Abstract
The hedgehog signaling pathway was first discovered in the 1980s. It is a stem cell-related pathway that plays a crucial role in embryonic development, tissue regeneration, and organogenesis. Aberrant activation of hedgehog signaling leads to pathological consequences, including a variety of human tumors such as pancreatic cancer. Multiple lines of evidence indicate that blockade of this pathway with several small-molecule inhibitors can inhibit the development of pancreatic neoplasm. In addition, activated hedgehog signaling has been reported to be involved in fibrogenesis in many tissues, including the pancreas. Therefore, new therapeutic targets based on hedgehog signaling have attracted a great deal of attention to alleviate pancreatic diseases. In this review, we briefly discuss the recent advances in hedgehog signaling in pancreatic fibrogenesis and carcinogenesis and highlight new insights on their potential relationship with respect to the development of novel targeted therapies.
INTRODUCTION
In 1980, hedgehog signaling was first discovered in the fruit fly by Nusslein-Volhard,1 and has since been found in vertebrates within various organs. Hedgehog signaling, a pathway characterized by being conserved but considerably multifunctional,2,3 is involved in a variety of developmental and physiological processes, such as body axis formation, angiogenesis, and stem cell homeostasis. As a result, the developing tissues grow into the correct size with the appropriate cell types, orientation, and vascularization.4,5
According to the World Cancer Report in 2012, pancreatic cancer was ranked as the seventh most common cause of cancer deaths, with 330,000 deaths globally and a 5-year survival of less than 5%.6 Pancreatic cancer cells exhibit tenacious growth, early dissemination, metastatic ability, and resistance to radiotherapy and chemotherapy, all of which contribute to high mortality. Without proper and early diagnosis, delayed detection is common. In this case, most patients are diagnosed with end-stage pancreatic carcinoma. Thus, only 10%–15% patients are able to receive surgery, even though an operation is still the most valid therapeutic method; the 5-year survival of these patients is approximately 10%. The patients who are unable to undergo surgery will inevitably suffer through chemotherapy and radiotherapy. The standard remedy for pancreatic cancer established by Burris et al7 has been updated to include gemcitabine with erlotinib.8 When compared with gemcitabine alone, the significantly improved 0.3-month survival advantage seems to have no obvious effect on clinical treatment. Hedgehog boosts the initiation and development of pancreatic cancers.9 Studies indicate that the inhibition of hedgehog can cure malignant diseases.10–12 Currently, the underlying mechanism of hedgehog signaling in carcinoma is being increasingly studied, as such a somber condition as pancreatic cancer warrants the development of novel and effective methods.
Pancreatic tissue fibrosis is a terminal and distinguishing feature of pathological changes with diverse means of inflicting harm. The formation of pancreatic fibrosis is a complicated and long-term process in which multiple factors interact with each other. Injuries (apoptosis and necrosis) of the pancreas can induce the synthesis and release of proinflammatory factors, chemokines and growth factors such as PDGF, TGF-β1, and angiotensin II,13–15 resulting in the activation of pancreatic stellate cells (PSCs) and the accumulation of myofibroblasts. Myofibroblasts are terminally differentiated cells that are responsible for the synthesis and deposition of extracellular matrix (ECM) components such as type I and III collagens.16,17 If repair mechanisms are disrupted or ineffective, excessive deposition of ECM components will form a barrier around the original pathological injury, leading to the intensive resistance to radiotherapy and chemotherapy.18–20 Hedgehog signaling is an important pathway involved in the activation of PSCs. Inhibition of hedgehog signaling can reduce or even reverse PSCs activation, leading to improved outcomes in chronic pancreatitis. For example, resveratrol, a botanical compound derived mainly from the skins of red grapes, may have antifibrotic effects on the pancreas by antagonizing the hedgehog pathway.21 Therefore, screening of highly effective pharmaceutical agents to inhibit the activation of hedgehog signaling provides a great opportunity for the development of antifibrotic drugs.
Hedgehog Signaling: Structure and Function
The hedgehog signaling pathway is classified into 2 modalities: canonical and noncanonical. Noncanonical hedgehog signaling refers to hedgehog signaling receptor dependent signals that do not operate via Gli or Smo. Noncanonical hedgehog signaling is divided into 2 types: Type I acts through Ptch,22–24 while type II acts through Smo without being regulated by Gli.23,25 In total, hedgehog signaling molecules include 3 ligands (Sonic hedgehog (Shh), Indian hedgehog (Ihh), and Desert hedgehog (Dhh)),26–28 2 receptors (Ptch1 and Ptch2),29,30 a signal transducer Smoothened (Smo),4,31 and 3 transcription factors (Gli1, Gli2, and Gli3).32–34 Each ligand functions in various ways.
As a secretory signal protein, the hedgehog ligand acts as the initiating event in a signaling cascade. When the signaling pathway is activated, hedgehog ligand undergoes autoprocessing and lipid modification reactions to generate the hedgehog signaling peptide with N-terminal palmitoylation and C-terminal cholesterylation, and thereby activates itself.35–38 Mature hedgehog peptide with lipid modifications will be released from secreting cells by multitransmembrane transporter-like proteins that are dispatched39 and transduced to target cells through the multiple-transmembrane receptor Ptch.40
Ptch is encoded by the Patched gene and is a 12-transmembrane protein that acts as the receptor for hedgehog signaling.35Patched is a tumor suppressor gene and mutations in this gene can lead to Gorlin syndrome, medulloblastoma, and esophageal squamous cancer.41,42 Ptch1, which is confined to target cells, is upregulated in response to hedgehog-signaling proteins.43 It is the subtype that is definitively involved in the activation of hedgehog signaling. The transcription of Ptch2 is independent of pathway activation and is coexpressed with hedgehog proteins.44 Goodrich et al45 found in Ptch1 knockout mice that the inhibition of Smo activity was abolished. Further studies confirmed that Ptch inhibits Smo activity when hedgehog ligands are absent.22,23
When hedgehog peptides are released and activate the hedgehog receptors of target cells, Ptch activity is suppressed, leading to Smo translocation to the plasma membrane, interaction with Cos2, and increased autophosphorylation. Smo is encoded by a protooncogene (Smo gene). It is a 7-transmembrane protein that is coupled with a heterotrimeric G-protein.46 As previously reported, the small molecule cyclopamine targets the Smo protein to inhibit hedgehog signaling.47 In Drosophilae, Smo directly recruits the cytoplasmic complex Cos2/Ci/Fu through Cos2 and then influences their activity.48 In the absence of Smo, the complex Cos2/Ci/Fu phosphorylates and binds to the zinc finger-like transcription factor Gli.
In summary, the hedgehog ligand protein spindles Ptch, which is bound to the ciliary, thereby inducing the release of Smo; then, the Gli protein is released from Smo and is translocated to the nucleus where it functions as a transcriptional activator.49,50 Only the repressed form of Gli can enter the nucleus and induce the transcription of target genes.51 The aberrant expression of Gli1 or the dominant activating mutant Gli2 in keratinocytes regulates overlapping transcriptional processes of these 2 proteins (Figure 1).52
FIGURE 1.
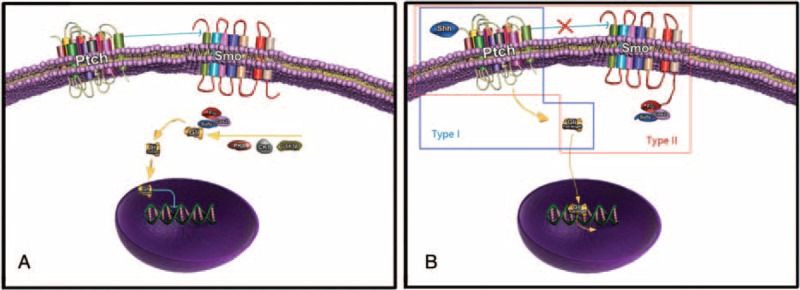
Hedgehog signaling in vertebrates. A, In the absence of hedgehog ligand (e.g., Shh), Ptch inhibits Smo from reaching the plasma membrane. In this case, the microtubule-associated Cos2–Fu–SuFu complex can bind full-length Gli, which can be phosphorylated by glycogen synthase kinase-3β (GSK-3β), protein kinase A (PKA), and casein kinase 1 (CK1). Phosphorylated Gli is cleaved to an N-terminal form and then will translocate the nucleus to suppress transcription. B, In the presence of hedgehog ligand, Ptch activity is suppressed, and thereby Smo translocates to the plasma membrane and interacts with Cos2. In this state, the Cos2–Fu–SuFu complex cannot bind Gli, and Gli is able to enter the nucleus and induce transcription of target genes. In the blue and orange frames there are 2 types of noncanonical hedgehog signaling. Blue frame: Type I requires only binding of a hedgehog isoform to Ptch and is mediated by novel functions of Ptch unrelated to Smo repression, and it is by definition insensitive to Smo modulators. Orange frame: Type II is dependent on Smo and in some cases it has been shown to rely on signaling through Gli proteins, and it is both mimicked by Smo agonists and inhibited by Smo antagonists.
