Abstract
The quality of collateral circulation affects the severity and prognosis of stroke patients. The effect of the circle of Willis, which is the primary collateral circulation, on ischemic stroke has attracted significant attention. This study was designed to investigate the effect of different circles of Willis types on stroke severity and prognosis in patients with noncardiac stroke.
A total of 376 patients with noncardiac ischemic stroke, who were treated by the specialty team of cerebrovascular diseases at the First Affiliated Hospital of Sun Yat-sen Hospital, were successively enrolled in this study. The detailed clinical characteristics of the patients were recorded upon admission, including risk factors of vascular disease and National Institutes of Health Stroke Scale (NIHSS) scores. The patients were divided into groups of different circles of Willis types based on magnetic resonance angiography (MRA) that was performed within 3 days of admission—type I: complete circle of Willis; type II: complete anterior half of the circle of Willis and incomplete posterior half of the circle of Willis; type III: incomplete anterior half of the circle of Willis and complete posterior half of the circle of Willis; and type IV: incomplete anterior and posterior halves of the circle of Willis. Patients were re-evaluated for NIHSS scores at discharge and after discharge. The modified Rankin score (mRS) was recorded for 90 days, and stroke recurrence and death after 90 days were also recorded until the end of the study.
The 376 patients were divided into 4 groups based on the MRA—type I group: 92 patients (24.5%); type II group: 215 patients (57.2%); type III group: 12 patients (3.2%), and type IV group: 57 patients (15.2%). NIHSS scores at admission and discharge were significantly lower for the type I group compared with those for the type II and type IV groups (P < 0.05). NIHSS scores were higher in the groups with an incomplete circle of Willis compared with the group with a complete circle of Willis. A poor recovery rate was highest for the type IV group, whereas a good recovery rate was highest for the type I group. The logistic regression analysis showed that a complete circle of Willis was one of the predictors of suitable recovery (odds ratio [OR] = 0.708, 95% confidence interval [CI]: 0.554–0.906).
Circle of Willis type was associated with stroke severity and patient prognosis, whereas an incomplete circle of Willis was associated with more severe conditions and a higher 90-day poor diagnosis rate. A complete circle of Willis was an independent predictor of good prognosis.
INTRODUCTION
The collateral circulation of cerebral arteries is closely associated with the occurrence, development, and outcome of acute cerebral infarction. Studies have shown that1 acceptable intracranial collateral circulation effectively reduced the risk of cerebral infarction and that the rapid establishment of collateral circulation after cerebral artery occlusion may significantly reduce the extent of damage to brain function and structure. Multiple collateral circulation pathways exist for cerebral arteries, and the circle of Willis, which is the primary collateral circulation of cerebral arteries, is one of the most important intracranial collateral circulation compensatory pathway, followed by the ophthalmic artery, leptomeningeal vessels, and anastomosis among the perforating arteries of cortical arteries. Compensatory pathways, such as the ophthalmic artery and the leptomeningeal artery, will not be a factor unless a compensatory dysfunction of the circle of Willis is present.2,3 The compensatory capacity of the circle of Willis, which is a major intracranial collateral circulation pathway, is dependent on the presence of its constituent vessels4,5 and the size of the vessels.6,7
The circle of Willis is closely associated with the occurrence of acute cerebral infarction. As early as the 1960 s, Alpers and Berry discovered via autopsy that the rate of dysplasia of the circle of Willis was significantly higher in brains with signs of cerebral infarction (194 cases) than in normal brains (350 cases).8 However, Battacharji et al9 reported that the circle of Willis was closely associated with cerebral infarction for patients with severe stenosis of the internal carotid artery or vertebral-basilar artery. Studies of the relationship between the circle of Willis and cerebral ischemia4,10–13 tended to enroll patients with severe stenosis or occlusion of the internal carotid artery. Henderson and Eliasziw conducted a study of cases in the North American Symptomatic Carotid Endarterectomy Trial (NASCET) and discovered that the incidence of hemispheric cerebral infarction or transient cerebral ischemic attack was significantly reduced during the perioperative or long-term follow-up period for patients with > 70% stenosis of the internal carotid artery if the normal constituent vessels of the circle of Willis were present.12 However, additional research revealed that the relationship between the circle of Willis and ischemic stroke was extended beyond cases of severe stenosis (or occlusion) of the internal carotid artery. Hoksbergen et al2 conducted a case-control study and determined that the nonfunctional rate of the anterior communicating artery (AcoA) and posterior communicating artery (PcoA) was significantly higher in the cerebral infarction group than in the noncerebral infarction group when stenosis of the internal carotid artery was excluded as a factor and the incidence of an incomplete circle of Willis (hypoplasia or absence of the constitute vessels of the circle of Willis) was significant in patients with anterior circulation infarction. Miyazawa et al performed a logistic regression analysis and determined that the incidence of cerebral infarction was significant and that the number of lacunar infarctions significantly increased for patients with an incomplete anterior half of the circle of Willis.14
The majority of previous studies focused on patients with stenosis of the internal carotid artery, and reports on the general population are relatively scarce. Studies of the features of the circle of Willis and the severity of cerebral infarction and treatment outcome were frequently incomprehensive and only focused on certain features of the circle of Willis (e.g., A1 segment hypoplasia and poor development of PCoA) or patients with a certain limited condition (e.g., patients after intravenous thrombolysis), which affected the applicability of research findings. This study was designed to analyze the relationship between different circle of Willis types and the severity of cerebral infarction and treatment outcomes in patients with initial acute noncardiac cerebral infarction.
