Abstract
We investigated and compared 2 clinical strategies to prevent postendoscopic retrograde cholangiopancreatography (ERCP) pancreatitis (PEP).
We retrospectively reviewed data from patients who underwent ERCP between 2008 and 2014. Of 623 patients at high risk for PEP, 145 were treated with prophylactic pancreatic stent placement (PSP) only, and 478 were treated with rectal indomethacin (RI) only, for PEP prevention. Patients were matched by one-to-one propensity score matching (PSM) by risk factors, with overall PEP incidence as primary outcome, and moderate or severe PEP and complication rates as secondary outcomes.
Of 623 patients with high-risk factors, 145 pairs were generated after PSM. Thirty-two patients developed pancreatitis—10 (6.9 %) in the PSP group and 22 (15.2 %) in the RI group (P = 0.025). Moderate-to-severe pancreatitis developed in 5 patients (2.8%) in the PSP group and 14 patients (9.7 %) in the RI group (P = 0.047).
Although indomethacin represents an easy, inexpensive treatment, prophylactic PSP is still the better prevention strategy for PEP.
INTRODUCTION
Acute pancreatitis is a common and serious complication of endoscopic retrograde cholangiopancreatography (ERCP).1–3 Post-ERCP pancreatitis (PEP) accounts for substantial annual morbidity and health care expenditure, and occasional death.4 The prevention of PEP is an ongoing area of active research. Several proposed pharmacologic agents and therapeutic techniques have been proposed to reduce the risk of PEP.5–7
Prophylactic pancreatic stent placement (PSP) decreases the PEP incidence in high-risk and mixed-case groups, and nearly eliminates the risk of severe PEP (overall risk [OR]: 0.44; 95% confidence interval [CI]: 0.24–0.81; absolute risk reduction [RR]: 12.0%; 95% CI: 3.0–21.0),3,8–11 but its use is reportedly not widespread.2,12 Details of technique, including clarification of which patient populations are at significantly greater risk for PEP, and identification of patient- and procedure-related risk factors are important considerations in preventing or minimizing PEP.
Nonsteroidal antiinflammatory drugs (NSAIDs) reduce incidence of PEP in both high- and low-risk patients.13,14 In a recent multicenter RCT, PEP developed in 9.2% versus 16.9% of patients in the indomethacin versus placebo group, respectively (P = 0.005)15; its post hoc analysis suggests that indomethacin may obviate the need for prophylactic PSP.
In our center, prophylactic PSP was used to prevent PEP before 2012. In the last 2 years, NSAIDs became our first choice for prevention of PEP. However, as the rate of PEP increased gradually, which is the better clinical strategy? Is it premature to abandon PSP? Although studies comparing administration of indomethacin alone and PSP alone are needed, RCTs are difficult to conduct because of patient volume and ethical considerations—especially in ERCP-related procedures, which are affected by intraoperative decisions.16 Using observational data and case series, propensity score adjustment and matching can reduce bias and balance unequal chances of allocation to a treatment group.17,19
In this study, we tried to compare the efficacy and outcomes between prophylactic PSP alone and rectal indomethacin (RI) alone for prevention of PEP within a high-risk group.
MATERIALS AND METHODS
Patients
We analyzed the available data for patients with PEP risk factors who had undergone ERCP at a university-affiliated medical center, including their clinical characteristics, risk factors of PEP, clinical strategy for prevention of PEP and any complications of ERCP. The institutional review board at our hospital approved the study protocol; written informed consent was obtained from each patient before ERCP.
Selection of risk factors, and of inclusion and exclusion criteria, were determined after discussion by our group.20,21 Risk factors for PEP are defined in consideration of the European Society of Gastrointestinal Endoscopy (ESGE) guidelines.2,3
Definitions
PEP was defined by consensus criteria22,23: clinical evidence of pancreatitis; elevation of pancreatic enzymes to 3 times the upper limit of normal 24 hours after the procedure; and hospital admission for 2 to 3 days (mild pancreatitis), 4 to 9 days (moderate pancreatitis), or longer than 10 days (severe pancreatitis). The scoring system was complex for assessment of the severity of PEP.