In contrast to the canonical pathway referred to above, studies have demonstrated that not all hedgehog signaling proceeds through Gli activation; these subtypes of Gli-independent pathways were named noncanonical hedgehog signaling.52 The binding of a hedgehog isoform to Ptch is required in type I noncanonical hedgehog signaling, which is mediated by the novel functions of Ptch that are unrelated to Smo repression and are thus insensitive to modulators of Smo. In the absence of a hedgehog ligand, the ectopic expression of Ptch1 induces apoptosis.53 Several studies corroborate the hypothesis that the proapoptotic effect of Ptch1 is exerted through a type I noncanonical pathway. For example, the in vitro or in vivo disruption of detectable canonical hedgehog signaling,20,21 the absence of an effect of Smo antagonists to prevent the Shh-dependent reduction in caspase-3 activity and the inability of the Smo agonist to mimic the antiapoptotic effect of Shh,25 and Ptch1 regulation of the cell cycle through cyclin B1 in a Smo- and Gli-independent manner.52,54
Compared with type I noncanonical hedgehog signaling, type II signaling is defined by Smo-dependent and Gli-independent cascades.23,55 Hedgehog signaling responds to Smo agonists that are mediated through the activation of small GTPases to elicit cellular responses. Bijlsma et al56 found that Shh-induced fibroblast migration was Smo-dependent but Gli-independent. In Chinchilla study,23 hedgehog proteins in endothelial cells promoted actin stress fiber formation and endothelial cell tubulogenesis in a Smo-dependent manner.57 In axonal development, Shh signals stimulated the activity of Src family kinase members in a Smo-dependent manner. In a later study,58 a novel signaling cascade operating through SH3 domain-containing proteins was found to be directly stimulated by hedgehog ligands (Figure 1).
Hedgehog Signaling in the Pancreas: Development to Adult Tissue Remodeling
In humans, the pancreas is located in the abdominal cavity behind the stomach and acts as an endocrine and exocrine gland for the digestive and endocrine systems. Hedgehog signaling influences the development of both endocrine and exocrine functions from the newborn to the mature adult. Staining of embryos reveals that Shh signaling is expressed throughout the gut endoderm,59,60 with the exception of the pancreatic bud endoderm. During embryogenesis, the pancreas develops from the pancreas bud of the end endodermal foregut and its surrounding mesoderm.61–63 The function of pancreatic cells emerges from common precursors present in the early gut endoderm.64,65 Pancreatic bud endoderm expresses high levels of the homeodomain protein Ipf1/Pdx1 (insulin promoter factor 1/pancreatic and duodenal homeobox 1), an essential regulator of early pancreatic development.66–69 When pancreatic explants were exposed to Shh, Ipf1/Pdx1 induced constitutive Shh overexpression in the pancreatic bud, thus promoting pancreatic mesoderm differentiation into smooth muscle and interstitial Cajal cells, which are typical of the developing intestine rather than the pancreatic mesenchyme and spleen.70
Inhibition of Hedgehog signaling with the specific inhibitor cyclopamine would induce a variety of changes in the pancreas,9 for example, gastric explants expressing endocrine pancreatic markers such as insulin and glucagon,71 an increase in islet cell number and size and the high level expression of Ipf1. The experiment conducted by diIorio in zebrafish clarified that gene mutation of Shh and the relative signaling pathway had the specific dependence with endocrine function. These mutants confirmed that during gastrulation, the transient Shh signaling induced the pancreatic endoderm to differentiate into the islet tissue subsequently. In the later development, a second hedgehog-dependent activity appeared which was similar to role of Shh in the foregut endoderm assisting the localization of pancreas.72
The biological function of hedgehog signaling in the pancreatic epithelium remains controversial, while activation or inhibition of hedgehog signaling pathway only has slight effect on epithelial development. It suggests that hedgehog pathway might mainly focus in pancreatic mesenchyme.73–76 During embryo development, beta-cell was transiently delayed due to the Pdx1-driven loss of Smo that Lau and Hebrok73 discovered in pancreatic epithelial progenitor cells of Pdx1−/Smo−/− mice. After given birth, beta-cell numbers would restore; however they were dysfunctional. In this case, the phenomenon (reduced insulin secretion, slight insulin-dependent diabetes, and enhanced insulin sensitivity) occurred. The primary cilium that was structurally required as signaling downstream of Smo had close relationship with the mild responsiveness to the activation of hedgehog signaling in pancreatic epithelial cells. Overactivation of hedgehog pathway in mature epithelial cell specifically with resection of primary cilium caused the following results that pancreas tissue dedifferentiated, markers of progenitor cells reexpressed, and endocrine area decreased.77,78
Hedgehog Signaling: A Role in PSCs
PSCs are myofibroblast-like cells that can switch between quiescent and activated phenotypes, such as hepatic stellate cells, and reside in exocrine areas of the pancreas.16,17 They are also the effector cells that respond to pathogens. Hedgehog signaling influences PSCs to a certain degree. In the pancreas, hedgehog signaling is rigorously regulated. Quiescent hedgehog signaling is vitally important for proper differentiation and development of the pancreas. When pathogens lead to pancreatic fibrosis or carcinoma, the sensitive signaling of the hedgehog pathway is easily activated.
Activated PSCs will migrate to the injured location and participate in tissue repair activities by secreting ECM components.79,80 Research by Shinozaki et al81 revealed in an in vitro study that exogenous Ihh protein could enhance the migrational ability of PSCs by increasing membrane type-1 matrix metalloproteinase on the plasma membrane, while Gli1 negatively regulated Ihh stimulated PSCs migration by inhibiting active MT1-MMP. They also found that Ihh signaling did not modulate PSC activation or proliferation. However, the data from Sicklick et al82 were not consistent with this study. This could be because in different organs, hedgehog signaling may function differently.
Myofibroblasts were detected in capan-2 tumors, thus supporting the hypothesis that these represented pancreatic stellate cells within the tumor and proliferated in response to Shh stimulation. Shh can promote the differentiation and proliferation of PSCs. Jennifer and colleagues stimulated PSCs with recombinant Shh for 24 hours at 1 and 10 μg/mL, and observed an increase in the mesenchymal markers Sma, vimentin and desmin and a decrease in the epithelial marker CK19. This was the first study to show that Shh induces the differentiation of PSCs into myofibroblasts.83
However, in the stroma, tumor cells overexpressing Shh led to the consequences that activated the hedgehog signaling pathway in PSCs instead of themselves. Activated PSCs were crucial in enhancing neural invasion of pancreatic cancer along axons and nerve growth of cancer cell colonies that assisted pancreatic cancer cell migration. In vivo pancreatic cancer model, paracrine Shh activated the PSCs and finally resulted in the tumor cell invasion of the trunk, sciatic nerve dysfunction, and the growth and metastasis of the orthotopic xenograft tumor, while the inhibitor cyclopamine could impede these pathological changes.84
Expression of hedgehog signaling pathway components revealed that Smo and Gli were primarily observed in stromal-derived PSCs, whereas the ligands Shh and Ihh were limited to pancreatic tumor cells.85 The development of PDAC tumors was not affected when Smo was genetically ablated in the pancreatic epithelium of PDAC-susceptible mice.86 This suggested that there might be a paracrine signaling mechanism involved. Treatment of PSCs with Shh and Ihh activated the hedgehog signaling pathway (the upregulation of Gli mRNA supports this finding), and stimulated the proliferation of PSCs. Hedgehog signaling ligands secreted from neighboring cancer cells stimulated PSCs to produce soluble factors that then fed back on cancer cells to promote their activity.87
Hedgehog Signaling: A Role in Pancreatic Fibrosis
When the pancreas suffers injuries, PSCs undergo morphological and functional changes to become activated to express α-Smooth muscle actin.88,89 They will proliferate, migrate, and secrete ECM proteins, thus leading to pancreatic fibrosis.90,91 Fibrogenesis will appear approximately during the onset of a lesion. Hedgehog signaling induces progressive pancreatic fibrosis intermingled with proliferating ductal structures that are accompanied by the destruction of acinar structures. Paracrine hedgehog signaling activates myofibroblasts and leads to their proliferation, and it shows the restricted expression of hedgehog signaling downstream components including Ptch, Smo, and Gli1/2 in hedgehog-responsive cells, while hedgehog signaling ligands induce matrix metalloproteinases (MMPs) in all hedgehog-responsive cells, as shown in Figure 2.92
FIGURE 2.

