Abstract
Several studies have shown coronary artery bypass surgery (CABG) to be beneficial in patients with type 2 diabetes mellitus (T2DM) and multivessel coronary artery diseases. Patients with insulin-treated T2DM (ITDM) are usually patients with poor glycemic control and are expected to suffer more complications compared with patients with non-insulin-treated T2DM (NITDM). However, the adverse clinical outcomes in patients with ITDM and NITDM after CABG are still not very clear. Hence, to solve this issue, we aim to compare the short-and long-term adverse clinical outcomes in a larger number of patients with ITDM and NITDM after CABG, respectively.
Randomized controlled trials and observational studies comparing the adverse clinical outcomes such as mortality, major adverse events (MAEs), stroke, myocardial infarction, and repeated revascularization in patients with ITDM and NITDM after CABG have been searched from Medline, EMBASE, Cochrane, and PubMed databases. A short-term follow-up (≤30 days) and a long-term follow-up (≥1 year) were considered. Odds ratio (OR) with 95% confidence interval (CI) was used to express the pooled effect on discontinuous variables and the pooled analyses were performed with RevMan 5.3.
Eleven studies involving a total of 64,152 patients with T2DM (23,781 patients with ITDM and 40,371 patients with NITDM) have been included in this meta-analysis. During the short-term follow-up period, patients with ITDM had a significantly higher mortality (OR: 1.47; 95% CI: 1.33–1.61, P < 0.00001) and MAEs (OR: 1.66; 95% CI: 1.48–1.87, P < 0.00001). During the long-term follow-up period, patients with ITDM still had a significantly higher rate of mortality, MAEs, and stroke (OR: 1.23, 95% CI: 1.02–1.49, P = 0.03; OR: 1.50, 95% CI: 1.07–2.12, P = 0.02; OR: 1.39, 95% CI: 1.22–1.59, P < 0.00001, respectively) after CABG. However, our results showed similar repeated revascularization rate between the ITDM and NITDM groups after CABG (OR: 1.31, 95% CI: 0.81–2.12, P = 0.27).
According to this study, patients with ITDM had a significantly higher rate of mortality and MAEs compared with patients with NITDM after CABG. Stroke was also significantly higher in patients with ITDM during a long-term follow-up period. However, since the result for the long-term mortality had a higher heterogeneity as compared with the other subgroups, and because a similar revascularization rate was observed between the ITDM and NITDM groups after CABG maybe because of a limited number of patients analyzed, further studies still need to be conducted to completely solve this issue.
INTRODUCTION
Several studies have shown coronary artery bypass surgery (CABG) to be the most suitable revascularization procedure in patients with type 2 diabetes mellitus (T2DM) especially for those diabetic patients who suffer from multivessel coronary artery diseases.1,2 CABG has proved to be better compared with percutaneous coronary intervention (PCI) in these patients with T2DM.3 Patients with T2DM are either on diet control, oral hypoglycemic agents (OHAs) or/and on insulin therapy.
Patients with insulin-treated T2DM (ITDM) are expected to be associated with higher comorbidities and a longer duration of diabetic status compared with patients with non-insulin-treated T2DM (NITDM). Normally, insulin treatment is often reserved for patients with complications because of T2DM and in those patients whose blood glucose level cannot be controlled by OHA.
Recently, Bundhun et al4 who compared ITDM with NITDM showed adverse clinical outcomes to be significantly higher in patients with ITDM compared with diabetic patients without insulin therapy after PCI. But however, the outcomes and prognosis in similar patients after CABG are still not very clear.
The Emory Angioplasty versus Surgery Trial (EAST) reported no benefits of CABG in patients with T2DM at 3 years,5 whereas the Coronary Artery Bypass Revascularization Investigation (CABRI) found a benefit of CABG in similar patients.6 The FREEDOM trial, which compared CABG and PCI in patients with ITDM and NITDM found CABG to be beneficial compared with PCI in patients with NITDM; however, its results could not provide enough evidence to show any beneficial effect of CABG in the ITDM group because the study showed no interaction between treatment and T2DM type with respect to the composite reported outcomes and this may have been because a shortage of patients.7 Hence, to solve this issue, we aim to compare the short- and long-term adverse clinical outcomes in a larger number of patients with ITDM and NITDM after CABG, respectively.
METHODS
Data Source and Search Strategy
Medline, EMBASE, Cochrane, and PubMed databases as well as official websites of well-known and most suitable journals such as the New England Journal of Medicine (NEJM), Journal of American College of Cardiology (JACC), Circulation (AHA), and Cardiovascular Diabetology were searched for randomized controlled trials (RCTs) and observational studies by typing the words or phrases “diabetes mellitus and coronary artery bypass surgery/CABG” or “insulin-treated and non-insulin treated diabetes mellitus and CABG.” References of relevant studies were also reviewed for articles suitable for our meta-analysis. No language restriction was applied.
Inclusion and Exclusion criteria
Studies were included if:
They were either RCTs or observational studies.
They compared CABG in patients with ITDM and NITDM.
They reported adverse clinical outcomes as their endpoints in these patients with T2DM.
Studies were excluded if:
They were case studies, meta-analyses, review articles, and letter to editors.
They compared CABG in patients with T2DM and non-T2DM without further subdividing patients with T2DM into patients with ITDM and NITDM.
They did not report adverse clinical outcomes as their clinical endpoints.
They were duplicates.
Defining terms, outcomes and follow up periods
ITDM is defined as patients with T2DM who required insulin therapy with or without OHA as treatment.
NITDM is defined as patients with T2DM who were on diet control or on OHA, but without insulin therapy.
CABG is defined as an open heart surgery normally indicated in severe coronary artery disease or multivessel coronary diseases especially in patients with T2DM or in conditions whereby PCI was contraindicated.
