Supplemental Digital Content is available in the text
Abstract
Although distal pancreatectomy with en bloc celiac resection (DP-CAR) is used to treat locally advanced pancreatic cancer, the advantages and disadvantages of this surgical procedure remain unclear. The purpose of this study was to evaluate its clinical safety and efficacy.
Studies regarding DP-CAR were retrieved from the following databases: PubMed, EMBASE, Web of Science, Cochrane Library, and Chinese electronic databases. Articles were selected according to predesigned inclusion criteria, and data were extracted according to predesigned sheets. Clinical, oncologic, and survival outcomes of DP-CAR were systematically reviewed by hazard ratios (HRs) or odds ratio (OR) using fixed- or random-effects models.
Eighteen studies were included. DP-CAR had a longer operating time and greater intraoperative blood loss compared to distal pancreatectomy (DP). A high incidence of vascular reconstruction occurred in DP-CAR: 11.53% (95%CI: 6.88–18.68%) for artery and 33.28% (95%CI: 20.45–49.19%) for vein. The pooled R0 resection rate of DP-CAR was 72.79% (95% CI, 46.19–89.29%). Higher mortality and morbidity rates were seen in DP-CAR, but no significant differences were detected compared to DP; the pooled OR was 1.798 for mortality (95% CI, 0.360–8.989) and 2.106 for morbidity (95% CI, 0.828–5.353). The pooled incidence of postoperative pancreatic fistula (POPF) was 31.31% (95%CI, 23.69–40.12%) in DP-CAR, similar to that of DP (OR = 1.07; 95%CI, 0.52–2.20). The pooled HR against DP-CAR was 5.67 (95%CI, 1.48–21.75) for delayed gastric emptying. The pooled rate of reoperation was 9.74% (95%CI, 4.56–19.59%) in DP-CAR. The combined 1-, 2-, and 3-year survival rates in DP-CAR were 65.22% (49.32–78.34%), 30.20% (21.50–40. 60%), and 18.70% (10.89–30.13%), respectively. The estimated means and medians for survival time in DP-CAR patients were 24.12 (95%CI, 18.26–29.98) months and 17.00 (95%CI, 13.52–20.48) months, respectively. There were no significant differences regarding postoperative 1-, 2-, and 3-year survival rates between DP-CAR and DP, whereas DP-CAR had a better 1-year survival rate compared to palliative treatments. The pooled HR for overall survival between DP-CAR and DP was 1.36 (95%CI: 0.997–1.850); the pooled HR favoring DP-CAR was 0.38 (95%CI: 0.25–0.58) for overall survival compared to palliative treatments. The rate of cancer-related pain relief from DP-CAR was 89.20% (95%CI, 77.85–95.10%). The pooled incidence of postoperative diarrhea was 37.10% (95%CI, 20.79–57.00%); however, most diarrhea was effectively controlled.
DP-CAR is feasible and acceptable in terms of its survival benefits and improved quality of life. However, it should be performed with caution due to its high postoperative morbidity.
INTRODUCTION
Pancreatic body/tail cancer is usually diagnosed in its advanced stage, which is often considered unresectable1,2 because of the involvement of the celiac axis (CA) or the origin of the common hepatic artery (CHA).3 Chemo- and/or radiotherapies have been the only options for these locally advanced pancreatic cancers, but their effects have been dismal. The 2-year survival rate in unresectable pancreatic cancer is only 10%, with a median overall survival of 9.8 months.4 The reported 5-year survival rate of distal pancreatectomy (DP) with multimodal treatments is ∼29%, with a median overall survival5 of 35 months. Extended distal pancreatectomy with en bloc resection of the celiac artery (DP-CAR) may provide a chance for complete resection of locally advanced pancreatic cancer.6 However, data regarding DP-CAR are limited. It is unclear whether it is safe and effective, can provide survival benefits similar to DP, or can result in prolonged survival and better quality of life compared to supportive treatments.
Celiac axis resection without vascular reconstruction for gastric cancer was initially reported for total gastrectomy by Appleby.7 Since then, celiac axis resection has been applied to distal pancreatectomy, a procedure referred to as DP-CAR. DP-CAR is a difficult and complicated procedure that has been the subject of much debate. It is feasible in theory because the blood supply through the superior mesenteric artery, pancreatoduodenal arcades, and gastroduodenal artery can support the hepatobiliary system and stomach.8 However, postoperative ischemic problems continue to be a concern. Although DP-CAR dramatically increases tumor resectability,9 the associated postoperative morbidity rate is high. The value of DP-CAR has not been made clear. The results from current studies that compared short-term outcomes between DP-CAR and DP have been inconsistent. Postoperative survival and quality of life after DP-CAR are also controversial. Some authors reported no survival benefits from DP-CAR10–12 when compared with DP, whereas others have suggested that it resulted in prolonged disease-free survival in select patients.13 When compared with palliative treatments, patients might achieve significant survival benefits from DP-CAR.4,14 Hirano et al15 reported that the 5-year overall survival with DP-CAR was 42%, which was better than the 5-year survival rate with DP alone. Because of the dissection of the nerve plexus surrounding the common hepatic artery and/or celiac axis, DP-CAR may lead to severe postoperative diarrhea and malnutrition.15 Nonetheless, several studies have demonstrated that the diarrhea in DP-CAR was not severe2 and could be effectively controlled with medication.
DP-CAR is not routinely performed in most surgical clinical centers due to its complexity and high postoperative morbidity and mortality. It is extremely difficult to conduct a study with a large case series. The largest published case series included 422 patients and took almost 10 years to collect. Most of existing reports regarding DP-CAR include small case numbers without comparable data, which make it hard to determine the actual value of this procedure. Meta-analysis is an effective way to pool data from different studies to generate more stable and consolidated results. Therefore, the objective of this study was to use a meta-analysis to determine whether DP-CAR is a safe, feasible, and beneficial procedure.
MATERIALS AND METHODS
Search Strategy
MOOSE (Meta-Analysis of Observational Studies in Epidemiology) guidelines16 were adopted in our systematic review. A computerized search was conducted using PubMed, EMBASE, Web of Science, the Cochrane Library, and Chinese electronic databases (VIP database, WanFang database, and CNKI database). The ultimate search date was October18, 2014, and the language was restricted to English or Chinese. Search terms used were: “celiac resection, pancreatic neoplasm, and distal pancreatectomy.” Search details are described in the Supplemental Data (S1). Ethical approval was not required for this study, because it was a literature review and had no bad effects on patients.
Eligibility Criteria
Inclusion Criteria
Types of participants: locally advanced body/tail pancreatic cancer involving the origin of the common hepatic artery, the root of the splenic artery, or the celiac axis.
