Supplemental Digital Content is available in the text
Abstract
We evaluated whether age- and gender-based colorectal cancer screening is cost-effective.
Recent studies in the United States identified age and gender as 2 important variables predicting advanced proximal neoplasia, and that women aged <60 to 70 years were more suited for sigmoidoscopy screening due to their low risk of proximal neoplasia. Yet, quantitative assessment of the incremental benefits, risks, and cost remains to be performed.
Primary care screening practice (2008–2015).
A Markov modeling was constructed using data from a screening cohort. The following strategies were compared according to the Incremental Cost Effectiveness Ratio (ICER) for 1 life-year saved: flexible sigmoidoscopy (FS) 5 yearly; colonoscopy 10 yearly; FS for each woman at 50- and 55-year old followed by colonoscopy at 60- and 70-year old; FS for each woman at 50-, 55-, 60-, and 65-year old followed by colonoscopy at 70-year old; FS for each woman at 50-, 55-, 60-, 65-, and 70-year old. All male subjects received colonoscopy at 50-, 60-, and 70-year old under strategies 3 to 5.
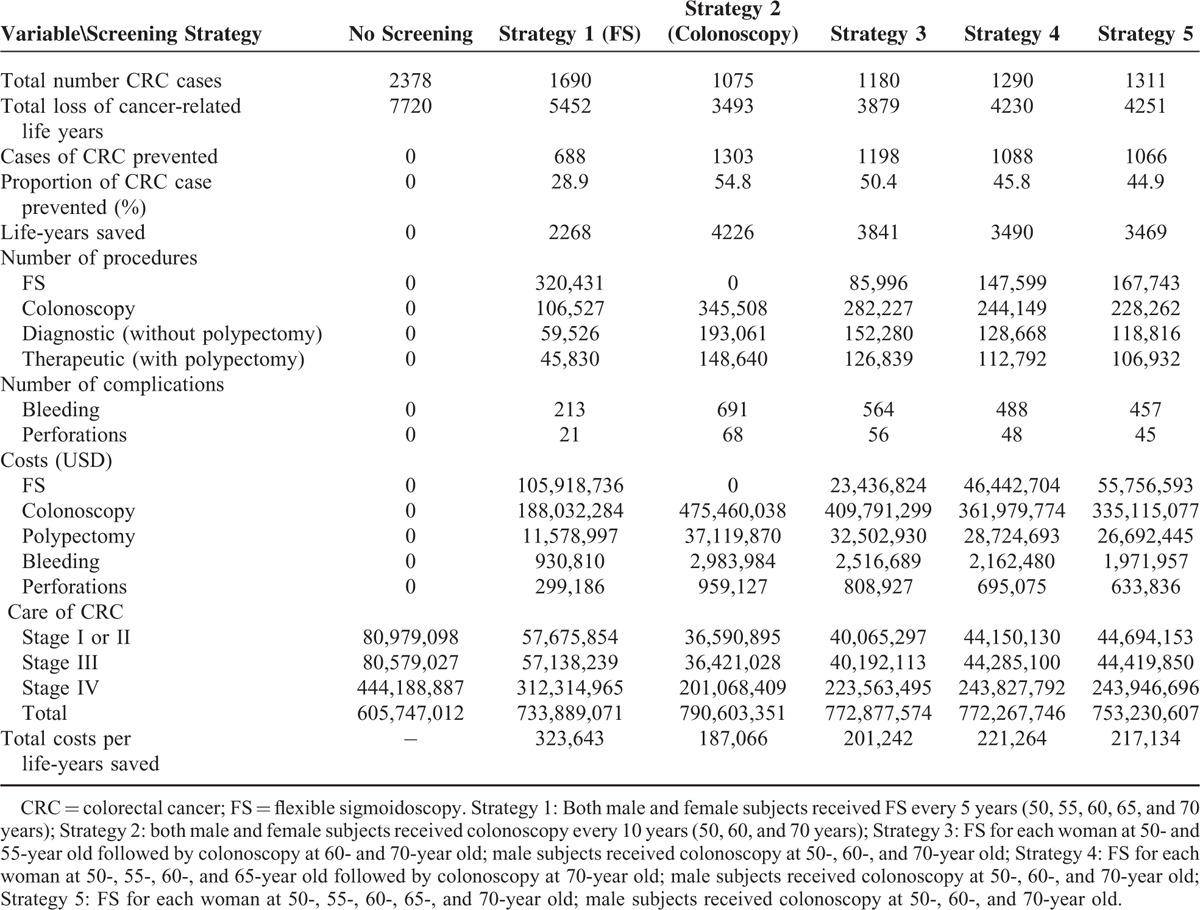
From a hypothetical population of 100,000 asymptomatic subjects, strategy 2 could save the largest number of life-years (4226 vs 2268 to 3841 by other strategies). When compared with no screening, strategy 5 had the lowest ICER (US$42,515), followed by strategy 3 (US$43,517), strategy 2 (US$43,739), strategy 4 (US$47,710), and strategy 1 (US$56,510). Strategy 2 leads to the highest number of bleeding and perforations, and required a prohibitive number of colonoscopy procedures. Strategy 5 remains the most cost-effective when assessed with a wide range of deterministic sensitivity analyses around the base case.
From the cost effectiveness analysis, FS for women and colonoscopy for men represent an economically favorable screening strategy. These findings could inform physicians and policy-makers in triaging eligible subjects for risk-based screening, especially in countries with limited colonoscopic resources. Future research should study the acceptability, feasibility, and feasibility of this risk-based strategy in different populations.
