Abstract
Bile acid metabolism was reported to be involved in glucose metabolism homeostasis. However, the exact relationship between bile acid and glucose metabolism as well as insulin sensitivity is not clarified. Therefore, we sought to investigate the association between insulin sensitivity and hyperbileacidemia in type 2 diabetic and nondiabetic population.
This community-based cross-sectional study included 9603 residents from Jiading, Shanghai, China, who were 40 years and older. Standardized questionnaire, anthropometric measurements and laboratory tests were conducted. Homeostasis model assessment of insulin resistance (HOMA-IR) ≥ 2.7 was defined as insulin resistance and fasting TBA ≥ 10 mmol/L was defined as hyperbileacidemia.
Multivariate stepwise regression analysis revealed that HOMA-IR, age, and male sex were positively associated with hyperbileacidemia in both nondiabetic and diabetic participants. In multivariate logistic models, participants with insulin resistance had significantly higher risk of hyperbileacidemia compared to those who have no insulin resistance, in both nondiabetic and diabetic population (nondiabetic: OR = 1.76; 95% CI 1.42–2.19; P < 0.001; diabetic: OR = 1.56; 95% CI 1.06 – 2.31; P = 0.025, respectively). Further adjustment for the HbA1c level in diabetic population did not change the significant association (OR = 1.59; 95% CI 1.06 − 2.40; P = 0.024). In nondiabetic participants, each 1-unit increment of HOMA-IR conferred an 18% higher risk of hyperbileacidemia (95% CI 1.04–1.35; P = 0.013), whereas in diabetic participants, this association was similar but not significant (95% CI 0.95–1.59; P = 0.117).
Insulin resistance was positively associated with hyperbileacidemia in both nondiabetic and diabetic population. The increase in the bile acid level in insulin-resistant population regardless of status of diabetes and glucose level indicated the important role of insulin resistance in the regulation of bile acid metabolism in human.
INTRODUCTION
Bile acid (BA) was demonstrated to exert variety of metabolic effects besides the known role in cholesterol homeostasis.1 Increasing amount of studies have proposed that the bile acid (BA) was involved in glucose metabolism homeostasis.1–4 Animal studies have denoted that glucose induces cholesterol bile acid synthesis via insulin signaling and epigenetic mechanisms.5 Circulating insulin was reported to induce the CYP7A1 gene in diabetic mice, a gene mainly controlled BA synthesis in hepatocytes, which leads to higher level of circulating BA.6–8 Meanwhile, BA could promote secretion of incretin in the digestive tract, which in turn improved glucose tolerance, promoted insulin release, and increased insulin sensitivity.9–12
It is important to note that to date most of these findings on the association between insulin sensitivity and bile acid have indeed been observed in mouse models.13,14 Therefore, it is not well known whether alterations in BAs also occur in humans; inconsistent results were seen in the few human studies. In some studies, total bile acid (TBA) in serum or plasma was unchanged in type 2 diabetes (T2D) in comparison to controls.13,15 Whereas in other study, BA were found increased in diabetic population.15,16 Hence, given the unclear interaction between circulating insulin and BA in the progression of diabetes, it is important to understand the role of circulating insulin in BA in human. Therefore, the aim of our study was to investigate the association between insulin sensitivity and TBA concentrations in a Chinese population. Furthermore, we investigated this correlation in both diabetic and nondiabetic participants.
METHODS
Survey Design and Subjects
A population-based cross-sectional research randomly recruited 10,375 participants from Jiading district, Shanghai metropolitan area, China, between March 2010 and August 2010. All the participants were 40 years and older. Participants with missing data on glucose and insulin levels have been excluded (N = 18). Further exclusion occurred if participant took any hypoglycemic agents such as sulfonylurea, metformin, thiazide, diuretic, acarbose, or insulin (N = 754), as those agents could influence the exact relationship between bile acid and insulin sensitivity. Thus, the diabetic individuals included in the analysis were mostly newly diagnosed through our study. Eventually, a total of 9603 individuals (8463 nondiabetic and 1140 diabetic participants) were enrolled in the current analysis.
The study protocol was approved by the Institutional Review Board of the Rui-Jin Hospital affiliated to Shanghai Jiao-Tong University School of Medicine. Written informed consent was obtained from each participant before data collection.
