Abstract
Uromodulin, released from tubular cells of the ascending limb into the blood, may be associated with kidney function. This work studies the relevance of plasma uromodulin as a biomarker for kidney function in an observational cohort of chronic kidney disease (CKD) patients and subjects without CKD (CKD stage 0). It should be further evaluated if uromodulin allows the identification of early CKD stages.
Plasma uromodulin, serum creatinine, cystatin C, blood-urea-nitrogen (BUN) concentrations, and estimated glomerular filtration rate (eGFR CKD-EPIcrea-cystatin) were assessed in 426 individuals of whom 71 were CKD stage 0 and 355 had CKD. Besides descriptive statistics, univariate correlations between uromodulin and biomarkers/eGFR were calculated using Pearson-correlation coefficient. Multiple linear regression modeling was applied to establish the association between uromodulin and eGFR adjusted for demographic parameters and pharmacologic treatment. Receiver-operating-characteristic (ROC) analysis adjusted for demographic parameters was performed to test if uromodulin allows differentiation of subjects with CKD stage 0 and CKD stage I.
Mean uromodulin plasma levels were 85.7 ± 60.5 ng/mL for all CKD stages combined. Uromodulin was correlated with all biomarkers/eGFR in univariate analysis (eGFR: r = 0.80, creatinine: r = −0.76, BUN: r = −0.72, and cystatin C: r = −0.79). Multiple linear regression modeling showed significant association between uromodulin and eGFR (coefficient estimate β = 0.696, 95% confidence interval [CI] 0.603–0.719, P < 0.001). In ROC analysis uromodulin was the only parameter that significantly improved a model containing demographic parameters to differentiate between CKD 0° and I° (area under the curve [AUC] 0.831, 95% CI 0.746–0.915, P = 0.008) compared to creatinine, cystatin C, BUN, and eGFR (AUC for creatinine: 0.722, P = 0.056, cystatin C: 0.668, P = 0.418, BUN: 0.653, P = 0.811, and eGFR: 0.634, P = 0.823).
Plasma uromodulin serves as a robust biomarker for kidney function and uniquely allows the identification of early stages of CKD. As a marker of tubular secretion it might represent remaining nephron mass and therefore intrinsic “kidney function” rather than just glomerular filtration, the latter only being of limited value to represent kidney function as a whole. It therefore gives substantial information on the renal situation in addition to glomerular filtration and potentially solves the problem of creatinine-blind range of CKD, in which kidney impairment often remains undetected.
INTRODUCTION
Uromodulin is a 95 kDa protein, also known as Tamm-Horsfall protein encoded by the UMOD gene located on chromosome 16p12.3.1–3 It represents the most abundant urinary protein exclusively produced in the tubular cells of the thick ascending limb and the early distal tubule.2 Most of the protein is released into the tubular lumen, forming a layer on the tubular cell surface.4,5 Its physiological role is hypothesized to protect tubular cells from ascending urinary tract infection6 and to be involved in chronic pyelonephritis7 and urolithiasis.8 Additionally to tubular secretion, uromodulin is also released on the basolateral side of the tubular cell into the interstitium,9 the physiological reason remains unclear up to now.10 A reduced number of tubular cells, due to for example, interstitial fibrosis/tubular atrophy in chronic kidney disease (CKD), is paralleled by reduced urinary and serum concentrations of uromodulin.11 Therefore, uromodulin might represent a promising biomarker for the number of intact nephrons and therefore renal mass rather than only reflecting “kidney function” by measuring glomerular filtration. In anephric patients, no uromodulin could be detected in the blood. Urinary uromodulin concentrations have been studied in the context of CKD and did show some correlation to kidney/graft function.12–14 Variants of the encoding UMOD gene were related to rare CKDs, but also urinary uromodulin levels were influenced by certain variants.15,16 Since more than 5 decades uromodulin is analyzed in urine samples, for example, applying quantitative radial immunodiffusion technique. However, due to severe preanalytic limitations and instability of the uromodulin conformation (monomer vs multimers) the diagnostic power of urine analyses is still questionable and unsuitable in patients with poly- or oligo-/anuria. The significance of serum/plasma uromodulin levels in the context of CKD has not been extensively studied so far. Several small studies delivered promising but inconsistent results indicating that serum uromodulin might reflect kidney function in CKD patients but not necessarily in healthy subjects.11,17,18 The largest study recently published involved only elderly healthy patients.19
In this study, we evaluated plasma uromodulin as a biomarker of kidney function in patients with different stages of CKD and individuals without CKD. We additionally questioned whether plasma uromodulin is able to identify early stages of kidney disease and distinguish non-CKD patients from individuals with CKD.
PATIENTS AND METHODS
Study Population
The cohort consisted of 426 patients with 71 patients without kidney disease (CKD 0°) serving as a control group and 355 patients of stages I°–V° of CKD. The study was based on a prospective, observational study concept. The study was approved by the local ethics committee of Klinikum rechts der Isar, Technische Universität, Munich, Germany and adheres to the declaration of Helsinki. All patients enrolled in this study gave their informed consent.
