Abstract
Rheumatoid arthritis (RA), a chronic, systemic inflammatory disorder, primarily affects joints. Several studies have indicated that early inflammation, cardiovascular disease, and depression in patients were associated with a considerably increased risk of dementia. Nonsteroidal anti-inflammatory drugs (NSAIDs) are commonly used for treating RA. NSAIDs facilitate alleviating RA-associated chronic pain, inflammation, and swelling. Therefore, we conducted this nationwide study for evaluating the association between the dementia risk and NSAID treatment in patients with RA.
The RA cohort comprised patients aged 20 years and older who were newly diagnosed with RA between 2000 and 2011, with data obtained from the Registry of Catastrophic Illnesses Patient Database (RCIPD). Patients without RA were frequency matched with the RA cohort at a 1:4 ratio according to age, sex, and year of RA diagnosis. The relative risks of dementia were estimated using Cox proportional hazard models.
The risk of dementia in the RA cohort was not significantly higher than that in the non-RA cohort (adjusted HR [hazard ratio] = 0.95, 95% confidence interval [CI] = 0.87–1.02). Regarding the duration of NSAID treatment, the risk of dementia was significantly lower when the RA cohort used NSAIDs for >2191 days (HR = 0.56, 95% CI = 0.45–0.68).
A longer duration of NSAID treatment possibly reduces the risk of dementia. Additional studies are warranted for verifying the association of dementia risk with NSAID treatment in patients with RA.
INTRODUCTION
Rheumatoid arthritis (RA), a chronic and systemic inflammatory disease, can cause severe disability and increase the mortality rate because of deformed and painful joints.1,2 Previous studies have indicated an increased risk of cerebrovascular and neurodegenerative diseases and depression in patients with RA.3–6 Furthermore, early inflammation, cardiovascular diseases, and depression in patients were associated with an increased risk of dementia.7–10 Nonsteroidal anti-inflammatory drugs (NSAIDs) are commonly used for treating RA, and several studies suggest that NSAIDs reduce the risk of Alzheimer disease (AD) or dementia in patients with RA.11–14 However, the association between RA and dementia remains unclear. Therefore, in this nationwide study, we evaluated the risk of dementia in and effect of NSAID treatment on patients with RA.
METHODS
Data Source
The National Health Insurance (NHI) program was implemented on March 1, 1995, and covers ∼99% of the Taiwanese population (∼23.74 million).15 The National Health Research Institutes audits and releases the NHI Research Database (NHIRD), described in detail in previous studies,16,17 for research purposes. Diseases were classified on the basis of International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes. This study analyzed depersonalized secondary data; thus, no informed consent was required. This study was exempted from review by the Institutional Review Board of China Medical University (CMUH104-REC2-115).
Patients
We used the Registry for Catastrophic Illness Patient Database (RCIPD), a subset of the NHIRD, for identifying patients with RA (ICD-9-CM 714) aged 20 years. The date of the first diagnosis of RA between 2000 and 2011 served as the index date. The NHI RCIPD was established for tracking patients with major or catastrophic illnesses, including cancer, end-stage renal disease, mental illness, congenital illness, and several autoimmune diseases such as RA. The application for a catastrophic illness card is scrutinized through peer review, and patients having this card can be exempted from copayment. We excluded patients with a history of dementia (ICD-9-CM 290, 294.1, and 331.0) before the index date and those with incomplete data on age and sex. Patients without RA (non-RA cohort) were randomly selected from the NHIRD and were frequency-matched with the RA cohort at a 1:4 ratio according to age (in 5-y intervals), sex, and year of RA diagnosis by using the same exclusion criteria.
Outcome and Comorbidities
The outcome variable was the development of dementia during follow-up. All patients were followed until the occurrence of dementia; death; withdrawal from the NHI program; or December 31, 2011. Baseline comorbidities for each patient included diabetes (ICD-9-CM 250), hypertension (ICD-9-CM 401-405), hyperlipidemia (ICD-9-CM 272), coronary artery disease (CAD: ICD-9-CM 410-414), head injury (ICD-9-CM 310.2, 800, 801, 803, 804, 850, 851, 853, and 854), depression (ICD-9-CM 296.2, 296.3, 296.82, 300.4, and 311), stroke (ICD-9-CM 430-438), chronic obstructive pulmonary disease (COPD: ICD-9-CM 491, 492, and 496), and congestive heart failure (CHF: ICD-9-CM 428).