MATERIALS AND METHODS
The study was approved by ethics committee of First Affiliated Hospital of SunYat-sen University. All patients or their legal representatives gave written informed consent before being included in this study.
Collection of Clinical Data
A total of 376 patients with acute noncardiac cerebral infarction, who were treated at the Department of Neurology, were successively enrolled in this study. All patients were observed by a neurology specialist, and general clinical data, such as gender, age, current medical history, family history, smoking and drinking, were collected. The patients received a thorough physical examination of their nervous systems. At admission, all patients were rated using the National Institutes of Health Stroke Scale (NIHSS).15 The inclusion criteria were as follows: (1) age 18 to 80; (2) time from onset to admission ≤ 7 days; (3) acute cerebral infarction diagnosed with head computed tomography (CT) and/or magnetic resonance imaging (MRI); (4) initial clinical onset; and (5) ability to cooperate with various examinations after admission, such as head MRI + magnetic resonance angiography (MRA), electrocardiography (ECG), echocardiography, Doppler vascular ultrasonography of the neck, chest x-ray, and laboratory tests. The exclusion criteria were as follows: (1) age < 18 or > 80; (2) time from onset to admission > 7 days; (3) cardiac cerebral embolism; (4) received intravenous thrombolysis, arterial thrombolysis, or stent; (5) previous history of cerebral infarction or cerebral hemorrhage; and (6) intracranial lesions, such as intracranial tumors, arteriovenous malformations, hydrocephalus, venous sinus thrombosis, multiple sclerosis, and congenital abnormalities.
Collection of MRI Data
All enrolled patients underwent a plain head MRI scan, diffusion-weighted imaging (DWI), and 3-dimensional time-of-flight (3D-TOF) MRA within 72 hours of admission. A 3.0 T superconductive MRI scanner (gradient field: 45 mT/m) and a 16-channel array head coil were used. Routine MRI sequences included axial T1-FLAIR, T2WI, and T2-FIAIR, and sagittal or coronal T2WI. Two radiologists with extensive experience in neuroimaging and diagnosis conducted a blinded and independent evaluation of all MRI images. In the case of discrepancy, the 2 radiologists discussed the images to obtain a diagnosis. The MRI images were primarily analyzed for the presence of the constituent vessels of the circle of Willis. According to the criteria outlined in the literature,6 if the start and end points of a vessel are present on the 3D-TOF source image, then the vessel is determined to be “present.” A vessel is defined as “normal” if the vessel diameter ≥ 0.8 mm or is indicative of dysplasia if the vessel diameter < 0.8 mm.
Criteria for the type of the circle of Willis are as follows: the typical circle of Willis is anatomically divided into the anterior half and the posterior half, in which the anterior half consists of ACoA, bilateral A1 segments, and bilateral terminal segments of the internal carotid artery (ICA) and the posterior half consists of bilateral PCoAs, bilateral P1 segments, and the basilar artery (BA). Based on the classification method of the Chinese researcher Zhang,16 the classification criteria of Hartkamp et al,17 and the actual clinical practice of this study, we classified 4 circles of Willis types—type I: complete circle of Willis-presence and no dysplasia of the constituent vessels of the circle of Willis (Figure 1A); type II: complete anterior half and incomplete posterior half of the circle of Willis-presence and no dysplasia of the constituent vessels of the anterior half of the circle of Willis (Figure 1B), and absence or dysplasia of at least 1 vessel of the posterior half of the circle of Willis; type III: incomplete anterior half and complete posterior half of the circle of Willis-absence or dysplasia of at least 1 vessel of the anterior half of the circle of Willis, and presence and no dysplasia of the constitute vessels of the posterior half of the circle of Willis (Figure 1C); and type IV: incomplete anterior and posterior halves of the circle of Willis-absence or dysplasia of at least 1 vessel of the anterior half of the circle of Willis, and absence or dysplasia of at least 1 vessel of the posterior half of the circle of Willis (Figure 1D).