Activation of hedgehog signaling promotes the myofibroblast phenotypes in pancreas.
The hedgehog signaling pathway can be expressed in the embryonic mouse pancreas and is also observed in the adult mouse pancreas.93 Ptch and Smo are also expressed in islet β cells and activated PSCs. Because Ihh expression is elevated in the pancreas of patients with chronic pancreatitis, Ihh is thought to participate in chronic pancreatic injury, especially pancreatic fibrosis.82,94 Islet cells of tissues undergoing chronic pancreatitis exhibit an abnormal localization pattern for Ihh. As a signal transducer, Smo is upregulated in cancer-associated stromal fibroblasts, and the expressed Smo could transduce the Shh to activate Gli1 expression.95
However, Ihh does not directly activate PSCs nor stimulate PSC proliferation; in other words, the parameter of PSCs transformation (expression of collagen-1 or alpha-smooth muscle actin) stays unresponsive to Ihh.82 Ihh has a powerful effect on PSC migration not only in chemotactic manner but also in chemokinetic way. When target cells get stimulated and need to migrate, Ihh raises the level of membrane-type 1 matrix metalloproteinase (MT1-MMP) and shifts them onto the plasma membrane from original location. According to the result from Satoshi Shinazaki and his colleagues, we could conclude that Ihh promote PSCs migration via Gli1-dependent signaling pathway.81 They applied adenovirus to cause Gli1 overexpression and RNA interference technique to reduce Gli1 expression.81 The results that overexpression of Gli1 nevertheless blocks MT1-MMP localization on the plasma membrane and PSCs migration while RNA interference-mediated knockdown of Gli1 augments PSCs migration indicated that there was a negative feedback loop in the relationship between Ihh and Gli1.81
During in vitro wound healing, Shh increases the ability of myofibroblasts to migrate into a wounded area as a monolayer. Shh significantly increases the ability of myofibroblasts to recover from the wound and promotes the migration and invasion of human pancreatic myofibroblasts in vitro.85
The receptor Ptch also influences this process substantially. There are 3 hedgehog signaling coreceptors, GAS1, BOC, and CDON, which are cell-surface-associated proteins that act as pathway activators96–98 and are expressed in cancer-associated fibroblasts.96,99 The deletion of 2 coreceptors (Gas1 and Boc) in fibroblasts leads to a reduction in hedgehog signaling responsiveness. Additionally, these fibroblasts promote greater tumor growth and tumor-associated vascularity. The deletion of all 3 coreceptors (Gas1, Boc, and Cdon) results in the almost complete abrogation of hedgehog signaling and a corresponding failure to promote tumorigenesis and angiogenesis. In general, the study by Mathew et al100 identified a role for hedgehog dosage in the promotion of pancreatic cancer and interpreted the clinical failure of blocking the hedgehog signaling pathway as a therapeutic approach in pancreatic cancer.
Hedgehog Signaling: A Role in Pancreatic Cancer and Crosstalk With Other Factors
Referring to tumorigenesis, hedegehog signaling has the nonnegligible relationship. The role of hedgehog signaling relating to carcinoma was first identified in patients with Gorlin syndrome caused by mutation of Ptch.101 Once loss-of-function mutations in Ptch or mutations in Smo, it leads to sustained activation of hedgehog pathway. Overexpression of Gli1 and Hedgehog proteins is associated with a variety of cancers and implicated in the onset of pancreatic ductal neoplasia and the maintenance of advanced cancers.102,103 Not as we expected, in pancreatic cancer, ligand-dependent activation of hedgehog signaling instead of genomic mutation was reported and overexpression of Shh was considered adequate to trigger the initiation of pancreatic cancer.104,105 As the vital effect of hedgehog signaling on pancreatic cancer, many other factors may interact with it to influence the development of the malignant disease.
Perineural invasion is a common pathologic feature in pancreatic cancer by which cancer cells invade the peripheral nerves and are disseminated.106 Shh overexpression is involved in perineural invasion in PSCs. Several studies showed that stromal PSCs may play a regulatory role in the interaction between cancer cells and nerves.107–109 Specific cell types and tissues that release bioactive Shh from pancreatic cancer cells can produce heparan sulfate (HS). According to in vivo knockdown and in vitro cell culture studies, glypican HS proteoglycans release bioactive Shh morphogens from the surface of transfected Bosc23 cells through HS chains in a cell autonomous manner. HS specifically modifies Shh processing on the cell surface, and purified glycosaminoglycans enhance the proteolytic removal of N- and C-terminal Shh peptides under cell-free conditions.110 The overexpression of Shh in tumor cells activates the hedgehog signaling pathway in PSCs in the stroma rather than activating tumor cells directly to augment the output of MMP2, MMP9, and NGF. These activated PSCs and associated molecules promote pancreatic cancer cell migration along nerve axons and nerve outgrowth to pancreatic cancer cell colonies. Coimplantation of PSCs activated by paracrine Shh induces tumor cell invasion of the trunk, stimulates nerve dysfunction, and promotes orthotropic xenograft tumor growth, metastasis, and perineural invasion in vivo.84
In recent years, several experiments proved that in both vivo and vitro, the hippo pathway exerted regulator ability of cell density.111–114 When the cell density exceeded the threshold, the hippo cascade would be turned on and lead to the activation of large tumor suppressor (LATS) kinases, finally causing the phosphorylation of Yes-associated protein and its paralog TAZ (YAP/TAZ). In cell culture, the activity of hedgehog signaling is dependent on cell-to-cell contact. When the cell intimately contacted with each other or the intensity reached high enough, hedgehog pathway activity would be regulated to higher level.115 It seems contact inhibition has no effect on hedgehog signaling in normal fibroblasts.116,117 Adenovirus-mediated YAP overexpression nevertheless prevents hedgehog signaling while the knockdown of YAP expression RNA interference augments hedgehog/Gli activity.118 However, hedgehog signaling enhances the post-transcription of YAP to promote its activity. It indicates that there is a negative feedback loop. However, in human and mouse pancreatic cancers, low hedgehog signaling pathway activity accompanies strong nuclear YAP immunoreactivity. But tumor could utilize both ocogenic pathway simultaneously on the condition there exist protease-activated receptors (PARs) which has the potential ability to override the Hippo/hedgehog pathway.118
As a converse effect of YAP, BRD4, a regulator of epigenetic proteins that can activate Shh members in a ligand-independent manner in PDAC cells, has recently appeared as an alternative therapeutic strategy. BRD4 induces PDAC cell proliferation and chemoresistance. In vitro, suppression of BRD4 damaged PDAC cell viability and proliferation and negatively influenced the tumor growth rate.119 Gemcitabine can increase the expression of BRD4. Combination treatment of gemcitabine and BRD4 silencing has a synergistic effect on chemotherapeutic efficacy and significantly promoted apoptosis in the PANC-1 and MIAPaCa-2 cell lines. This combination can overcome the side effects of gemcitabine as the single medication. This suggests that BRD4 is a promising target of the transcriptional program of PDACs.120
Not surprisingly, tissue repair occurs in pancreatic carcinoma. Gli1 was found as a central player in pancreatic tissue repair upon Kras inactivation. Its activity in pancreatic fibroblasts leads to the expression of IL-6, an inflammatory cytokine that activates Stat3 in pancreatic cancer cells.121 Improper stromal remodeling and persistence of the inflammatory infiltrate in pancreatic tumorigenesis is a consequence of the deletion of Gli1, while partial loss of Gli1 affects fibrogenesis and the recruitment of immune cells. IL-6, mIL-8, Mcp-1, and M-csf (Csf1), as a subset of cytokines, potentially direct Gli1 target genes to mediate this phenomenon.122
In recent years, noncoding RNA has become a popular in many fields. microRNAs (miRNAs) are a class of small noncoding RNAs that play important roles in carcinogenesis.123 Smo is a direct target of miR-125b, miR-193b, miR-324-5p miR-326,124 and miR-338-3p.125 Downregulation of miR-125b, miR-193b, miR-324-5p, miR-326, and miR-338-3p in human cancers derepress Smo and promote tumor proliferation and invasion through aberrant hedgehog signaling. miR-324-5p downregulation is caused by deletion of the miR-324-5p gene in a high percentage of MBs as a consequence of the loss of chromosome 17p. Deletion of chromosome 17p leads to the loss of certain genes, thus contributing to hedgehog induced tumorigenesis.126,127
The levels of tumor suppressor miR-let7b, which targets several genes (K-RAS, MUC4, NCOA3, HMGA2, TGFβR1, and STAT3 phosphorylation)128–130 involved in PDAC pathogenesis, are down-regulated.131,132 Kumar et al133 found that the combination therapy of miR-let7b and GDC-0449 effectively inhibited tumor growth when injected to athymic nude mice bearing ectopic tumors generated using MIA PaCa-2 cells compared with micelles carrying GDC-0449 or miR-let7b alone.