Adverse clinical outcomes included are as follows:
Mortality: including all-cause death (both cardiac and non-cardiac death)
Myocardial infarction (MI)
Repeated revascularization
Stroke: including cerebrovascular accidents (CVA)
Major adverse events (MAEs) including major adverse cardiovascular events (MACEs) and major adverse cardiovascular and cerebrovascular events (MACCEs), which, as a whole, comprised of all-cause death, MI, CVA, and repeated revascularization.
Follow-up periods included:
a short-term follow-up period, which was defined as a follow up-period of ≤30 days,
a long-term follow-up period, which was defined as a follow-up period of ≥1 year.
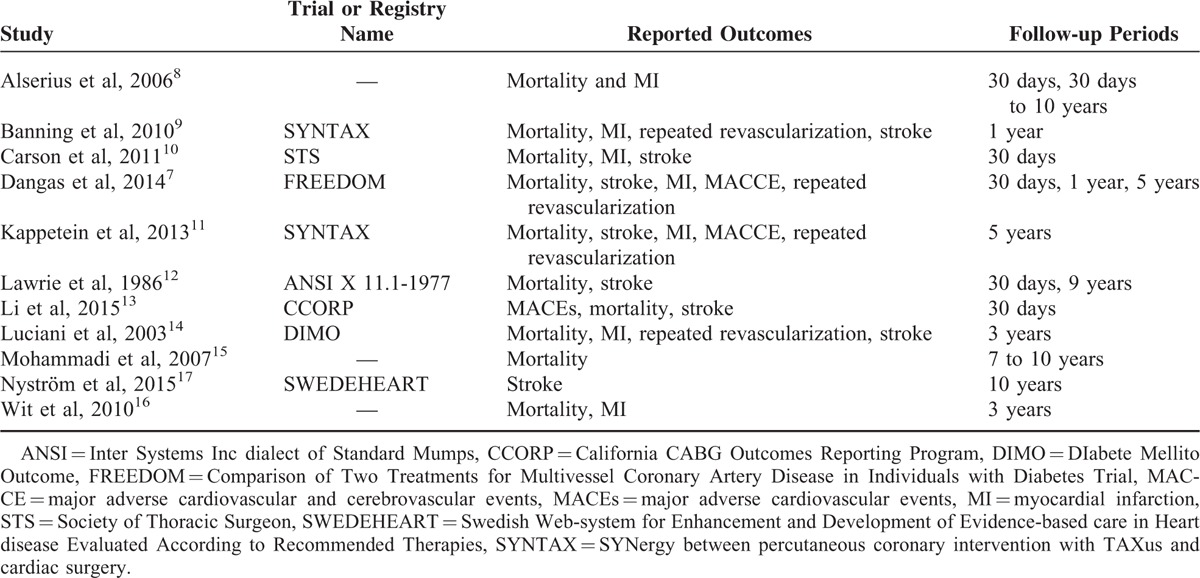
The adverse clinical outcomes reported in all the included studies as well as the follow-up periods have been listed in Table 1.
TABLE 1.
Reported Outcomes in Each Included Study
Data Extraction and Quality Assessment
Three authors (KM, PKB, and HQ) independently reviewed the data and assessed the eligibility and methodological quality as well as the type of each eligible study. Information regarding the year of publication, author names, number of patients with ITDM and NITDM, patient characteristics, the reported clinical outcomes as well as information concerning the follow-up periods was systematically extracted. If the authors disagreed about certain studies or data, or could not decide whether to accept or reject a study, disagreements were discussed among the authors, and if the authors could not reach a consensus, disagreements were resolved by the fourth author (ZT). The bias risk of trials was assessed with the components recommended by the Cochrane Collaboration.18 Our meta-analysis included RCTs and observational studies. The RCTs have been carefully assessed and a score ranging from 0 to 12 points has been allocated to the trials depending on whether they satisfied all the 6 components recommended by the Cochrane Collaboration. A score of 1 was allocated for unclear bias. Low risk of bias was allocated a score of 2 in each of these 6 components, whereas a score of 0 was given if this evaluation showed a high risk of bias. Therefore, if a trial showed “low risk bias” in all the 6 components, a total score of 2 × 6 = 12 would be given.
Statistical Analysis
Owing to the presence of RCTs along with observational studies in this meta-analysis, recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement (PRISMA) were used during the study selection.19 Heterogeneity across trials was assessed using the Cochrane Q-statistic whereby P ≤ 0.05 was considered statistically significant, and also using the I2-statistic whereby I2 described the percentage of total variation across studies. No heterogeneity was indicated by an I2 value of 0%. Larger values of I2 indicated increased heterogeneity. A fixed-effect model was used if I2 was <50%. However, if I2 was >50%, a random-effect model was used. Publication bias, which was also taken into consideration in our study, was visually estimated by assessing the funnel plots. We calculated odd ratios (ORs) and 95% confidence intervals (CIs) for categorical variables and the pooled analyses of data from our included studies were performed with RevMan 5.3 software.
Ethical approval was not necessary, as this study is a systematic review and meta-analysis.
RESULTS
Study Selection
A total of 4265 articles have been obtained from Medline, EMBASE, Cochrane, and PubMed databases. When official websites of selected journals mentioned above were searched for relevant studies, another 12 articles, which could possibly be suitable for our meta-analysis, were obtained. In addition, 24 suitable articles were obtained from the reference lists. After a careful check, 476 duplicates have been eliminated. Among the remaining 3825 articles, another 3748 articles were eliminated because they were not related to our topic/meta-analysis. Seventy-seven full-text articles were assessed for eligibility. Sixty-six articles were further eliminated because they were either meta-analyses, case studies, or letter to editors, adverse clinical outcomes were not among their clinical endpoints, or patients with T2DM were not further classified into patients with ITDM and NITDM. Finally, 11 studies, which satisfied our inclusion criteria, were selected for this meta-analysis. The flow diagram for the study selection has been represented in Figure 1.