INTRODUCTION
Worldwide, colorectal cancer (CRC) is a leading global burden of disease.1,2 Flexible sigmoidoscopy (FS) and colonoscopy could reduce CRC mortality by 41% and 68%, respectively.3 Guidelines from Western and Asia Pacific countries have endorsed both tests for population-based CRC screening.4,5 It was recommended that CRC screening should be performed 5 yearly based on FS and 10 yearly based on colonoscopy starting from 50 years of age.
Colonoscopy is the predominant screening test in the United States, whereas FS is more commonly used in Australia and European countries.6,7 Although colonoscopy has been found to be cost-effective,4 the provider and financial resources required are substantial.8,9 It could only be performed by specialists, and is relatively labor-intensive, expensive, and invasive.8,9 On the other hand, FS is an office-based procedure which is more acceptable, safe and can be performed by primary care physicians.5 It has emerged as an attractive alternative to colonoscopy.10
Previous studies have evaluated a few validated tools to predict the risk of advanced proximal neoplasia (APN),11–14 based on which an informed choice of colonoscopy versus FS could be made. However, all these risk scores require distal findings, which imply that they could not be used to tailor CRC screening among screening-naive subjects. A recent evaluation by Imperiale et al15 based on 10,124 adults aged ≥50 years found that the risk of APN is a function of age and gender only, without an absolute need to incorporate distal findings. It was recommended from their study that women aged under 60 or 70 should be considered FS. Nevertheless, the authors suggested an explicit quantitative assessment of the incremental benefits, risks, and cost.15
The primary objective of this study was to evaluate the cost-effectiveness of applying this age- and gender-based CRC screening strategy in reducing CRC mortality as compared with use of FS alone or colonoscopy alone. We also studied the resources and complications induced by these different endoscopy-based strategies.
METHODS
Decision Model Framework
This study adopted a similar methodology to that of a cost-effectiveness analysis on CRC screening,16 where recent studies also used similar assumptions or approaches.17,18 Based on a Markov process, a hypothetical population of 100,000 asymptomatic subjects aged 50 years was included in a decision analysis model. All subjects received 1 of the 5 different strategies and were followed up until the age of 70 years (2008–2015), based on the recommendations by Imperiale et al.15 This is according to a recent consensus recommending that 75 years is a reasonable age to stop screening for both men and women.5 The study was approved by the Clinical Research Ethics Committee of the Chinese University of Hong Kong.
The rationale of the following 5 strategies was based on the findings from Imperiale et al,15 where women aged under 60 or 70 could be recommended FS. However, since the age limit where women should receive FS has not been clearly specified, we tested FS screening for women 5 yearly from 50 to 55 years (strategy 3); 50 to 65 years (strategy 4), and 50 to 70 years (strategy 5).
Strategy 1: FS as a primary screening test. This strategy offers each asymptomatic subject FS every 5 yearly at 50, 55, 60, 65, 70 years. If polyps are found, the subject will receive a colonoscopy.
Strategy 2: Colonoscopy as a primary screening test. This strategy offers each asymptomatic subject colonoscopy every 10 yearly at 50, 60, and 70 years.
Strategy 3: This strategy offers FS to each female subject every 5 yearly at 50 and 55 years. If polyps are found, the subject will receive a colonoscopy. If a colonoscopy finding is normal, FS is resumed after 10 years before aged 70. Each female subject aged 60 and male subject will be offered colonoscopy every 10 yearly up to 70 years.
Strategy 4: This strategy offers FS to each female subject every 5 yearly at 50, 55, 60, and 65 years. If polyps are found, the subject will receive a colonoscopy. If a colonoscopy finding is normal, FS is resumed after 10 years before aged 70. Each female subjects aged 70 and male subject will be offered colonoscopy every 10 yearly up to 70 years.
Strategy 5: This strategy offers FS to each female subject every 5 yearly at 50, 55, 60, 65, and 70 years. If polyps are found, the subject will receive a colonoscopy. If a colonoscopy finding is normal, FS is resumed after 10 years before aged 70. Male subject will be offered colonoscopy every 10 yearly up to 70 years.
In these 5 strategies, based on a published cost-effectiveness analysis,16 we assumed polyps detected by colonoscopy are removed, and the screening participant will receive repeated colonoscopy every 3 years, until no more polyp detection. If a colonoscopy shows normal finding, it will be repeated after 10 years.
Transition Probabilities
We employed data from >10,000 Chinese subjects who were aged 50 years or older who underwent colonoscopy in the study period 2008 to 2014, in a population-based screening program for Hong Kong residents. More details could be found elsewhere.19–21 We assumed the rate of compliance with colonoscopy was 98.9%. The overall age-adjusted rate of polypectomy was 43.5% (50.3% for male and 37.3% for female), cumulative over 20 years when the screening participants join the program at 50 years till 70-year old. The rate of bleeding (0.2%) and perforation (0.02%) was also based on observations from the cohort. Ten percent was assumed to be the mortality rate induced by bowel perforation.22 Since the screening subjects were not offered FS, we made reference to published studies,23,24 where the sensitivity and specificity of FS was estimated as 75% to 80% and 60% to 90%, respectively. The specificity of FS refers to detection of polyps in the distal colon by the index FS but subsequent colonoscopy failed to detect a screen-relevant lesion. Our screening program also offered fecal occult blood tests but these were not taken into the present analysis as the strategies compared did not involve fecal tests.