MiR-212 is upregulated in PDAC tissues and cells.134 Gain-of-function and loss-of-function experiments show that a pro-oncogenic function of miR-212 exists in PDAC. Ptch1 is a direct target of miR-212 in nonsmall lung cancer.135 A new study shows that this also applies in PDAC.136 Upregulation of Ptch1 can attenuate the effect induced by miR-212. This suggests that miR-212 could facilitate PDAC progression and metastasis by targeting Ptch1, implicating a novel mechanism for the progression of PDAC.136
miRNA is not the only relevant nucleic acid, as some discoveries in DNA have been made as well. Twenty-five members have been reported to belong to the S100 family in humans. Twenty-one of them are known as the epidermal differentiation complex (EDC) involved in epithelial-derived cell differentiation, which are coded by genes clustered at chromosome locus 1q21.137 S100A2, S100A4, S100A6, S100A11, and S100A14 from the S100 gene family are found to be significantly down-regulated due to Gli1 knockdown. Gli1 primarily regulates S100A family members via cis-acting elements. S100A4 and vimentin genes are up-regulated significantly by increased Shh/Gli1 expression, while E-cadherin is significantly reduced at the same time. Migration of pancreatic cancer cells is increased significantly with dose-dependent increases in Gli1 expression. Silencing of S100A4 significantly reversed the response of pancreatic cancer cells induced by L-Shh transduction.138
Hedgehog signaling pathway inhibitors accelerate rather than delay the progression of oncogenic Kras-driven disease. This finding was substantiated in 3 distinct genetically engineered mouse models used to study the effects of genetic or pharmacologic inhibition of the hedgehog signaling pathway activity. The balance between epithelial and stromal elements is notably influenced by pharmacologic inhibition of hedgehog, thus suppressing stromal desmoplasia. This inhibition also accelerated the growth of the PanIN epithelium. In contrast, the activation of this pathway using a small molecule agonist causes stromal hyperplasia and reduces epithelial proliferation. The stromal response to hedgehog signaling is protective against PDAC, and the pharmacologic activation of the pathway can slow tumorigenesis. Thus, it may offer an explanation for the failure of hedgehog inhibitors in clinical trials and may offer new insights into novel therapeutic interventions.139
Hedgehog Signaling: A Diagnostic Tool in Pancreatic Cancer
Pancreatic cancer is a highly aggressive carcinoma with an ultrahigh case fatality rate due to poor diagnosis and the lack of proper therapies. Considering the unique role hedgehog signaling plays in pancreatic cancer, many strategies are focused on utilizing this pathway as a diagnostic tool for pancreatic carcinoma. Clinical studies showed that the levels of Shh in human blood were lower in patients with pancreatitis and pancreatic cancer compared with healthy individuals. However, hematopoietic cells do not express Shh. This finding suggests that hedgehog is secreted into the bloodstream. However, hedgehog activity is blocked by plasma proteins. Reduced plasma levels of hedgehog were found in pancreatic cancer patients, but they were insufficient alone to predict pancreatic cancer.140
The epithelial-to-mesenchymal transition (EMT) is a common phenomenon in various cancers. Hedgehog signaling controls EMT, enhances cell proliferation in an MAPK- and PI3-kinase-dependent manner, decreases apoptosis through the regulation of Bcl-2 and Bcl-X, and induces the proliferation of cancer stem cells. Gli1 is positively associated with MMP9. Patients with Gli1 and MMP9 coexpression have poor overall survival. Silencing of Gli1 alone without external stimulus had no effect on EMT, but inhibited TGF-β1 and EGF-induced EMT. It performs a protumor role in the aggressive invasion of PSCs by promoting TGF-β1 and EGF-induced EMT.141
Shh and the clinical outcome in esophageal cancer demonstrated that Gli1 was a strong and independent prognostic factor for a poor outcome.142 Furthermore, Gli1 and Shh are independent of the major clinical features known to influence prognosis and could serve as an adjunct to current staging systems. Importantly, no interaction was observed among Gli1, Shh, and adjuvant treatment activity, ensuring that this confounding factor will not affect the relationship between Gli1 and Shh. In resected pancreatic ductal adenocarcinoma (PDAC), the protein abundance of Shh and Gli1 was an independent prognostic factor. Gli1 expression is essential for PDAC cell survival by facilitating the migration and invasion of cells by promoting EMT.143–145 Lower expression of Gli1 or Shh in tumor cells contributed to longer disease free survival (DFS) and overall survival (OS) times. The combination of Shh and Gli1 levels is thought to be the most significant predictor for overall survival of OS.146
Previous studies demonstrated that activation of both the NF-κB and hedgehog signaling pathways played prominent roles in the initiation and progression of pancreatic cancer by acting not only in the cancer cell itself (autocrine) but also in stromal cells (paracrine),11,86,104,147 while the expression of either nuclear or cytoplasmic NF-κB was a poor prognostic factor for pancreatic cancer.148 Nuclear expression of either Gli1 or NF-κB (RelA/p65) is associated with poor overall survival in pancreatic cancer patients, and nuclear expression of NF-κB was the only significant prognostic factor according to multivariate analysis.149
In conclusion, NF-κB expression, MMP9 expression, and Gli1 expression may have prognostic significance in advanced pancreatic cancer and could be used as biomarkers to guide therapies for pancreatic adenocarcinoma in the future.
PROSPECTIVE
Most pancreatic diseases have poor outcomes such as pancreatic fibrosis and carcinoma. As a physiologic and pathologic process, fibrosis in the pancreas has a firm relationship with pancreatic cancer. Due to considerable roles in 2 lethal pathologies, the hedgehog signaling pathway has become a focus of new treatment strategies. With an increased understanding of this pathway in relevant fields, more useful and accurate methods to detect and treat these terrible diseases may improve the 5-year survival rate. Hedgehog signaling transduction and its regulatory factors are the basis of this promising field. Currently, the mechanism underlying the failures in the clinical application of this pathway is being discovered, and these findings will go a long way toward assisting doctors in the development of new therapies.
Footnotes
Abbreviations: DFS = disease free survival, Dhh = desert hedgehog, ECM = extracellular matrix, EDC = epidermal differentiation complex, EMT = epithelial-to-mesenchymal transition, HS = heparan sulfate, Ihh = Indian hedgehog, Ipf1/Pdx1 = insulin promoter factor 1/pancreatic and duodenal homeobox 1, LATS = large tumor suppressor, miRNAs = microRNAs, MMPs = matrix metalloproteinases, OS = overall survival, PARs = protease-activated receptors, PDAC = pancreatic ductal adenocarcinoma, PSCs = pancreatic stellate cells, Shh = Sonic hedgehog, Smo = smoothened, YAP = yes-associated protein
The ethical approval is not necessary for the reasons that this is a review of current knowledge of hedgehog signaling in pancreas and this paper has no relation with animal experiment or patient.