Patient's Demographic Data, Inclusion Criteria, Definition of CKD, and Laboratory Parameters
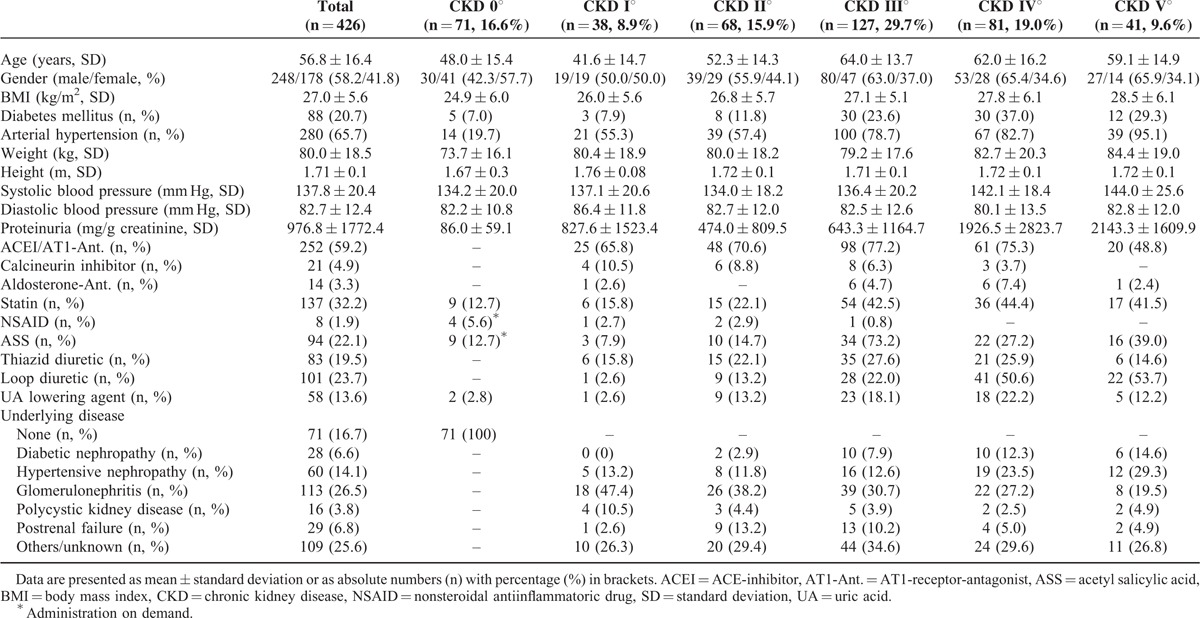
We included 355 patients presenting to our nephrological outpatient clinic at Klinikum rechts der Isar, Munich, Germany. Inclusion criteria followed the definitions for CKD according to the last KDIGO guidelines20: “CKD is defined as abnormalities of kidney structure or function, present for >3 months, with implications for health.” Therefore, the diagnosis of CKD was made when either estimated glomerular filtration rate (eGFR) was <60 mL/min and/or apparent signs of kidney damage were present. As apparent signs of kidney damage we considered proteinuria with a cut-off >150 mg/g creatinine on spot urine specimen and/or histologically proven kidney disease and/or abnormalities detected in imaging techniques (ultrasound, computed tomography, magnetic resonance imaging, or nuclear imaging). Calculation of eGFR was based on both serum creatinine and cystatin C concentration (CKD-EPIcrea-cystatin).21 Once the diagnosis of CKD was established, patients were assigned to a certain stage of CKD according to the KDIGO recommendation: CKD I° when eGFR >90 mL/min, CKD II° 61 to 90 mL/min, CKD 3° 31 to 60 mL/min, CKD IV° 16 to 30 mL/min, and CKD V° 0 to 15 mL/min. Patients with a single kidney (e.g., due to nephrectomy) were assumed to have CKD despite an eGFR >60 mL/min. Furthermore, we included 71 individuals without medical history for CKD and an eGFR >60 mL/min (CKD 0°). Besides uromodulin, creatinine, blood-urea-nitrogen (BUN), and cystatin C were measured in the plasma samples and eGFR (CKD-EPIcrea-cystatin) was calculated. Protein-to-creatinine ratio of spot urine sample was measured. The following patient data were assessed: age, gender, body mass index (BMI), systolic/diastolic blood pressure, history of diabetes mellitus, arterial hypertension and underlying disease (UD), serum c-reactive protein, sodium, potassium, total protein, uric acid, glutamic-pyruvic-transaminase, and complete blood count.
Primary analysis was the correlation of plasma uromodulin with eGFR (CKD-EPIcrea-cystatin) in the whole cohort and advanced stages of CKD separately. Secondary analyses were correlation of plasma uromodulin with serum creatinine, cystatin C, BUN, and proteinuria. Additionally, we evaluated the difference of plasma uromodulin, serum creatinine, BUN, and cystatin C concentrations as well as eGFR between patients at CKD I°/II° and CKD 0°. Furthermore, we analyzed if plasma uromodulin differs significantly between CKD 0° and all stages of CKD and CKD III°–V°, respectively. Patients’ demographics are given in Table 1.
TABLE 1.