Clinical Data Collection and Biochemistry Measurements
A standard questionnaire was administered to all participants for information collection on disease history, medication administration, and lifestyle risk factors.
All anthropometric measurements were performed by trained clinical staff adhered to standard protocols. Height and weight were recorded to the nearest 0.1 cm and 0.1 kg while participants were standing on the electronic weight device with light clothing and no shoes. BMI was then calculated as weight (kg) divided by squared height (kg/m2). Waist circumference (WC) was measured to the nearest 0.1 cm at the umbilical level while participants were standing facing the staff with normal breathing. After 10 minutes of rest, blood pressure was measured in a sitting position 3 times with 1 minute interval. The averages of the triplicate readings of blood pressure were used for data analysis.
Glucose metabolism was measured using a simplified oral glucose tolerance test (OGTT) as described in our previous study.17 Plasma glucose and insulin were measured by autoanalyzer Modular P800 (Roche, Basel, Switzerland) and Modular E170 (Roche), respectively. The insulin resistance index HOMA-IR was calculated as followed: HOMA-IR = fasting insulin (μIU/mL) × fasting glucose (mmol/L)/22.5. Glycated hemoglobin (HbA1c) was measured by VARIANT II Hemoglobin Testing System (Bio-Rad Laboratories). Lipid profile including triglycerides (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) were examined by Modular E170 (Roche). Fasting total bile acid was examined using the enzyme cycle method by Bayer-1650 (German).
Definitions
In this study, diabetic participants were defined either with confirmed history of type 2 diabetes or in accordance with the 1999 World Health Organization (WHO) diagnostic criteria18 (fasting plasma glucose [FPG] ≥ 7.0 mmol/L, and/or OGTT-2 h postload plasma glucose [PPG] ≥ 11.1 mmol/L). Impaired glucose tolerance (IGR) was defined as the FPG level between 6.0 mmol/L and 7.0 mmol/L, and/or OGTT-2 h PPG level between 7.8 mmol/L and 11.0 mmol/L. Dyslipidemia was defined as one or more of the following condition: hypercholesterolemia was defined as the TC level ≥ 6.22 mmol/L; high LDL-C level was ≥ 4.14 mmol/L; low HDL-C was < 1.04 mmol/L; hypertriglyceridemia was defined as TG level ≥ 2.26 mmol/L.19 General obesity was defined as a BMI of 30.0 or higher.20 Central obesity was defined as waist circumference 90 cm or more in men and 80 cm or more in women.21
HOMA-IR >2.7 was defined as insulin resistance.22 As diabetic population were all insulin resistant by definition, in diabetic population, HOMA-IR >2.7 was described as high insulin resistance, if <2.7, described as low insulin resistance.23
TBA between 0 and 10 μmol/L was considered normal level24 and higher than 10 μmol/L was defined as hyperbileacidemia.25
Statistical Analysis
Skewed variables including TG and HOMA-IR were logarithmic transformed before analyses and presented as medians (interquartile range). Other normalized-distributed continuous variables were presented as mean ± standard deviation (SD). Categorical variables were expressed as numbers (proportions). Comparisons of proportions were performed with the χ2 test.
Pearson's correlation and multiple stepwise linear regression were used to analyze the associations between influencing factors and TBA. The unadjusted (model 1) and multivariate adjusted logistic regression analyses were used to investigate association between TBA and insulin sensitivity. Model 1 included no covariates whereas model 2 included age, sex, BMI, hypertension, and hyperlipemia. Sex (male/female), hypertension status (yes/no), and dyslipidemia (yes/no) were used as categorical variables. All statistical tests were 2-sided, and P value < 0.05 was considered statistically significant. Statistical analysis was performed using SAS version 9.3 (SAS Institute Inc, Cary, NC).
RESULTS
Characteristics of the Study Population
The participants’ characteristics were presented in Table 1. Among the total 9603 participants, 3632 (37.82%) were men. In nondiabetic population, the mean ( ± SD) age is 57.97 ± 9.64 years, the median of TBA is 4.3 μmol/L, and the median of HOMA-IR is 1.47. In the diabetic population, the mean age is 61.23 ± 9.71 years, the median of TBA level is 4.9 μmol/L, and the median of HOMA-IR is 2.78.