FIGURE 1.
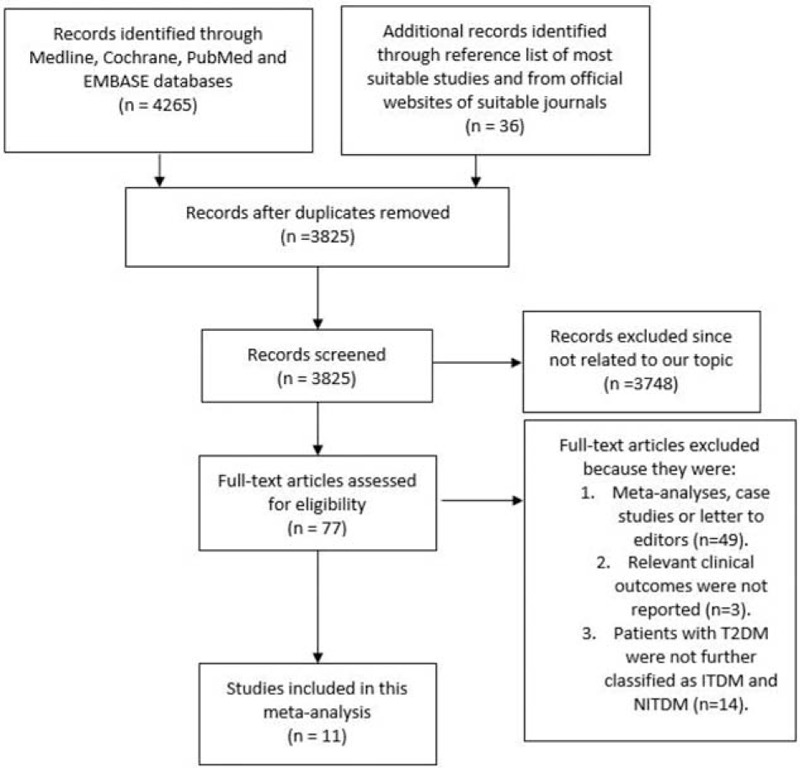
Flow diagram for the study selection.
General and Baseline Features
A total of 64,152 patients with T2DM involving 23,781 patients with ITDM and 40,371 patients with NITDM were included in this analysis. The total number of patients included and the types of study have been listed in Table 2.
TABLE 2.
The Types of Study and Number of Patients Included
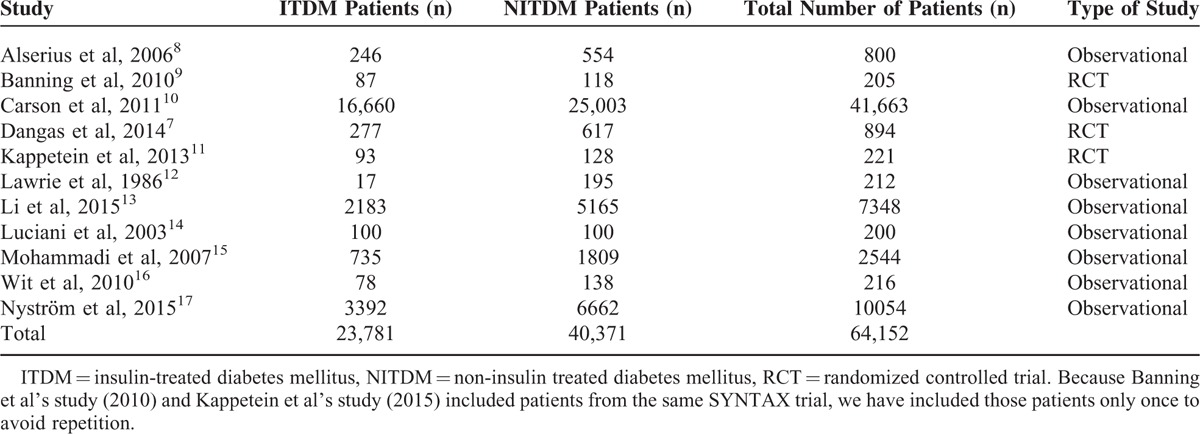
Because Banning et al's study (2010) and Kappetein et al's study (2013) included patients from the same SYNTAX trial, we have included those patients only once to avoid repetition.
The baseline features of these included studies have been shown in Tables 3 and 4.
TABLE 3.
Baseline Characteristics of the Included Studies (Part 1)
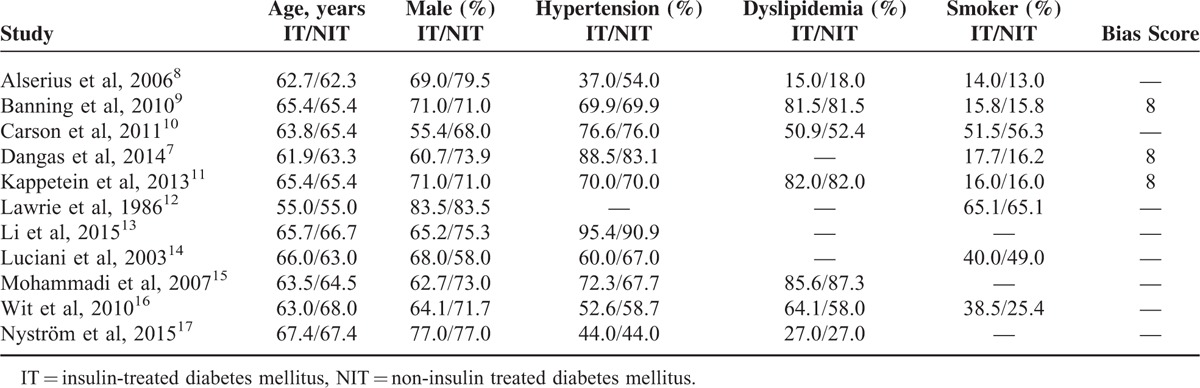
TABLE 4.