Demographic Characteristics and Laboratory Parameters
Biomarker Measurements and eGFR Equations
All plasma samples were stored at – 80 °C before measurements were performed. Plasma uromodulin measurements were performed using a commercially available assay (Euroimmun AG, Lübeck, Germany). Short performance characteristics of the ELISA for plasma samples given by the manufacturer: detection limit for plasma samples 2 ng/mL; mean linearity recovery 97% (83%–107% at 59–397 ng/mL); intraassay precision 1.8–3.2% (at 30–214 ng/mL), interassay precision 6.6% to 7.8% (at 35–228 ng/mL), and interlot precision 7.2% to 10.1% (at 37–227 ng/mL). Plasma samples were diluted 1:101 using dilution buffer. A total of 100 μL of calibrators, controls, or diluted samples were pipetted into coated wells of the microtiter plate (MTP), subsequently 100 μL of biotinylated detection antibody (final concentration 50 ng/mL) were added. The MTP was covered with foil and incubated for 2 hours at 450 rotations per minute (rpm) and room temperature on a rotary shaker. After 2 hours the MTP was washed 3 times using 300 μL washing buffer, then the wells were tapped gently. A total of 100 μL of steptavidin-polyperoxidase (SPO, final concentration 67 ng/mL) were pipetted into each well followed by another incubation for 30 minutes at 450 rpm. Subsequently, the SPO was soaked and the MTP washed 3 times with 300 μL of washing buffer. Consequently, 100 μL of substrate solution (containing the chromogen tetramethylbenzidin and hydrogen peroxide as the substrate for SPO) were pipetted into each well. The MTP was incubated in the dark for 15 minutes at room temperature. The reaction was terminated by adding 100 μL of stop solution. This causes a color change from blue to yellow. Finally, the substrate solution was measured using a photometer at a wavelength of 450 nm and reference wavelength of 620 nm. Data analysis was performed using the program Magellan (Tecan). Creatinine and BUN were measured by photometric techniques (creatinine: normal range 0.7–1.3 mg/dL in males and 0.5–1.1 mg/dL in females; BUN: normal range 7–18 mg/dL). Cystatin C levels were assessed using a nephelometric immunoassay (normal range 0.50–0.96 mg/L). The eGFR equation was calculated based on serum creatinine and cystatin C concentrations as published.21
Statistics
For statistical analysis IBM SPSS 20 and R 3.1.022 were used. Continuous data are expressed as mean with standard deviation. Categorical variables are reported in absolute numbers and percentages. Differences between mean uromodulin values in adjacent CKD stages were analyzed using student's t-test. For calculation of univariate correlations between demographic variables and biomarker concentrations Pearson correlation coefficient was applied with parameters transformed into logarithmic scales. Pearson correlation coefficient was also used to calculate univariate correlations between plasma uromodulin and creatinine, cystatin C, BUN, eGFR (CKD-EPIcrea-cystatin), and proteinuria, all parameters were transformed into logarithmic scales. In order to adjust for age, gender, BMI, UD, and prescription of various medications that might affect tubular function (ACE-inhibitors [ACEI]/AT1-receptor blockers [ARB], calcineurininhibitors, aldosterone antagonists, statins, nonsteroidal-antiinflammatory drugs [NSAID], acetylsalicylic acid [ASA], thiazide and loop diuretics, and uric acid lowering agents, Table 1) multiple linear regression modeling using was performed to assess the association between uromodulin (independent variable) and eGFR (CKD-EPIcrea-cystatin)/proteinuria (dependent variable). For this purpose, uromodulin, eGFR, and proteinuria were transformed into logarithmic scales. To evaluate if the association of uromodulin and eGFR varies in different stages of CKD, both univariate correlation and multivariate linear regression analysis (the latter adjusted for the same parameters as in the previously described analysis) was performed between (log) uromodulin (independent variable) and eGFR (dependent variable) in patients at CKD stage IV°–V°. Finally, the diagnostic characteristics of all parameters to distinguish patients with CKD 0° and stage I° and II° CKD as well as CKD 0° versus all CKD stages and stage III°–V°, respectively, were evaluated using univariate Wilcoxon rank test and/or receiver-operating-characteristic (ROC) analysis. To take into account the effect of age, gender, and BMI, a logistic regression model was fitted to “stage” with these covariates in an ROC analysis performed based on this model using pROC.23 Then the biomarkers/eGFR was added one by one to this model assessing whether the addition of variables improved the diagnostic ability in terms of ROC curve and increase of area under the curve (AUC).24 The optimal cut-off to distinguish CKD stages from each other was calculated using univariate ROC analysis, since the multivariate analysis is based on a linear combination of variables from a logistic regression model so its optimal cut-off value is not meaningful.
All reported P-values are 2-sided, with a significance level of 0.05, and have not been adjusted for multiple testing. The statistical analyses performed have been evaluated by an expert in the field.
RESULTS
Patients’ Demographics
The mean age of all subjects was 56.8 ± 16.4 years, 248 (58.2%) were male. Glomerulonephritis of different causes was the most frequent UD (26.5%), 20.7% had an accompanying diabetic disease, 65.7% suffered from arterial hypertension. Postrenal failure (n = 29) was due to chronic postrenal obstruction (e.g., retroperitoneal fibrosis), chronic pyelonephritis, or hereditary abnormalities of the urogenital tract but not acute postrenal obstruction. Thus, patients had stable eGFR over at least the last 3 months. Detailed baseline characteristics of the participants are presented in Table 1.
Uromodulin Concentrations at Different Stages of CKD
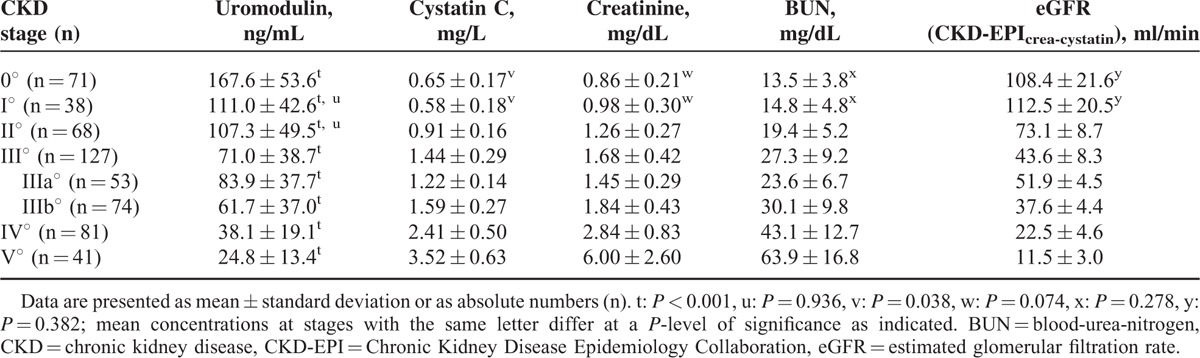
Uromodulin concentrations gradually decreased with progressive impairment of kidney function, ranging from 281 in CKD II° to 3 ng/mL in CKD V°. The mean level in CKD I° was 111.0 ng/mL, 107.3 ng/mL in CKD II°, 71.0 ng/mL in CKD III°, 38.1 ng/mL in CKD IV°, and 24.8 in CKD V°. The highest value was detected in a patient with CKD 0° (312 ng/mL). The difference between uromodulin plasma concentrations for all pairs of CKD stages (P < 0.001) was significant except for stages I° and II° (P = 0.936, Table 2).