TABLE 1.
Baseline Characteristics of the Study Population According to Insulin Sensitivity
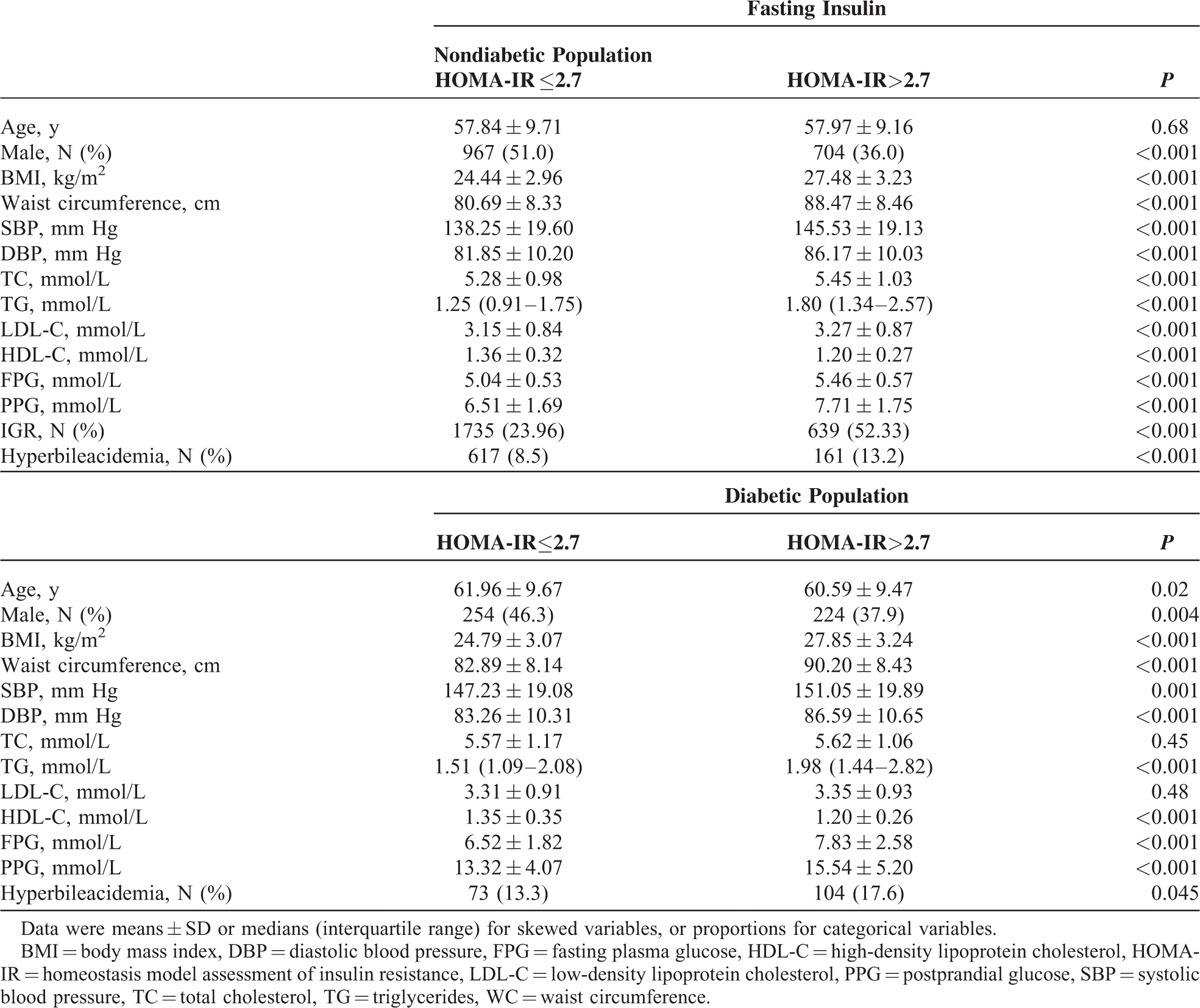
Both nondiabetic and diabetic populations were divided into 2 groups according to insulin sensitivity. Among nondiabetic population, those who had insulin resistance had significantly higher levels of BMI, waist circumference, SBP, DBP, TC, TG, LDL, FPG, PPG, proportion of IGR, and proportion of hyperbileacidemia than those who were not insulin resistant (all P < 0.05). Conversely, the level of HDL-c was higher in the noninsulin-resistant group (P < 0.05). There were no differences of age between the groups. Similar trend was also presented in diabetic patients except that there were no differences of TC or LDL-c level between the low-insulin-resistant and high-insulin-resistant counterparts.