Statistical Analysis
We used the chi-square test for determining the categorical differences in demographic variables and comorbidities between the RA and non-RA cohorts and compared the mean age and follow-up period between the cohorts by using Student's t test. We calculated the incidence (per 1000 person-y) of dementia by using different risk factors and calculated the relative risk of dementia between the cohorts by using demographic variables. Univariate and multivariate Cox proportional hazards regression analyses were used for estimating the risk of dementia associated with RA. Hazard ratios (HRs) and 95% confidence intervals (CIs) were estimated using the Cox model. The multivariate model was adjusted for sex, age, and comorbidities, revealing a significant difference with the univariate model. We also evaluated the effect of the duration of NSAID use (≤730, 731–1460, 1461–2190, and >2190 d) on the risk of dementia among patients with RA. All analyses were conducted using SAS statistical software (Version 9.4 for Windows; SAS Institute, Inc, Cary, NC). A 2-tailed P value of 0.05 was considered statistically significant.
RESULTS
Our study included 33,229 and 132,916 patients in the RA and non-RA cohorts, respectively. In the RA cohort, 39.3% of patients were aged <49 years and 77.6% of them were women (Table 1). The mean age of the RA and non-RA cohorts was 53.9 ± 14.2 years and 53.3 ± 14.2 years, respectively. The RA cohort had a higher prevalence of preexisting comorbidities, namely hypertension, coronary artery disease (CAD), head injury, depression, stroke, chronic obstructive pulmonary disease (COPD), and congestive heart failure (CHF) (all P < 0.05).
TABLE 1.
Characteristics of Patients Between Patients With Rheumatoid Arthritis and Patients Without Rheumatoid Arthritis
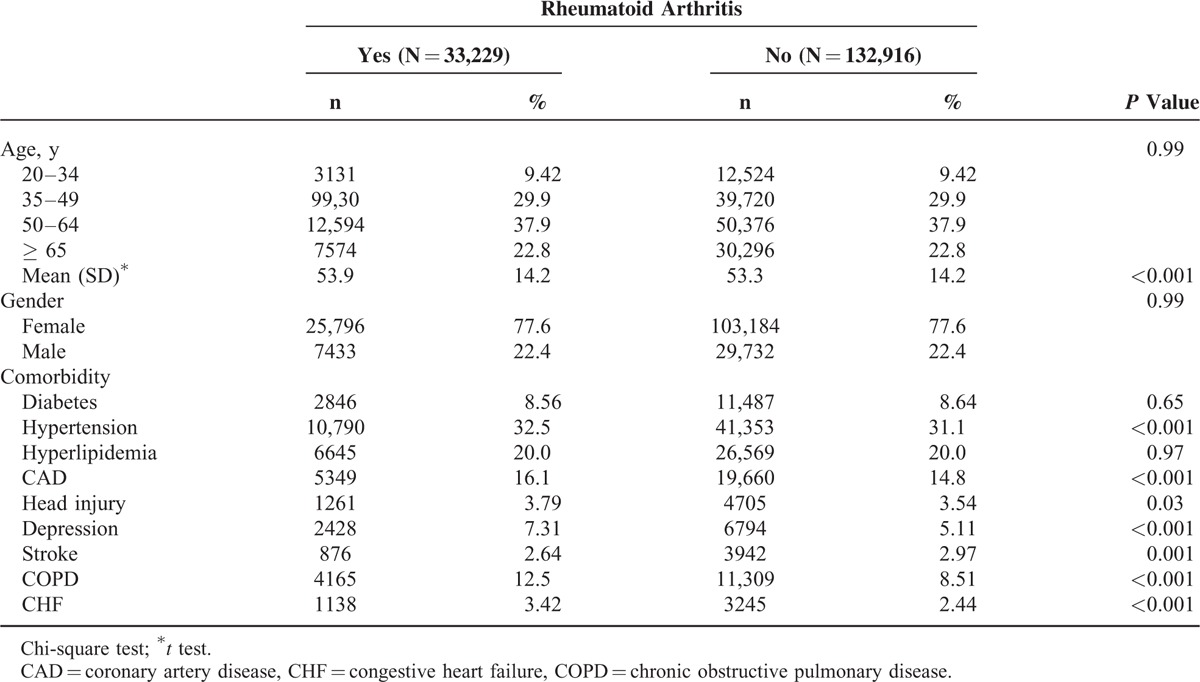
During the mean follow-up period of 6.65 years and 6.70 years for the RA and non-RA cohorts, respectively, the overall incidence of dementia was non-significantly higher (0.89-fold) in the RA cohort than in the non-RA cohort (3.34 vs 3.74 per 1000 person-y), with an adjusted HR of 0.95 (95% CI = 0.87–1.02) after adjustment for age, sex, and comorbidity (Table 2). The incidence of dementia increased with increasing age and comorbidities.