Yongyu Bai and Yongheng Bai contributed equally to this work. Study concept and design: Yongyu Bai, Yongheng Bai, MZ. Drafting of the manuscript: Yongyu Bai, Yongheng Bai, JD, QL, YJ. Study supervision: BC, MZ.
This study was supported by grants from Zhejiang Provincial Natural Science Foundation of China (LY16H050007, LQ12H05001 and No. LY12H03005), Bureau of Chinese Medicine of Zhejiang Province (2011ZA073), China National Natural Science Foundation (No. 81370563), and the Wenzhou Municipal Science and Technology Plan Project (Y20150037).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Nusslein-Volhard C, Wieschaus E. Mutations affecting segment number and polarity in Drosophila. Nature 1980; 287:795–801. [DOI] [PubMed] [Google Scholar]
- 2.Pan YB, Gong Y, Ruan HF, et al. Sonic hedgehog through Gli2 and Gli3 is required for the proper development of placental labyrinth. Cell Death Dis 2015; 6:e1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arimoto A, Tagawa K. Hedgehog expression during development and regeneration in the hemichordate, ptychodera flava. Zoolog Sci 2015; 32:33–37. [DOI] [PubMed] [Google Scholar]
- 4.Maeda Y, Schipani E, Densmore MJ, et al. Partial rescue of postnatal growth plate abnormalities in Ihh mutants by expression of a constitutively active PTH/PTHrP receptor. Bone 2010; 46:472–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feng R, Xiao C, Zavros Y. The role of Sonic Hedgehog as a regulator of gastric function and differentiation. Vitam Horm 2012; 88:473–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013; 63:11–30. [DOI] [PubMed] [Google Scholar]
- 7.Burris HA, 3rd, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 1997; 15:2403–2413. [DOI] [PubMed] [Google Scholar]
- 8.Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 2007; 25:1960–1966. [DOI] [PubMed] [Google Scholar]
- 9.Kayed H, Kleeff J, Osman T, et al. Hedgehog signaling in the normal and diseased pancreas. Pancreas 2006; 32:119–129. [DOI] [PubMed] [Google Scholar]
- 10.Feldmann G, Habbe N, Dhara S, et al. Hedgehog inhibition prolongs survival in a genetically engineered mouse model of pancreatic cancer. Gut 2008; 57:1420–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feldmann G, Dhara S, Fendrich V, et al. Blockade of hedgehog signaling inhibits pancreatic cancer invasion and metastases: a new paradigm for combination therapy in solid cancers. Cancer Res 2007; 67:2187–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joost S, Almada LL, Rohnalter V, et al. GLI1 inhibition promotes epithelial-to-mesenchymal transition in pancreatic cancer cells. Cancer Res 2012; 72:88–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jaster R. Molecular regulation of pancreatic stellate cell function. Mol Cancer 2004; 3:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Menke A, Adler G. TGFbeta-induced fibrogenesis of the pancreas. Int J Gastrointest Cancer 2002; 31:41–46. [DOI] [PubMed] [Google Scholar]
- 15.Apte MV, Wilson JS. Mechanisms of pancreatic fibrosis. Dig Dis 2004; 22:273–279. [DOI] [PubMed] [Google Scholar]
- 16.Apte MV, Haber PS, Applegate TL, et al. Periacinar stellate shaped cells in rat pancreas: identification, isolation, and culture. Gut 1998; 43:128–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bachem MG, Schneider E, Gross H, et al. Identification, culture, and characterization of pancreatic stellate cells in rats and humans. Gastroenterology 1998; 115:421–432. [DOI] [PubMed] [Google Scholar]
- 18.Olive KP, Jacobetz MA, Davidson CJ, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science 2009; 324:1457–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Netti PA, Berk DA, Swartz MA, et al. Role of extracellular matrix assembly in interstitial transport in solid tumors. Cancer Res 2000; 60:2497–2503. [PubMed] [Google Scholar]
- 20.Cordes N, Meineke V. Cell adhesion-mediated radioresistance (CAM-RR). Extracellular matrix-dependent improvement of cell survival in human tumor and normal cells in vitro. Strahlenther Onkol 2003; 179:337–344. [DOI] [PubMed] [Google Scholar]
- 21.Bai Y, Lu H, Wu C, et al. Resveratrol inhibits epithelial-mesenchymal transition and renal fibrosis by antagonizing the hedgehog signaling pathway. Biochem Pharmacol 2014; 92:484–493. [DOI] [PubMed] [Google Scholar]
- 22.Pola R, Ling LE, Silver M, et al. The morphogen Sonic hedgehog is an indirect angiogenic agent upregulating two families of angiogenic growth factors. Nat Med 2001; 7:706–711. [DOI] [PubMed] [Google Scholar]
- 23.Chinchilla P, Xiao L, Kazanietz MG, et al. Hedgehog proteins activate pro-angiogenic responses in endothelial cells through non-canonical signaling pathways. Cell Cycle 2010; 9:570–579. [DOI] [PubMed] [Google Scholar]
- 24.Lavine KJ, Kovacs A, Ornitz DM. Hedgehog signaling is critical for maintenance of the adult coronary vasculature in mice. J Clin Invest 2008; 118:2404–2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brennan D, Chen X, Cheng L, et al. Noncanonical Hedgehog signaling. Vitam Horm 2012; 88:55–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krauss S, Concordet JP, Ingham PW. A functionally conserved homolog of the Drosophila segment polarity gene hh is expressed in tissues with polarizing activity in zebrafish embryos. Cell 1993; 75:1431–1444. [DOI] [PubMed] [Google Scholar]
- 27.Riddle RD, Johnson RL, Laufer E, et al. Sonic hedgehog mediates the polarizing activity of the ZPA. Cell 1993; 75:1401–1416. [DOI] [PubMed] [Google Scholar]
- 28.Roelink H, Augsburger A, Heemskerk J, et al. Floor plate and motor neuron induction by vhh-1, a vertebrate homolog of hedgehog expressed by the notochord. Cell 1994; 76:761–775. [DOI] [PubMed] [Google Scholar]
- 29.St-Jacques B, Dassule HR, Karavanova I, et al. Sonic hedgehog signaling is essential for hair development. Curr Biol 1998; 8:1058–1068. [DOI] [PubMed] [Google Scholar]
- 30.Stone DM, Hynes M, Armanini M, et al. The tumour-suppressor gene patched encodes a candidate receptor for Sonic hedgehog. Nature 1996; 384:129–134. [DOI] [PubMed] [Google Scholar]
- 31.Pathi S, Pagan-Westphal S, Baker DP, et al. Comparative biological responses to human Sonic, Indian, and Desert hedgehog. Mech Dev 2001; 106:107–117. [DOI] [PubMed] [Google Scholar]
- 32.Rohatgi R, Milenkovic L, Scott MP. Patched1 regulates hedgehog signaling at the primary cilium. Science 2007; 317:372–376. [DOI] [PubMed] [Google Scholar]
- 33.Svard J, Heby-Henricson K, Persson-Lek M, et al. Genetic elimination of suppressor of fused reveals an essential repressor function in the mammalian Hedgehog signaling pathway. Dev Cell 2006; 10:187–197. [DOI] [PubMed] [Google Scholar]
- 34.Varjosalo M, Li SP, Taipale J. Divergence of hedgehog signal transduction mechanism between Drosophila and mammals. Dev Cell 2006; 10:177–186. [DOI] [PubMed] [Google Scholar]
- 35.Lum L, Beachy PA. The Hedgehog response network: sensors, switches, and routers. Science 2004; 304:1755–1759. [DOI] [PubMed] [Google Scholar]
- 36.Hooper JE, Scott MP. Communicating with Hedgehogs. Nat Rev Mol Cell Biol 2005; 6:306–317. [DOI] [PubMed] [Google Scholar]
- 37.Pasca di Magliano M, Hebrok M. Hedgehog signalling in cancer formation and maintenance. Nat Rev Cancer 2003; 3:903–911. [DOI] [PubMed] [Google Scholar]
- 38.Gallet A, Rodriguez R, Ruel L, et al. Cholesterol modification of hedgehog is required for trafficking and movement, revealing an asymmetric cellular response to hedgehog. Dev Cell 2003; 4:191–204. [DOI] [PubMed] [Google Scholar]