Baseline Features of the Included Studies (Part 2)
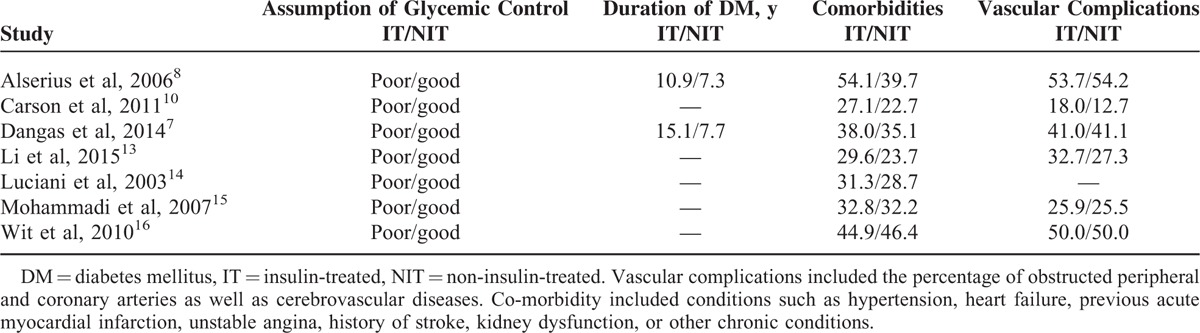
Patients in the ITDM and NITDM groups were almost similar in age. The percentage of males was higher compared with females in both groups. Moreover, the risk factors such as hypertension, dyslipidemia, and smoking were almost similar between the ITDM and NITDM groups. Overall, there were no significant differences in baseline features among patients with T2DM treated with insulin therapy and those not treated with insulin therapy except for comorbidities and length of duration of T2DM, which were higher in ITDM compared to NITDM as shown in Table 4.
Results of This Meta-analysis
The results of this meta-analysis have been represented in Table 5.
TABLE 5.
Results of this Meta-analysis
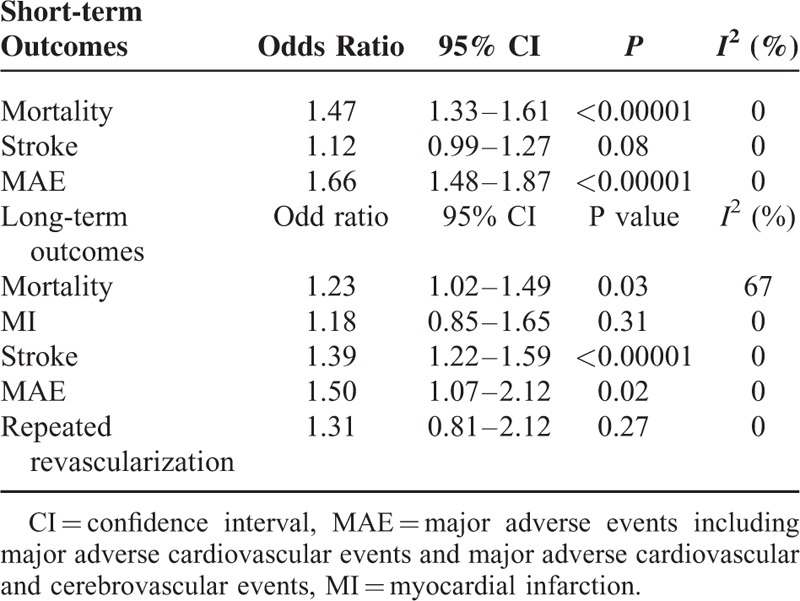
Our results showed that during a short-term follow up-period after CABG, mortality was significantly higher in the ITDM group (OR: 1.47, 95% CI: 1.33–1.61, P < 0.00001). MAE also significantly favored NITDM (OR: 1.66, 95% CI: 1.48–1.87, P < 0.00001). However, the short-term stroke was not significantly different between the ITDM and NITDM groups (OR: 1.12, 95% CI: 0.99–1.27, P = 0.08). The short-term (≤30 days) clinical outcomes between ITDM and NITDM after CABG have been illustrated in Figure 2.
FIGURE 2.
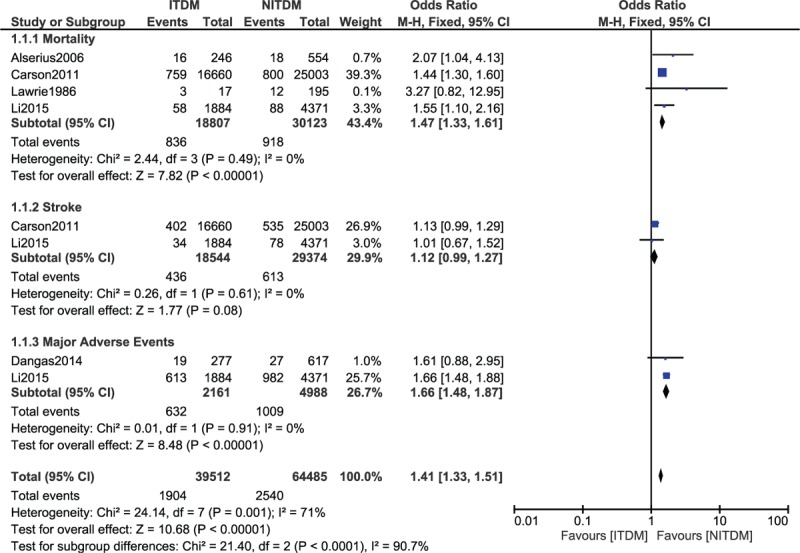
Forest plot comparing the short term adverse clinical outcomes between insulin-treated diabetes mellitus (ITDM) and non-insulin-treated diabetes mellitus (NITDM) after coronary artery bypass surgery.