FIGURE 1.
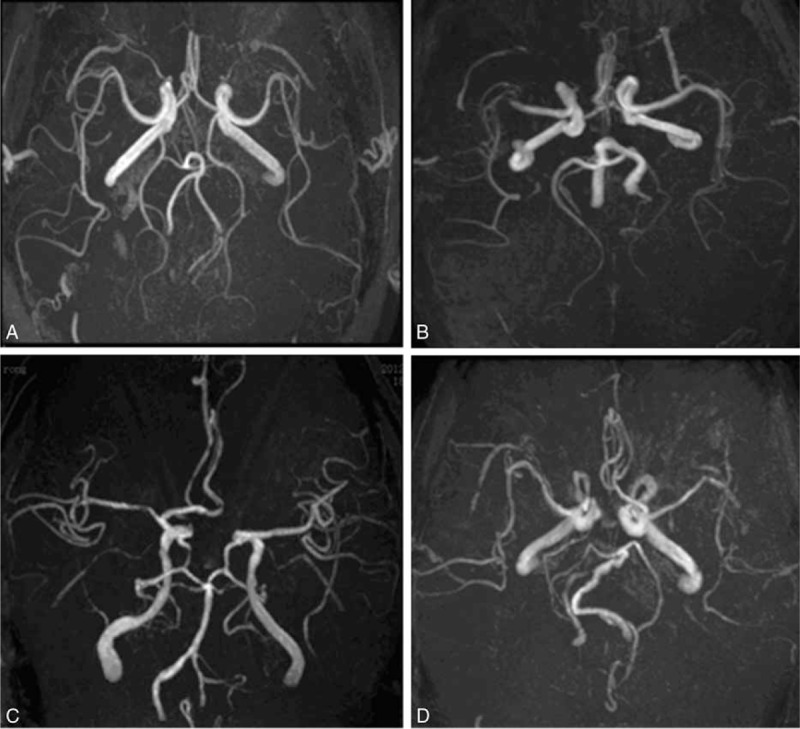
The samples of different types of circle of Willis on MRA. A shows type I circle: complete circle of Willis; B shows type II circle: complete anterior half and incomplete posterior half of the circle of Willis; C shows type III circle: incomplete anterior half and complete posterior half of the circle of Willis; and D shows type IV circle: incomplete anterior and posterior halves of the circle of Willis. MRA = magnetic resonance angiography.
Measurement of the Severity of Cerebral Infarction
In this study, NIHSS scores were used to assess the severity of neurological impairments in patients with acute cerebral infarction.15
Treatment Outcome and Follow-up
All patients received standard treatment as outlined in the guidelines.18 NIHSS scores were assessed at discharge, and the patients received follow-up for 90 days to obtain modified Rankin scores (mRSs). The patients continued receiving follow-up exams for 2 to 3 years to record information about stroke recurrence (including ischemic and hemorrhagic stroke) and death.
Collection of Laboratory Data
Venous blood samples were collected from all patients on the next morning after admission; the samples were sent to the Department of Clinical Laboratory Testing of our hospital for routine blood tests and blood chemistry and glycosylated hemoglobin (HbAlc) analysis. The blood chemistry analysis included the assessment of total cholesterol (CHOL), triglyceride (TG), low-density lipoprotein (LDL-c), high-density lipoprotein (HDL-c), fasting blood glucose (GLu), serum creatinine (Cr), and uric acid (UA). All patients underwent routine 12-lead ECG, echocardiography, Doppler vascular ultrasonography of the neck, and chest x-rays within 72 hours of admission.
Statistical Analysis
IBM SPSS 20.0 for windows was used to analyze all data. Measurement data are expressed as x ± s. A t test was performed to compare the mean values between 2 groups; a univariate analysis of variance was performed to compare the mean values of multiple groups; the Bonferroni method was used for the pairwise comparison of the baseline data; and the least-significant difference (LSD) method was used for a pairwise comparison of the NIHSS scores. A χ2 test was performed to compare the count data, and an R × C contingency table χ2 test was performed to analyze multirow and multicolumn count data, where P < 0.05 was considered to be statistically significant. A logistic regression method was used for the multivariate analysis.