TABLE 2.
The Incidence (Per 1000 Person-Years) and Risk Factors for Dementia
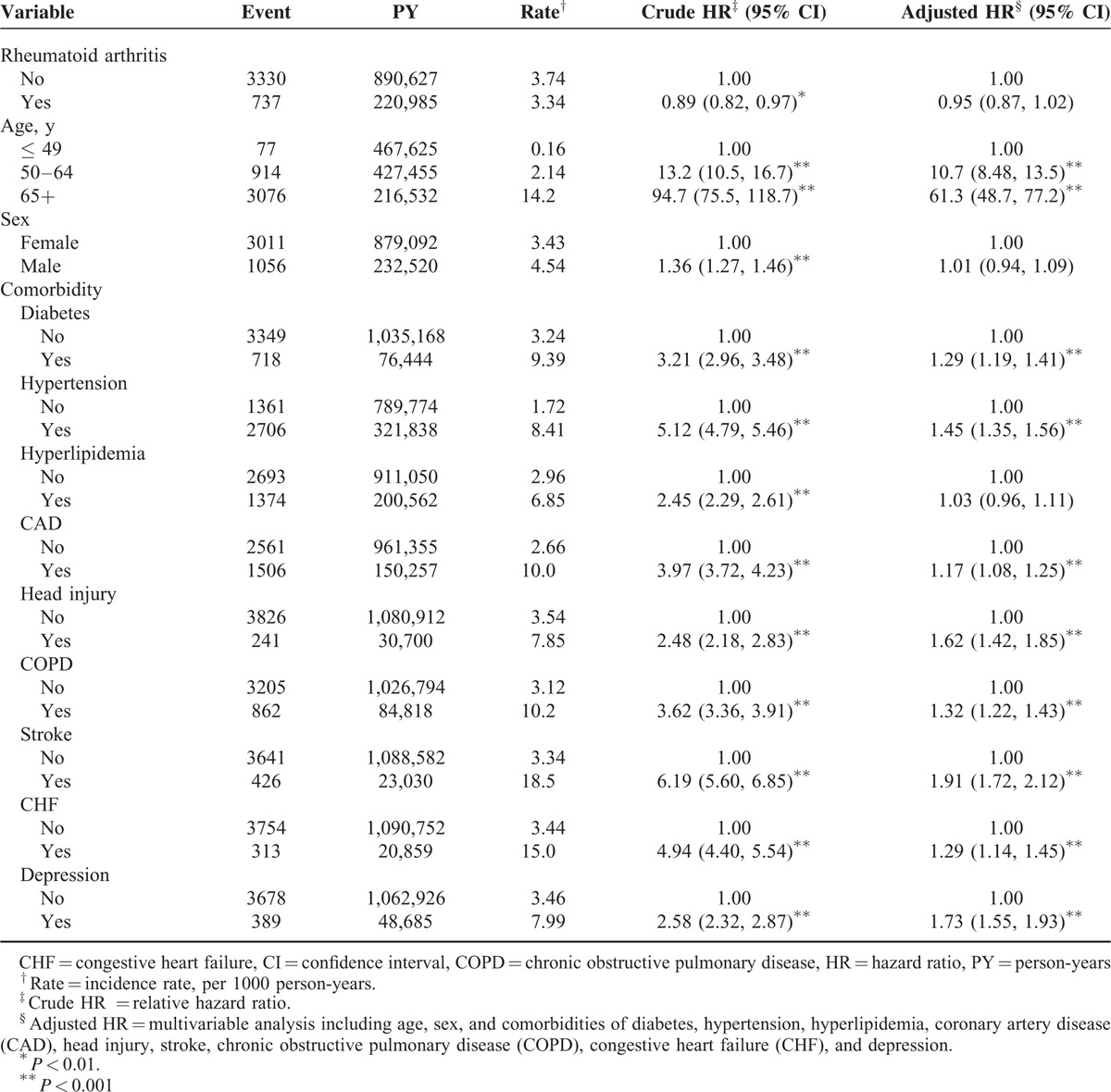
In the multivariate Cox model, compared with patients aged ≤ 49 years, the risk of dementia was 10.7-fold higher in patients aged 50 to 64 years (95% CI = 8.48–13.5) and 61.3-fold higher in those aged ≥ 65 years (95% CI = 48.7–77.2). The risk of dementia was higher in patients with diabetes (HR = 1.29, 95% CI = 1.19–1.41), hypertension (HR = 1.45, 95% CI = 1.35–1.56), CAD (HR = 1.17, 95% CI = 1.08–1.25), head injury (HR = 1.62, 95% CI = 1.42–1.85), COPD (HR = 1.32, 95% CI = 1.22–1.43), stroke (HR = 1.91, 95% CI = 1.72–2.12), CHF (HR = 1.29, 95% CI = 1.14–1.45), and depression (HR = 1.73, 95% CI = 1.55–1.93). The dementia incidence increased with age in both cohorts; however, the relative risk of dementia in the age-specific RA and non-RA cohorts was significantly lower for older patients (HR = 0.89; 95% CI = 0.71–0.97; Table 3).