The following conditions are considered to represent high risk for PEP3: endoscopic ampullectomy, known or suspected sphincter of Oddi dysfunction (SOD), pancreatic sphincterotomy (SPT), precut biliary SPT, pancreatic guidewire-assisted biliary cannulation, endoscopic balloon sphincteroplasty, or presence of >3 risk factors listed in the ESGE guidelines. Procedures and patient conditions that do not fulfill these criteria are considered to represent low risk for PEP.
We excluded patients in whom ERCP was unsuitable, and those who had active pancreatitis, previous endoscopic SPT or papillary balloon dilation, chronic pancreatitis, pancreatic-head mass; tumor of papilla of Vater, pancreas divisum, or postpancreaticoduodenectomy.15,16
Intervention
All procedure-related interventions were dictated by the performing endoscopist. Two endoscopists performed the ERCP procedures. ERCP procedures performed by trainees were excluded. Prophylactic antibiotics were routinely given. To stop duodenal peristalsis, 10 mg anisodamine was administered intramuscularly just before. All ERCP procedures were performed under intravenous anesthesia with propofol, with continuous monitoring of blood pressure, heart rates, and oxygen saturation, using a therapeutic duodenoscope (TJF-150 or TJF-160; Olympus Optical Co., Tokyo, Japan). The kinds of ERCP devices used (ie, sphincterotome or guidewire), were not limited to any specific types. For stent placement, we mostly used the Jagwire guidewire (0.035 in., Boston Scientific Corp, Natick, MA). Among our ERCPs, the proportion of therapeutic procedures was approximately 85%.
Pancreatic Stent Placement and Rectal Indomethacin
PSP was attempted at the surgeon's discretion, having considered that the case had become a high-risk case of PEP. We used a 5 F single-pigtail stent,24 3 to 5 cm in length (Cook Endoscopy, Inc., Winston-Salem, NC). In all cases, abdominal radiographs were taken on day 7 after surgery. If it had not dislodged, the stent was then removed by duodenoscopy.
NSAIDs were reportedly rarely used in clinical practice to prevent PEP before 2012. The proportion of endoscopists who used NSAIDs had increased to 40% in our center over the past 2 years. Patients received 100-mg indomethacin suppositories within 30 minutes after ERCP when the operator considered that the case was at high risk for PEP.
Statistical Analysis
Differences in clinical characteristics between the 2 groups were analyzed by Chi-square test or Fisher exact test for categorical variables. P < 0.05 was considered significant. To investigate whether prophylactic PSP or RI was the better clinical strategy for PEP prevention, propensity score analysis was performed. This analysis can generate a quasi-randomized comparison from retrospective data.17,18 Propensity score was calculated with identified variables (age, sex, suspected SOD, previous PEP, cannulation attempts >10 minutes, precut SPT, pancreatic SPT, pancreatic guidewire passages >1, pancreatic injection, intraductal ultrasound (IDUS), and ampullectomy) that were not equally distributed between the PSP and RI groups.16 We chose the patient after one-to-one matching with nearest neighbor approach The c-statistic was calculated by the receiver operating characteristic curve. Analyses were performed on SPSS statistical software, version 19.0 (SPSS Institute, Cary, NC).