Types of interventions: distal pancreatectomy with en bloc celiac artery resection (DP-CAR).
Outcomes: postoperative mortality, postoperative morbidity, or postoperative survival.
Types of studies: randomized controlled trials (RCTs), cohort studies, case-control studies, and case series (case numbers >5).
Exclusion Criteria
Abstracts, letters, comments, editorials, expert opinions, and reviews without original data.
Studies with small case numbers (DP-CAR<6).
Non-English or non-Chinese language articles, nonhuman studies, and duplicates published by the same center.
Data Extraction
Two authors identified and screened the search findings. The titles and abstracts were screened for potentially eligible studies. Full-text articles were obtained for detailed evaluation. When studies were conducted in the same institution, we included either the study of better quality or the more recent publication. Two reviewers independently extracted the following data from each identified study: first author, year of publication, details of where the studies were conducted, study period, sample size, baseline characteristics of the studies, neoadjuvant therapy, vascular resections and reconstruction, operative time, intraoperative blood loss, reoperation, morbidity, mortality, hospital stay, survival, duration of follow-ups.
The Centre for Reviews and Dissemination partial checklist and the National Institute of Clinical Excellence (NICE) checklist17 were implemented to assess the risk of bias and the methodological quality of the included studies.
Statistical Analysis
This meta-analysis was conducted using Comprehensive Meta-analysis Software (version 2.0). The mean difference (MD), standardized mean difference (SMD), odds ratios (OR) and hazard ratios (HR) with 95% confidence intervals were computed using fixed- or random-effects models to evaluate relevant clinical outcomes. Statistical heterogeneity between trials was evaluated by the χ2 test (P < 0.100 was considered to be significant) and I2 values. An I2 value of 50% or greater indicated the presence of heterogeneity.18 In the absence of statistically significant heterogeneity, the fixed-effect method was utilized to combine the results. If heterogeneity was confirmed, the random-effect model was used. Publication bias was assessed using funnel plots and tested by Egger's test and Begg's test. P < 0.05 was considered to be statistically significant.
RESULTS
Description of the Studies
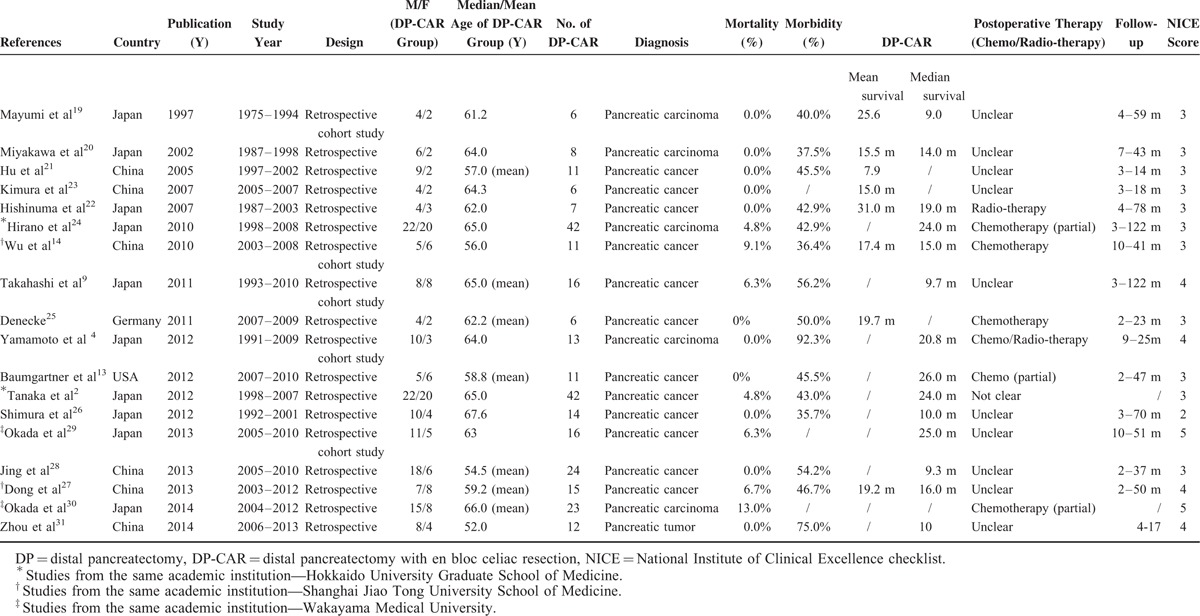
Database and manual searches identified 570 potentially relevant abstracts after excluding duplicates using EndNote X6 software. After screening the titles and abstracts, 54 articles were retrieved for a comprehensive review. Of these, 36 articles were excluded, resulting in 18 articles2,4,9,13,14,19–31 suitable for inclusion in the systematic review (Figure 1). Ten studies were conducted in Japan,2,4,9,19,20,22,24,26,29,30 1 in the United States,13 1 in Germany,25 and 6 in China.14,21,23,27,28,31 Details are shown in Table 1. Moreover, some articles from the same institutions were also included because they focused on different outcomes (Hirano's24 study and Tanaka's study2 from Hokkaido University Graduate School of Medicine, Okada et al's 201329 and Okada et al's 201430 from Wakayama Medical University, and Wu et al's study14 and Dong et al's study27 from the Shanghai Jiao Tong University School of Medicine).
FIGURE 1.
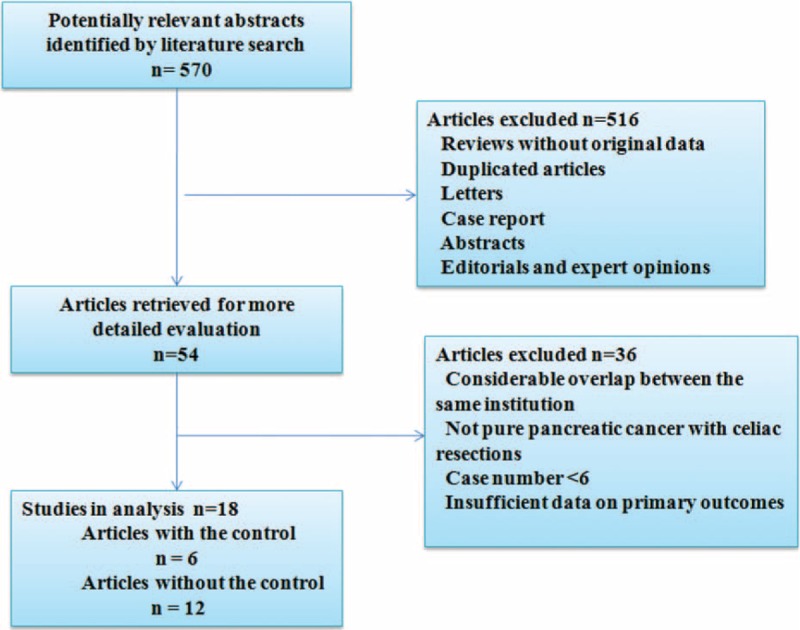
Flowchart of identification of eligible studies.