RESULTS
A total of 376 patients were enrolled in this study, including 254 men and 122 women, whose ages ranged from 29 to 80 (mean: 62.7 ± 12.4 years). The patients were divided into 4 groups based on circle of Willis type—type I: 92 patients (24.5%), including 68 men and 24 women, with a mean age of 58.3 ± 13.2 years; type II: 215 patients (57.2%), including 140 men and 75 women, with a mean age of 64.0 ± 11.7 years; type III: 12 patients (3.2%), including 8 men and 4 women, with a mean age of 66.8 ± 7.6 years; and type IV: 57 patients (15.2%), with 38 men and 19 women, with a mean age of 63.8 ± 13.1 years.
Comparison of the Baseline Clinical Characteristics of Patients With Different Circles of Willis Types
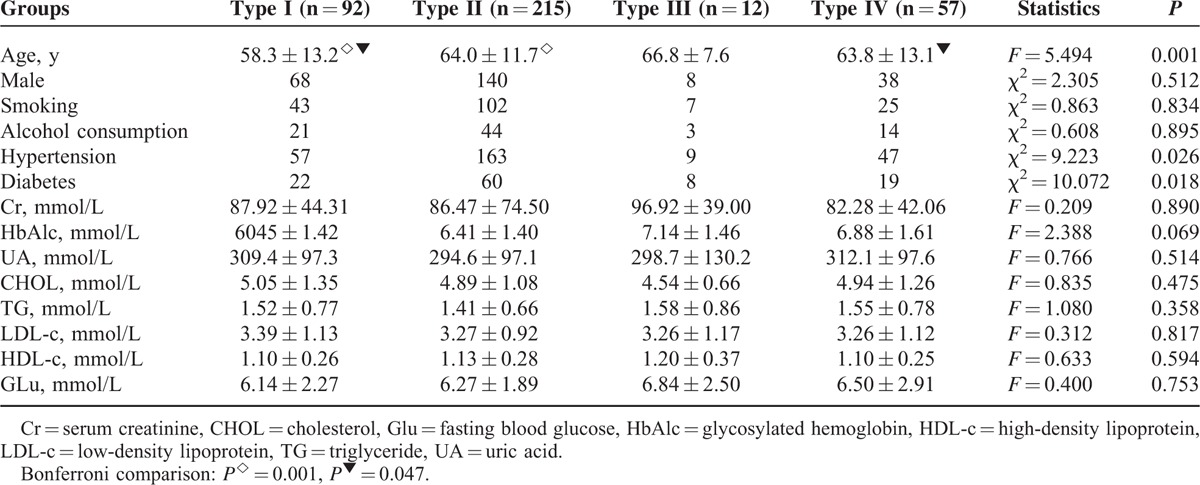
Patients in the type I group were younger than patients in the type II group and the type IV group, statistically significant (P < 0.05). No significant difference in gender, smoking, alcohol consumption, serum Cr, HbAlc, UA, CHOL, TG, LDL-c, HDL-c, and Glu was observed among the groups (P > 0.05). A significant difference in history of hypertension and diabetes among the groups (P < 0.05) (Table 1) was noted.
TABLE 1.
Clinical Characteristics Among Patients With Different Types of Circles of Willis
Comparison of the Severity of Neurological Impairments of Patients With Different Circles of Willis Types
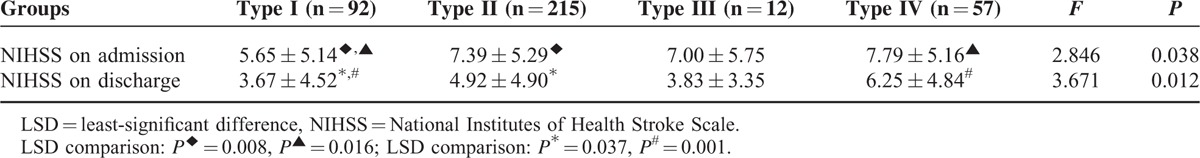
Significant differences in NIHSS scores at admission and at discharge between the patients with different circles of Willis types (P < 0.05) were observed (Table 2). The pairwise comparison using the LSD method showed that NIHSS scores were significantly lower in the type I group than in the type II group and the type IV group (P < 0.05).
TABLE 2.