TABLE 2.
Kidney Function Parameters in Different Stages of Kidney Disease
Correlation of Uromodulin With Conventional Biomarkers, eGFR, and Proteinuria
In univariate analysis, plasma uromodulin concentrations were significantly associated with all biomarkers and eGFR calculations, being highest for eGFR (CKD-EPIcrea-cystatin, r = 0.80, P < 0.001) followed by cystatin C (r = −0.79, P < 0.001), creatinine (r = −0.76, P < 0.001), and BUN (r = −0.72, P < 0.001, Figure 1). The correlation between uromodulin and proteinuria was weaker, however still significant (r = −0.273, P < 0.001). As uromodulin had inverse kinetics compared to conventional biomarkers of decreasing concentrations with progressive impairment of kidney function the correlations with eGFR calculations were positive, whereas inverse correlations were found with other biomarkers. In multiple linear regression modeling adjusting for age, gender, BMI, UD, and pharmacological treatment (all dependent variables) the coefficient estimate between uromodulin (log, independent variable) and eGFR (CKD-EPIcrea-cystatin, log, and dependent variable) was 0.696 (95% confidence interval [CI] 0.603–0.719, P < 0.001, Figure 2), resulting in R2 = 0.837. Thus, 83.7% variation of eGFR (log) can be explained by the parameters included in the study. The coefficient estimate between uromodulin (log, independent variable) and proteinuria (log, dependent variable) adjusted for the same parameters as the calculation between uromodulin and eGFR was −0.393 (95% CI (−0.922)–(−0.555), P < 0.001, Figure 2), resulting in R2 = 0.523. We further tested if plasma uromodulin correlates with eGFR in advanced stages of CKD (IV° and V°) using univariate correlation and multivariate regression analysis, since in these stages the variation of conventional biomarkers such as creatinine shows a wider range. In both univariate correlation and multivariate regression analysis, the correlation/the regression estimate remained significant (univariate correlation r = 0.487, P < 0.001; multivariate regression coefficient estimate β = 0.420, 95% CI 0.170–0.421, P < 0.001).
FIGURE 1.
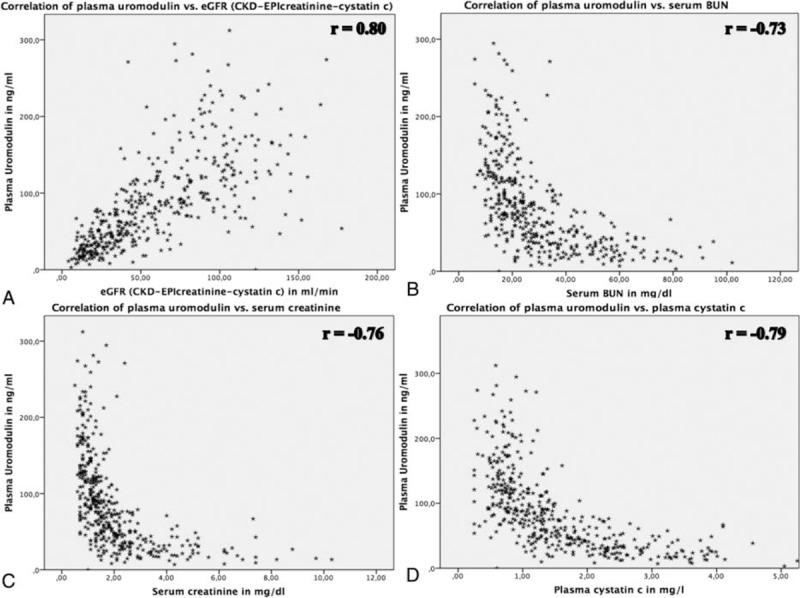
Relationship between plasma uromodulin and (A) serum creatinine, (B) serum cystatin C, (C) blood urea nitrogen, and (D) eGFR (CKD-EPIcrea-cystatin). r = correlation coefficient of univariate analysis. CKD = chronic kidney disease, CKD-EPI = Chronic Kidney Disease Epidemiology Collaboration, eGFR = estimated glomerular filtration rate.
FIGURE 2.
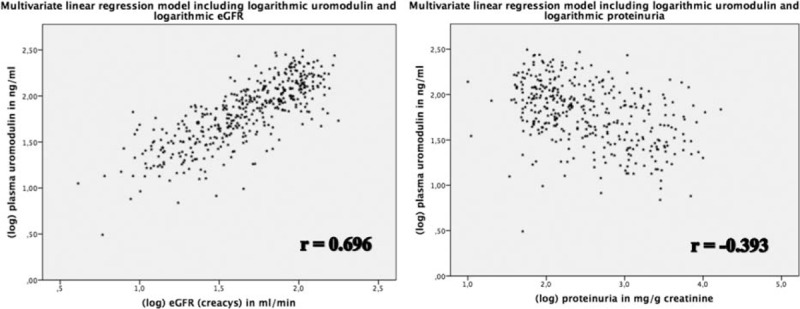
Relationship between logarithmic plasma uromodulin and logarithmic eGFR (CKD-EPIcrea-cystatin). eGFR = estimated glomerular filtration rate. CKD = chronic kidney disease, CKD-EPI = Chronic Kidney Disease Epidemiology Collaboration, eGFR = estimated glomerular filtration rate.
Since diabetes was underrepresented in our cohort in comparison to the general population we analyzed the association between plasma (log) uromodulin and (log) eGFR only in this subcohort (n = 28) using univariate Pearson correlation coefficient and multivariate linear regression analysis. Univariate correlation was 0.716 (P < 0.001), in multivariate regression analysis the coefficient estimate was 0.666 (95%CI 0.273–0.976, P = 0.002). Both analyses of the subcohort delivered comparable results, which were found during calculations performed using data from whole cohort.