TABLE 1.
Study Characteristics
Methodological Quality
The Centre for Reviews and Dissemination Quality Assessment Checklist was utilized to address selection bias, attrition bias, and detection bias;17 the results are shown in Table s1. Using the National Institute of Clinical Excellence checklist (NICE) criteria, no studies were of multicenter prospective designs. None of the 18 studies stratified the outcomes. Only 3 studies reported consecutive case recruitment. Overall, all studies had a NICE total score of < 8. A total NICE score of 4 or greater is considered to be “higher quality”. Six studies had a NICE total score of ≥4 (Table s1).
Intraoperative Outcomes
Operating Time
Data on operating time were available from 8 studies4,9,19,25,27,28,30,31 including115 patients undergoing DP-CAR. The mean operating time varied from 200 to 612 minutes. Significant statistical heterogeneity was observed among these studies (I2 = 95.69%). The integrated mean operating time was 335.55 minutes (95%CI: 270.71–400.39) using random models. Meta-analysis of the 5 studies4,9,14,19,29 that provided comparative data revealed that the operating time for DP-CAR was significantly prolonged compared to that of DP (SMD:1.497, 95%CI: 0.538–2.456, P = 0.002; heterogeneity: P < 0.001, I2 = 87.40%).
Intraoperative Blood Loss
Seven studies4,9,13,27,28,30,31 including 114 patients undergoing DP-CAR reported intraoperative blood loss. The mean blood loss varied from702 mL to 1867.5 mL. Because of significant heterogeneity between these studies, random models were adopted to combine data; the pooled intraoperative blood loss was 1319.88 mL (95%CI: 938.84–1700.92). Meta-analysis of 5 studies4,9,14,19,29 with comparative data showed a significantly higher intraoperative blood loss in DP-CAR compared to DP (SMD:0.839, 95% CI:0.304–1.374, P = 0.002; heterogeneity: P = 0.021, I2 = 65.30%).
Vascular Reconstruction
Vascular reconstruction includes arterial and venous reconstruction. Nine studies2,9,13,20,21,23,26,27,30 reported the incidence of arterial reconstruction during DP-CAR. The pooled incidence of arterial reconstruction in DP-CAR was 11.53% (95%CI: 6.88–18.68%), without significant heterogeneity (P = 0.55, I2 = 0%). Superior mesenteric vein (SMV)/portal vein (PV) resections in DP-CAR were reported in 11 studies2,4,9,13,14,19–22,25,30 involving 154 DP-CAR cases. The pooled incidence of vein resections in DP-CAR was 33.28% (95%CI: 20.45–49.19%) using random models. Only 3 studies4,9,29 compared the incidence of vein resections between DP-CAR and DP. Pooled data of the 3 studies showed a higher incidence of vein resections in DP-CAR compared to DP (odds ratio: 4.619, 95% CI: 1.48–14.40, P = 0.008) (Figure 2).
FIGURE 2.
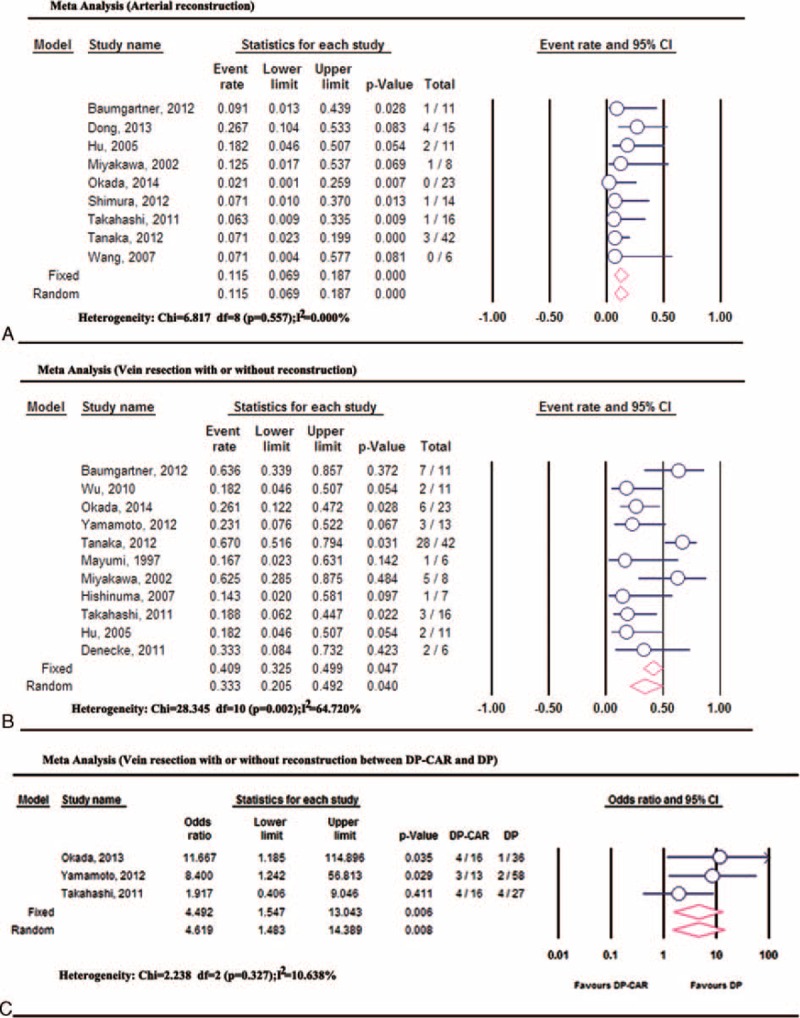
Vascular resection and reconstruction during DP-CAR: (A) The incidence of arterial reconstruction; (B) the incidence of venous resection; (C) the incidence of venous resection between DP-CAR and DP.DP = distal pancreatectomy, DP-CAR = distal pancreatectomy with en bloc celiac resection.
Intraoperative Combined Resection of Other Organs During DP-CAR
The majority of combined organ resections in DP-CAR were due to tumor invasion. The pooled combined rates of gallbladder resection,13,20,22 gastrectomy,4,9,13,19–24 colon resection,9,19,21–24 left kidney resection,9,21–23 and small bowel resection9,24 were 46.28%, 36.29% (22.54% for partial gastrectomy and 29.42% for total gastrectomy), 15.89%, 14.66%, and 6.91%, respectively.