TABLE 3.
Incidence and Hazard Ratio of Dementia Between Patients With Rheumatoid Arthritis and Without Rheumatoid Arthritis
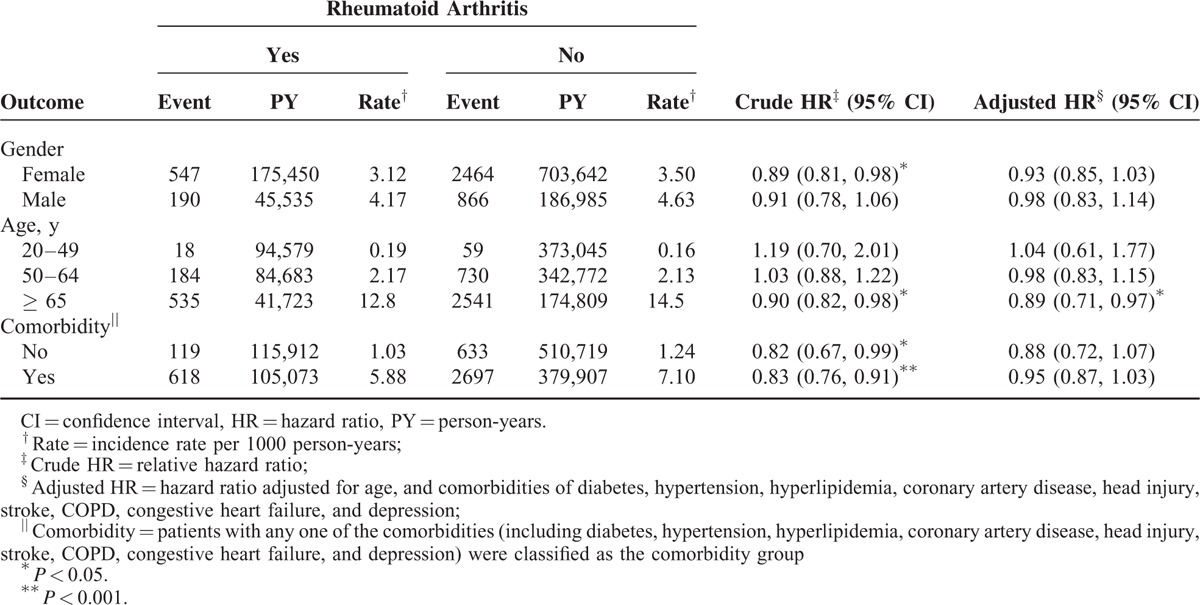
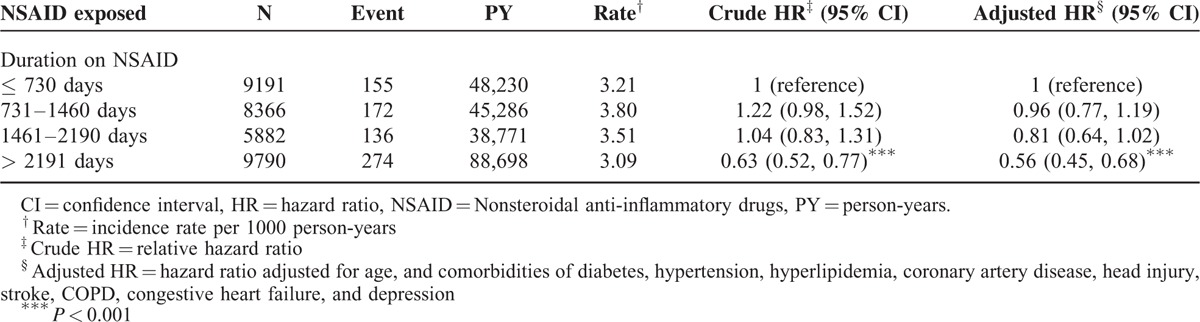
Table 4 presents the risk of dementia stratified according to the duration of NSAID treatment. Compared with patients with RA who used NSAIDs for ≤730 days, the risk of dementia was significantly lower in those who used NSAIDs for>2191 days (HR = 0.56, 95% CI = 0.45–0.68).