Based on reports from the Hong Kong Cancer Registry, the age-stratified incidence of CRC of the Hong Kong population was estimated assuming no CRC screening was offered.25 From a US study, FS was found to reduce the CRC incidence by 34%.26 Moreover, colonoscopy screening could attain a 76% to 90% reduction in the incidence of CRC, as implied by a former study27 From a published cost-effectiveness study,16 the reduction in the incidence of CRC was discounted since a 10% noncompliance rate with screening was built in. Colonoscopy and FS could decrease CRC incidence by 54% and 29%, respectively. The compliance rate of FS was different than that of colonoscopy. Since our CRC screening program offered colonoscopy but not FS, we assumed compliance figures for colonoscopy from our program but that of FS from published literature.
The annual mortality rate of CRC was extrapolated from the Hong Kong Cancer Registry.25 Patients with stage I and II CRC are managed by surgical procedures which aim to attain survival with no recurrence for a maximum period of 5 years. Stage III CRC patients are treated by surgery followed by adjuvant chemotherapy, with an estimated cure rate of 70%, and death rate of 30% due to CRC recurrence. Subjects with CRC at stage IV receive palliative treatment, and half of them require additional operation due to liver metastasis.28,29 It was assumed that those with CRC at stage III and IV receive FOLFOX and bevacizumab as detailed in a previous study.16 The details of the chemotherapy treatments are also presented in Table 1.16
TABLE 1.
Estimates for the Costs Based on Different Screening Scheme and Treatment Methods
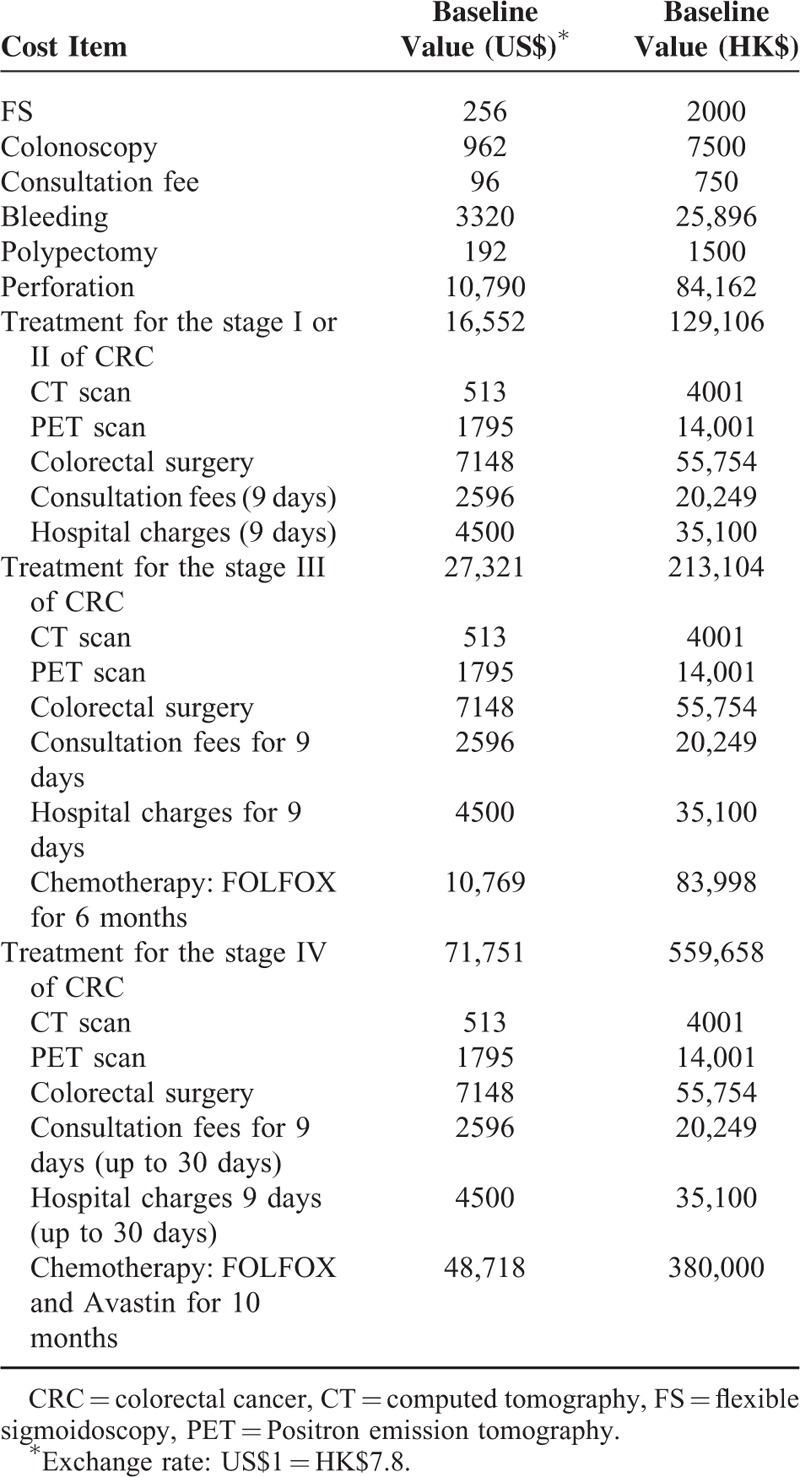
Cost Estimates
We used the Hong Kong Government Gazette to derive all costs (in $US)30 The components of the decision model consisted of screening cost; investigation costs; CRC staging; as well as costs incurred by treatment and hospital admission. In addition, we included the costs of admission for possible complications, like bleeding or perforation in endoscopic procedures (Table 2). Indirect costs were excluded for simpler analysis. Labor costs for daily hospital care and disposable instruments were considered in the hospitalization costs, while CT and PET scans, surgical procedures and consultations were taken into account separately. The average hospital length of stay (LOS) for surgical patients with CRC was assumed as 9 days.31 Chemotherapy costs were only deduced for stage III and IV CRC patients. Among patients with CRC at stage IV, the choice of treatment and LOS are contingent on progression of diseases. Hence, we performed a sensitivity analysis where the maximum cost is US$100,000. The maximum LOS is 30 days. Furthermore, we assumed 50% of these subjects require liver resection,28,29 with a unit cost is $12,962. Overall, each patient incurs $6,481, without accounting for costs due to blood transfusions and follow-up visits. Expenditure items were discounted at an annual rate of 3%.32
TABLE 2.