- 39.Burke R, Nellen D, Bellotto M, et al. Dispatched, a novel sterol-sensing domain protein dedicated to the release of cholesterol-modified hedgehog from signaling cells. Cell 1999; 99:803–815. [DOI] [PubMed] [Google Scholar]
- 40.Ingham PW, Taylor AM, Nakano Y. Role of the Drosophila patched gene in positional signalling. Nature 1991; 353:184–187. [DOI] [PubMed] [Google Scholar]
- 41.Kansal A, Brueton L, Lahiri A, et al. Hypoplastic thumb in Gorlin's syndrome. J Plast Reconstr Aesthet Surg 2007; 60:440–442. [DOI] [PubMed] [Google Scholar]
- 42.Lindstrom E, Shimokawa T, Toftgard R, et al. PTCH mutations: distribution and analyses. Hum Mutat 2006; 27:215–219. [DOI] [PubMed] [Google Scholar]
- 43.Milenkovic L, Goodrich LV, Higgins KM, et al. Mouse patched1 controls body size determination and limb patterning. Development 1999; 126:4431–4440. [DOI] [PubMed] [Google Scholar]
- 44.Fabian SL, Penchev RR, St-Jacques B, et al. Hedgehog-Gli pathway activation during kidney fibrosis. Am J Pathol 2012; 180:1441–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goodrich LV, Milenkovic L, Higgins KM, et al. Altered neural cell fates and medulloblastoma in mouse patched mutants. Science 1997; 277:1109–1113. [DOI] [PubMed] [Google Scholar]
- 46.Riobo NA, Manning DR. Pathways of signal transduction employed by vertebrate Hedgehogs. Biochem J 2007; 403:369–379. [DOI] [PubMed] [Google Scholar]
- 47.King RW. Roughing up Smoothened: chemical modulators of Hedgehog signaling. J Biol 2002; 1:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Malpel S, Claret S, Sanial M, et al. The last 59 amino acids of Smoothened cytoplasmic tail directly bind the protein kinase Fused and negatively regulate the Hedgehog pathway. Dev Biol 2007; 303:121–133. [DOI] [PubMed] [Google Scholar]
- 49.Choy SW, Cheng SH. Hedgehog signaling. Vitam Horm 2012; 88:1–23. [DOI] [PubMed] [Google Scholar]
- 50.Varjosalo M, Taipale J. Hedgehog: functions and mechanisms. Genes Dev 2008; 22:2454–2472. [DOI] [PubMed] [Google Scholar]
- 51.Gill PS, Rosenblum ND. Control of murine kidney development by sonic hedgehog and its GLI effectors. Cell Cycle 2006; 5:1426–1430. [DOI] [PubMed] [Google Scholar]
- 52.Jenkins D. Hedgehog signalling: emerging evidence for non-canonical pathways. Cell Signal 2009; 21:1023–1034. [DOI] [PubMed] [Google Scholar]
- 53.Thibert C, Teillet MA, Lapointe F, et al. Inhibition of neuroepithelial patched-induced apoptosis by sonic hedgehog. Science 2003; 301:843–846. [DOI] [PubMed] [Google Scholar]
- 54.Barnes EA, Kong M, Ollendorff V, et al. Patched1 interacts with cyclin B1 to regulate cell cycle progression. EMBO J 2001; 20:2214–2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Polizio AH, Chinchilla P, Chen X, et al. Heterotrimeric Gi proteins link Hedgehog signaling to activation of Rho small GTPases to promote fibroblast migration. J Biol Chem 2011; 286:19589–19596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bijlsma MF, Borensztajn KS, Roelink H, et al. Sonic Hedgehog induces transcription-independent cytoskeletal rearrangement and migration regulated by arachidonate metabolites. Cell Signal 2007; 19:2596–2604. [DOI] [PubMed] [Google Scholar]
- 57.Yam PT, Langlois SD, Morin S, et al. Sonic Hedgehog guides axons through a noncanonical, Src-family-kinase-dependent signaling pathway. Neuron 2009; 62:349–362. [DOI] [PubMed] [Google Scholar]
- 58.Chang H, Li Q, Moraes RC, et al. Activation of Erk by sonic hedgehog independent of canonical hedgehog signalling. Int J Biochem Cell Biol 2010; 42:1462–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Echelard Y, Epstein DJ, St-Jacques B, et al. Sonic hedgehog, a member of a family of putative signaling molecules, is implicated in the regulation of CNS polarity. Cell 1993; 75:1417–1430. [DOI] [PubMed] [Google Scholar]
- 60.Bitgood MJ, McMahon AP. Hedgehog and Bmp genes are coexpressed at many diverse sites of cell-cell interaction in the mouse embryo. Dev Biol 1995; 172:126–138. [DOI] [PubMed] [Google Scholar]
- 61.Haffen K, Kedinger M, Simon-Assmann P. Mesenchyme-dependent differentiation of epithelial progenitor cells in the gut. J Pediatric Gastroenterol Nutr 1987; 6:14–23. [DOI] [PubMed] [Google Scholar]
- 62.Golosow N, Grobstein C. Epitheliomesenchymal interaction in pancreatic morphogenesis. Dev Biol 1962; 4:242–255. [DOI] [PubMed] [Google Scholar]
- 63.Wessells NK, Cohen JH. Early pancreas organogenesis: morphogenesis, tissue interactions, mass effects. Dev Biol 1967; 15:237–270. [DOI] [PubMed] [Google Scholar]
- 64.Le Douarin NM. On the origin of pancreatic endocrine cells. Cell 1988; 53:169–171. [DOI] [PubMed] [Google Scholar]
- 65.Li H, Arber S, Jessell TM, et al. Selective agenesis of the dorsal pancreas in mice lacking homeobox gene Hlxb9. Nat Genet 1999; 23:67–70. [DOI] [PubMed] [Google Scholar]
- 66.Ohlsson H, Karlsson K, Edlund T. IPF1, a homeodomain-containing transactivator of the insulin gene. EMBO J 1993; 12:4251–4259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jonsson J, Carlsson L, Edlund T, et al. Insulin-promoter-factor 1 is required for pancreas development in mice. Nature 1994; 371:606–609. [DOI] [PubMed] [Google Scholar]
- 68.Ahlgren U, Jonsson J, Edlund H. The morphogenesis of the pancreatic mesenchyme is uncoupled from that of the pancreatic epithelium in IPF1/PDX1-deficient mice. Development 1996; 122:1409–1416. [DOI] [PubMed] [Google Scholar]
- 69.Offield MF, Jetton TL, Labosky PA, et al. PDX-1 is required for pancreatic outgrowth and differentiation of the rostral duodenum. Development 1996; 122:983–995. [DOI] [PubMed] [Google Scholar]
- 70.Apelqvist A, Ahlgren U, Edlund H. Sonic hedgehog directs specialised mesoderm differentiation in the intestine and pancreas. Curr Biol 1997; 7:801–804. [DOI] [PubMed] [Google Scholar]
- 71.van den Brink GR, Hardwick JC, Tytgat GN, et al. Sonic hedgehog regulates gastric gland morphogenesis in man and mouse. Gastroenterology 2001; 121:317–328. [DOI] [PubMed] [Google Scholar]
- 72.diIorio PJ, Moss JB, Sbrogna JL, et al. Sonic hedgehog is required early in pancreatic islet development. Dev Biol 2002; 244:75–84. [DOI] [PubMed] [Google Scholar]
- 73.Lau J, Hebrok M. Hedgehog signaling in pancreas epithelium regulates embryonic organ formation and adult beta-cell function. Diabetes 2010; 59:1211–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Thomas MK, Rastalsky N, Lee JH, et al. Hedgehog signaling regulation of insulin production by pancreatic beta-cells. Diabetes 2000; 49:2039–2047. [DOI] [PubMed] [Google Scholar]
- 75.Kawahira H, Scheel DW, Smith SB, et al. Hedgehog signaling regulates expansion of pancreatic epithelial cells. Dev Biol 2005; 280:111–121. [DOI] [PubMed] [Google Scholar]
- 76.Mfopou JK, Bouwens L. Hedgehog signals in pancreatic differentiation from embryonic stem cells: revisiting the neglected. Differentiation 2008; 76:107–117. [DOI] [PubMed] [Google Scholar]
- 77.Landsman L, Parent A, Hebrok M. Elevated Hedgehog/Gli signaling causes beta-cell dedifferentiation in mice. Proc Natl Acad Sci U S A 2011; 108:17010–17015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cervantes S, Lau J, Cano DA, et al. Primary cilia regulate Gli/Hedgehog activation in pancreas. Proc Natl Acad Sci U S A 2010; 107:10109–10114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Masamune A, Watanabe T, Kikuta K, et al. Roles of pancreatic stellate cells in pancreatic inflammation and fibrosis. Clin Gastroenterol Hepatol 2009; 7 (11 Suppl):S48–54. [DOI] [PubMed] [Google Scholar]