R0 Resection Rate
The R0 resection rate was reported by 8 studies,2,4,13,22,23,25,28,30 with significant heterogeneity between the studies. The R0 resection rate during DP-CAR ranged from 30.8% to 100%, whereas the pooled R0 resection rate was 72.79% (95% CI, 46.19%–89.29%). Meta-analysis of the results from 3 comparative studies4,9,29 involving 146 patients showed a lower R0 resection rate in DP-CAR, but no significant difference was identified between DP-CAR and DP (OR: 0.36, 95% CI, 0.05–2.67, P = 0.32; heterogeneity: I2 = 85.19%).
Postoperative Outcomes
Ischemic Complications and Preoperative Embolization
Postoperative ischemic complications after DP-CAR commonly include gastric, gallbladder, and hepatic ischemic problems. Postoperative gastric ischemic events were reported in 12 studies.2,13,14,20,22,23,25–28,30,31 The pooled incidence of gastric ischemic events was 12.87% (95%CI: 8.30–19.42%), without significant heterogeneity between the studies (P = 0.64, I2 = 0%). Preoperative embolization, advocated by several clinical centers,2,24,25,29,30 might decrease the incidence of postoperative ischemic complications by developing an extra blood supply. There were 3 studies2,25,30 using the preoperative embolization technique to prevent postoperative gastric ischemic events. The pooled incidence of gastric ischemic complications with or without preoperative embolization was 10.74% (95%CI: 5.19–20.92%) and 14.38% (95%CI: 8.25–23.90%),13,22,23,26–28,31 respectively. Fewer gastric ischemic complications were observed in the preoperative embolization group, but no significant difference was identified compared to the nonpreoperative embolization group (RR: 0.74, 95%CI: 0.30–1.80, P = 0.51) (Figure 3).
FIGURE 3.
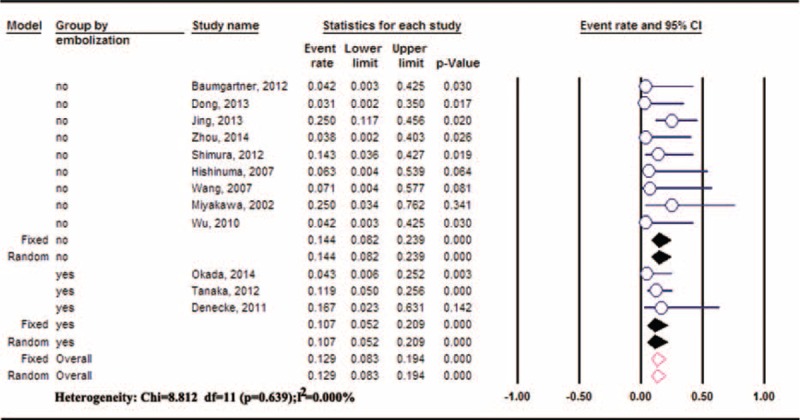
Meta-analysis of gastric ischemic events after DP-CAR. DP-CAR = distal pancreatectomy with en bloc celiac resection.
The incidence of transient abnormal AST or ALT levels, up to 40%,27 after DP-CAR was reported in some studies.22,23,27 However, relevant liver ischemic problems, including liver dysfunction, hepatic infarction, and liver abscess, were not frequent. The pooled incidence of these ischemic problems involving 152 patients was 5.14% (95%CI, 2.46–10.42%). No significant difference in relevant liver ischemic problems was observed between the nonembolization group and the embolization group. The incidence for nonembolization was 6.45% (2.82–14.09%), compared to 2.28% (0.46–10.52%) for embolization (RR: 2.83, 95%CI: 0.48–16.48, P = 0.25).
Regarding gallbladder ischemic problems, 2 cases of gallbladder perforation were reported by Shimura et al26 and Miyakawa et al.20 The other 4 studies reported no gallbladder ischemic problems.13,23,27,31 The combined incidence of this problem was 7.31% (2.72–18.17%).
Postoperative Mortality
The mortality rate for DP-CAR ranged between 2% and 16.7%. Nine of 221 patients died during the postoperative hospital stay: 2 cardiac infarction,2,30 2 multiple organ failure,2,21 1 respiratory failure secondary to severe methicillin-resistant staphylococcus aureus (MRSA) pneumonia;9 the causes of death included 1 serious intra-abdominal infection secondary to severe POPF and gastrointestinal leakage,27 1 hypoglycemia,23 1 acute respiratory distress syndrome,30 and 1 portal venous bleeding.30 The overall postoperative mortality rate was 6.7% (95% CI, 4%–11.2%; P < 0.001; I2 = 0.00%). Five comparative studies4,9,19,22,29 showed higher mortality rates in DP-CAR, but no significant difference was identified compared to DP (OR: 1.798, 95%CI, 0.360–8.989, P = 0.475; heterogeneity: I2 = 0.000%) (Figure 4A). Publication bias based on the mortality rate from comparative studies was not observed using Egger's test (P = 0.36) and Begg's test (P = 0.81).
FIGURE 4.
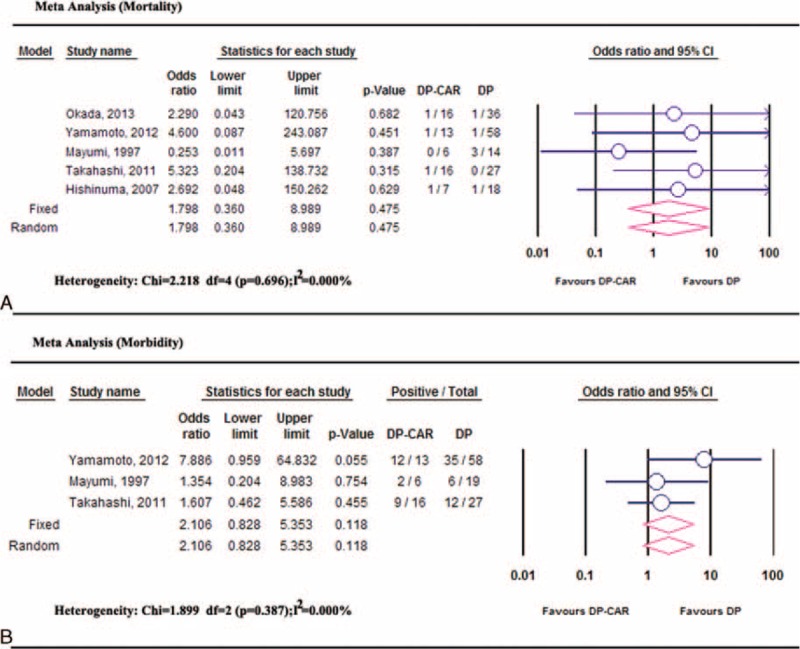
Meta-analysis of mortality and morbidity: (A) comparable mortality rates between DP-CAR and DP; (B) comparable morbidity rates between DP-CAR and DP. DP = distal pancreatectomy, DP-CAR = distal pancreatectomy with en bloc celiac resection.