TABLE 4.
Incidence and Adjusted Hazard Ratio of Dementia Stratified by Duration of NSAID in Patients With Rheumatoid Arthritis
DISCUSSION
In this nationwide retrospective cohort study, we enrolled all Taiwanese patients with RA over a 10-year follow-up period. The study explored the risk of dementia in patients with RA and evaluated the effects of NSAIDs on reducing the aforementioned risk. The major finding was that a longer duration of NSAID treatment reduced the risk of dementia in patients with RA.
We observed that the prevalence of hypertension, CAD, stroke, and depression was significantly higher in the RA cohort than in the non-RA group (Table 1). This finding was consistent with that of previous studies stating that patients with RA have a higher risk of cardiovascular and cerebrovascular diseases.18,19 Diabetes, hypertension, CAD, head injury, COPD, stroke, CHF, and depression are also known risk factors for dementia (Table 2).7,20,21
The tumor necrosis factor (TNF) plays a crucial role in the pathogenesis of RA.22,23 Previous studies have indicated that the dysregulation of TNF production is implicated in AD.24,25 A characteristic of AD is β-amyloid aggregation, and deposition of β-amyloid activates glial cells, subsequently inducing TNF production.26–28 However, we did not observe any significant association between the risk of dementia and development of RA, possibly because of the effect of the NSAID treatment. In the present study, we enrolled all patients with RA having severe disabilities in Taiwan from the RCIPD during 2000 and 2011. The major symptom of RA is pain,29 and the main aim of the treatment for RA is to reduce the symptoms.30 In the RA and non-RA cohorts, NSAID treatment had unequal probabilities of causing the reverse result (HR = 0.95, 95% CI = 0.87–1.02); however, this difference was not significant. This finding can be explained by the results presented in Table 4. A longer duration of NSAID treatment reduces the risk of dementia, particularly in the patient group administered NSAIDs for >2191 days. Because of the limitation of the NHIRD, the risk of dementia in patients with RA remains unclear and warrants experimental studies.
Cigarette smoking is a risk factor for both RA and dementia, but information on smoking is unavailable in the NHIRD. Therefore, COPD has been used widely as a proxy variable for cigarette smoking in previous studies.31,32 Furthermore, the lack of clinical data, such as data from blood tests, electroencephalograms, and neuroimaging, was the main limitation of this NHIRD-based study. Nevertheless, we identified patients with RA according to ICD-9-CM codes, which are recorded by well-trained physicians. Finally, the NSAID treatment might have a low potency because patients with RA and dementia were less likely to receive the treatment. This factor might have caused the efficacy of NSAIDs in preventing the development of dementia to be underestimated.
This study has several strengths. First, we conducted the longitudinal and nationwide study design by population-based data and NHIRD records in the present study. Both of study and control cohorts were with low loss to follow-up. We enrolled all patients with RA aged ≥20 years from 2000 to 2011 in Taiwan without sampling. Second, the frequencies of clinical visits were different between the patients with and without RA because of differences in disease severity. However, this potential bias might not occur over an 11-year follow-up period. Third, we adjusted for many confounders for determining the risk of dementia in patients with RA, namely diabetes, hypertension, hyperlipidemia, CAD, head injury, depression, stroke, COPD, and CHF, which were risk factors for both RA and dementia. In addition, NHIRD covers ∼99% of the Taiwanese population, due to the reimbursement policy, Taiwan government is the single-buyer. The medical reimbursement specialists and peer review scrutinized all insurance claims based on the standard diagnosed criteria. If these doctors or hospitals make wrong diagnoses or coding, they will be punished with a lot of penalties. Therefore, the diagnostic criteria of dementia based on ICD-9 codes in this study were highly reliable.