RESULTS
Patients
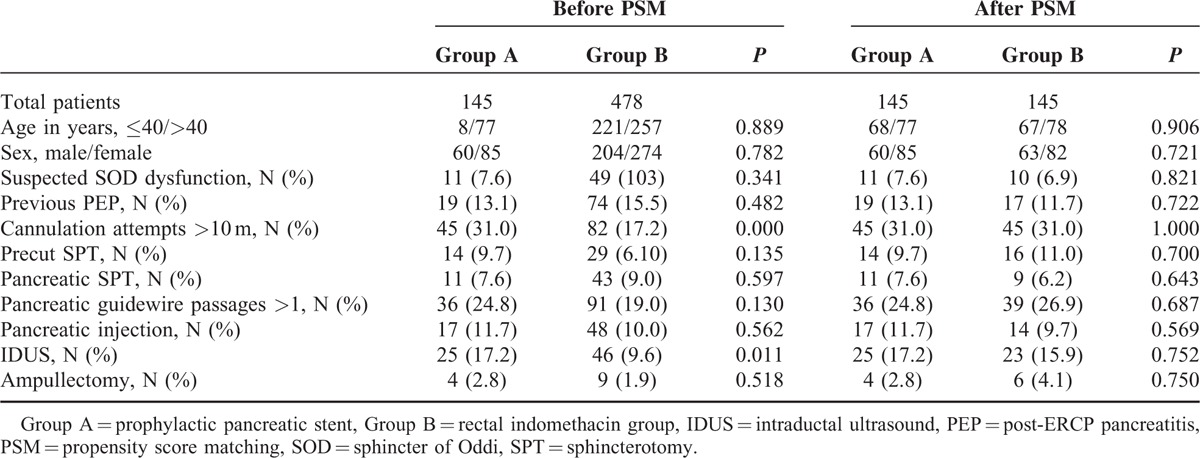
We identified 623 patients who had undergone ERCP from 2008 and 2014 (Table 1). There were significant differences in annulation attempts >10 minutes (P < 0.0001), and IDUS (P = 0.011) before propensity score matching. The propensity score was calculated for each patient based on a logistic regression analysis25,26 of the probability of prophylactic PSP using clinical characteristics. The c-statistic of our model was 0.737, which showed that our model had good ability to distinguish PSP patients from RI patients. After propensity score matching, the patient distributions were closely balanced between the 2 groups.
TABLE 1.
Characteristics of the Patients Before and After PSM
Study Outcomes
The overall frequency of PEP was 8.0% (50/623). Before PSM, 10 of 145 (6.9%) PEP cases occurred in the PSP group and 40 of 478 (8.4%) occurred in the RI group (P = 0.568; Figure 1A). Moderate or severe PEP developed in 5 patients in the PSP group (2.8%) and in 19 patients in the RI group (4.0%) (P = 0.773). To minimize the effect of selection bias between the 2 groups, propensity score matching analysis was performed. After the matching, the clinical characteristics of the patients did not significantly differ between the 2 groups, including for the risk factors of PEP (Figure 1B and Table 1). The incidences of PEP and of moderate or severe PEP in the matched PSP group were significantly lower than those in the matched RI group (6.9% vs. 15.2%, P = 0.025; 2.8% vs. 9.7%, P = 0.047, respectively). Two patients in the PSP group and 3 patients in the RI group had severe PEP, but no patient had pancreatic necrosis according to CT scans.
FIGURE 1.
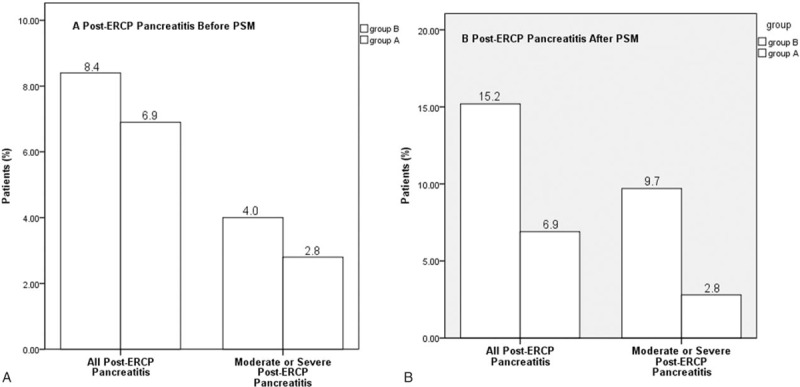
Incidence of primary and secondary end points. (A) Before PSM, post-ERCP pancreatitis developed in 10 of 145 patients (6.9%) in group A and in 40 of 478 patients (8.4%) in group B (P = 0.568). Moderate or severe post-ERCP pancreatitis developed in 5 patients in group A (2.8%) and in 19 patients in group B (4.0%) (P = 0.773). (B) After PSM, post-ERCP pancreatitis developed in 10 of 145 patients (6.9%) in group A and in 22 of 145 patients (15.2%) in group B (P = 0.025). Moderate or severe post-ERCP pancreatitis developed in 5 patients in group A (2.8%) and in 14 patients in group B (9.7%) (P = 0.047). Group A: patients who received prophylactic pancreatic duct stent; Group B: patients who received rectal indomethacin; PSM: propensity score matching.