Association Between Insulin Sensitivity and Total Bile Acid
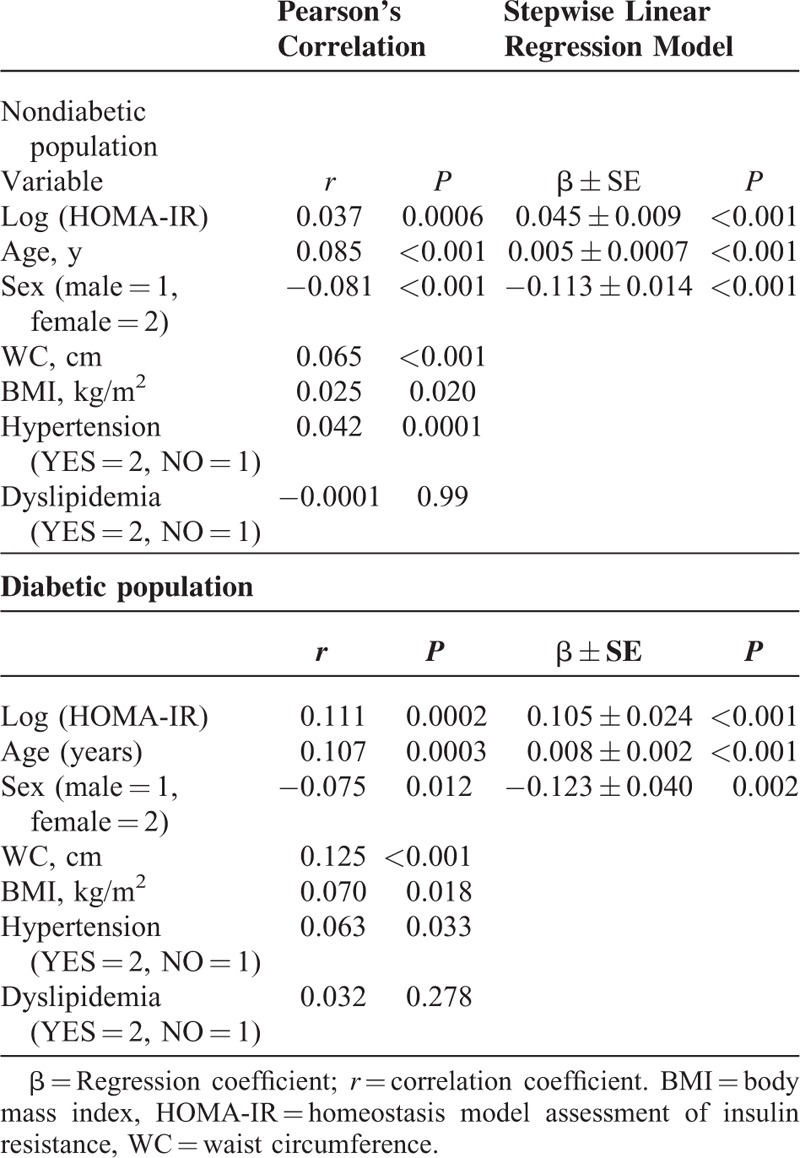
The variables associated with TBA level demonstrated by Pearson's correlation and stepwise linear regression was shown in Table 2. In nondiabetic population, Pearson's correlation indicated that HOMA-IR, age, male sex, WC, BMI, and hypertension were positively correlated with level of TBA. After performing multivariate stepwise linear regression analysis, HOMA-IR (β=0.045, P < 0.001), age (β=0.005, P < 0.001), and male sex (β=0.113, P < 0.001) were demonstrated to be positively associated with TBA (Table 2). Similarly in diabetic population, HOMA-IR, age, male sex, WC, BMI and hypertension showed positive significant correlation with TBA. Multivariate stepwise linear regression analysis demonstrated that HOMA-IR (β=0.105, P < 0.001), age (β=0.008, P < 0.001), and male sex (β=0.123, P = 0.002) were positively associated with TBA.