Postoperative Morbidity
There was a similar incidence of postoperative complications among 12 studies2,4,9,13,20–22,25–28,31 involving179 DP-CAR patients. The pooled postoperative morbidity rate was 0.494 (95%CI, 41.8–57.0%, heterogeneity: I2 = 5.28%). Data from the 3 controlled studies4,9,19 indicated no difference in the morbidity rates between DP-CAR and DP (OR, 2.106, 95%CI 0.828–5.353, P = 0.118; heterogeneity: P = 0.387, I2 = 0.000%) (Figure 4B), and no significant publication bias was identified.
POPF
The incidence of POPF was reported in 14 studies2,4,9,13,19,21–23,25–28,30,31 including 206 patients. POPF was defined by the ISGFP (the International Study Group on Pancreatic Fistula)32 in 8 studies.4,9,13,25,27,28,30,31 It was graded according to the definition reported by Okano in 1 study,26 whereas it was not clearly defined in 5 others.2,19,21–23 The pooled incidence of POPF was 31.31% (95%CI: 23.69–40.12%). Meta-analysis of 4 comparative studies4,9,14,29 showed no significant difference in the incidence of POPF between DP-CAR and DP (OR:1.07, 95%CI:0.52–2.20; heterogeneity: P = 0.640, I2 = 0.000%); no significant publication bias was detected.
DGE
Clinically relevant DGE (CR-DGE) was reported by 2 studies;4,30 the pooled incidence of CR-DGE was 30.56% (95%CI, 17.80–47.21%). The pooled odds rate regarding CR-DGE between DP-CAR and DP from small samples4,29 was 5.67 (95%CI, 1.48–21.75, P = 0.01). Therefore, the Bayes estimator was also performed using WinBUGS14; the OR was 6.91 (95%CI, 1.75–28.53).
Re-operation
Zero incidence of reoperation was reported in 3 studies.13,14,31 Three of 23 patients underwent reoperation was reported by Okada et al:30 1 for gastric leakage, and the cause of the other 2 was unclear. Takahashi et al9 reported that 2 of 16 patients were re-operated on due to bile peritonitis that developed from a stump of partial resection of the liver and a pseudoaneurysm that developed from the stump of the common hepatic artery (CHA). The pooled rate of reoperation in 73 patients9,13,14,30,31 was 9.74% (95%CI, 4.56–19.59%; heterogeneity: P = 0.793, I2 = 0.000%).
Hospital Stay
High heterogeneity was found among the studies9,13,19,21,27,28,31 in terms of hospital stay (I2 = 93.45%); the combined mean hospital stay using a random model was 24.16 days (95%CI, 17.50–30.82). The study by Yamamoto et al4 favored a longer hospital stay with DP-CAR compared to DP, whereas the other 3 studies 9,14,19 did not detect a significant difference between DP-CAR and DP in hospital stay data. Pooled results showed a longer hospital stay in DP-CAR, but no significant difference was observed compared to DP (SMD: 0.301, 95%CI −0.286 to 0.887, P = 0.315; heterogeneity: P = 0.043, I2 = 63.077%).
Survival and Quality of Life After DP-CAR
Survival
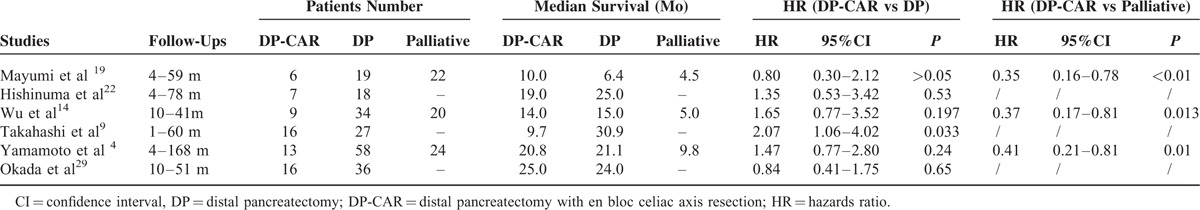
The combined 1-, 2-, and 3-year survival rates after DP-CAR were 65.22% (49.32–78.34%),4,9,19,22,25–29 30.20% (21.50–40. 60%),4,9,19,22,26–29 and 18.70% (10.89–30.13%),4,9,19,22,26–28 respectively. The5-year survival rates varied from 0% to 25% in the included studies,2,21,22,26 with a median survival time of 9 to 25 months for DP-CAR patients. Detailed individual survival data with DP-CAR were available from 8 articles13,19,20,22,23,25–27 involving71 patients. Pooled data from these patients revealed that the estimated means and medians for survival time of DP-CAR were 24.12 (95%CI, 18.26–29.98) months and 17.00 (95%CI, 13.52–20.48) months, respectively. Survival curves are shown in Figure 5. Six studies4,9,14,19,22,29 reported comparative data in postoperative survival. There was no significant difference regarding postoperative 1-, 2-, and 3-year survival rates between DP-CAR and DP (Table 2), whereas a better 1-year survival rate was observed in DP-CAR when compared to palliative treatments (Table 3). Pooled HR for overall survival between DP-CAR and DP was 1.36 (95%CI:0.997–1.850, P = 0.052).4,9,14,19,22,29 Pooled HR for overall survival between DP-CAR and palliative support was 0.38 (95%CI:0.25–0.58, P < 0.01)4,14,19 (Table 4).
FIGURE 5.