In summary, our findings reveal no significant association between the risk of dementia and RA. Nevertheless, it is suggested that prolonged use of NSAIDs reduces the risk of dementia in patients with RA. Because of the limitations of the NHIRD, it is necessary to conduct an experimental study determining the mechanism underlying the association of dementia risk with NSAID treatment in patients with RA.
Footnotes
Abbreviations: RA = rheumatoid arthritis, NSAIDs = nonsteroidal anti-inflammatory drugs, HR = hazard ratio, RCIPD = Registry of Catastrophic Illnesses Patient Database, AD = Alzheimer disease, NHI = National Health Insurance, NHIRD = NHI Research Database, CAD = coronary artery disease, COPD = chronic obstructive pulmonary disease, CHF = congestive heart failure, TNF = tumor necrosis factor
M-CL and C-HK contributed equally to this study.
Author contributions—conception and design: K-HC, C-HK; administrative support: C-HK; collection and assembly of data: all authors; data analysis and interpretation: all authors; manuscript writing: all authors; final approval of manuscript: all authors.
Funding: this study is supported in part by the Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW105-TDU-B-212-133019), China Medical University Hospital, Academia Sinica Taiwan Biobank Stroke Biosignature Project (BM10501010037), NRPB Stroke Clinical Trial Consortium (MOST 104-2325-B-039-005), Tseng-Lien Lin Foundation, Taichung, Taiwan, Taiwan Brain Disease Foundation, Taipei, Taiwan, and Katsuzo and Kiyo Aoshima Memorial Funds, Japan; and CMU under the Aim for Top University Plan of the Ministry of Education, Taiwan. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. No additional external funding received for this study.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Choy EH, Panayi GS. Cytokine pathways and joint inflammation in rheumatoid arthritis. N Engl J Med 2001; 344:907–916. [DOI] [PubMed] [Google Scholar]
- 2.Helmick CG, Felson DT, Lawrence RC, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part I. Arthritis Rheum 2008; 58:15–25. [DOI] [PubMed] [Google Scholar]
- 3.Solomon DH, Goodson NJ, Katz JN, et al. Patterns of cardiovascular risk in rheumatoid arthritis. Ann Rheum Dis 2006; 65:1608–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liou TH, Huang SW, Lin JW, et al. Risk of stroke in patients with rheumatism: a nationwide longitudinal population-based study. Sci Rep 2014; 4:5110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Margaretten M, Yelin E, Imboden J, et al. Predictors of depression in a multiethnic cohort of patients with rheumatoid arthritis. Arthritis Rheum 2009; 61:1586–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Margaretten M, Barton J, Julian L, et al. Socioeconomic determinants of disability and depression in patients with rheumatoid arthritis. Arthritis Care Res (Hoboken) 2011; 63:240–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Knopman DS. Cerebrovascular disease and dementia. Br J Radiol 2007; 80 (Spec No 2):S121–S127. [DOI] [PubMed] [Google Scholar]
- 8.O’Brien JT. Vascular cognitive impairment. Am J Geriatr Psychiatry 2006; 14:724–733. [DOI] [PubMed] [Google Scholar]
- 9.Li G, Wang LY, Shofer JB, et al. Temporal relationship between depression and dementia: findings from a large community-based 15-year follow-up study. Arch Gen Psychiatry 2011; 68:970–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muliyala KP, Varghese M. The complex relationship between depression and dementia. Ann Indian Acad Neurol 2010; 13:S69–S73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McGeer PL, Schulzer M, McGeer EG. Arthritis and anti-inflammatory agents as possible protective factors for Alzheimer's disease: a review of 17 epidemiologic studies. Neurology 1996; 47:425–432. [DOI] [PubMed] [Google Scholar]
- 12.Stewart WF, Kawas C, Corrada M, et al. Risk of Alzheimer's disease and duration of NSAID use. Neurology 1997; 48:626–632. [DOI] [PubMed] [Google Scholar]