Baseline Estimates for the Screening Scheme
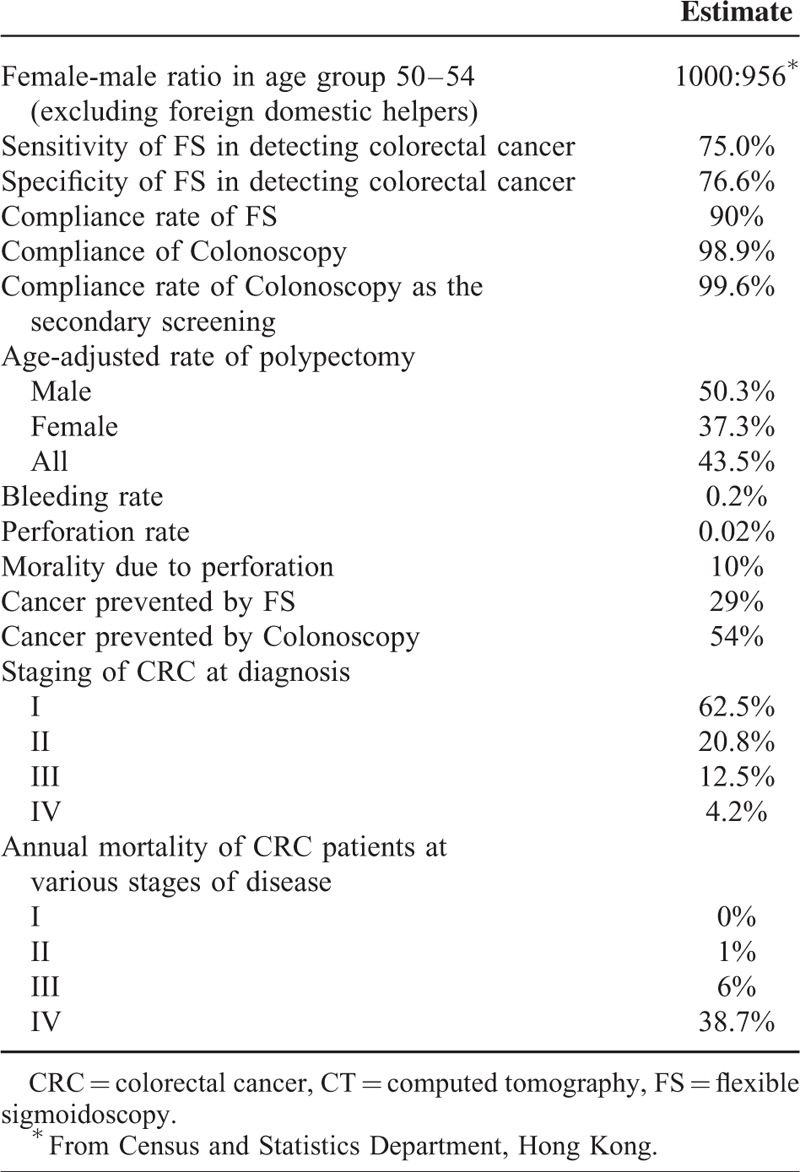
The Cost-Effectiveness Analysis
Life-year saved was used to measure the effectiveness of CRC screening as a result of CRC prevention and better prognosis due to early diagnosis of CRC. It was measured by the difference in life-years lost arising from cancer-specific mortality between screening-present and screening-absent Markov modeling. We used the standard life table of Hong Kong to project life-years lost.33 For premature deaths due to CRC, we accumulated the life-years lost based on age-specific proportions in each cycle throughout their expected lifetime. The incremental cost effectiveness ratio (ICER) among the screening strategies is the major outcome. It is quantitatively measuring the extra cost needed for 1 life-year saved, and is computed by dividing the difference in cost by the differentials in effectiveness among various screening methods.
Sensitivity Analyses
We performed 1-way sensitivity analyses in the modeling on the ICERs with respect to different screening strategies based on various variables. The specificity of FS ranged between 60% to 90%.16 Furthermore, as differences in program compliance are relatively significant,34–36 we assessed compliance rate in the range of 10% to 100%.16 We also evaluated colonoscopy costs in the range US$100–US$1000. The cost of FS ranged from US$100–US$500.16 A probabilistic analysis was performed by Monte Carlo simulation. All analyses and simulation of data were performed in the excel.