Comparison of NIHSS Among Patients With Different Types of Circle of Willis
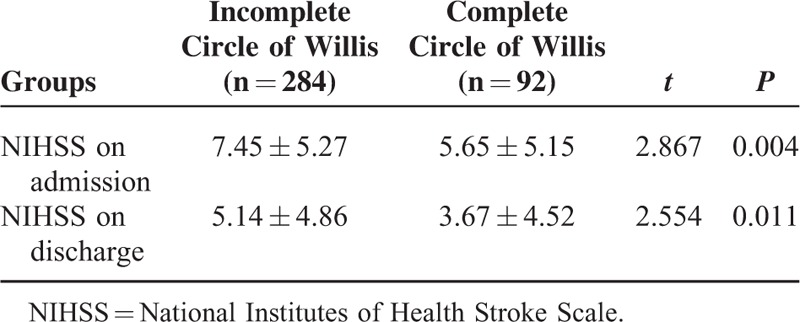
The patients with acute cerebral infarction were divided into 2 groups based on the integrity of the circle of Willis: complete circle of Willis group (type I of the circle of Willis) and incomplete circle of Willis group (type II + type III + type IV of the circle of Willis). NIHSS scores at admission and discharge were significantly higher in the incomplete circle of Willis group than in the complete circle of Willis group (Table 3).
TABLE 3.
Completeness of Circle of Willis and NIHSS on Admission and Discharge
Comparison of Disability During 90-Day Follow-up Among Patients With Different Circle of Willis Types
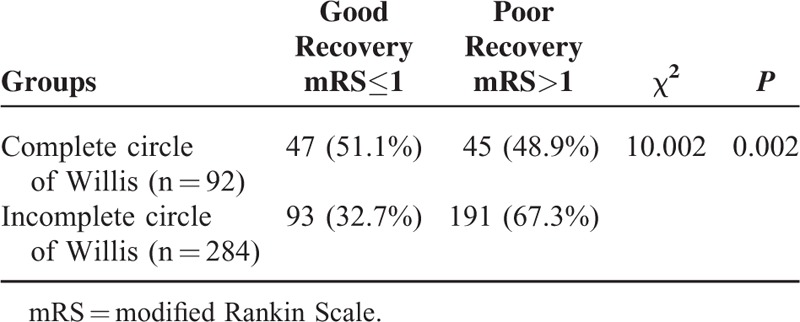
At the end of the 90-day follow-up, we compared the mRS among different groups with complete circle of Willis and incomplete circle of Willis. We found that the mRS in patients with complete circle of Willis had high rate of lower mRS than those with incomplete circle of Willis (Figure 2). Then, the patients were determined to have good neurological recovery (mRS ≤ 1) or poor neurological recovery (mRS > 1). Circle of Willis type was significantly correlated with the disability of the patients with acute cerebral infarction. A poor recovery rate was highest in type IV patients, whereas a good recovery rate was highest in type I patients (Table 4). As previously described, the patients with acute cerebral infarction were divided into 2 groups based on the integrity of the circle of Willis: the complete circle of Willis group and the incomplete circle of Willis group. A higher percentage of patients in the complete circle of Willis group had good neurological recovery at the end of the 90-day follow-up compared with that of the patients in the incomplete circle of Willis group (Table 5). The patients were further divided into the good recovery group and the poor recovery group; the logistic regression analysis showed that a complete circle of Willis was one of the predictors of good recovery (odds ratio [OR] = 0.708, 95% confidence interval [CI]: 0.554–0.906).
FIGURE 2.

The comparison of mRS in patients with complete circle of Willis and with incomplete circle of Willis. mRS = modified Rankin score.
TABLE 4.
mRS in Patients With Different Types of Circle of Willis

TABLE 5.
Completeness of Circle of Willis and mRS on Discharge
Follow-Up Results of Stroke Recurrence and Death
In this study, only 187 patients completed the 2 years follow-up. Fifteen patients had recurrent stroke, including 12 cases of ischemic stroke, 3 cases of hemorrhagic stroke, 5 cases of complete circle of Willis, and 10 cases of an incomplete circle of Willis. Twenty-nine patients died, including 3 cases of a complete circle of Willis and 26 cases of an incomplete circle of Willis. The difference was statistically significant (P < 0.05).
DISCUSSION
This study showed that circle of Willis type was closely associated with the severity and treatment outcome of cerebral infarction. Compared with patients with acute cerebral infarction in the incomplete circle of Willis group, patients in the complete circle of Willis group had lower NIHSS scores at admission and discharge, less-severe neurological impairments, and better recovery of neurological function at the end of the 90-day follow-up.