- 80.Omary MB, Lugea A, Lowe AW, et al. The pancreatic stellate cell: a star on the rise in pancreatic diseases. J Clin Invest 2007; 117:50–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shinozaki S, Ohnishi H, Hama K, et al. Indian hedgehog promotes the migration of rat activated pancreatic stellate cells by increasing membrane type-1 matrix metalloproteinase on the plasma membrane. J Cell Physiol 2008; 216:38–46. [DOI] [PubMed] [Google Scholar]
- 82.Sicklick JK, Li YX, Choi SS, et al. Role for hedgehog signaling in hepatic stellate cell activation and viability. Lab Invest 2005; 85:1368–1380. [DOI] [PubMed] [Google Scholar]
- 83.Bailey JM, Swanson BJ, Hamada T, et al. Sonic hedgehog promotes desmoplasia in pancreatic cancer. Clin Cancer Res 2008; 14:5995–6004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li X, Wang Z, Ma Q, et al. Sonic hedgehog paracrine signaling activates stromal cells to promote perineural invasion in pancreatic cancer. Clin Cancer Res 2014; 20:4326–4338. [DOI] [PubMed] [Google Scholar]
- 85.Tian H, Callahan CA, DuPree KJ, et al. Hedgehog signaling is restricted to the stromal compartment during pancreatic carcinogenesis. Proc Natl Acad Sci U S A 2009; 106:4254–4259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nolan-Stevaux O, Lau J, Truitt ML, et al. GLI1 is regulated through Smoothened-independent mechanisms in neoplastic pancreatic ducts and mediates PDAC cell survival and transformation. Genes Dev 2009; 23:24–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hwang RF, Moore TT, Hattersley MM, et al. Inhibition of the hedgehog pathway targets the tumor-associated stroma in pancreatic cancer. Mol Cancer Res 2012; 10:1147–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Casini A, Galli A, Pignalosa P, et al. Collagen type I synthesized by pancreatic periacinar stellate cells (PSC) co-localizes with lipid peroxidation-derived aldehydes in chronic alcoholic pancreatitis. J Pathol 2000; 192:81–89. [DOI] [PubMed] [Google Scholar]
- 89.Schneider E, Schmid-Kotsas A, Zhao J, et al. Identification of mediators stimulating proliferation and matrix synthesis of rat pancreatic stellate cells. Am J Physiol Cell Physiol 2001; 281:C532–543. [DOI] [PubMed] [Google Scholar]
- 90.Apte MV, Haber PS, Darby SJ, et al. Pancreatic stellate cells are activated by proinflammatory cytokines: implications for pancreatic fibrogenesis. Gut 1999; 44:534–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hu L, Lin X, Lu H, et al. An overview of hedgehog signaling in fibrosis. Mol Pharmacol 2015; 87:174–182. [DOI] [PubMed] [Google Scholar]
- 92.Jung IH, Jung DE, Park YN, et al. Aberrant Hedgehog ligands induce progressive pancreatic fibrosis by paracrine activation of myofibroblasts and ductular cells in transgenic zebrafish. PLoS One 2011; 6:e27941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang LW, Lin H, Lu Y, et al. Sonic hedgehog expression in a rat model of chronic pancreatitis. World J Gastroenterol 2014; 20:4712–4717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kayed H, Kleeff J, Keleg S, et al. Distribution of Indian hedgehog and its receptors patched and smoothened in human chronic pancreatitis. J Endocrinol 2003; 178:467–478. [DOI] [PubMed] [Google Scholar]
- 95.Walter K, Omura N, Hong SM, et al. Overexpression of smoothened activates the sonic hedgehog signaling pathway in pancreatic cancer-associated fibroblasts. Clin Cancer Res 2010; 16:1781–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Allen BL, Tenzen T, McMahon AP. The Hedgehog-binding proteins Gas1 and Cdo cooperate to positively regulate Shh signaling during mouse development. Genes Dev 2007; 21:1244–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Martinelli DC, Fan CM. Gas1 extends the range of Hedgehog action by facilitating its signaling. Genes Dev 2007; 21:1231–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tenzen T, Allen BL, Cole F, et al. The cell surface membrane proteins Cdo and Boc are components and targets of the Hedgehog signaling pathway and feedback network in mice. Dev Cell 2006; 10:647–656. [DOI] [PubMed] [Google Scholar]
- 99.Allen BL, Song JY, Izzi L, et al. Overlapping roles and collective requirement for the coreceptors GAS1, CDO, and BOC in SHH pathway function. Devel Cell 2011; 20:775–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mathew E, Zhang Y, Holtz AM, et al. Dosage-dependent regulation of pancreatic cancer growth and angiogenesis by hedgehog signaling. Cell Rep 2014; 9:484–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Unden AB, Holmberg E, Lundh-Rozell B, et al. Mutations in the human homologue of Drosophila patched (PTCH) in basal cell carcinomas and the Gorlin syndrome: different in vivo mechanisms of PTCH inactivation. Cancer Res 1996; 56:4562–4565. [PubMed] [Google Scholar]
- 102.Hezel AF, Kimmelman AC, Stanger BZ, et al. Genetics and biology of pancreatic ductal adenocarcinoma. Genes Dev 2006; 20:1218–1249. [DOI] [PubMed] [Google Scholar]
- 103.Taipale J, Beachy PA. The Hedgehog and Wnt signalling pathways in cancer. Nature 2001; 411:349–354. [DOI] [PubMed] [Google Scholar]
- 104.Thayer SP, di Magliano MP, Heiser PW, et al. Hedgehog is an early and late mediator of pancreatic cancer tumorigenesis. Nature 2003; 425:851–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Berman DM, Karhadkar SS, Maitra A, et al. Widespread requirement for Hedgehog ligand stimulation in growth of digestive tract tumours. Nature 2003; 425:846–851. [DOI] [PubMed] [Google Scholar]
- 106.Marchesi F, Piemonti L, Mantovani A, et al. Molecular mechanisms of perineural invasion, a forgotten pathway of dissemination and metastasis. Cytokine Growth Factor Rev 2010; 21:77–82. [DOI] [PubMed] [Google Scholar]
- 107.Ceyhan GO, Demir IE, Rauch U, et al. Pancreatic neuropathy results in “neural remodeling” and altered pancreatic innervation in chronic pancreatitis and pancreatic cancer. Am J Gastroenterol 2009; 104:2555–2565. [DOI] [PubMed] [Google Scholar]
- 108.Cornell RJ, Rowley D, Wheeler T, et al. Neuroepithelial interactions in prostate cancer are enhanced in the presence of prostatic stroma. Urology 2003; 61:870–875. [DOI] [PubMed] [Google Scholar]
- 109.Okada Y, Eibl G, Duffy JP, et al. Glial cell-derived neurotrophic factor upregulates the expression and activation of matrix metalloproteinase-9 in human pancreatic cancer. Surgery 2003; 134:293–299. [DOI] [PubMed] [Google Scholar]
- 110.Ortmann C, Pickhinke U, Exner S, et al. Sonic hedgehog processing and release are regulated by glypican heparan sulfate proteoglycans. J Cell Sci 2015; 128:2374–2385. [DOI] [PubMed] [Google Scholar]
- 111.Camargo FD, Gokhale S, Johnnidis JB, et al. YAP1 increases organ size and expands undifferentiated progenitor cells. Curr Biol 2007; 17:2054–2060. [DOI] [PubMed] [Google Scholar]
- 112.Zhao B, Tumaneng K, Guan KL. The Hippo pathway in organ size control, tissue regeneration and stem cell self-renewal. Nat Cell Biol 2011; 13:877–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhao B, Wei X, Li W, et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev 2007; 21:2747–2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yu FX, Guan KL. The Hippo pathway: regulators and regulations. Genes Dev 2013; 27:355–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Taipale J, Chen JK, Cooper MK, et al. Effects of oncogenic mutations in Smoothened and Patched can be reversed by cyclopamine. Nature 2000; 406:1005–1009. [DOI] [PubMed] [Google Scholar]