RESULTS
Table 3 shows the outcomes of 5 screening strategies and no screening. With no screening, the cohort will have 2378 CRC cases with a total loss of 7720 cancer-related life years. The proportion of CRC cases prevented is the highest with strategy 2 (54.8%), followed by strategy 3 (50.4%), strategy 4 (45.8%), strategy 5 (44.9%), and strategy 1 (28.9%). Similarly, the life-years saved are the highest with strategy 2 (4226 years), but at a substantially higher cost. When compared with no screening, all 5 strategies lead to much lower costs of treatment at each CRC stage (Table 3). The total number of colonoscopy procedures required (n = 345,508), bleeding (n = 691), and perforation (n = 68) is the highest with strategy 2.
TABLE 3.
Outcome of a Cohort of 100,000 Average-Risk Individuals Aged 50–70 Years With Various Screening Strategies for Colorectal Cancer
When compared with no screening, the ICERs of strategy 1 to 5 are US$56,510, US$43,739, US$43,517, US$47,710, and US$42,515, respectively. Therefore, strategy 5 is the most cost-effective strategy in the prevention and treatment of CRC (Table 4 and Figure 1).
TABLE 4.
Incremental Cost-Effectiveness Ratios Switching From Screening Method 1 to 2
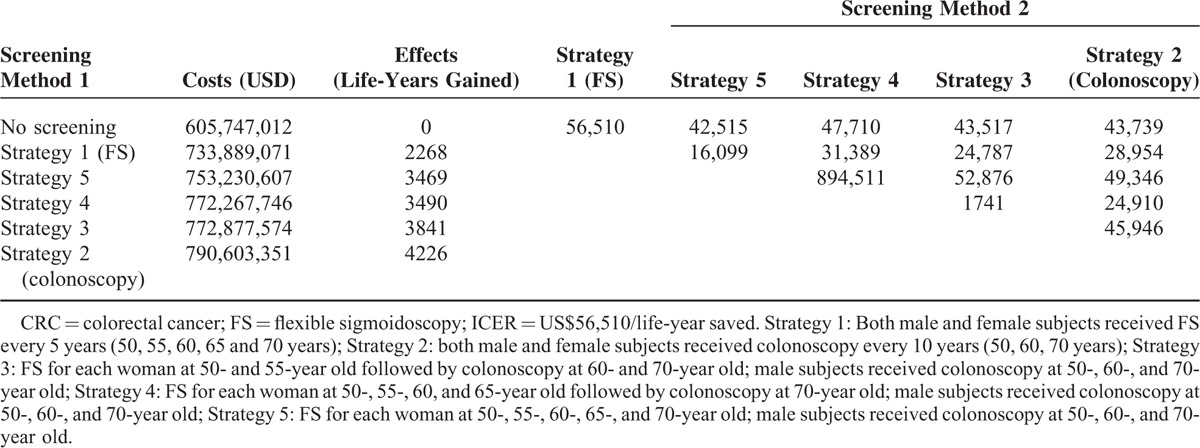
FIGURE 1.
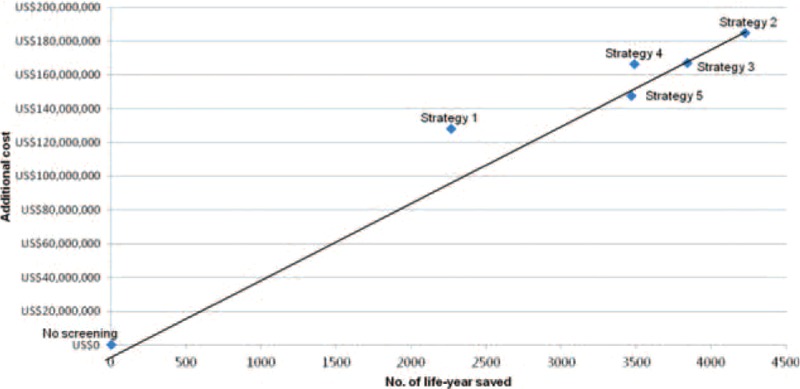
The additional costs for a life-year saved on the screening tests compared with no screening.
A sensitivity analysis was performed with varying FS compliance and fixed colonoscopy compliance (Supplementary Figure 1a). With a reduced FS compliance rate, ICER rises indicating that extra costs are required for a life-year saved. Strategy 5 remains the lowest in ICER over FS compliance of range of 10% to 96%. Strategy 2 is more cost-effective beyond 96% FS compliance when the ICER of other strategies is greater than that of colonoscopy (represented by the gray area). In conclusion, if compliance with FS were very high, colonoscopy would be preferred. Otherwise, strategy 5 would be better.
The ICERs decrease with increasing specificity of FS from 60% to 90% at fixed sensitivity of FS of 75% (Supplementary Figure 1b). Colonoscopy is more cost-effective when specificity is less than 73.2%. Strategy 5 performs the best beyond 73.2% as its ICER is the lowest; that is, out of the gray area. Similar trends were found in higher sensitivities of FS up to 80%. At the range of US$100 to $450 per colonoscopy (Supplementary Figure 1c), ICERs of strategies 2 to 5 are cost-effective as their ICERs are negative compared to no screening. ICER of strategy 2 remains the lowest at $902 or lower. When the cost of colonoscopy rises above $902, strategy 5 is the most cost-effective among all screening strategies. At the range of US$100 to $139 per FS (Supplementary Figure 1d), ICER of strategy 1 is the lowest in all screening. Strategy 5 is the most cost-effective in the range of $139 to $275. Beyond $275, the higher cost of FS renders all other FS-included strategies less cost effective than strategy 2 as a primary screening tool. A wide range of deterministic sensitivity analyses around the base case showed that strategy 5 is the most cost-effective.