TABLE 2.
Pearson's Correlation and Multiple Stepwise Linear Regression Analysis of Risk Factors Associated With log (tba) Level
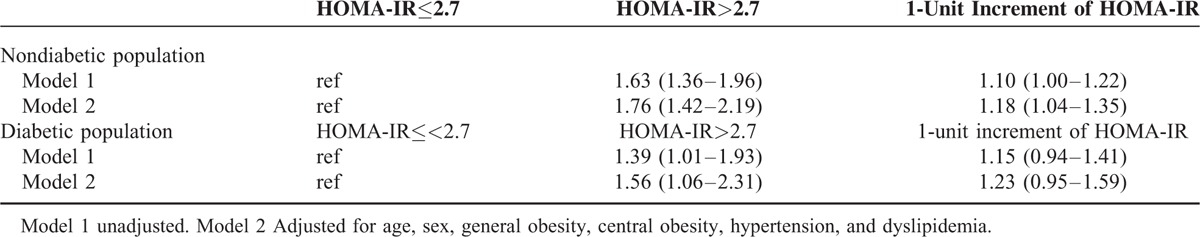
Multivariate-adjusted ORs of hyperbileacidemia with insulin resistance were presented in Table 3. Among nondiabetic participants, the unadjusted model showed that insulin resistance conferred a 63% higher risk of hyperbileacidemia compared to those without insulin resistance (OR = 1.63; 95% CI 1.36–1.96; P < 0.0001). After adjusting for age, sex, WC, BMI, hypertension, and dyslipidemia, this association remained significant (OR = 1.76; 95% CI 1.42–2.19; P < 0.0001). Each quartile increment of HOMA-IR conferred an 18% (95% CI 1.04–1.35; P = 0.013) higher risk of hyperbileacidemia (Table 3). In diabetic participants, those with insulin resistance also conferred an increased risk of hyperbileacidemia (OR = 1.39; 95% CI 1.01–1.93; P = 0.046). After adjusting for confounding factors, those with insulin resistance still yield a 56% higher risk of hyperbileacidemia compared to those without insulin resistance (95% CI 1.06–2.31; P = 0.025) (Table 3). Interestingly, further adjustment of HbA1c level in diabetic population did not affect this significant association (OR = 1.59; 95% CI 1.06–2.40; P = 0.024). Each quartile increment of HOMA-IR conferred a 23% higher risk, although it has not reached statistical significance (95% CI 0.95–1.59; P = 0.117).
TABLE 3.
The Risk of Hyperbileacidemia According to Insulin Sensitivity in Nondiabetic and Diabetic Participants
DISCUSSION
This study demonstrated that the total bile acid level was positively associated with insulin resistance in both nondiabetic and diabetic population aged 40 years or older. In the multiple stepwise regression analysis, insulin resistance was demonstrated to be positively associated with hyperbileacidemia in both groups. Furthermore, this association was demonstrated to be independent of status of diabetes, indicating the important role of insulin resistance in the regulation of bile acid metabolism in both diabetic and non-diabetic participants.
At present, the physiologic mechanism of relationship between insulin resistance and hyperbileacidemia has not been fully clarified. In rodents model, insulin signaling is known to inactivate the forkhead box transcription factor O1 (FoxO1),26 the later were reported to inhibit cholesterol 7α-hydroxylase (CYP7A1), a key enzyme in bile acid synthesis, by blocking the HNF4 interaction with PGC-127 suggesting that insulin inhibit FoxO1 binding to the CYP7A1 gene promoter and results in induction of CYP7A1 gene expression and bile acid synthesis.5 Other studies demonstrated that insulin would increase level of BA due to insulin resistance characteristic of diabetes through elevating the hydroxylase.5,14,28 Furthermore, some bile salt hydrolase, which was reported to specifically degrade bile acid,29 is inactive in the gut microbial environment of mice with obesity and diabetes.30 In turn, BA could improve insulin sensitivity via reducing endoplasmic reticulum (ER) stress.31 Insulin were also reported to induce the CYP7A1 gene and bile acid synthesis in primary human hepatocytes,6–8 which were in line with our results in population research. The result of our study that the bile acid level increases in insulin-resistant population indicated the important role of insulin resistance in the regulation of bile acid metabolism in human.