Comparison of Patients Without CKD and CKD I°/CKD II°/CKD I°–V° and III°–V°
When comparing early stages of CKD and subjects without CKD, uromodulin concentrations differed significantly between both CKD 0° and I° (167.6 vs 111.0 ng/mL, P < 0.001, Table 2) and CKD 0° and CKD II° (167.6 vs 107.3 ng/mL, P < 0.001, Table 2). None of the other parameters differed significantly between CKD 0° and I° in univariate analysis (creatinine: P = 0.074; BUN: P = 0.278; eGFR [CKD-EPIcrea-cystatin]: P = 0.382, Table 2). Cystatin C levels were higher in the CKD 0° group compared to CKD I° (P = 0.038, Table 2). All parameters were significantly different between CKD 0° and II° (P < 0.001 resp.). However for creatinine, cystatin C, and eGFR this finding was obvious since the classification of CKD was based on eGFR calculated from creatinine and cystatin C.
In the ROC analysis, age, gender, and BMI alone resulted in an AUC of 0.632 to discriminate between CKD 0° and I° (representing adjustment for these parameters). Adding uromodulin to the model resulted in a significant increase of the AUC to 0.831 (95% CI 0.746–0.915, P = 0.008, Figure 3) at an optimal cut-off of 142.3 ng/mL with 64.8% sensitivity and 83.8% specificity (the latter data from univariable ROC analysis). None of the other biomarkers/eGFR contributed to the model regarding increase of the AUC (creatinine: AUC 0.722, 95% CI 0.612–0.831, P = 0.056, cystatin C: AUC 0.668, 95% CI 0.556–0.781, P = 0.418, BUN: AUC 0.653, 95% CI 0.529–0.776, P = 0.811, eGFR: AUC 0.634, 95% CI 0.517–0.751, P = 0.823, Figure 3), meaning that none of the biomarkers added to age, gender, and BMI significantly helped to discriminate between CKD 0° and I°. Combining eGFR and uromodulin in an ROC analysis adjusted for age, gender, and BMI did not improve the AUC compared to the model of age, gender, BMI, and uromodulin alone (AUC 0.829, 95% CI 0.744–0.914, P = 0.009).
FIGURE 3.
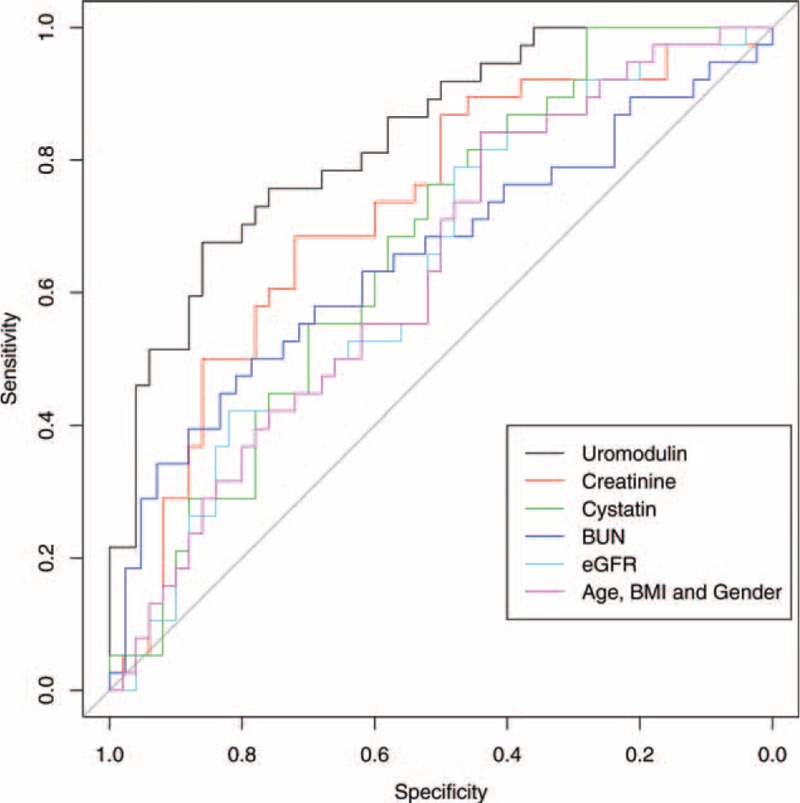
Multivariate ROC analysis evaluating the ability of different parameters to distinguish patients without CKD and CKD stage I°; all parameters (uromodulin, creatinine, cystatin C, BUN, and eGFR) adjusted for age, gender, and BMI; purple line indicates the ability of age, gender, and BMI alone to differentiate between both groups. BMI = body mass index, BUN = blood-urea-nitrogen, CKD = chronic kidney disease, eGFR = estimated glomerular filtration rate, ROC = receiver-operating-characteristic.
In order to assess the ability of plasma uromodulin to differentiate between non-CKD subjects and patients of all CKD stages we performed multivariable ROC analysis. Uromodulin, in addition to gender, BMI, and age resulted in an increased AUC of 0.924 (95% CI 0.892–0.956, P < 0.001) compared to the AUC for gender, BMI, and age alone (AUC 0.743, 95% CI 0.677–0.808). Limiting the CKD group to patients with CKD III°–V° further increased the AUC to 0.971 (95% CI 0.951–0.992, P < 0.001) from 0.817 (95% CI 0.756–0.878).
DISCUSSION
This is to our knowledge the largest study assessing the performance of plasma uromodulin, a marker representing tubular function and therefore differing substantially from all conventional glomerular filtration biomarkers, concentration as marker for kidney function in both CKD patients as well as patients without CKD. Plasma uromodulin seems to be working as a marker for kidney function in both CKD patients as well as patients without CKD. Unlike other conventional biomarkers for kidney function, uromodulin concentrations were significantly higher in individuals without CKD compared to patients at all stages of CKD, especially in patients at early stages of kidney disease (CKD I°), presumably due to the fact that no evasion mechanism for tubular function exists in contrary to glomerular filtration. Uromodulin allowed to distinguish between patients without CKD and patients at all stages of CKD by simply assessing plasma concentrations, at a reasonable level of sensitivity and specificity outperforming all other biomarkers tested in this study.