Less than 50% (or significantly <50%) of the general population have a normal circle of Willis due to congenital abnormalities or atherosclerosis and other factors. Previous studies of the circle of Willis patterns were dependent on autopsy. An autopsy study of 1000 brain specimens showed that 45.2% of the specimens exhibited a normal circle of Willis and 54.8% of the specimens exhibited variation in the circle of Willis.19 Advances in medical imaging techniques have enabled additional studies of the physiological state of the circle of Willis. Some researchers investigated the integrity of the circle of Willis in 160 healthy Chinese using head computed tomography angiography (CTA). The results showed that 79% of the subjects had a complete anterior half of the circle of Willis, 31% had a complete posterior half of the circle of Willis, 27% had a complete circle of Willis, and 9.4% had embryonic posterior cerebral artery.20 Therefore, the variation in the pattern of the circle of Willis is common, and the compensatory capacity of different variations of the circle of Willis (as intracranial collateral circulation) also varies. In this study, the MRA showed that 24.5% of the patients with acute cerebral infarction had a complete circle of Willis, 81.7% had a complete anterior half of the circle of Willis, and 27.7% had a complete posterior half of the circle of Willis. Compared with foreign studies,2,14,21 lower rates for a complete circle of Willis and a complete posterior half of the circle of Willis were obtained in this study, whereas the rates for a complete anterior half of the circle of Willis were comparable to this study. Previous studies have shown that2,15,16 the integrity of the circle of Willis is significantly associated with the incidence of cerebral infarction and that an incomplete circle of Willis is associated with a higher incidence of cerebral infarction. This difference in the integrity of the circle of Willis between studies in China and other countries may explain why the incidence of cerebral infarction in China is significantly higher than the global average of the incidence of cerebral infarction.
The severity of cerebral infarction is significantly correlated with the collateral circulation of cerebral arteries.3,22 Good collateral circulation enables the timely reperfusion of blood flow to the ischemic penumbra, which reduces the volume of cerebral infarction, minimizes neurological impairments, and improves prognosis. A complete circle of Willis was an independent predictor of good recovery. According to the past studies, the protective effect of a complete circle of Willis on acute cerebral infarction may be attributed to several factors. First, a complete circle of Willis has better collateral circulation and higher compensatory capacity in the case of cerebral ischemia, enabling a greater chance of collateral reperfusion to the ischemic penumbra and minimizing neurological impairments. Second, a complete circle of Willis indicates that more blood flow is available to form collateral pathways via the circle of Willis, creating the conditions for reperfusion to the cerebral ischemic lesions23 and helps to improve the hemodynamics of artery anastomoses in the pia mater, improving the compensatory capacity of collateral anastomoses in the pia mater to the infarct vessels. Third, a complete circle of Willis reduces cerebral ischemia-reperfusion injury24 and counteracts the hemodynamic changes of cerebral ischemia and reperfusion, reducing brain damage.25,26
This study showed that patients in the complete circle of Willis group had better neurological recovery, which was consistent with the study results of Chuang et al.23 However, Chuang et al investigated patients with acute noncardiac cerebral infarction who received intravenous thrombolysis. The enrolled subjects had to satisfy specific inclusion criteria. In this study, the inclusion criterion was patients with initial acute noncardiac cerebral infarction, producing a more inclusive study. Chuang et al showed that the mean NIHSS score at admission was lower in the complete circle of Willis group than in the incomplete circle of Willis group, but the difference was not statistically significant, which was inconsistent with this study. This finding is most likely attributed to the following factors: (1) the onset time—Chuang et al23 evaluated patients within 3 hours of onset, whereas this study evaluated patients within 7 days of onset. Some time is required for acceptable collateral circulation to serve a protective role on cerebral ischemia, and (2) intravenous thrombolysis—Chuang et al23 evaluated patients who received intravenous thrombolysis, which may alter the integrity of the circle of Willis. Compared with the study conducted by Chuang et al, this study was more inclusive and representative with broader clinical significance.
This study showed that circle of Willis type is associated with stroke severity and prognosis; patients with an incomplete circle of Willis have more severe conditions and a higher 90-day poor prognosis rate than patients with a complete circle of Willis. A complete circle of Willis is an independent predictor of good prognosis. However, this study has some drawbacks. First, although 3D-TOF MRA combined with raw images is highly sensitive and accurate for analyzing the circle of Willis and is easy to operate, MRA is insensitive for detecting tiny blood vessels with slow blood flow17 and has certain limitations. Some constituent vessels of the circle of Willis, especially communicating arteries, did not show on the MRA due to slow blood flow. As a result, this study may have slightly underestimated the percentage of patients with a complete circle of Willis. Second, a significant difference in age was observed among the groups in this study. Previous studies6,27,28 showed that younger age was associated with a higher rate of a complete circle of Willis. The younger the age, the greater are the diameters of the constituent vessels of the circle of Willis. Age is one of the most important factors that affects the severity of neurological impairments, neurological recovery and clinical prognosis.29,30 Therefore, the difference in age among the groups in this study inevitably affected the assessment of the severity of a patient's condition and the clinical prognosis. Third, for 2-year follow-up, the lost rate was as high as 50%. This may have some influence on our results about the correlation between completeness of circle of Willis and stroke recurrence. But in our study, all of the patients finished the 90-day follow-up and the main findings that patients with complete circle of Willis would be with good recovery still make sense.