Our result was also consistent with other human studies. It has been reported that bile acid pool and fecal bile acids are elevated in diabetic patients with uncontrolled hyperglycemia.32 Haeusler et al33 revealed that in their healthy subjects, insulin resistance was associated with increased BA (cholic acid, deoxycholic acid, and their conjugated forms). In T2DM patients, BA was revealed to be nearly 2-fold higher compared with healthy subjects. Interestingly the fasting insulin had no significant relationship with BA in 35 diabetic patients of their study. In our study, TBA was positively associated with insulin resistance. The possible explanation of this difference may be attributable to the different sample size and variability of study population. Meanwhile, in Haeusler's study, diabetic patients were taking hypoglycemic medications, which may affect insulin sensitization, whereas in our study, we excluded participants using hypoglycemic agents, leaving those with newly diagnosed diabetes in order to control the effect of hypoglycemic agents on this relationship. More importantly, our results were partially in accordance with Taylor's study4 which demonstrated a positive correlation between urinary total bile acid excretion and HbA1c in diabetes patients. Moreover, in our study, the association between insulin resistance and TBA in diabetic population were not affected by the HbA1c level, indicating that the change of bile acid level in dysglycemic status largely due to insulin sensitivity rather than the glucose level. Therefore, our results, together with Taylor's, indicated that there might be a compensatory increase in bile acid signaling to maintain glucose homeostasis. Further studies were warranted to elucidate the exact role of insulin resistance in BA levels and more importantly.
The results of the Pearson's correlation showed the positive relationship between hypertension and bile acid level. So far, no studies have been found concerning the exact relationship between bile acid and hypertension. Previous studies showed that LDL-c can be reduced through increasing the fecal bile acid waste and by compensatory hepatic upregulation of bile acid synthesis to protect against atherosclerosis.28,34 Thus, it could be deducted that the real connection between bile acid and hypertension may be mediated by the relationship between atherosclerosis and hypertension.
We also note several limitations in our study. First, limitation of such an association study resides in its correlative nature not allowing conclusion on causality. Prospective studies are needed to clarify their precise interrelationship. Second, the secondary BA types were not available in our study, the exact association between insulin sensitivity and each secondary BA type was not clearly known. Some researchers deduced that the effects of BA type changes may be dissociable from the effects of altering total BA levels, further studies to provide information of BA types should be conducted to clarify the detailed relationship. Third, antibodies of diabetes, including antibodies to insulin (IAA), glutamic acid decarboxylase (GAA or GAD) and protein tyrosine phosphatase (IA2 or ICA512), were not examined in our study. Thus, we could not clearly distinguish type 1 from type 2 diabetes; thus the association in T2DM patients might be underestimated, as Type 1 diabetes (T1DM) has been shown to be a disease characterized by immune-mediated destruction of the insulin-secreting cells of the pancreas. However, the prevalence of type 1 diabetes is very low worldwide; it comprises the majority of cases of diabetes seen in childhood and ∼5% to 10% of all cases of diabetes mellitus in the USA.35 Zhou et al reported that prevalence of latent autoimmune diabetes in adult (LADA) distinguished from type 2 diabetes in China is only 6.1% in men and 5.5% in women.36 Furthermore, we excluded patients who used hypoglycemic agents; therefore less patients with type 1 diabetes could be included in the final analysis.
In conclusion, our study revealed that insulin resistance was significantly positively associated with hyperbileacidemia in middle-aged and elderly Chinese population, independent of status of diabetes and glucose levels. This study provides additional evidence to the correlation of TBA with insulin resistance in both diabetic and nondiabetic participants, indicating the important role of insulin resistance in regulation of bile acid metabolism. It warrants further experimental and longitudinal studies to determine the exact causal relationship between bile acid and insulin resistance.
Footnotes
Abbreviations: BMI = body mass index, CI = confidence interval, DBP = diastolic blood pressure, FPG = fasting plasma glucose, HDL-c = high-density lipoprotein cholesterol, HOMA-IR = homoeostasis model assessment of insulin resistance, IGR = impaired glucose regulation, LDL-c = low-density lipoprotein cholesterol, OR = odds ratio, PPG = post-prandial glucose, SBP = systolic blood pressure, TC = total cholesterol, WC = waist circumference
WS, DZ, and ZW contributed equally to this work.