Prophylactic Pancreatic Stent and Adverse Events
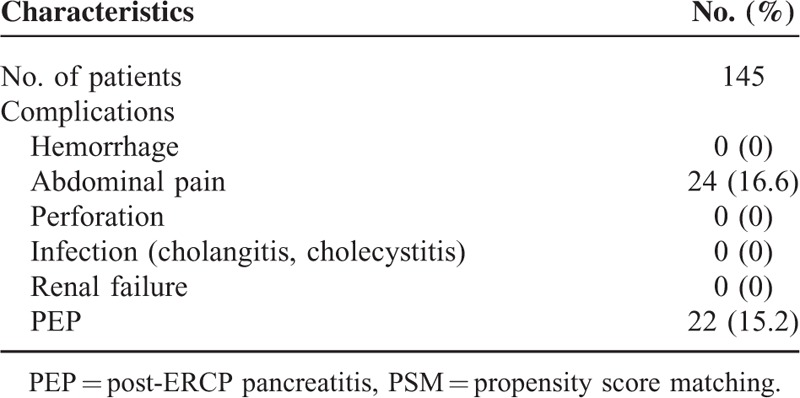
Tables 2 and 3 summarize the prophylactic pancreatic duct stent placements and proportions of adverse events in the 2 groups. The rate of spontaneous dislodgment was 96.4% (135/140) in the PSP group, with a mean dislodgment time of 2.4 days (range: 0–6 days). No major complications (such as stent migration, hemorrhage, perforation, severe biliary infection, or renal failure) were seen in either group. Abdominal pain, the most common complication, occurred in 20% (29/145) of the PSP group and 16.6% (24/145) of the RI group (P = 0.447).
TABLE 2.
Summary of Prophylactic Pancreatic Duct Stent Placement
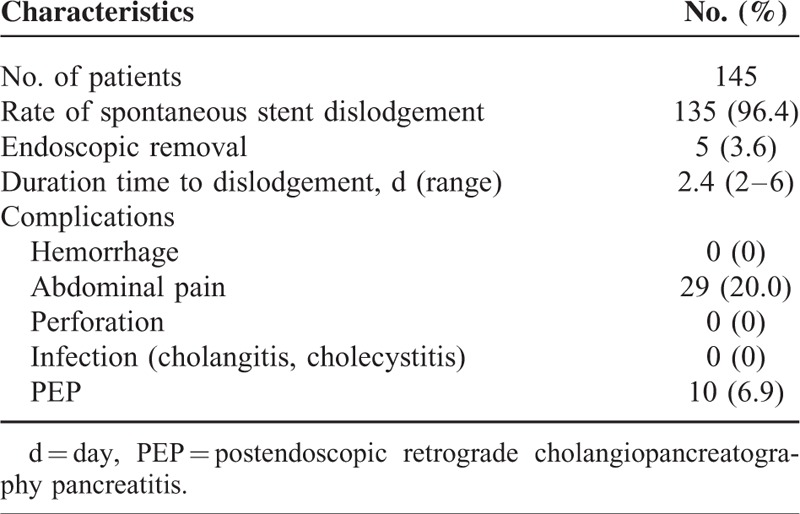
TABLE 3.