Footnotes
Abbreviations: 3D-TOF = 3-dimensional time-of-flight, AcoA = anterior communicating artery, BA = basilar artery, CHOL = cholesterol, CI = confidential interval, Cr = serum creatinine, CT = computed tomography, CTA = computed tomography angiography, ECG = electrocardiography, Glu = fasting blood glucose, HbAlc = glycosylated hemoglobin, HDL-c = high-density lipoprotein, ICA = internal carotid artery, LDL-c = low-density lipoprotein, MRA = magnetic resonance angiography, MRI = magnetic resonance imaging, mRS = modified Rankin score, NASCET = North American Symptomatic Carotid Endarterectomy Trial, NIHSS = National Institutes of Health Stroke Scale, OR = odds ratio, PcoA = posterior communicating artery, TG = triglyceride, UA = uric acid
HZ and JS contributed equally to this study.
Financial support: Natural Science Foundation of Guangdong Province (No. S2013010015840) and Guangdong province science and technology plan (2015A030302013).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Christoforidis GA, Mohammad Y, Kehagias D, et al. Angiographic assessment of pial collaterals as a prognostic indicator following intra-arterial thrombolysis for acute ischemic stroke. AJNR Am J Neuroradiol 2005; 26:1789–1797. [PMC free article] [PubMed] [Google Scholar]
- 2.Hoksbergen AW, Legemate DA, Csáti G, et al. Absent collateral function of the circle of Willis as risk factor for ischemic stroke. Cerebrovasc Dis 2003; 16:191–198. [DOI] [PubMed] [Google Scholar]
- 3.Liebeskind DS. Collateral circulation. Stroke 2003; 34:2279–2284. [DOI] [PubMed] [Google Scholar]
- 4.Schomer DF, Marks MP, Steinberg GK, et al. The anatomy of the posterior communicating artery as a risk factor for ischemic cerebral infarction. N Engl J Med 1994; 330:1565–1570. [DOI] [PubMed] [Google Scholar]
- 5.Miralles M, Dolz JL, Cotillas J, et al. The role of the circle of Willis in carotid occlusion: assessment with phase contrast MR angiography and transcranial duplex. Eur J Vasc Endovasc Surg 1995; 10:424–430. [DOI] [PubMed] [Google Scholar]
- 6.Krabbe-Hartkamp MJ, van der Grond J, de Leeuw FE, et al. Circle of Willis: morphological variation on MR angiograms. Radiology 1998; 207:103–111. [DOI] [PubMed] [Google Scholar]
- 7.Barboriak DP, Provenzale JM. Pictorial review: magnetic resonance angiography of arterial variants at the circle of Willis. Clin Radiol 1997; 52:429–436. [DOI] [PubMed] [Google Scholar]
- 8.Alpers BJ, Berry RG. Circle of Willis in cerebral vascular disorders. Arch Neurol 1963; 8:398–402. [DOI] [PubMed] [Google Scholar]
- 9.Battacharji SK, Hutchinson EC, McCall AJ. The circle of Willis: The incidence of developmental abnormalities in normal and infracted brains. Brain 1967; 90:747–758. [DOI] [PubMed] [Google Scholar]
- 10.Ringelstein EB, Weiller C, Weckesser M, et al. Cerebral vasomotor reactivity is significantly reduced in low-flow as compared to thromboembolic infarctions: the key role of the circle of Willis. J Neurol Sci 1994; 121:103–109. [DOI] [PubMed] [Google Scholar]
- 11.Hedera P, Bujdakova J, Traubner P. Effect of collateral flow patterns on outcome of carotid occlusion. Eur Neurol 1995; 35:212–216. [DOI] [PubMed] [Google Scholar]
- 12.Henderson RD, Eliasziw M, Fox AJ, et al. Barnett HJM, for the North American Symptomatic Carotid Endarterectomy Trial (NASCET) Group: angiographically defined collateral circulation and risk of stroke in patients with severe carotid artery stenosis. Stroke 2000; 31:128–132. [DOI] [PubMed] [Google Scholar]
- 13.Vernieri F, Pasqualetti P, Matteis M, et al. Effect of collateral blood flow and cerebral vasomotor reactivity on the outcome of carotid artery occlusion. Stroke 2001; 32:1552–1558. [DOI] [PubMed] [Google Scholar]