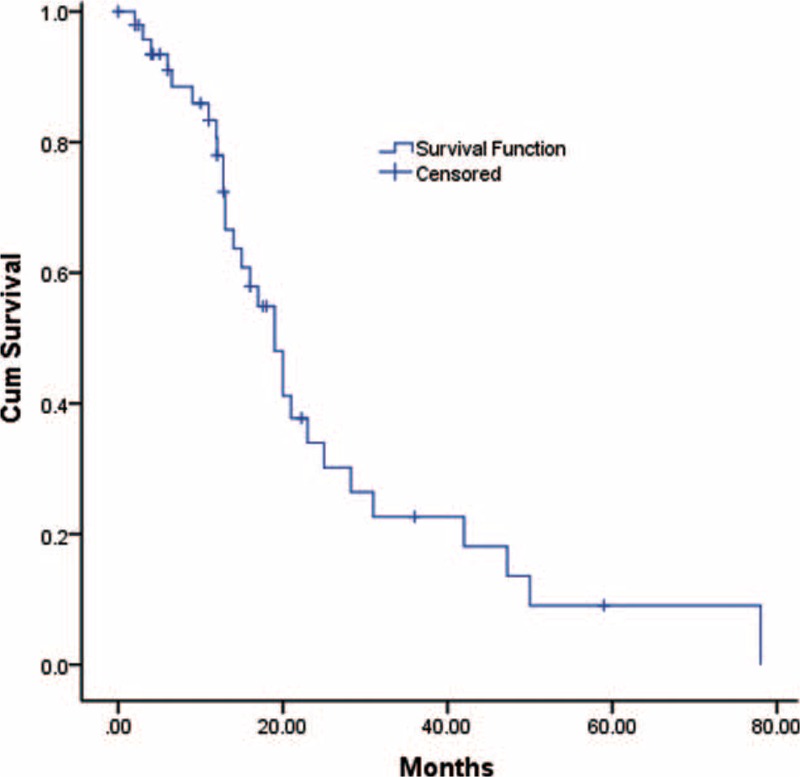
Postoperative overall survival of 71 patients who underwent DP-CAR. The estimated means and medians for survival time of DP-CAR were 24.12 (95%CI, 18.26–29.98) months and 17.00 (95%CI, 13.52–20.48) months, respectively. DP-CAR = distal pancreatectomy with en bloc celiac resection.
TABLE 2.
1-, 2-, and 3-Year Survival Rates Between DP-CAR and DP
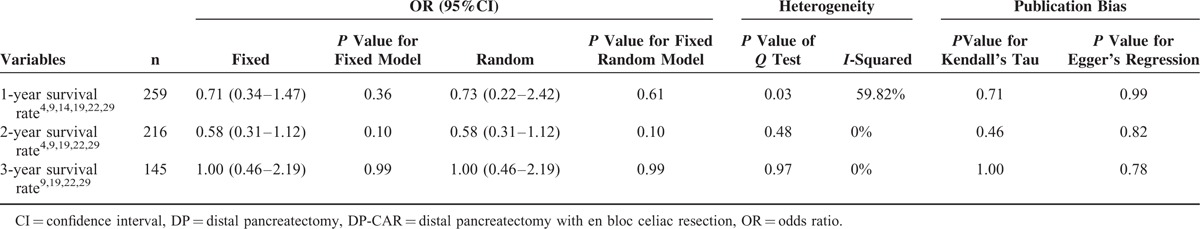
TABLE 3.
1-, 2-, and 3-Year Survival Rates Between DP-CAR and Palliative Treatments
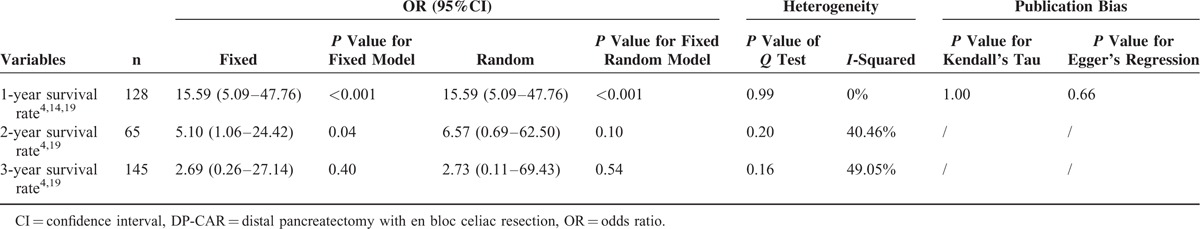
TABLE 4.
Survival Summary in 6 Comparative Studies
Postoperative Analgesia
DP-CAR alleviates epigastric and/or back pain due to the resection of the celiac plexus. A high incidence (83–100%) of cancer-related pain relief was achieved after DP-CAR as reported in 6 studies.21–23,27,28,31 The combined proportion of cancer-related pain relief was 89.20% (95%CI, 77.85–95.10%).
Postoperative Diarrhea
The resection of celiac ganglia in DP-CAR may result in postoperative diarrhea. Eight studies4,9,21–24,27,28 reported the rate of postoperative diarrhea. DP-CAR rarely induced intractable diarrhea; most diarrhea could be effectively controlled using loperamide and/or opium tincture or autorelieved. Baumgartner et al reported that 1 of 11 patients required readmission due to postoperative diarrhea and dehydration,13 but they did not report the detailed incidence of postoperative diarrhea. Five patients with refractory diarrhea were reported by Yamamoto et al,4 but their management was not reported. The highest incidence of mild postoperative diarrhea, up to 100%, was reported by Dong et al,27 but all cases were auto-alleviated in 1 to 6 months. In order to avoid bias, the Dong study27 was excluded when pooling the incidence of postoperative diarrhea. The incidence of postoperative diarrhea ranged from 8.30% to 100%; the pooled incidence of postoperative diarrhea was 37.10% (95%CI, 20.79–57.00%) with significant heterogeneity among the studies.
DISCUSSION
Advanced pancreatic body/tail cancer is often considered unresectable because the celiac artery is usually invaded by the time of diagnosis. DP-CAR dramatically increases the tumor respectability because of the complete resection of tumors and involved vessels. Until now, only sporadic retrospective studies of DP-CAR with small samples have been available. We have lacked convincing evidence comparing postoperative outcomes between DP-CAR and standard DP. The advantages and disadvantages of DP-CAR remain unclear. Therefore, a systematic review and meta-analysis comparing DP-CAR to DP was performed; the results indicated that DP-CAR is a complex surgical procedure with high postoperative morbidity but with acceptable postoperative survival and quality of life.
DP-CAR can dramatically increase the R0 resection rate to72.79% but with a high incidence of vascular reconstruction. Wedge resection with primary closure, end-to-end anastomosis or graft interposition for the management of involved vessels is often utilized during DP-CAR. The pooled incidence of arterial reconstruction in DP-CAR was 11.53%, where it was 33.28% for vein resection and reconstruction. Meanwhile, the incidence of intraoperative combined resection of other organs during DP-CAR was also high. Partial gastrectomy, total gastrectomy, left adrenal gland removal, and colon resections were commonly performed to remove the tumors in en bloc. Prolonged operating times and increased intraoperative blood loss were identified more often in DP-CAR compared with DP. A higher incidence of postoperative mortality and morbidity was seen in DP-CAR, but no significant differences were detected compared to DP. Data on 21,482 pancreatectomies from the National Cancer Data Base (NCDB)33 showed that the unadjusted 90-day mortality rate was 7.4% (95%CI: 7.0–7.8). Our data showed that the overall postoperative mortality rate of DP-CAR was 6.7% (95%CI: 4–11.2%), and the pooled postoperative morbidity rate was 49.4% (95% CI: 41.8–57.0%). POPF and DGE are the most common postoperative complications. The present study demonstrated that the incidence of POPF after DP-CAR was 31.31% (95% CI: 23.69–40.12%), which was similar to that of DP. However, clinically relevant DGE was higher in DP-CAR compared to DP.