During the long-term (≥1 year) follow-up period, ITDM was associated with a significantly higher mortality (OR: 1.23, 95% CI: 1.09–1.42, P = 0.03), and a significantly higher rate of MAE (OR: 1.50, 95% CI: 1.07–2.12, P = 0.02). Long-term stroke also significantly favored the NITDM after CABG (OR: 1.39, 95% CI: 1.22–1.59, P < 0.00001). However, our results showed similar MI and repeated revascularization rates between the ITDM and NITDM groups (OR: 1.18, 95% CI: 0.85–1.65, P = 0.31 and OR: 1.31, 95% CI 0.81–2.12, P = 0.27, respectively) during this long-term follow-up period. The long-term clinical outcomes between ITDM and NITDM after CABG have been illustrated in Figure 3.
FIGURE 3.
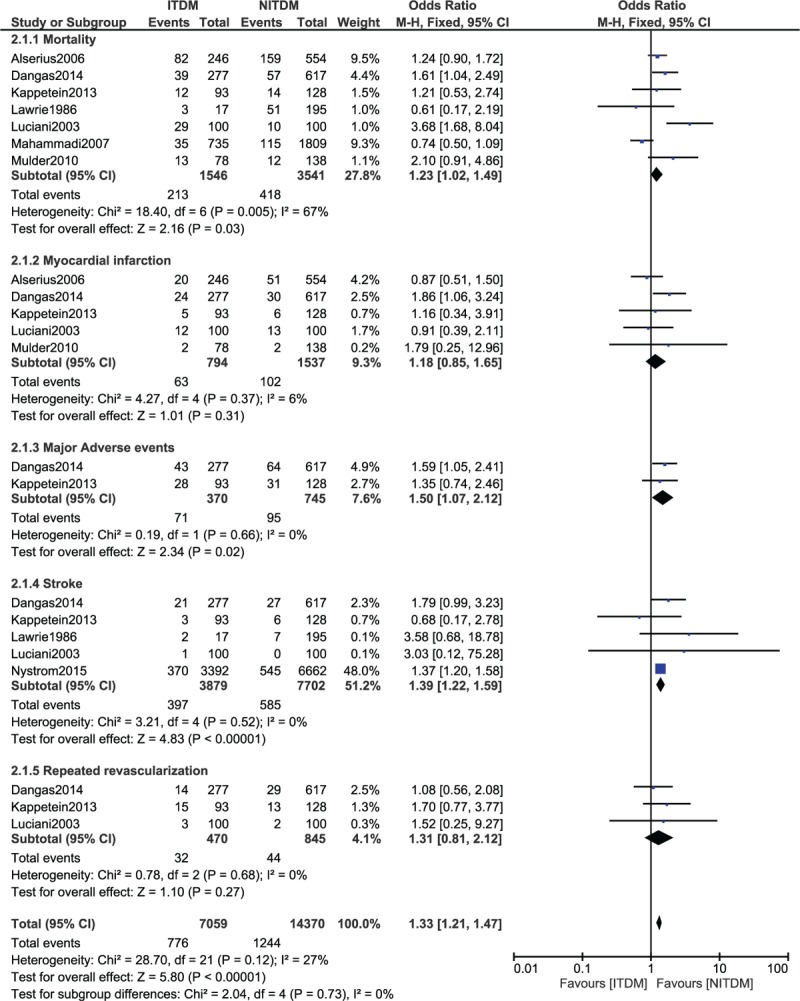
Forest plot comparing the long-term adverse clinical outcomes between insulin-treated diabetes mellitus (ITDM) and non-insulin-treated diabetes mellitus (NITDM) after coronary artery bypass surgery.
For all of the above analyses, sensitivity analyses yielded consistent results. Based on a visual inspection of the funnel plots, there has been no evidence of publication bias for the included studies that assessed all clinical endpoints in patients with ITDM and NITDM after CABG. The funnel plots have been illustrated in Figures 4 A and B.
FIGURE 4.
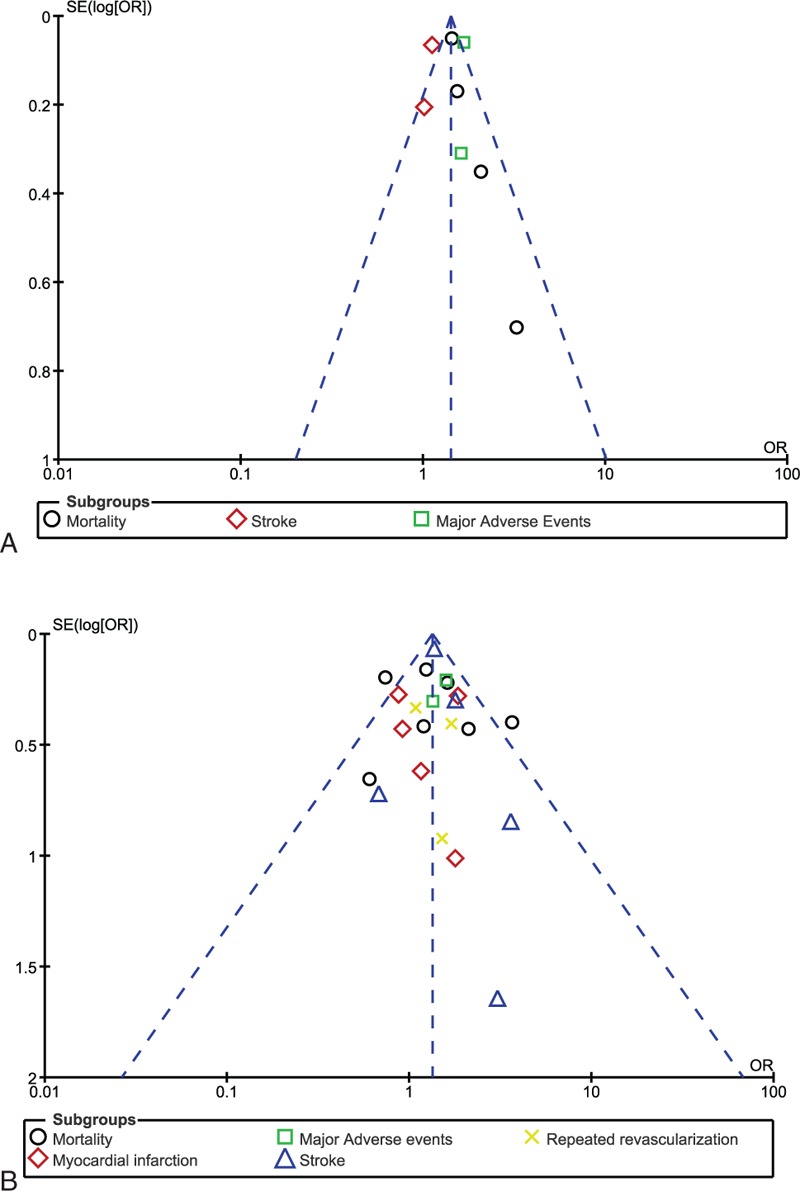
Funnel plots showing sensitivity analysis.
