associated biliary infection.13
What are the implications of these findings for clinical practice? We argue against empiric treatment using fluoroquinolones because of the high resistance observed in patients referred to a tertiary care center. Instead, we favor the use of ureidopenicillins, which cover over 80% of the gram-negative strains that still have reasonable rates of susceptibility among Enterococcus spp. However, the combination of carbapenems and vancomycin is effective in severely ill patients, and the use of tigecycline warrants further study.
One of the main findings of this study is the clear benefit of routine bile sampling during ERCs. Bile cultures can be used to identify bacterial strains and, more importantly, reveal a patient's susceptibility profile if performed routinely. Blood cultures are far less sensitive for detecting this information. The inclusion of this information from bile sampling led to changes in the antibiotic treatment in more than half of the clinical cases. This was also proposed by another recent study,21 suggesting that bile sampling and culture could narrow down treatment options, thereby helping prevent further resistance development, or expand treatment to target-resistant bacteria already present in the patient.
In conclusion, bactobilia and fungobilia can frequently be detected by routine microbiological sampling, thereby allowing optimized, strain-specific antibiotic treatment. A previous endoscopic intervention, clinical and laboratory signs of cholangitis, and age are independent risk factors for bactobilia and fungobilia and should be considered when devising treatment regimens. MR bacteria and fungobilia are an evolving problem in cholangitis, especially in immunocompromised patients, and further study on antibiotic susceptibility in those patients is warranted.
Acknowledgment
We thank Tom Brucker, Department of Medical Biometry and Informatics, for critical review of the statistic analyses.
Footnotes
Abbreviations: CRP = C-reactive protein, ERC = endoscopic retrograde cholangiography, ESBL = E coli with extended spectrum beta-lactamase resistance, MR = multi-resistant, VRE = vancomycin-resistant Enterococcus faecalis
DNG and KHW were supported by the postdoctoral program of the Medical Faculty of the University of Heidelberg. DNG was also supported by a grant from the Deutsche Forschungsgemeinschaft.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Wada K, Takada T, Kawarada Y, et al. Diagnostic criteria and severity assessment of acute cholangitis: Tokyo Guidelines. J Hepatobiliary Pancreat Surg 2007; 14:52–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lai EC, Mok FP, Tan ES, et al. Endoscopic biliary drainage for severe acute cholangitis. N Engl J Med 1992; 326:1582–1586. [DOI] [PubMed] [Google Scholar]
- 3.Deviere J, Motte S, Dumonceau JM, et al. Septicemia after endoscopic retrograde cholangiopancreatography. Endoscopy 1990; 22:72–75. [DOI] [PubMed] [Google Scholar]
- 4.Ehrenstein BP, Salamon L, Linde HJ, et al. Clinical determinants for the recovery of fungal and mezlocillin-resistant pathogens from bile specimens. Clin Infect Dis 2002; 34:902–908. [DOI] [PubMed] [Google Scholar]
- 5.Lorenz R, Herrmann M, Kassem AM, et al. Microbiological examinations and in-vitro testing of different antibiotics in therapeutic endoscopy of the biliary system. Endoscopy 1998; 30:708–712. [DOI] [PubMed] [Google Scholar]
- 6.Lee JG. Diagnosis and management of acute cholangitis. Nat Rev Gastroenterol Hepatol 2009; 6:533–541. [DOI] [PubMed] [Google Scholar]
- 7.Nomura T, Shirai Y, Hatakeyama K. Enterococcal bactibilia in patients with malignant biliary obstruction. Dig Dis Sci 2000; 45:2183–2186. [DOI] [PubMed] [Google Scholar]
- 8.Rosch T, Triptrap A, Born P, et al. Bacteriobilia in percutaneous transhepatic biliary drainage: occurrence over time and clinical sequelae. A prospective observational study. Scand J Gastroenterol 2003; 38:1162–1168. [DOI] [PubMed] [Google Scholar]
- 9.Lenz P, Conrad B, Kucharzik T, et al. Prevalence, associations, and trends of biliary-tract candidiasis: a prospective observational study. Gastrointest Endosc 2009; 70:480–487. [DOI] [PubMed] [Google Scholar]
- 10.Rudolph G, Gotthardt D, Kloters-Plachky P, et al. Influence of dominant bile duct stenoses and biliary infections on outcome in primary sclerosing cholangitis. J Hepatol 2009; 51:149–155. [DOI] [PubMed] [Google Scholar]
- 11.Leung JW, Ling TK, Chan RC, et al. Antibiotics, biliary sepsis, and bile duct stones. Gastrointest Endosc 1994; 40:716–721. [PubMed] [Google Scholar]
- 12.Tanaka A, Takada T, Kawarada Y, et al. Antimicrobial therapy for acute cholangitis: Tokyo Guidelines. J Hepatobiliary Pancreat Surg 2007; 14:59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Solomkin JS, Mazuski JE, Bradley JS, et al. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Clin Infect Dis 2010; 50:133–164. [DOI] [PubMed] [Google Scholar]
- 14.Pohl J, Ring A, Stremmel W, et al. The role of dominant stenoses in bacterial infections of bile ducts in primary sclerosing cholangitis. Eur J Gastroenterol Hepatol 2006; 18:69–74. [DOI] [PubMed] [Google Scholar]
- 15.Kullman E, Borch K, Lindstrom E, et al. Bacteremia following diagnostic and therapeutic ERCP. Gastrointest Endosc 1992; 38:444–449. [DOI] [PubMed] [Google Scholar]
- 16.Sauter G, Grabein B, Huber G, et al. Antibiotic prophylaxis of infectious complications with endoscopic retrograde cholangiopancreatography. A randomized controlled study. Endoscopy 1990; 22:164–167. [DOI] [PubMed] [Google Scholar]
- 17.Beger HG, Bittner R, Block S, et al. Bacterial contamination of pancreatic necrosis. A prospective clinical study. Gastroenterology 1986; 91:433–438. [DOI] [PubMed] [Google Scholar]
- 18.Rupp C, Friedrich K, Folseraas T, et al. Fut2 genotype is a risk factor for dominant stenosis and biliary candida infections in primary sclerosing cholangitis. Aliment Pharmacol Ther 2014; 39:873–882. [DOI] [PubMed] [Google Scholar]
- 19.Rupp C, Bode KA, Chahoud F, et al. Risk factors and outcome in patients with primary sclerosing cholangitis with persistent biliary candidiasis. BMC Infect Dis 2014; 14:562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gotthardt DN, Rudolph G, Kloters-Plachky P, et al. Endoscopic dilation of dominant stenoses in primary sclerosing cholangitis: outcome after long-term treatment. Gastrointest Endosc 2010; 71:527–534. [DOI] [PubMed] [Google Scholar]
- 21.Negm AA, Schott A, Vonberg RP, et al. Routine bile collection for microbiological analysis during cholangiography and its impact on the management of cholangitis. Gastrointest Endosc 2010; 72:284–291. [DOI] [PubMed] [Google Scholar]