Postoperative ischemic problems continue to be a difficult issue in DP-CAR. Celiac axis resection may result in ischemia of the hepatoduodenal region, leading to ischemic gastropathy2 and ischemic hepatopathy. Our pooling results showed that the incidence of gastric ischemic problems was 12.87%. Preservation of the stomach during DP-CAR remains debatable and needs to be clarified in the future. Relevant liver ischemic problems are rare, mild, and acceptable. Transitory abnormal ALT or AST levels were identified after DP-CAR, but they resolved fully within ∼1 to 2 weeks. No significant difference between the DP-CAR and DP was detected regarding postoperative liver function. Prophylactic cholecystectomy might be unnecessary unless the arterial blood supply is compromised. Preoperative coil embolization was reported to be an effective method of avoiding postoperative ischemic complications6 because of enhanced collateral arterial flow. However, whether preoperative embolization decreases the incidence of postoperative ischemic events is unclear. The present study showed a lower incidence of postoperative ischemic complications in the preoperative embolization group, but no significant difference was identified compared to nonembolization. Therefore, preoperative embolization might be beneficial, if not required. Regardless of preoperative embolization, it is very important to confirm the hepatic arterial inflow with intraoperative ultrasonography13 or palpating the pulse28 of the proper hepatic artery (PHA) after temporary blocking of the celiac axis. Weak pulsation of the PHA hints at insufficient arterial blood supply, suggesting hepatic artery reconstruction.
Most of the studies showed that better quality of life was achieved after DP-CAR, but overall survival remains under debate.21 The 5-year survival rate varied from 0% to 42%15,24 after DP-CAR, with a median survival time of 9 to 26 months for advanced pancreatic body/tail cancer. Our data showed that the estimated means and medians for survival time of DP-CAR patients were 24.12 months and 17 months, respectively. DP-CAR had shorter overall survival compared to DP, but no significant difference was identified. This trend was not unexpected because patients who underwent DP-CAR were normally at a more advanced stage. When compared to palliative treatments, DP-CAR had a better 1-year survival rate, and it reduced the risk of all-cause death by 62%. Moreover, DP-CAR dramatically improves patients’ quality of life through immediate and complete relief of back pain. Resections of the celiac plexus and celiac ganglia as well as the retroperitoneal tissues might result in severe diarrhea and malnutrition. Unlike pancreatoduodenectomy, DP-CAR preserves the continuity of the digestive tracts; therefore, it might be less associated with uncontrollable diarrhea. Actually, the patients who underwent DP-CAR had a reasonable nutritional status, which made postoperative chemotherapy possible.
The present study has several limitations, so its conclusions should be interpreted with caution. No randomized controlled trials of DP-CAR are available in the existing studies. All data in our study came from retrospective studies with small samples, and the clinical evidence level is low. It takes several years, possibly even more than a decade, to obtain series with >10 cases of DP-CAR in the clinical setting. The mean interval of research time in the included studies is (8.89+5.43) years. Therefore, the results might be affected by different treatment protocols and perioperative management techniques over this long interval. Evidence of heterogeneity and possible publication bias were detected in the analysis of some outcomes. Moreover, a high incidence of vascular resection and reconstruction was observed in DP-CAR. Different types of vascular resections have different portal vein occlusion times, which might affect postoperative liver function. Ischemic liver problems after DP-CAR should be evaluated separately according to the presence or absence of vascular reconstruction, which was not done in the current studies. Because of the small samples in the included comparative studies, bias, and possibly even errors, might have occurred in data transformations regarding survival analysis. Quality of life was reported in a few trials; this should be further investigated in well-designed future studies.
In conclusion, DP-CAR is a reasonable treatment choice for locally advanced pancreatic body/tail cancer. It is acceptable in terms of its high resectability rate, postoperative survival benefits and excellent postoperative pain control. However, it is a complicated procedure with high risks and should only be performed by experienced hands. Because of the limitations of the present study, many controversial issues remain in DP-CAR, and its conclusions should be interpreted with caution.
Supplementary Material
Footnotes
Abbreviations: DGE = delayed gastric emptying, DP = distal pancreatectomy, DP-CAR = distal pancreatectomy with en bloc celiac resection, ISGPS = International Study Group of Pancreatic Surgery, PD = pancreaticoduodenectomy, OR = odds ratio, POPF = postoperative pancreatic fistula, SMD = standard mean difference, WMD = weighted mean difference
(PROSPERO registration number: CRD42015025702)
GH and MR contributed equally to this study.