DISCUSSION
Recently, Bundhun et al4 showed adverse clinical outcomes in patients with ITDM to be significantly higher compared to patients with NITDM after PCI. When CABG was compared to PCI, the adverse outcomes in patients revascularized by CABG were significantly lower compared to those revascularized by PCI.20 Another most recent meta-analysis again conducted by Bundhun et al21 comparing CABG and PCI in patients with ITDM showed CABG to be associated with significantly lower adverse outcomes compared to PCI. However, even if CABG is considered a better option in patients with T2DM, the adverse clinical outcomes in patients with ITDM and NITDM post-CABG are still controversial. Therefore, this study aimed to compare the short-term and long-term adverse clinical outcomes in a large number of patients with ITDM and NITDM after CABG.
CABG is a better option and is associated with significantly lower adverse clinical events compared with PCI in patients with or without T2DM. The SYNTAX trial, which involved patients with and without T2DM, showed that during a 5-year follow-up period, CABG was associated with a significantly lower rate of composite outcomes and repeated revascularization in both categories of patients when compared with PCI.22
A meta-analysis including 10 RCTs comparing CABG and PCI in patients with T2DM again showed CABG to be associated with better outcomes during the long-term follow-up periods.23 Moreover, the American College of Cardiology Foundation and the Society of Thoracic Surgeons Collaboration on the Comparative Effectiveness of Revascularization Strategies (ASCERT) study, which compared patients with multivessel diseases who underwent CABG and PCI respectively, showed CABG to be associated with a significantly lower rate of mortality compared with PCI in patients with T2DM treated with or without insulin therapy. However, our study compared the adverse clinical outcomes in patients with ITDM and NITDM, respectively, after CABG.24
Our results showed that even during a short-term follow-up period or a long-term follow-up period, ITDM was associated with a significantly higher rate of mortality, and major adverse cardiovascular and cerebrovascular events after CABG. The long-term rate of stroke was also significantly higher in the ITDM group, whereas MI and repeated revascularization were not significantly different between these 2 groups after CABG.
All the different subgroups whether for the short-term or the long-term follow-up periods had a low heterogeneity except for the long-term mortality, which had a higher heterogeneity during the comparison of outcomes.
Similar to our results, the FREEDOM trial also showed a significantly higher 5-year mortality, stroke, and MI in the ITDM group after CABG compared with the NITDM group.7 The 30-day MAEs also significantly favored the NITDM group after CABG in the FREEDOM trial. In the study by Deaton et al,25 the authors also concluded that patients with ITDM had worse clinical outcomes after CABG because of a poor glycemic control and a poor health status in such patients. Moreover, results from the PRoject of Ex-vivo Vein graft ENgineering via Transfection IV Trial, which included a total of 3014patients (with and without T2DM) also supported our results by showing that patients with ITDM had worse adverse clinical outcomes compared to patients with NITDM after CABG.26
Several reasons have been suggested to be responsible for such outcomes. Normally patients with T2DM who are treated by insulin therapy have several comorbidities such as hypertension, heart failure, and cerebrovascular events or are even associated with several diabetic complications. They have a poor glycemic control.25 This could be among the reasons for such outcomes after CABG.
Moreover, similar to what was reported in the FREEDOM Trial, patients in the NITDM group were more often only in angina NYHA class 0 or I. These patients experienced less comorbidities and had a good control of their blood glucose level compared with the ITDM group. Patients with ITDM had T2DM for a longer period of time as mentioned in the baseline features. Moreover, they had a higher level of hemoglobin A1c, they were more at risk of chronic renal insufficiency, and had a high level of blood urea nitrogen, had worse NYHA class, and had a higher rate of hypoglycemia post CABG.7
However, a worse prognosis in patients with ITDM after CABG could also be because of the adverse effects of insulin during treatment. Studies have shown several mechanisms that are involved during insulin treatment to also be equally responsible for such adverse outcomes after coronary revascularization. In patients with ITDM, iatrogenic hyperinsulinemia controls high blood glucose level. Endogenous hyperinsulinemia in patients with T2DM is expected to be associated with an increased production of triglycerides and cholesterol by the liver.27 Patients with T1DM who maintain a good control of their blood sugar level often require exogenous insulin in a far much greater quantity compared with the amount synthesized by normal beta cells. The relationship between high insulin level and hepatic markers of atherogenesis was demonstrated by Wang et al28 in a murine model of T1DM. Their study showed insulin injection to significantly increase the levels of PCSK-9 in the blood, but, this rise in level of PCSK-9 in plasma did not exceed that of nondiabetic mice, which had lower insulin levels. On the contrary, injection of insulin seemed to induce the release of the proinflammatory mediator tumor necrosis factor (TNF) and interleukin-1 in mice with diabetes to levels higher than that seen in mice without diabetes.