In our study, plasma uromodulin confirmed the previously documented19 inverse behavior compared to other biomarkers resulting in lower plasma uromodulin concentrations at advanced stages of CKD. From the current pathophysiological understanding this circumstance is due to the fact that uromodulin may not be, like creatinine and BUN, an indirect marker for glomerular filtration but a direct marker for the amount of intact tubular cells of the ascending limb (where it is exclusively produced) and therefore may represent a marker for the number of remaining functional nephrons/renal tissue/tubular secretion. This is a novel approach to measure “kidney function,” potentially helping the treating physician to assess the remaining renal mass and therefore kidney function in the phase when conventional markers/glomerular filtration fails to indicate deterioration of kidney function.
The scatterplot in Figure 1 illustrates another important finding: unlike conventional glomerular filtration markers (creatinine, BUN, and cystatin C), which show hyperbolic correlation to eGFR, uromodulin exhibits linear correlation to eGFR. Therefore, there is no need to take into account the stage of CKD, when for example, for creatinine changes between 1 and 2 mg/dL represent pronounced changes of kidney function, whereas changes between 5 and 6 mg/dL do not. Therefore, it appears that changes of uromodulin concentrations are much easier to be interpreted.
Concerning confounding factors influencing plasma uromodulin concentrations, we detected that age and BMI were weakly, but statistically significantly inversely correlated with serum uromodulin (lower uromodulin levels correlate with higher age and BMI). Risch et al19 also detected in their study that age was inversely correlated to uromodulin serum concentrations.
The fact that plasma uromodulin concentrations differ between subjects without CKD, and early stages of CKD might bridge the gap of current biomarkers for kidney function. Due to the curvilinear relationship between serum creatinine and eGFR based on the mathematical relationship GFR × serum creatinine = 24 hour urine creatinine excretion (equation applying to a steady state), creatinine serum concentrations tend to rise in serum only when approximately 40% to 50% of renal parenchyma is reversibly or irreversibly damaged. Therefore, early stages of acute or chronic kidney failure are often overlooked, a circumstance that delays possible diagnostic and therapeutic interventions, for example, kidney biopsy followed by immunosuppressive treatment in autoimmune or inflammatory diseases. Our data suggest that impaired kidney function of any reason impairs tubular function of the ascending limb already in early phases of the disease. This leads to decreased uromodulin plasma concentrations. Whether this also holds true for situations of acute kidney failure needs to be evaluated in the future.
Our data are in contrast to data from Prajczer et al.11 Applying their own assay, these authors describe a weak but positive correlation between a decrease in GFR and a rise of serum uromodulin, where statistical significance was not achieved. Similar to our study, mainly patients with glomerulonephritis were included, thus, the reason for these discrepant findings remains unknown. However, Thornley et al17 also detected the correlation of serum uromodulin and GFR, measured by creatinine clearance. However, they failed to show a correlation in healthy subjects. We assume that the results obtained in this study that dates back to 1980 are not unrestrictedly comparable to our results, since diagnostic accuracy of uromodulin measurements might have changed over 30 years of research. Dawnay and Cattell18 and Risch et al19 achieved similar results to our study showing that serum uromodulin is related to kidney function. However, they did not perform analyses comparing subjects without CKD with patients at various stages of CKD. Risch et al included patients who felt subjectively healthy but did not undergo proper nephrologic evaluation in the form of quantification of proteinuria or sonographic evaluation, so the actual nephrological situation in these patients remained unclear. Another limitation of the study is that only diabetic patients aged over 60 years were included. Also a cruder classification of CKD was performed in the study, with patients at an eGFR below 45 mL/min being classified as 1 group. Therefore, we think our results are more generalizable and add substantial impact to the topic. Future studies need to further validate our results of plasma uromodulin being a marker for the detection of early stages of CKD. Other biomarker studies identifying early stages of CKD are not available. Cystatin C was beneficial to identify early stages of diabetic nephropathy25 but not in the general CKD population.
Since proteinuria is known to be a strong predictor of CKD progression26 we evaluated the association of plasma uromodulin with proteinuria and detected a significant association in multivariate analysis. This raises the hypothesis whether uromodulin might also serve as a predictor for CKD progression. This might be evaluated in future studies.
Since research up to now focused mainly on urinary uromodulin as a marker of CKD with a recent publication also documenting the predictive value of urinary uromodulin in CKD,27 we compared the diagnostic accuracy of plasma versus urinary uromodulin. For this purpose we compared plasma and urine samples of 335 patients of the cohort. In univariate analysis using Pearson correlation coefficient urinary uromodulin correlated eGFR (r = 0.581, P < 0.001) to a lesser extent than plasma uromodulin (r = 0.786, P < 0.001). When we applied the similar multivariable linear regression analysis as in the main study using logarithmic data with eGFR being the dependent variable and uromodulin being the independent one adjusted for age, BMI, gender, UD, and pharmacologic treatment results were similar (urinary uromodulin: β = 0.509, 95% CI 0.278–0.688, P < 0.001; plasma uromodulin: β = 0.699, 95% CI 0.601–0.733, P < 0.001). At last, in univariable ROC analysis performed to assess the ability of the biomarkers to discriminate between non-CKD and CKD I° patients, the AUC value of plasma uromodulin (AUC = 0.847, 95% CI 0.750–0.945, P < 0.001) was again higher than the AUC value of urinary uromodulin, which was not able to differentiate between CKD 0° and CKD I° (AUC = 0.546, 95% CI 0.393–0.699, P = 0.542). Comparing CKD 0° and the group of patients with CKD III°–V° pooled in ROC analysis, plasma uromodulin delivered a better AUC value (0.971, 95% CI 0.946–0.995, P < 0.001) compared to urinary uromodulin (0.784, 95% CI 0.712–0.857, P < 0.001) to discriminate these groups. Taken the results of these analyses together, plasma uromodulin appears to outperform urinary uromodulin in terms of correlation/association with eGFR, identification of early stages of CKD, and discrimination of non-CKD versus advanced CKD stages.