Summary of Rectal Indomethacin Group After PSM Tent
DISCUSSION
The most frequent and feared complication of ERCP is PEP, which is associated with significant postprocedure morbidity and mortality. The incidence of PEP is about 3.5% in unselected patients,3 and as high as 18% to 26% in certain high-risk populations.27,28 The prevention of PEP has been and remains an ongoing area of active research.5 RCTs and meta-analyses have demonstrated that prophylactic PSP significantly reduces PEP incidence in patients at high risk for PEP (OR: 0.44; 95% CI: 0.24–0.81; absolute RR: 12.0%; 95% CI: 3.0–21.0).10,11 However, the use of prophylactic PSP was reportedly less widespread in a European investigation,29 which seemed counterintuitive, considering the scientific evidence. Although the details of technique (type, size, and length of stent) have been clarified,24 prophylactic PSP seems controversial.29
NSAIDs are the only drug class with proven efficacy for prevention of PEP.3 Several RCTs and meta-analyses have shown NSAIDs to reduce PEP incidence in both high- and low-risk PEP groups. In a multicenter, placebo-controlled, double-blind clinical trial, a single dose of RI was associated with a lower rate of PEP in high-risk patients.
Compared with prophylactic PSP, NSAIDs are inexpensive, easily administered and have a favorable risk profile when given as a one-time dose, making them an attractive option in the pharmacological prevention of PEP.15 So, how does one translate all these findings into clinical practice?29 We think that it is premature to abandon stent placement. Studies comparing administration of indomethacin alone and prophylactic PSP alone are needed. However, an RCT to compare use of indomethacin alone to PSP alone can be difficult to arrange in light of the number of patients needed and the ethical considerations, especially in ERCP-related procedures, which are decided intraoperatively.16
Therefore, we conducted a retrospective study to evaluate the efficacy of prophylactic PSP alone versus RI alone for the prevention of PEP in high-risk patients. We used a propensity score analysis, which can balance the effects of confounding risk factors.30
Our findings showed no significant differences in PEP incidence (6.9% vs 8.4%, P = 0.568) or moderate or severe PEP (2.8% vs 4.0%, P = 0.773) before PSM. However, incidences of PEP and moderate or severe PEP in the matched PSP group were significantly lower than those in the matched RI group (6.9% vs 15.2%, P = 0.025; and 2.8% vs 9.7%, P = 0.047, respectively). The significant differences are a result of the PSM method, which minimizes the effect of selection bias between the 2 groups, and reduces the effects of confounding and assesses average treatment effects.
Abdominal pain was the most common complication. Interestingly, the proportion of abdominal pain in the matched PSP group was higher than that in the matched RI group (20% vs 16.6%, P = 0.447). NSAIDs are potent inhibitors of phospholipase A2, cyclooxygenase, and neutrophil–endothelial interactions—all believed to have important functions in PEP pathogenesis.15 Administration of RI can reduce abdominal pain.
The present study has some limitations. First, this is a retrospective study, and although the PSM algorithm produced fairly comparable groups, the study was not randomized. Second, we cannot adjust for unknown covariates. Third, we cannot show the preventive efficacy of some risk factors because of the limited number of patients.
In conclusion, prophylactic PSP is more effective than RI for prevention of post-ERCP pancreatitis for high-risk patients. Would this clinical strategy be improved by combining it with RI? Further investigations are needed.
Acknowledgment
We are indebted to Zilong Lu of the Shandong Center for Disease Control and Prevention for his help with the statistical data analysis.