- 14.Miyazawa N, Shinohara T, Yamagata Z. Association of incompleteness of the anterior part of the circle of Willis with the occurrence of lacunes in the basal ganglia. Eur J Neurol 2011; 18:1358–1360. [DOI] [PubMed] [Google Scholar]
- 15.Brott T, Adams HP, Jr, Olinger CP, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke 1989; 20:864–870. [DOI] [PubMed] [Google Scholar]
- 16.Zhang ZS. Correlative Anatomy and Clinic of the Human Cerebral Blood Vessel. 2nd Edition2003; Beijing:Scientific and Technological Literature Publishing House, 397–408. [Google Scholar]
- 17.Hartkamp MJ, van Der Grond J, van Everdingen KJ, et al. Circle of Willis collateral flow investigated by magnetic resonance angiography. Stroke 1999; 30:1678–2671. [DOI] [PubMed] [Google Scholar]
- 18.Guideline Writing Group of Acute Ischemic Stroke, Cerebrovascular Group of Chinese Society of Neurology of Chinese Medical Association. Chinese Guideline of Diagnosis and Treatment of Acute Ischemic Stroke (2010). Chin J Neurol 2010; 43:1–8. [Google Scholar]
- 19.Kapoor K, Singh B, Dewan LI. Variations in the configuration of the circle of Willis. Anat Sci Int 2008; 83:96–106. [DOI] [PubMed] [Google Scholar]
- 20.Li Q, Li J, Lv F, et al. A multidetector CT angiography study of variations in the circle of Willis in a Chinese population. J Clin Neurosci 2011; 18:379–383. [DOI] [PubMed] [Google Scholar]
- 21.Chuang YM, Liu CY, Pan PJ, et al. Posterior communicating artery hypoplasia as a risk factor for acute ischemic stroke in the absence of carotid artery occlusion. J Clin Neurosci 2008; 15:1376–1381. [DOI] [PubMed] [Google Scholar]
- 22.Silvestrini M, Vernieri F, Troisi E, et al. Cerebrovascular reactivity in carotid artery occlusion: possible implications for surgical management of selected groups of patients. Acta Neurol Scand 1999; 99:187–191. [DOI] [PubMed] [Google Scholar]
- 23.Chuang YM, Chan L, Lai YJ, et al. Configuration of the circle of Willis is associated with less symptomatic intracerebral hemorrhage in ischemic stroke patients treated with intravenous thrombolysis. J Crit Care 2013; 28:166–172. [DOI] [PubMed] [Google Scholar]
- 24.Liang F, Fukasaku K, Liu H, et al. A computational model study of the influence of the anatomy of the circle of Willis on cerebral hyperperfusion following carotid artery surgery. Biomed Eng Online 2011; 10:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chuang YM, Lin CP, Wong HF, et al. Plasticity of circle of Willis: a longitudinal observation of flow patterns in the circle of Willis one week after stenting for severe internal carotid artery stenosis. Cerebrovasc Dis 2009; 27:572–578. [DOI] [PubMed] [Google Scholar]
- 26.Chuang YM, Chang YJ, Chang CH, et al. Correlation between the flow pattern of the circle of Willis and segmental perfusion asymmetry after carotid artery revascularization. Eur J Neurol 2011; 18:1132–1138. [DOI] [PubMed] [Google Scholar]
- 27.Macchi C, Catini C, Federico C, et al. Magnetic resonance angiographic evaluation of circulus arteriosus cerebri (circle of Willis): a morphologic study in 100 human healthy subjects. Ital J Anat Embryol 1996; 101:115–123. [PubMed] [Google Scholar]
- 28.Hoksbergen AW, Legemate DA, Ubbink DT, et al. Collateral variations in circle of Willis in atherosclerotic population assessed by means of transcranial color-coded duplex ultrasonography. Stroke 2000; 31:1656–1660. [DOI] [PubMed] [Google Scholar]
- 29.Wahlgren N, Ahmed N, Dávalos A, et al. Thrombolysis with alteplase for acute ischaemic stroke in the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST): an observational study. Lancet 2007; 369:275–282. [DOI] [PubMed] [Google Scholar]
- 30.Ogubgbo BI, Ojinni FI, Okor D, et al. Predicting major neurological improvement with intravenous recombinant tissue plasminogen activator treatment of stroke. Stroke 2004; 35:147–150. [DOI] [PubMed] [Google Scholar]