Funding: this project was sponsored by the grants from the Key Laboratory for Endocrine and Metabolic Diseases of Ministry of Health [1994DP131044], the Sector Funds of Ministry of Health [201002002], the National Key New Drug Creation and Manufacturing Program of Ministry of Science and Technology [2012ZX09303006–001], National Nature Science Foundation of China [81170739,81170719,81222008], Science and Technology Commission of Shanghai Municipality [11DJ1400200], 863 Project [2012AA02A509], Shanghai Municipal Education Commission–Gaofeng Clinical Medicine Grant Support [20152202].
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Lefebvre P, Cariou B, Lien F, et al. Role of bile acids and bile acid receptors in metabolic regulation. Physiol Rev 2009; 89:147–191. [DOI] [PubMed] [Google Scholar]
- 2.Sinal CJ, Tohkin M, Miyata M, et al. Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell 2000; 102:731–744. [DOI] [PubMed] [Google Scholar]
- 3.Roberts RE, Glicksman C, Alaghband-Zadeh J, et al. The relationship between postprandial bile acid concentration, GLP-1, PYY and ghrelin. Clin Endocrinol (Oxf) 2011; 74:67–72. [DOI] [PubMed] [Google Scholar]
- 4.Taylor DR, Alaghband-Zadeh J, Cross GF, et al. Urine bile acids relate to glucose control in patients with type 2 diabetes mellitus and a body mass index below 30 kg/m2. PLoS One 2014; 9:e93540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li T, Francl JM, Boehme S, et al. Glucose and insulin induction of bile acid synthesis: mechanisms and implication in diabetes and obesity. J Biol Chem 2012; 287:1861–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li T, Kong X, Owsley E, et al. Insulin regulation of cholesterol 7α-hydroxylase expression in human hepatocytes: roles of forkhead box O1 and sterol regulatory element-binding protein 1c. J Biol Chem 2006; 281:28745–28754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Song KH, Chiang JY. Glucagon and cAMP inhibit cholesterol 7α-hydroxylase (CYP7A1) gene expression in human hepatocytes: discordant regulation of bile acid synthesis and gluconeogenesis. Hepatology 2006; 43:117–125. [DOI] [PubMed] [Google Scholar]
- 8.Li T, Chanda D, Zhang Y, et al. Glucose stimulates cholesterol 7alpha-hydroxylase gene transcription in human hepatocytes. J Lipid Res 2010; 51:832–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thomas C, Gioiello A, Noriega L, et al. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab 2009; 10:167–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fiorucci S, Mencarelli A, Palladino G, et al. Bile-acid-activated receptors: targeting TGR5 and farnesoid-X-receptor in lipid and glucose disorders. Trends Pharmacol Sci 2009; 30:570–580. [DOI] [PubMed] [Google Scholar]
- 11.Duran-Sandoval D, Cariou B, Percevault F, et al. The farnesoid X receptor modulates hepatic carbohydrate metabolism during the fasting-refeeding transition. J Biol Chem 2005; 280:29971–29979. [DOI] [PubMed] [Google Scholar]
- 12.Ma K, Saha PK, Chan L, et al. Farnesoid X receptor is essential for normal glucose homeostasis. J Clin Invest 2006; 116:1102–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herrema H, Meissner M, van Dijk TH, et al. Bile salt sequestration induces hepatic de novo lipogenesis through farnesoid X receptor- and liver X receptor alpha-controlled metabolic pathways in mice. Hepatology 2010; 51:806–816. [DOI] [PubMed] [Google Scholar]
- 14.Uchida K, Makino S, Akiyoshi T. Altered bile acid metabolism in nonobese, spontaneously diabetic (NOD) mice. Diabetes 1985; 34:79–83. [DOI] [PubMed] [Google Scholar]