Footnotes
Abbreviations: ERCP = endoscopic retrograde cholangiopancreatography, PEP = post-ERCP pancreatitis, PSM = propensity score matching, PSP = prophylactic pancreatic stent placement, RI = rectal indomethacin
Authors’ contributions: G-DL, X-YJ, and C-YQ designed the study. G-DL, Q-PP, H-LZ, and X-JZ performed ERCP procedures. H-YD, RG, and Y-CD followed the patients and provided all figures and data for the paper. G-DL, X-YJ, and C-YQ wrote the paper.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Freeman ML. Pancreatic stents for prevention of post-endoscopic retrograde cholangiopancreatography pancreatitis. Clin Gastroenterol Hepatol 2007; 5:1354–1365. [DOI] [PubMed] [Google Scholar]
- 2.Dumonceau JM, Andriulli A, Deviere J, et al. European Society of Gastrointestinal Endoscopy (ESGE) Guideline: prophylaxis of post-ERCP pancreatitis. Endoscopy 2010; 42:503–515. [DOI] [PubMed] [Google Scholar]
- 3.Dumonceau JM, Andriulli A, Elmunzer BJ, et al. Prophylaxis of post-ERCP pancreatitis: European Society of Gastrointestinal Endoscopy (ESGE) Guideline—updated. Endoscopy 2014; 46:799–815. [DOI] [PubMed] [Google Scholar]
- 4.Joseph Elmunzer B, Scheiman JM, Lehman GA, et al. A randomized trial of rectal indomethacin to prevent post-ERCP pancreatitis. N Engl J Med 2012; 366:1414–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feurer ME, Adler DG. Post-ERCP pancreatitis: review of current preventive strategies. Curr Opin Gastroenterol 2012; 28:280–286. [DOI] [PubMed] [Google Scholar]
- 6.Parsi MA. NSAIDs for prevention of pancreatitis after endoscopic retrograde cholangiopancreatography: ready for prime time? World J Gastroenterol 2012; 18:3936–3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choudhary A, Bechtold ML, Arif M, et al. Pancreatic stents for prophylaxis against post-ERCP pancreatitis: a meta-analysis and systematic review. Gastrointest Endosc 2011; 73:275–282. [DOI] [PubMed] [Google Scholar]
- 8.Andriulli A, Forlano R, Napolitano G, et al. Pancreatic duct stents in the prophylaxis of pancreatic damage after endoscopic retrograde cholangiopancreatography: a systematic analysis of benefits and associated risks. Digestion 2007; 75:156–163. [DOI] [PubMed] [Google Scholar]
- 9.Singh P, Das A, Isenberg G, et al. Does prophylactic pancreatic stent placement reduce the risk of post-ERCP acute pancreatitis? A meta analysis of controlled trials. Gastrointest Endosc 2004; 60:544–550. [DOI] [PubMed] [Google Scholar]
- 10.Sofuni A, Maguchi H, Itoi T, et al. Prophylaxis of post-endoscopic retrograde cholangiopancreatography pancreatitis by an endoscopic pancreatic spontaneous dislodgement stent. Clin Gastroenterol Hepatol 2007; 5:1339–1346. [DOI] [PubMed] [Google Scholar]
- 11.Das A, Singh P, Sivak MV, et al. Pancreatic-stent placement for prevention of post-ERCP pancreatitis: a cost-effectiveness analysis. Gastrointest Endosc 2007; 65:960–968. [DOI] [PubMed] [Google Scholar]
- 12.Dumonceau JM, Rigaux J, Kahaleh M, et al. Prophylaxis of post-ERCP pancreatitis: a practice survey. Gastrointest Endosc 2010; 71:934–939. [DOI] [PubMed] [Google Scholar]
- 13.Dai HF, Wang XW, Zhao K. Role of nonsteroidal anti-inflammatory drugs in the prevention of post-ERCP pancreatitis: a meta-analysis. Hepatobiliary Pancreat Dis Int 2009; 8:11–16. [PubMed] [Google Scholar]
- 14.Elmunzer B, Waljee A, Elta G, et al. A meta-analysis of rectal NSAIDs in the prevention of post-ERCP pancreatitis. Gut 2008; 57:1262–1267. [DOI] [PubMed] [Google Scholar]