- 13.Wang J, Tan L, Wang HF, et al. Anti-inflammatory drugs and risk of Alzheimer's disease: an updated systematic review and meta-analysis. J Alzheimers Dis 2015; 44:385–396. [DOI] [PubMed] [Google Scholar]
- 14.Jaturapatporn D, Isaac MG, McCleery J, et al. Aspirin, steroidal and non-steroidal anti-inflammatory drugs for the treatment of Alzheimer's disease. Cochrane Database Syst Rev 2012; 2:CD006378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Health Insurance Research database, Taiwan. http://nhird.nhri.org.tw/en/index.html (cited in 2015) [Google Scholar]
- 16.Chang KH, Hsu YC, Chang MY, et al. A large-scale study indicates increase in the risk of epilepsy in patients with different risk factors, including rheumatoid arthritis. Medicine (Baltimore) 2015; 94:e1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hsu WY, Lane HY, Lin CL, et al. A population-based cohort study on deep vein thrombosis and pulmonary embolism among schizophrenia patients. Schizophr Res 2015; 162:248–252. [DOI] [PubMed] [Google Scholar]
- 18.Solomon DH, Karlson EW, Rimm EB, et al. Cardiovascular morbidity and mortality in women diagnosed with rheumatoid arthritis. Circulation 2003; 107:1303–1307. [DOI] [PubMed] [Google Scholar]
- 19.del Rincón ID, Williams K, Stern MP, et al. High incidence of cardiovascular events in a rheumatoid arthritis cohort not explained by traditional cardiac risk factors. Arthritis Rheum 2001; 44:2737–2745. [DOI] [PubMed] [Google Scholar]
- 20.Kivipelto M, Helkala EL, Laakso MP, et al. Midlife vascular risk factors and Alzheimer's disease in later life: longitudinal, population based study. BMJ 2001; 322:1447–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ott A, Stolk RP, van Harskamp F, et al. Diabetes mellitus and the risk of dementia: the Rotterdam Study. Neurology 1999; 53:1937–1942. [DOI] [PubMed] [Google Scholar]
- 22.Feldmann M, Brennan FM, Maini RN. Role of cytokines in rheumatoid arthritis. Ann Rev Immunol 1996; 14:397–440. [DOI] [PubMed] [Google Scholar]
- 23.Tam LS, Tomlinson B, Chu TT, et al. Impact of TNF inhibition on insulin resistance and lipids levels in patients with rheumatoid arthritis. Clin Rheumatol 2007; 26:1495–1498. [DOI] [PubMed] [Google Scholar]
- 24.Swardfager W, Lanctôt K, Rothenburg L, et al. A meta-analysis of cytokines in Alzheimer's disease. Biol Psychiatry 2010; 68:930–941. [DOI] [PubMed] [Google Scholar]
- 25.Wang T. TNF-alpha G308A polymorphism and the susceptibility to Alzheimer's disease: an updated meta-analysis. Arch Med Res 2015; 46:24–30.e1. [DOI] [PubMed] [Google Scholar]
- 26.Cummings BJ, Cotman CW. Image analysis of beta-amyloid load in Alzheimer's disease and relation to dementia severity. Lancet 1995; 346:1524–1528. [DOI] [PubMed] [Google Scholar]
- 27.Blasko I, Marx F, Steiner E, et al. TNFalpha plus IFNgamma induce the production of Alzheimer beta-amyloid peptides and decrease the secretion of APPs. FASEB J 1999; 13:63–68. [DOI] [PubMed] [Google Scholar]
- 28.Laws SM, Perneczky R, Wagenpfeil S, et al. TNF polymorphisms in Alzheimer disease and functional implications on CSF beta-amyloid levels. Hum Mutat 2005; 26:29–35. [DOI] [PubMed] [Google Scholar]
- 29.McKenna F, Wright V. Pain and rheumatoid arthritis. Ann Rheum Dis 1985; 44:805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tuncay R, Eksioglu E, Cakir B, et al. Factors affecting drug treatment compliance in patients with rheumatoid arthritis. Rheumatol Int 2007; 27:743–746. [DOI] [PubMed] [Google Scholar]
- 31.Chang KH, Chang MY, Muo CH, et al. Increased risk of dementia in patients exposed to nitrogen dioxide and carbon monoxide: a population-based retrospective cohort study. PLoS One 2014; 9:e103078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chang KH, Chang MY, Muo CH, et al. Exposure to air pollution increases the risk of osteoporosis: a nationwide longitudinal study. Medicine (Baltimore) 2015; 94:e733. [DOI] [PMC free article] [PubMed] [Google Scholar]