These findings could probably suggest that exogenous insulin promotes the stimulation and increases proinflammatory macrophage responses and induces dysfunction of the signal transduction pathway by stimulating overactivation of hormones.29 This in turn could probably reduce the progression of atherogenesis, thus disrupting the balanced production and release of endothelial mediators and affect the hemodynamic control and cardiovascular function of the body resulting in essential hypertension, pathological cardiovascular manifestations, and could therefore result in heart failure.30
However, even if an increased rate of mortality was observed in the ITDM group after CABG, a few studies had results that were partly different from our meta-analysis. For example, the study published by Zhang et al31 in 2014 showed a higher rate of in hospital mortality associated with the NITDM (1.0% in the ITDM compared with 1.1% in patients on OHA and 1.3% in patients on diet control). Long-term mortality was also higher in the NITDM group with a percentage death of 14.0% associated with those patients on diet control and 11.2% associated with patients on oral hypoglycemic agents compared with 9.1% in patients with insulin therapy. However, except mortality, the rates of stroke, MAEs, and revascularization were all higher in the ITDM group. Mortality rate in his results was not similar to our analysis maybe because his study was not only showing the influence of diabetes mellitus on clinical outcomes after CABG, but also concentrating more on the influence of diabetes on the economic outcomes after CABG.
Recently, many studies have compared PCI with CABG in patients with T2DM. Other studies have also compared the adverse clinical outcomes in patients with ITDM and NITDM after PCI. However, very few researches have been conducted involving the comparison of adverse clinical outcomes in patients with ITDM and NITDM post-CABG and this study is maybe most probably the first meta-analysis to have done so. Hence, this fact could contribute to the novelty of this study.
Limitations
This study has several limitations. First of all, owing to the limited number of patients, maybe the results could have been affected to an extent. Second, the heterogeneity for the long-term mortality was high >50%. We were supposed to use a random-effect model instead of a fixed-effect model; however, because the subgroup mortality was combined with the other subgroups with low heterogeneity, and for a better result that can match the real theory of medicine, we have again used a fixed-effect model for this long-term mortality. This could also be a limitation in this study. Moreover, one study, Zhang et al31 (2014), which also satisfied the inclusion criteria of our meta-analysis, could not be included in this study because results concerning mortality in this study were completely different from other studies. Also, the inclusion of observational studies along with RCTs could be another limitation in our study.
CONCLUSION
According to this study, patients with ITDM had a significantly higher rate of mortality and MAEs compared to patients with NITDM post-CABG. Stroke was also significantly higher in patients with ITDM during a long-term follow-up period. However, as the result for the long term mortality had a higher heterogeneity as compared to the other subgroups, and because a similar revascularization rate was observed between the ITDM and NITDM groups after CABG which could be due to a limited number of patients, further studies still need to be conducted to completely solve this issue.
Footnotes
Abbreviations: CABG = coronary artery bypass surgery, ITDM = insulin-treated diabetes mellitus, MAEs = major adverse events, NITDM = non-insulin treated diabetes mellitus, PCI = percutaneous coronary intervention, T2DM = type 2 diabetes mellitus
KM is the first author and PKB is the second author.
The authors report no conflicts of interest.
REFERENCES
- 1.Luthra S, Leiva-Juárez MM, Taggart DP. Systematic review of therapies for stable coronary artery disease in diabetic patients. Ann Thorac Surg 2015; 100:2383–2397. [DOI] [PubMed] [Google Scholar]
- 2.Aronson D, Edelman ER. Coronary artery disease and diabetes mellitus. Heart Fail Clin 2016; 12:117–133. [DOI] [PubMed] [Google Scholar]
- 3.Luo Y, Yu X, Chen F, et al. Impact of diabetes mellitus on patients with unprotected left main coronary artery lesion disease treated with either percutaneous coronary intervention or coronary-artery bypass grafting. Coron Artery Dis 2012; 23:322–329. [DOI] [PubMed] [Google Scholar]
- 4.Bundhun PK, Li N, Chen MH. Adverse cardiovascular outcomes between insulin-treated and non-insulin treated diabetic patients after percutaneous coronary intervention: a systematic review and meta-analysis. Cardiovasc Diabetol 2015; 14:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.King SB, 3rd, Lembo NJ, Weintraub WS, et al. A randomized trial comparing coronary angioplasty with coronary bypass surgery. Emory Angioplasty versus Surgery Trial (EAST). N Engl J Med 1994; 331:1044–1050. [DOI] [PubMed] [Google Scholar]
- 6.CABRI. Long-term follow-up of European revascularization trial. Presented at the 68th Scientific Sessions, Plenary Session XII, of the American Heart Association; November 16, 1995; Anaheim, Calif. [Google Scholar]
- 7.Dangas GD, Farkouh ME, Sleeper LA, et al. Long-term outcome of PCI versus CABG in insulin and non-insulin-treated diabetic patients: results from the FREEDOM trial. J Am Coll Cardiol 2014; 64:1189–1197. [DOI] [PubMed] [Google Scholar]