- 15.Elmunzer BJ, Scheiman JM, Lehman GA, et al. A randomized trial of rectal indomethacin to prevent post-ERCP pancreatitis. N Engl J Med 2012; 366:1414–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takenaka M, Fujita T, Sugiyama D, et al. What is the most adapted indication of prophylactic pancreatic duct stent within the high-risk group of post-endoscopic retrograde cholangiopancreatography pancreatitis? Using the propensity score analysis. J Hepatobiliary Pancreat Sci 2014; 21:275–280. [DOI] [PubMed] [Google Scholar]
- 17.Griswold ME, Localio AR, Mulrow C. Propensity score adjustment with multilevel data: setting your sites on decreasing selection bias. Ann Intern Med 2010; 152:393–395. [DOI] [PubMed] [Google Scholar]
- 18.Sugihara T, Yasunaga H, Horiguchi H, et al. Does mechanical bowel preparation improve quality of laparoscopic nephrectomy? Propensity score-matched analysis in Japanese series. Urology 2013; 81:74–79. [DOI] [PubMed] [Google Scholar]
- 19.Chang JS, Keum KC, Kim NK, et al. Preoperative chemoradiotherapy effects on anastomotic leakage after rectal cancer resection. Ann Surg 2013; 00:1–6. [DOI] [PubMed] [Google Scholar]
- 20.Sofuni A, Maguchi H, Mukai T, et al. Endoscopic pancreatic duct stents reduce the incidence of post-ERCP pancreatitis in high-risk patients. Clin Gastroenterol Hepatol 2011; 9:851–858. [DOI] [PubMed] [Google Scholar]
- 21.Chahal P, Tarnasky PR, Petersen BT, et al. Short 5Fr vs long 3Fr pancreatic stents in patients at risk for post-endoscopic retrograde cholangiopancreatography pancreatitis. Clin Gastroenterol Hepatol 2009; 7:834–839. [DOI] [PubMed] [Google Scholar]
- 22.Cotton PB, Lehman G, Vennes J, et al. Endoscopic sphincterotomy complications and their management: an attempt at consensus. Gastrointest Endosc 1991; 37:383–393. [DOI] [PubMed] [Google Scholar]
- 23.Mallery JS, Baron TH, Dominitz JA, et al. Complications of ERCP. Gastrointest Endosc 2003; 57:633–638. [DOI] [PubMed] [Google Scholar]
- 24.Zolotarevsky E, Fehmi SM, Anderson MA, et al. Prophylactic 5-Fr pancreatic duct stents are superior to 3-Fr stents: a randomized controlled trial. Endoscopy 2011; 43:325–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gum PA, Thamilarasan M, Watanabe J, et al. Aspirin use and all-cause mortality among patients being evaluated for known or suspected coronary artery disease: a propensity analysis. JAMA 2001; 286:1187–1194. [DOI] [PubMed] [Google Scholar]
- 26.Abidov A, Rozanski A, Hachamovitch R, et al. Prognostic significance of dyspnea in patients referred for cardiac stress testing. N Engl J Med 2005; 353:1889–1898. [DOI] [PubMed] [Google Scholar]
- 27.Vandervoort J, Soetikno RM, Tham TC, et al. Risk factors for complications after performance of ERCP. Gastrointest Endosc 2002; 56:652–656. [DOI] [PubMed] [Google Scholar]
- 28.Masci E, Toti G, Mariani A, et al. Complications of diagnostic and therapeutic ERCP: a prospective multicenter study. Am J Gastroenterol 2001; 96:417–423. [DOI] [PubMed] [Google Scholar]
- 29.Ahmad NA, Brenner DM, Chan AT, et al. Pancreatic duct stents for the prevention of post-ERCP pancreatitis: for all or some? Gastroenterology 2012; 143:493–500. [DOI] [PubMed] [Google Scholar]
- 30.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res 2011; 46:399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]