Our study has certain limitations. At first we used eGFR but did not compare plasma uromodulin to a reference method of kidney function such as inulin clearance due to feasibility reasons. However, we used the most accurate eGFR currently available, incorporating both creatinine and cystatin C.21 Furthermore, since we evaluated a marker for tubular function it was not necessary to use the gold standard for glomerular filtration but only evaluate correlation to glomerular filtration in general. The diagnosis of proteinuria was established using protein/creatinine ratio in a spot urine sample. As estimation of proteinuria/creatinine ratio from spot urine was documented to be less reliable than proteinuria/creatinine ratio from a 24 hour-urine collection in lupus nephritis patients, it might have been more accurate to establish the diagnosis of proteinuria based on protein/creatinine ratio from a 24 hour-urine collection.28 We also included mainly Caucasian subjects, a circumstance that limits the transfer of the results to other subjects with a different ethnic background. Concerning UD chronic glomerulonephritis was predominant. Since diabetic nephropathy is the main cause of CKD in the general CKD population, the results of the study might not be simply transferred to the general population. To account for this we analyzed the association of plasma uromodulin and eGFR using univariate correlation and multivariate regression analysis exclusively in the subcohort with diabetic nephropathy. In both analyses, the association was similar as in the total cohort, so we consider that the specific composition of our cohort does not hamper the results significantly. However, especially the value of uromodulin to detect early stages of CKD needs to be validated in other cohorts with a higher proportion of patients with diabetic nephropathy. Demographic data especially between CKD 0° and I° were different; therefore, we used adjusted multivariate regression modeling and adjusted ROC analysis to eliminate possible demographic confounders. We did not perform an analysis of the uromodulin gene in the patients of our cohort. Mutations in the uromodulin gene may end in a misfolding of the uromodulin molecule, which in turn leads to decreased secretion and accumulation in the tubular cell29–31 and possibly reduced uromodulin plasma concentrations. However, since diseases based on mutations in the uromodulin genes are reported to be very rare (reported prevalence of 1.7 cases in 1,000,000) we do not think that this possible confounder affected our results substantially.32 In addition, since the aim of the study was to evaluate uromodulin as a universal biomarker of kidney function/remaining functional renal parenchyma it would have been a methodological problem to specifically address this issue. Although the total number of patients was adequate to run a meaningful statistical analysis, the number of patients in the particular CKD groups (except CKD III°) was below 100. Nevertheless significant preliminary results could be achieved, so this study laid the basis for future studies addressing specific subgroup analyses with bigger cohorts. At last, our study was performed cross-sectional; therefore, we cannot make a statement on the prognostic value of plasma uromodulin levels and on the development of plasma uromodulin concentrations in the course of changes of kidney function in single patients.
In conclusion, we consider plasma uromodulin a promising biomarker for the assessment of “kidney function,” defined as the remaining number of functional tubular cells in patients with and without CKD. It uniquely allows the identification of early stages of CKD when conventional biomarkers of kidney function, which are all markers of glomerular filtration, are still within normal range. Due to its site of production in tubular cells of the ascending limb, uromodulin might represent a marker quantifying the amount of intact renal parenchyma as useful tool amending established biomarkers only reflecting glomerular filtration. Based on our findings, future evaluation of “kidney function” might consist of a combination of measurement of glomerular filtration AND tubular secretion capacities. Whether uromodulin is important in other clinical settings, such as acute kidney failure needs to be addressed in future studies.
Acknowledgements
The authors thank Prof Hans-Joachim Anders, Medical Clinic IV, Hospital of the Ludwig-Maximilians-University Munich, Germany, for critical proof-reading of the manuscript. The authors also thank the support from the German Research Foundation (DFG) and the Technische Universität München within the funding programme Open Access Publishing.
Footnotes
Abbreviations: AUC = area under the curve, BMI = body mass index, BUN = blood-urea-nitrogen, CKD = chronic kidney disease, eGFR = estimated glomerular filtration rate, ROC = receiver-operating-characteristic, UD = underlying disease
This work was supported by the German Research Foundation (DFG) and the Technische Universität München within the funding programme Open Access Publishing.
MB, VH, and WS are currently employed at Euroimmun Medizinische Labordiagnostika AG, Lübeck, Germany.
The remaining authors have no conflicts of interest to disclose.