DISCUSSION
From this cost-effectiveness analysis based on real-life screening data and published literature, it was found that 10-yearly colonoscopy starting at aged 50 years was more cost-effective than 5-yearly FS. However, screening all women by FS and all men by colonoscopy starting at 50 years was even more cost-effective than colonoscopy for both genders. FS screening for all women aged at 50 and 55 years followed by colonoscopy at 60 and 70 years, while screening all men by colonoscopy, was also more cost-effective than colonoscopy for all. These remain true when the level of compliance with FS is <96%; the specificity of FS is >73.2%, the cost of colonoscopy is >US$902; and the cost of FS is <US$275. Probabilistic analysis also supported this strategy. Although colonoscopy for all could render the longest life-years saved, the colonoscopy and polypectomy procedures required is prohibitively huge. Its use as a primary screening tool is challenged by the substantially higher number of bleeding and perforations compared to other strategies. These findings support the risk tailoring strategy proposed by Imperiale et al,15 where FS could be considered sufficient screening among women aged under age 60.
We used a method similar to a cost-effectiveness study,16 which concluded FIT as the most cost-effective test, and findings were similar in sensitivity analysis with different rates of compliance. At the year of Tsoi and colleagues’ study, the recommendations by Imperiale and colleagues have not yet been released. Current studies on the cost-effectiveness of the various CRC screening modalities yielded mixed results.26,37–43 Some reported that screening based on FS was the most cost-effective,37–39 whilst other studies found that colonoscopy screening had greater cost-effectiveness.26,40,41 Yet one study reported that a hybrid method using yearly rehydrated faecal occult blood tests coupled with FS was amongst the most cost-effective.42 Our study is novel and unique as it is based on age and gender, and the data were from observations from actual screening practices.
The cost-effectiveness of using this age- and gender-based risk stratification tool was found to be the highest in this hypothetical population of screening participants.15 Imperiale and colleagues recruited 10,124 adults aged ≥50 years, with an average age of 57.5 years (SD 6.0) and were comprised of 44% women. The study population was derived from 2 company-based CRC prevention programs in Indianapolis, and included employees, retirees, and their dependents. The risk of APN increased markedly across the age categories (50–59 years: 1.47%; 60–69 years: 2.45%; ≥70 years: 6.64%). Among all subjects with no distal neoplasia, they found that men had nearly twice the risk of APN (relative risk = 1.91, 95% CI: 1.32–2.77). The risk for APN was very low among women in the 50 to 59 years (0.85%) and 60- to 69-year-old age groups (0.88%) among those with no distal neoplasia. The Number Needed to Screen (NNS) to detect on proximal cancer was 3221 in women aged 50 to 59 years, and this figure decreased markedly to 853 in men aged <60 years. It has been proposed that this age and gender-based strategy could optimize screening efficiency and increase colonoscopy yield. Our study demonstrated the need for a cost-effectiveness study to explicitly quantify the benefits and harms among tailored screening strategies for these groups. Although colonoscopy had the lowest ICER in other situations with different compliance levels, specificity of FS and costs of tests, it is arguably not practical to use it as the primary screening test for every eligible subject in the population as reflected by the large number of colonoscopic procedures required. Our study findings therefore supported the strategy proposed by Imperiale et al, where FS could be used as an alternative to colonoscopy in women without loss of cost-effectiveness. The screening strategy is especially relevant to resource-limited countries where colonoscopic resources are limited. In countries where colonoscopic resources are adequate, using colonoscopy as the primary screening tool can achieve the greatest life-years saved—but the potential complications including bleeding and perforations should be considered with caution.
There are several study limitations which should be mentioned. Firstly, the participants are self-referred and might not be representative of the general public. However, one might expect a high refusal rate if random sampling was used to recruit and select subjects for screening. Also, although we used actual data in the Markov models, some variables made reference to published studies due to the relatively short period (5 years) of follow-up of the screening cohort. For instance, the reduction of mortality due to colonoscopy was based on an observational study,27 and studies in the United States, Canada, and Germany suggested that the reduction in mortality or incidence were almost entirely in the right colon. Furthermore, more conservative screening strategies may refer only 3% to 7% of patients with advanced distal neoplasia at index FS for colonoscopy follow-up,44 and even lower proportions in subsequent FS screening tests. Another example is that a certain proportion of cancer polyps might only be treated with endoscopy but not surgery, and this may bear implications on the cost estimates. Similar arguments exist for the compliance rates for FS and colonoscopy, which might be relatively high when compared to other population-based screening programs. Also, since our modeling and cost estimates were based on CRC instead of advanced neoplasia (AN), the distribution of AN in subgroups might be more applicable in future modeling studies which compared strategies with AN as an outcome variable. Lastly, this study has not evaluated the cost-effectiveness of some hybrid strategies, like the combination of using fecal immunochemical tests (FITs) and FS.42
In summary, this study highlighted the higher cost-effectiveness of implementing tailored CRC screening programs based on age and gender. These findings could inform physicians and policy-makers in triaging eligible subjects for risk-based screening—especially in countries with limited colonoscopic resources. Future studies should explore the acceptability, feasibility, and patient preference of receiving FS versus colonoscopy in various patient subgroups, and assess the benefits and costs of using other candidate feature for tailoring.
Supplementary Material
Acknowledgments
We expressed our sincere gratitude for the participation of the screening participants in this study. We acknowledged the full funding support from the Hong Kong Jockey Club Charities Trust. There is no writing assistance.