- 15.Cariou B, Chetiveaux M, Zair Y, et al. Fasting plasma chenodeoxycholic acid and cholic acid concentrations are inversely correlated with insulin sensitivity in adults. Nutr Metab (Lond) 2011; 8:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brufau G, Bahr MJ, Staels B, et al. Plasma bile acids are not associated with energy metabolism in humans. Nutr Metab (Lond) 2010; 7:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun J, Zhang Y, Xu B, et al. Autonomic dysfunction assessed by EZSCAN and subclinical atherosclerosis. J Diabetes 2014; 6:409–416. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization. Definition, Diagnosis and Classification of Diabetes Mellitus and Its Complications. Report of WHO Consultation. World Health Organization, 1999 [Google Scholar]
- 19.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002; 106:3143–3421. [PubMed] [Google Scholar]
- 20.World Health Organization, World Health Organization. Obesity: Preventing and Managing the Global Epidemic; 1997. 2013; http://www.who.int/nutrition/publications/obesity_executive_summary [PubMed] [Google Scholar]
- 21.The World Health Organization Western Pacific Region, Region TWHOWP. The Asia-Pacific Perspective: Redefining Obesity and its Treatment. 2013; http://www.wpro.who.int/nutrition/documents/docs/Redefiningobesity [Google Scholar]
- 22.Geloneze B, Repetto EM, Geloneze SR, et al. The threshold value for insulin resistance (HOMA-IR) in an admixtured population IR in the Brazilian Metabolic Syndrome Study. Diabetes Res Clin Pract 2006; 72:219–220. [DOI] [PubMed] [Google Scholar]
- 23.Manjoo P, Dannenbaum D, Joseph L, et al. Utility of current obesity thresholds in signaling diabetes risk in the James Bay Cree of Eeyou Istchee. BMJ Open Diabetes Res Care 2015; 3:e000114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Long-ling C. Evaluation of serum total bile acid (TBA) and cholinesterase (CHE) in patients with liver disease. J Hainan Med Coll 2009; 15:362–363. [Google Scholar]
- 25.People's Medical Publishing House; 2006; Cao Z. The Obstetrics and Gynecology. Vol 2. 504–505. [Google Scholar]
- 26.Nakae J, Kitamura T, Silver DL, et al. The forkhead transcription factor Foxo1 (Fkhr) confers insulin sensitivity onto glucose-6-phosphatase expression. J Clin Invest 2001; 108:1359–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li T, Owsley E, Matozel M, et al. Transgenic expression of cholesterol 7alpha-hydroxylase in the liver prevents high-fat diet-induced obesity and insulin resistance in mice. Hepatology 2010; 52:678–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Charach G, Grosskopf I, Rabinovich A, et al. The association of bile acid excretion and atherosclerotic coronary artery disease. Therap Adv Gastroenterol 2011; 4:95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jones BV, Begley M, Hill C, et al. Functional and comparative metagenomic analysis of bile salt hydrolase activity in the human gut microbiome. Proc Natl Acad Sci U S A 2008; 105:13580–13585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cani PD, Bibiloni R, Knauf C, et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 2008; 57:1470–1481. [DOI] [PubMed] [Google Scholar]
- 31.Ozcan U, Yilmaz E, Ozcan L, et al. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science 2006; 313:1137–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bennion LJ, Grundy SM. Effects of diabetes mellitus on cholesterol metabolism in man. N Engl J Med 1977; 296:1365–1371. [DOI] [PubMed] [Google Scholar]
- 33.Haeusler RA, Astiarraga B, Camastra S, et al. Human insulin resistance is associated with increased plasma levels of 12α-hydroxylated bile acids. Diabetes 2013; 62:4184–4191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bhat BG, Rapp SR, Beaudry JA, et al. Inhibition of ileal bile acid transport and reduced atherosclerosis in apoE-/- mice by SC-435. J Lipid Res 2003; 44:1614–1621. [DOI] [PubMed] [Google Scholar]
- 35.WHO, WHO. 10 facts about diabetes. 2015; www.who.int/features/factfiles/diabetes/facts/en/index4.html. [Google Scholar]
- 36.Zhou Z, Xiang Y, Ji L, et al. Frequency, immunogenetics, and clinical characteristics of latent autoimmune diabetes in China (LADA China study): a nationwide, multicenter, clinic-based cross-sectional study. Diabetes 2013; 62:543–550. [DOI] [PMC free article] [PubMed] [Google Scholar]