REFERENCES
- 1.Rindler MJ, Naik SS, Li N, et al. Uromodulin (Tamm-Horsfall glycoprotein/uromucoid) is a phosphatidylinositol-linked membrane protein. J Biol Chem 1990; 265:20784–20789. [PubMed] [Google Scholar]
- 2.Serafini-Cessi F, Malagolini N, Cavallone D. Tamm-Horsfall glycoprotein: biology and clinical relevance. Am J Kidney Dis 2003; 42:658–676. [DOI] [PubMed] [Google Scholar]
- 3.Devuyst O, Dahan K, Pirson Y. Tamm-Horsfall protein or uromodulin: new ideas about an old molecule. Nephrol Dial Transplant 2005; 20:1290–1294. [DOI] [PubMed] [Google Scholar]
- 4.Malagolini N, Cavallone D, Serafini-Cessi F. Intracellular transport, cell-surface exposure and release of recombinant Tamm-Horsfall glycoprotein. Kidney Int 1997; 52:1340–1350. [DOI] [PubMed] [Google Scholar]
- 5.Cavallone D, Malagolini N, Serafini-Cessi F. Mechanism of release of urinary Tamm-Horsfall glycoprotein from the kidney GPI-anchored counterpart. Biochem Biophys Res Commun 2001; 280:110–114. [DOI] [PubMed] [Google Scholar]
- 6.Säemann MD, Weichhart T, Hörl WH, et al. Tamm-Horsfall protein: a multilayered defence molecule against urinary tract infection. Eur J Clin Invest 2005; 35:227–235. [DOI] [PubMed] [Google Scholar]
- 7.Andriole VT. The role of Tamm-Horsfall protein in the pathogenesis of reflux nephropathy and chronic pyelonephritis. Yale J Biol Med 1985; 58:91–100. [PMC free article] [PubMed] [Google Scholar]
- 8.Hess B. Tamm-Horsfall glycoprotein and calcium nephrolithiasis. Miner Electrolyte Metab 1994; 20:393–398. [PubMed] [Google Scholar]
- 9.Bachmann S, Koeppen-Hagemann I, Kriz W. Ultrastructural localization of Tamm-Horsfall glycoprotein (THP) in rat kidney as revealed by protein A-gold immunocytochemistry. Histochemistry 1985; 83:531–538. [DOI] [PubMed] [Google Scholar]
- 10.El-Achkar TM, Wu XR. Uromodulin in kidney injury: an instigator, bystander, or protector? Am J Kidney Dis 2012; 59:452–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prajczer S, Heidenreich U, Pfaller W, et al. Evidence for a role of uromodulin in chronic kidney disease progression. Nephrol Dial Transplant 2010; 25:1896–1903. [DOI] [PubMed] [Google Scholar]
- 12.Reznichenko A, van Dijk MC, van der Heide JH, et al. Uromodulin in renal transplant recipients: elevated urinary levels and bimodal association with graft failure. Am J Nephrol 2011; 34:445–451. [DOI] [PubMed] [Google Scholar]
- 13.Rampoldi L, Scolari F, Amoroso A, et al. The rediscovery of uromodulin (Tamm-Horsfall protein): from tubulointerstitial nephropathy to chronic kidney disease. Kidney Int 2011; 80:338–347. [DOI] [PubMed] [Google Scholar]
- 14.Lynn KL, Marshall RD. Excretion of Tamm-Horsfall glycoprotein in renal disease. Clin Nephrol 1984; 22:253–257. [PubMed] [Google Scholar]
- 15.Gudbjartsson DF, Holm H, Indridason OS, et al. Association of variants at UMOD with chronic kidney disease and kidney stones-role of age and comorbid diseases. PLoS Genet 2010; 6:e1001039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olden M, Corre T, Hayward C, et al. Common variants in UMOD associate with urinary uromodulin levels: a meta-analysis. J Am Soc Nephrol 2014; 25:1869–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thornley C, Dawnay A, Cattell WR. Human Tamm-Horsfall glycoprotein: urinary and plasma levels in normal subjects and patients with renal disease determined by a fully validated radioimmunoassay. Clin Sci (Lond) 1985; 68:529–535. [DOI] [PubMed] [Google Scholar]
- 18.Dawnay AB, Cattell WR. Serum Tamm-Horsfall glycoprotein levels in health and in renal disease. Clin Nephrol 1981; 15:5–8. [PubMed] [Google Scholar]
- 19.Risch L, Lhotta K, Meier D, et al. The serum uromodulin level is associated with kidney function. Clin Chem Lab Med 2014; 52:1755–1761. [DOI] [PubMed] [Google Scholar]
- 20.KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney International Supplements 2013; volume 3: issue 1. [DOI] [PubMed] [Google Scholar]
- 21.Inker LA, Schmid CH, Tighiouart H, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 2012; 367:20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.R Core Team (2014). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL http://www.r-project.org/ Accessed March 4, 2015. [Google Scholar]
- 23.Robin X, Turck N, Hainard A, et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics 2011; 12:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Janes H, Longton G, Pepe M. Accommodating covariates in ROC analysis. Stata J 2009; 9:17–39. [PMC free article] [PubMed] [Google Scholar]
- 25.Suzuki Y, Matsushita K, Seimiya M, et al. Serum cystatin C as a marker for early detection of chronic kidney disease and grade 2 nephropathy in Japanese patients with type 2 diabetes. Clin Chem Lab Med 2012; 50:1833–2183. [DOI] [PubMed] [Google Scholar]
- 26.Cozzolino M, Gentile G, Mazzaferro S, et al. Blood pressure, proteinuria, and phosphate as risk factors for progressive kidney disease: a hypothesis. Am J Kid Dis 2013; 62:984–992. [DOI] [PubMed] [Google Scholar]
- 27.Garimella PS, Biggs ML, Katz R, et al. Urinary uromodulin kidney function, and cardiovascular in elderly adults. Kidney Int 2015; 88:1126–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Birmingham DJ, Shidham G, Perna A, et al. Spot PC ratio estimates of 24-hour proteinuria are more unreliable in lupus nephritis than in other forms of chronic glomerular disease. Ann Rheum Dis 2014; 73:475–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bernascone I, Janas S, Ikehata M, et al. A transgenic mouse model for uromodulin-associated kidney diseases shows specific tubulo-interstitial damage, urinary concentrating defect and renal failure. Hum Mol Genet 2010; 19:2998–3010. [DOI] [PubMed] [Google Scholar]
- 30.Rampoldi L, Caridi G, Santon D, et al. Allelism of MCKD, FJHN and GCKD caused by impairment of uromodulin export dynamics. Hum Mol Genet 2003; 12:3369–3384. [DOI] [PubMed] [Google Scholar]
- 31.Dahan K, Devuyst O, Smaers M, et al. A cluster of mutations in the UMOD gene causes familial juvenile hyperuricemic nephropathy with abnormal expression of uromodulin. J Am Soc Nephrol 2003; 14:2883–2893. [DOI] [PubMed] [Google Scholar]
- 32.Scolari F, Izzi C, Ghiggeri GM. Uromodulin: from monogenic to multifactorial diseases. Nephrol Dial Transplant 2015; 30:1250–1256. [DOI] [PubMed] [Google Scholar]