Footnotes
Abbreviations: APN = advanced proximal neoplasia, CRC = colorectal cancer, FS = flexible sigmoidoscopy, ICER = incremental cost effectiveness ratio
This study received full funding support from the Hong Kong Jockey Club Charities Trust.
The authors have no funding and conflicts of interest to disclose.
Authors’ contributions: MCSW participated in design of the study, analysis of the results, and writing of the first draft of the manuscript; JYLC and VCWC participate in design of study and analysis of results; AKCL and TYTL conducted the study and analysis of the results; SSHW, SCN, SSMN JCYW, FKLC, and JJYS participated in design and performance of the study, analysis, and discussion; All authors read and approved the final manuscript. All authors included on a paper fulfill the criteria of authorship. There is no one else who fulfills the criteria but has not been included as an author.
REFERENCES
- 1.GLOBOCAN 2012 [Internet]. Cancer Fact Sheet. Colorectal Cancer Incidence, Mortality and Prevalence Worldwide in 2012. Available from: http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx Accessed on 23 September, 2015.
- 2.Sung JJY, Lau JYW, Goh KL, et al. Increasing incidence of colorectal cancer in Asia: implications for screening. Lancet Oncol 2005; 6:871–876. [DOI] [PubMed] [Google Scholar]
- 3.Nishihara R, Wu K, Lochhead P, et al. Long-term colorectal-cancer incidence and mortality after lower endoscopy. N Engl J Med 2013; 369:1095–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. CA Cancer J Clin 2008; 58:130–160. [DOI] [PubMed] [Google Scholar]
- 5.Sung JJ, Ng SC, Chan FK, et al. An updated Asia Pacific consensus recommendations on colorectal cancer screening. Gut 2015; 64:121–132. [DOI] [PubMed] [Google Scholar]
- 6.Benson VS, Patnick J, Davies AK, et al. Colorectal cancer screening: a comparison of 35 initiatives in 17 countries. Int J Cancer 2008; 122:1357–1367. [DOI] [PubMed] [Google Scholar]
- 7.Klabunde CN, Lanier D, Nadel MR, et al. Colorectal cancer screening by primary care physicians: recommendations and practices, 2006–2007. Am J Prev Med 2009; 37:8–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seeff LC, Manninen DL, Dong FB, et al. Is there endoscopic capacity to provide colorectal cancer screening to the unscreened population in the United States? Gastroenterology 2004; 127:1661–1669. [DOI] [PubMed] [Google Scholar]
- 9.Seeff LC, Richards TB, Shapiro JA, et al. How many endoscopies are performed for colorectal cancer screening? Results from CDC's survey of endoscopic capacity. Gastroenterology 2004; 127:1670–1677. [DOI] [PubMed] [Google Scholar]
- 10.Segnan N, Patnick J, von Karsa L. Publications Office of the European Union, European guidelines for quality assurance in colorectal cancer screening and diagnosis. Luxembourg:2010. [DOI] [PubMed] [Google Scholar]
- 11.Atkin WS, Edwards R, Kralj-Hans I, et al. Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: a multicentre randomised controlled trial. Lancet 2010; 375:1624–1633. [DOI] [PubMed] [Google Scholar]
- 12.Segnan N, Armaroli P, Bonelli L, et al. Once-only sigmoidoscopy in colorectal cancer screening: follow-up findings of the Italian Randomized Controlled Trial—SCORE. J Natl Cancer Inst 2011; 103:1310–1322. [DOI] [PubMed] [Google Scholar]
- 13.Bretthauer M, Gondal G, Larsen IK, et al. Design, organization and management of a controlled population screening study for detection of colorectal neoplasia attendance rates in the NORCCAP Study (Norwegian Colorectal Cancer Prevention). Scand J Gastroenterol 2002; 37:568–573. [DOI] [PubMed] [Google Scholar]
- 14.Imperiale TF, Wagner DR, Lin CY, et al. Using risk for advanced proximal colonic neoplasia to tailor endoscopic screening for colorectal cancer. Ann Intern Med 2003; 139:959–965. [DOI] [PubMed] [Google Scholar]
- 15.Imperiale TF, Glowinski EA, Lin-Cooper C, et al. Tailoring colorectal cancer screening by considering risk of advanced proximal neoplasia. Am J Med 2012; 125:1181–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsoi KK, Ng SS, Leung MC, et al. Cost-effectiveness analysis on screening for colorectal neoplasm and management of colorectal cancer in Asia. Aliment Pharmacol Ther 2008; 28:353–363. [DOI] [PubMed] [Google Scholar]
- 17.Wong MC, Ching JY, Chan VC, et al. The comparative cost-effectiveness of colorectal cancer screening using faecal immunochemical test vs. colonoscopy. Sci Rep 2015; 5:13568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wong MC, Ching JY, Chan VC, et al. Screening strategies for colorectal cancer among patients with nonalcoholic fatty liver disease and family history. Int J Cancer 2015; 138:576–583. [DOI] [PubMed] [Google Scholar]
- 19.Wong MC, Ching JY, Ng SC, et al. Prediction of proximal advanced neoplasia: a comparison of four existing sigmoidoscopy-based strategies in a Chinese population. Gut 2015; 64:776–783. [DOI] [PubMed] [Google Scholar]
- 20.Wong MC, Ching JY, Chan VC, et al. Factors associated with false positive and false negative faecal immunochemical test results for colorectal cancer screening. Gastrointest Endosc 2015; 81:596–607. [DOI] [PubMed] [Google Scholar]
- 21.Wong MC, Lam TYT, Tsoi KKF, et al. A validated tool to predict colorectal neoplasia and inform screening choice for asymptomatic subjects. Gut 2014; 63:1130–1136. [DOI] [PubMed] [Google Scholar]
- 22.Wu GH, Wang YM, Yen AM, et al. Cost-effectiveness analysis of CRC screening with stool DNA testing in intermediate-incidence countries. BMC Cancer 2006; 6:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sung JJY, Chan FK, Leung WK, et al. Screening for colorectal neoplasms in Chinese: fecal occult blood test, flexible sigmoidoscopy or colonoscopy? Gastroenterology 2003; 124:608–614. [DOI] [PubMed] [Google Scholar]
- 24.Soon MS, Kozarek RA, Ayub K, et al. Screening colonoscopy in Chinese and Western patients: a comparative study. Am J Gastroenterol 2005; 100:2749–2755. [DOI] [PubMed] [Google Scholar]
- 25.Hospital Authority: Hong Kong Cancer Registry [Internet]. Available form: http://www.ha.org.hk/cancereg Accessed on 14 September, 2015.