Funding: this work was supported by the Scientific Research Foundation for the Returned Overseas Chinese Scholars, Chinese Ministry of Education.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Takahashi T, Ishikura H, Motohara T, et al. Perineural invasion by ductal adenocarcinoma of the pancreas. J Surg Oncol 1997; 65:164–170. [DOI] [PubMed] [Google Scholar]
- 2.Tanaka E, Hirano S, Tsuchikawa T, et al. Important technical remarks on distal pancreatectomy with en-bloc celiac axis resection for locally advanced pancreatic body cancer (with video). J Hepatobiliary Pancreat Sci 2012; 19:141–147. [DOI] [PubMed] [Google Scholar]
- 3.Buchs NC, Chilcott M, Poletti PA, et al. Vascular invasion in pancreatic cancer: imaging modalities, preoperative diagnosis and surgical management. World J Gastroenterol 2010; 16:818–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yamamoto Y, Sakamoto Y, Ban D, et al. Is celiac axis resection justified for T4 pancreatic body cancer? Surgery 2012; 151:61–69. [DOI] [PubMed] [Google Scholar]
- 5.Paye F, Micelli Lupinacci R, Bachellier P, et al. Distal pancreatectomy for pancreatic carcinoma in the era of multimodal treatment. Brit J Surg 2015; 102:229–236. [DOI] [PubMed] [Google Scholar]
- 6.Alizai PH, Mahnken AH, Klink CD, et al. Extended distal pancreatectomy with en bloc resection of the celiac axis for locally advanced pancreatic cancer: a case report and review of the literature. Case Rep Med 2012; 2012:543167–1543167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Appleby LH. The coeliac axis in the expansion of the operation for gastric carcinoma. Cancer 1953; 6:704–707. [DOI] [PubMed] [Google Scholar]
- 8.Kondo S, Katoh H, Hirano S, et al. Results of radical distal pancreatectomy with en bloc resection of the celiac artery for locally advanced cancer of the pancreatic body. Langenbeck's Arch Surg 2003; 388:101–106. [DOI] [PubMed] [Google Scholar]
- 9.Takahashi Y, Kaneoka Y, Maeda A, et al. Distal pancreatectomy with celiac axis resection for carcinoma of the body and tail of the pancreas. World J Surg 2011; 35:2535–2542. [DOI] [PubMed] [Google Scholar]
- 10.Yamaguchi K, Nakano K, Kobayashi K, et al. Appleby operation for pancreatic body-tail carcinoma: report of three cases. Surg Today 2003; 33:873–878. [DOI] [PubMed] [Google Scholar]
- 11.Konishi M, Kinoshita T, Nakagori T, et al. Distal pancreatectomy with resection of the celiac axis and reconstruction of the hepatic artery for carcinoma of the body and tail of the pancreas. J Hepatobiliary Pancreat Surg 2000; 7:183–187. [DOI] [PubMed] [Google Scholar]
- 12.Mizutani S, Shioya T, Maejima K, et al. Two successful curative operations using stomach-preserving distal pancreatectomy with celiac axis resection for the treatment of locally advanced pancreatic body cancer. J Hepatobiliary Pancreat Surg 2009; 16:229–233. [DOI] [PubMed] [Google Scholar]
- 13.Baumgartner JM, Krasinskas A, Daouadi M, et al. Distal pancreatectomy with en bloc celiac axis resection for locally advanced pancreatic adenocarcinoma following neoadjuvant therapy. J Gastrointest Surg 2012; 16:1152–1159. [DOI] [PubMed] [Google Scholar]
- 14.Wu X, Tao R, Lei R, et al. Distal pancreatectomy combined with celiac axis resection in treatment of carcinoma of the body/tail of the pancreas: a single-center experience. Ann Surg Oncol 2010; 17:1359–1366. [DOI] [PubMed] [Google Scholar]
- 15.Hirano S, Kondo S, Hara T, et al. Distal pancreatectomy with en bloc celiac axis resection for locally advanced pancreatic body cancer: long-term results. Ann Surg 2007; 246:46–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000; 283:2008–2012. [DOI] [PubMed] [Google Scholar]
- 17.Kassam Z, Lee CH, Yuan Y, et al. Fecal microbiota transplantation for Clostridium difficile infection: systematic review and meta-analysis. Am J Gastroenterol 2013; 108:500–508. [DOI] [PubMed] [Google Scholar]
- 18.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003; 327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mayumi T, Nimura Y, Kamiya J, et al. Distal pancreatectomy with en bloc resection of the celiac artery for carcinoma of the body and tail of the pancreas. Int J Pancreatol 1997; 22:15–21. [DOI] [PubMed] [Google Scholar]
- 20.Miyakawa S, Horiguchi A, Hanai T, et al. Monitoring hepatic venous hemoglobin oxygen saturation during Appleby operation for pancreatic cancer. Hepatogastroenterology 2002; 49:817–821. [PubMed] [Google Scholar]
- 21.Hu CG, Jin G, Liu R, et al. Distal pancreatectomy combined with resection of celiac axis for pancreatic body and tail carcinoma: a report of 11 cases. Acad J Sec Mil Med Univ 2005; 26:871–872. [Google Scholar]
- 22.Hishinuma S, Ogata Y, Tomikawa M, et al. Stomach-preserving distal pancreatectomy with combined resection of the celiac artery: radical procedure for locally advanced cancer of the pancreatic body. J Gastrointest Surg 2007; 11:743–749. [DOI] [PubMed] [Google Scholar]
- 23.Kimura W, Huang XF, Sun Y, et al. Appleby operation for carcinoma of the body and tail of the pancreas. Chin J Gen Surg 2007; 22:32–34. [Google Scholar]
- 24.Hirano S, Kondo S, Tanaka E, et al. Postoperative bowel function and nutritional status following distal pancreatectomy with en-bloc celiac axis resection. Dig Surg 2010; 27:212–216. [DOI] [PubMed] [Google Scholar]
- 25.Denecke T, Andreou A, Podrabsky P, et al. Distal pancreatectomy with en bloc resection of the celiac trunk for extended pancreatic tumor disease: an interdisciplinary approach. Cardiovasc Interv Radiol 2011; 34:1058–1064. [DOI] [PubMed] [Google Scholar]
- 26.Shimura M, Ito M, Horiguchi A, et al. Distal pancreatectomy with en bloc celiac axis resection performed while monitoring hepatic arterial flow by using a transonic flowmeter during operation. Hepatogastroenterology 2012; 59:1498–1500. [DOI] [PubMed] [Google Scholar]
- 27.Dong Y, Jin JB, Peng CH, et al. Clinical effect of modified Appleby operation in the treatment of carcinoma of body and tail of the pancreas. J Surg Concepts Pract 2013; 18:142–146. [Google Scholar]
- 28.Jing W, Zhu G, Hu X, et al. Distal pancreatectomy with en bloc celiac axis resection for the treatment of locally advanced pancreatic body and tail cancer. Hepatogastroenterology 2013; 60:187–190. [DOI] [PubMed] [Google Scholar]
- 29.Okada K, Kawai M, Tani M, et al. Surgical strategy for patients with pancreatic body/tail carcinoma: who should undergo distal pancreatectomy with en-bloc celiac axis resection? Surgery 2013; 153:365–372. [DOI] [PubMed] [Google Scholar]
- 30.Okada KI, Kawai M, Tani M, et al. Preservation of the left gastric artery on the basis of anatomical features in patients undergoing distal pancreatectomy with celiac axis en-bloc resection (DP-CAR). World J Surg 2014; 38:2980–2985. [DOI] [PubMed] [Google Scholar]
- 31.Zhou YM, Zhang XF, Li XD, et al. Distal pancreatectomy with en bloc celiac axis resection for pancreatic body-tail cancer: is it justified? Med Sci Monit 2014; 20:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bassi C, Dervenis C, Butturini G, et al. Postoperative pancreatic fistula: an International Study Group (ISGPF) definition. Surgery 2005; 138:8–13. [DOI] [PubMed] [Google Scholar]
- 33.Swanson RS, Pezzi CM, Mallin K, et al. The 90-day mortality after pancreatectomy for cancer is double the 30-day mortality: more than 20,000 resections from the national cancer data base. Ann Surg Oncol 2014; 21:4059–4067. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