- 116.Lauth M, Bergstrom A, Shimokawa T, et al. Inhibition of GLI-mediated transcription and tumor cell growth by small-molecule antagonists. Proc Natl Acad Sci U S A 2007; 104:8455–8460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kimura H, Stephen D, Joyner A, et al. Gli1 is important for medulloblastoma formation in Ptc1+/- mice. Oncogene 2005; 24:4026–4036. [DOI] [PubMed] [Google Scholar]
- 118.Tariki M, Dhanyamraju PK, Fendrich V, et al. The Yes-associated protein controls the cell density regulation of Hedgehog signaling. Oncogenesis 2014; 3:e112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sahai V, Kumar K, Knab LM, et al. BET bromodomain inhibitors block growth of pancreatic cancer cells in three-dimensional collagen. Mol Cancer Ther 2014; 13:1907–1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wang YH, Sui YN, Yan K, et al. BRD4 promotes pancreatic ductal adenocarcinoma cell proliferation and enhances gemcitabine resistance. Oncol Rep 2015; 33:1699–1706. [DOI] [PubMed] [Google Scholar]
- 121.Mills LD, Zhang Y, Marler RJ, et al. Loss of the transcription factor GLI1 identifies a signaling network in the tumor microenvironment mediating KRAS oncogene-induced transformation. J Biol Chem 2013; 288:11786–11794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mathew E, Collins MA, Fernandez-Barrena MG, et al. The transcription factor GLI1 modulates the inflammatory response during pancreatic tissue remodeling. J Biol Chem 2014; 289:27727–27743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer 2006; 6:857–866. [DOI] [PubMed] [Google Scholar]
- 124.Ferretti E, De Smaele E, Miele E, et al. Concerted microRNA control of Hedgehog signalling in cerebellar neuronal progenitor and tumour cells. EMBO J 2008; 27:2616–2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Huang HJ, Liu J, Hua H, et al. MiR-214 and N-ras regulatory loop suppresses rhabdomyosarcoma cell growth and xenograft tumorigenesis. Oncotarget 2014; 5:2161–2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Argenti B, Gallo R, Di Marcotullio L, et al. Hedgehog antagonist REN(KCTD11) regulates proliferation and apoptosis of developing granule cell progenitors. J Neurosci 2005; 25:8338–8346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Briggs KJ, Corcoran-Schwartz IM, Zhang W, et al. Cooperation between the Hic1 and Ptch1 tumor suppressors in medulloblastoma. Genes Dev 2008; 22:770–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Johnson SM, Grosshans H, Shingara J, et al. RAS is regulated by the let-7 microRNA family. Cell 2005; 120:635–647. [DOI] [PubMed] [Google Scholar]
- 129.Trang P, Medina PP, Wiggins JF, et al. Regression of murine lung tumors by the let-7 microRNA. Oncogene 2010; 29:1580–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Patel K, Kollory A, Takashima A, et al. MicroRNA let-7 downregulates STAT3 phosphorylation in pancreatic cancer cells by increasing SOCS3 expression. Cancer Lett 2014; 347:54–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Li Y, VandenBoom TG, 2nd, Kong D, et al. Up-regulation of miR-200 and let-7 by natural agents leads to the reversal of epithelial-to-mesenchymal transition in gemcitabine-resistant pancreatic cancer cells. Cancer Res 2009; 69:6704–6712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Singh S, Chitkara D, Kumar V, et al. Mahato RI. miRNA profiling in pancreatic cancer and restoration of chemosensitivity. Cancer Lett 2013; 334:211–220. [DOI] [PubMed] [Google Scholar]
- 133.Kumar V, Mondal G, Slavik P, et al. Codelivery of small molecule hedgehog inhibitor and miRNA for treating pancreatic cancer. Mol Pharm 2015; 12:1289–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Park JK, Henry JC, Jiang J, et al. miR-132 and miR-212 are increased in pancreatic cancer and target the retinoblastoma tumor suppressor. Biochem Biophys Res Commun 2011; 406:518–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Li Y, Zhang D, Chen C, et al. MicroRNA-212 displays tumor-promoting properties in non-small cell lung cancer cells and targets the hedgehog pathway receptor PTCH1. Mol Biol Cell 2012; 23:1423–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ma C, Nong K, Wu B, et al. miR-212 promotes pancreatic cancer cell growth and invasion by targeting the hedgehog signaling pathway receptor patched-1. J Exp Clin Cancer Res 2014; 33:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Henry J, Toulza E, Hsu CY, et al. Update on the epidermal differentiation complex. Front Biosci 2012; 17:1517–1532. [DOI] [PubMed] [Google Scholar]
- 138.Xu X, Su B, Xie C, et al. Sonic hedgehog-Gli1 signaling pathway regulates the epithelial mesenchymal transition (EMT) by mediating a new target gene, S100A4, in pancreatic cancer cells. PLoS One 2014; 9:e96441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lee JJ, Perera RM, Wang H, et al. Stromal response to Hedgehog signaling restrains pancreatic cancer progression. Proc Natl Acad Sci U S A 2014; 111:E3091–3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.El-Zaatari M, Daignault S, Tessier A, et al. Plasma Shh levels reduced in pancreatic cancer patients. Pancreas 2012; 41:1019–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Liu Q, Sheng W, Dong M, et al. Gli1 promotes transforming growth factor-beta1- and epidermal growth factor-induced epithelial to mesenchymal transition in pancreatic cancer cells. Surgery 2015; 158:211–224. [DOI] [PubMed] [Google Scholar]
- 142.Yoshikawa R, Nakano Y, Tao L, et al. Hedgehog signal activation in oesophageal cancer patients undergoing neoadjuvant chemoradiotherapy. Br J Cancer 2008; 98:1670–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Dennler S, Andre J, Alexaki I, et al. Induction of sonic hedgehog mediators by transforming growth factor-beta: Smad3-dependent activation of Gli2 and Gli1 expression in vitro and in vivo. Cancer Res 2007; 67:6981–6986. [DOI] [PubMed] [Google Scholar]
- 144.Inaguma S, Kasai K, Ikeda H. GLI1 facilitates the migration and invasion of pancreatic cancer cells through MUC5AC-mediated attenuation of E-cadherin. Oncogene 2011; 30:714–723. [DOI] [PubMed] [Google Scholar]
- 145.Rajurkar M, De Jesus-Monge WE, Driscoll DR, et al. The activity of Gli transcription factors is essential for Kras-induced pancreatic tumorigenesis. Proc Natl Acad Sci U S A 2012; 109:E1038–E1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Marechal R, Bachet JB, Calomme A, et al. Sonic hedgehog and Gli1 expression predict outcome in resected pancreatic adenocarcinoma. Clin Cancer Res 2015; 21:1215–1224. [DOI] [PubMed] [Google Scholar]
- 147.Yamasaki A, Kameda C, Xu R, et al. Nuclear factor kappaB-activated monocytes contribute to pancreatic cancer progression through the production of Shh. Cancer Immunol Immunother 2010; 59:675–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Weichert W, Boehm M, Gekeler V, et al. High expression of RelA/p65 is associated with activation of nuclear factor-kappaB-dependent signaling in pancreatic cancer and marks a patient population with poor prognosis. Br J Cancer 2007; 97:523–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Yang SH, Hsu CH, Lee JC, et al. Nuclear expression of glioma-associated oncogene homolog 1 and nuclear factor-kappaB is associated with a poor prognosis of pancreatic cancer. Oncology 2013; 85:86–94. [DOI] [PubMed] [Google Scholar]