- 26.Sonnenberg A, Delco F, Inadomi JM. Cost-effectiveness of colonoscopy in screening for colorectal cancer. Ann Intern Med 2000; 133:573–584. [DOI] [PubMed] [Google Scholar]
- 27.Winawer SJ, Zauber AG, Ho MN, et al. Prevention of colorectal cancer by colonoscopic polypectomy. New Engl J Med 1993; 329:1977–1981. [DOI] [PubMed] [Google Scholar]
- 28.Kemeny N. Presurgical chemotherapy in patients being considered for liver resection. Oncologist 2007; 12:825–839. [DOI] [PubMed] [Google Scholar]
- 29.Borner MM. Neoadjuvant chemotherapy for unresectable liver metastases of colorectal cancer—too good to be true? Ann Oncol 1999; 10:623–626. [DOI] [PubMed] [Google Scholar]
- 30.Hospital Authority: List of Charges: S.S. No. 4 to Gazette No. 44/1996. Hong Kong, 562 People's Republic of China: Government Printers, 1996, 2003, 2008. [Google Scholar]
- 31.Leung KL, Kwok SP, Lam SC, et al. Laparoscopic resection of rectosigmoid carcinoma: prospective randomised trial. Lancet 2004; 363:1187–1192. [DOI] [PubMed] [Google Scholar]
- 32.Drummond MF, Jefferson TO. Guidelines for authors and peer reviewers of economic submissions to the BMJ. The BMJ Economic Evaluation Working Party. BMJ 1996; 313:275–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hong Kong Census and statistics Department: Hong Kong Life Tables 1996–2031. Hong Kong, People's Republic of China: Government of the Hong Kong Special Administrative Region, Government Printers, 2008. [Google Scholar]
- 34.Hou SI, Chen PH. Home-administered fecal occult blood test for colorectal cancer screening among worksites in Taiwan. Prev Med 2004; 38:78–84. [DOI] [PubMed] [Google Scholar]
- 35.Yang KC, Liao CS, Chiu YH, et al. Colorectal cancer screening with faecal occult blood test within a multiple disease screening programme: an experience from Keelung, Taiwan. J Med Screen 2006; 13:S8–S13. [PubMed] [Google Scholar]
- 36.Li S, Nie Z, Li N, et al. Colorectal cancer screening for the natural population of Beijing with sequential fecal occult blood test: a multicenter study. Chin Med J (Engl) 2003; 116:200–202. [PubMed] [Google Scholar]
- 37.McGrath JS, Ponich TP, Gregor JC. Screening for colorectal cancer: the cost to find an advanced adenoma. Am J Gastroenterol 2002; 97:2902–2907. [DOI] [PubMed] [Google Scholar]
- 38.Allameh Z, Davari M, Emami MH. Cost-effectiveness analysis of colorectal cancer screening methods in Iran. Arch Iran Med 2011; 14:110–114. [PubMed] [Google Scholar]
- 39.Sharp L, Tilson L, Whyte S, et al. Cost-effectiveness of population-based screening for colorectal cancer: a comparison of guaiac-based faecal occult blood testing, faecal immunochemical testing and flexible sigmoidoscopy. Br J Cancer 2012; 106:805–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sharaf RN, Ladabaum U. Comparative effectiveness and cost-effectiveness of screening colonoscopy vs. sigmoidoscopy and alternative strategies. Am J Gastroenterol 2012; 108:120–132. [DOI] [PubMed] [Google Scholar]
- 41.Ouakrim DA, Boussioutas A, Lockett T, et al. Cost-effectiveness of family history-based colorectal cancer screening in Australia. BMC Cancer 2014; 14:261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Frazier AL, Colditz GA, Fuchs CS, et al. Cost-effectiveness of screening for colorectal cancer in the general population. JAMA 2000; 284:1954–1961. [DOI] [PubMed] [Google Scholar]
- 43.Lansdorp-Vogelaar I, Knudsen AB, Brenner H. Cost-effectiveness of colorectal cancer screening. Epidemiol Rev 2011; 33:88–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wong MC, Ching JY, Chan VC, et al. Diagnostic accuracy of a qualitative fecal immunochemical test varies with location of neoplasia but not number of specimens. Clin Gastroenterol Hepatol 2015; 13:1472–1479. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