- 8.Alserius T, Hammar N, Nordqvist T, et al. Risk of death or acute myocardial infarction 10 years after coronary artery bypass surgery in relation to type of diabetes. Am Heart J 2006; 152:599–605. [DOI] [PubMed] [Google Scholar]
- 9.Banning AP, Westaby S, Morice MC, et al. Diabetic and nondiabetic patients with left main and/or 3-vessel coronary artery disease: comparison of outcomes with cardiac surgery and paclitaxel-eluting stents. J Am Coll Cardiol 2010; 55:1067–1075. [DOI] [PubMed] [Google Scholar]
- 10.Carson JL, Scholz PM, Chen AY, et al. Diabetes mellitus increases short-term mortality and morbidity in patients undergoing coronary artery bypass graft surgery. J Am Coll Cardiol 2002; 40:418–423. [DOI] [PubMed] [Google Scholar]
- 11.Kappetein AP, Head SJ, Morice MC, et al. Treatment of complex coronary artery disease in patients with diabetes: 5-year results comparing outcomes of bypass surgery and percutaneous coronary intervention in the SYNTAX trial. Eur J Cardiothorac Surg 2013; 43:1006–1013. [DOI] [PubMed] [Google Scholar]
- 12.Lawrie GM, Morris GC, Jr, Glaeser DH. Influence of diabetes mellitus on the results of coronary bypass surgery. Follow-up of 212 diabetic patients ten to 15 years after surgery. JAMA 1986; 256:2967–2971. [PubMed] [Google Scholar]
- 13.Li Z, Amsterdam EA, Young JN, et al. Contemporary outcomes of coronary artery bypass grafting among patients with insulin-treated and non-insulin-treated diabetes. Ann Thorac Surg 2015; 100:2262–2269. [DOI] [PubMed] [Google Scholar]
- 14.Luciani N, Nasso G, Gaudino M, et al. Coronary artery bypass grafting in type II diabetic patients: a comparison between insulin-dependent and non-insulin-dependent patients at short- and mid-term follow-up. Ann Thorac Surg 2003; 76:1149–1154. [DOI] [PubMed] [Google Scholar]
- 15.Mohammadi S, Dagenais F, Mathieu P, et al. Long-term impact of diabetes and its comorbidities in patients undergoing isolated primary coronary artery bypass graft surgery. Circulation 2007; 116 (11 Suppl):I220–I225. [DOI] [PubMed] [Google Scholar]
- 16.Wit MA, de Mulder M, Jansen EK, et al. Diabetes mellitus and its impact on long-term outcomes after coronary artery bypass graft surgery. Acta Diabetol 2013; 50:123–128. [DOI] [PubMed] [Google Scholar]
- 17.Nyström T, Holzmann MJ, Sartipy U. Long-term risk of stroke in patients with type 1 and type 2 diabetes following coronary artery bypassgrafting. J Am Heart Assoc 2015; 4: pii: e002411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wiley, Higgins JPT, Altman DG. Higgins JPT, Green S. Assessing risk of bias in included studies. Cochrane handbook for systematic reviews of interventions 2008; 187–241. [Google Scholar]
- 19.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcareinterventions: explanation and elaboration. BMJ 2009; 339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kapur A, Hall RJ, Malik IS, et al. Randomized comparison of percutaneous coronary intervention with coronary artery bypass grafting in diabeticpatients. 1-year results of the CARDia (Coronary Artery Revascularization in Diabetes) trial. J Am Coll Cardiol 2010; 55:432–440. [DOI] [PubMed] [Google Scholar]
- 21.Bundhun PK, Wu ZJ, Chen MH. Coronary artery bypass surgery compared with percutaneous coronary interventions in patients with insulin-treated type 2 diabetes mellitus: a systematic review and meta-analysis of 6 randomized controlled trials. Cardiovasc Diabetol 2016; 15:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Serruys PW, Morice MC, Kappetein AP, et al. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med 2009; 360:961–972. [DOI] [PubMed] [Google Scholar]
- 23.Hlatky MA, Boothroyd DB, Bravata DM, et al. Coronary artery bypass surgery compared with percutaneous coronary interventions for multivessel disease: acollaborative analysis of individual patient data from ten randomised trials. Lancet 2009; 373:1190–1197. [DOI] [PubMed] [Google Scholar]
- 24.Weintraub WS, Grau-Sepulveda MV, Weiss JM, et al. Comparative effectiveness of revascularization strategies. N Engl J Med 2012; 366:1467–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deaton C, Thourani V. Patients with type 2 diabetes undergoing coronary artery bypass graft surgery: predictors of outcomes. Eur J Cardiovasc Nurs 2009; 8:48–56. [DOI] [PubMed] [Google Scholar]
- 26.Koshizaka M, Lopes RD, Reyes EM, et al. Long-term clinical and angiographic outcomes in patients with diabetes undergoing coronary artery bypass graftsurgery: results from the Project of Ex-vivo Vein Graft Engineering via Transfection IV trial. Am Heart J 2015; 169:175–184. [DOI] [PubMed] [Google Scholar]
- 27.Unger RH, Orci L. Paracrinology of islets and the paracrinopathy of diabetes. Proc Natl Acad Sci USA 2010; 107:16009–16012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang M-Y, Yu X, Lee L, et al. Iatrogenic hyperinsulinemia in type 1 diabetes: its effect on atherogenic risk markers. J Diabetes Complications 2013; 27:70–74. [DOI] [PubMed] [Google Scholar]
- 29.Muniyappa R, Montagnani M, Koh KK, et al. Cardiovascular actions of insulin. Endocr Rev 2007; 28:463–491. [DOI] [PubMed] [Google Scholar]
- 30.Ingelsson E, Sundstrom J, Arnlov J, et al. Insulin resistance and risk of congestive heart failure. JAMA 2005; 294:334–341. [DOI] [PubMed] [Google Scholar]
- 31.Zhang H, Yuan X, Osnabrugge RL, et al. Influence of diabetes mellitus on long-term clinical and economic outcomes after coronary artery bypass grafting. Ann Thorac Surg 2014; 97:2073–2079. [DOI] [PubMed] [Google Scholar]


