Abstract
Background
Light therapy is a known treatment for patients with seasonal affective disorder. However, the efficacy of light therapy in treating patients with non-seasonal depression remains inconclusive.
Aims
To provide the current state of evidence for efficacy of light therapy in non-seasonal depressive disorders.
Method
Systematic review of randomised controlled trials (RCTs) was conducted by searching MEDLINE, EMBASE, PsycINFO, CINAHL, and CENTRAL from their inception to September 2015. Study selection, data abstraction and risk of bias assessment were independently conducted in duplicate. Meta-analyses were performed to provide a summary statistic for the included RCTs. The reporting of this systematic review follows the PRISMA guidelines.
Results
A meta-analysis including 881 participants from 20 RCTs demonstrated a beneficial effect of light therapy in non-seasonal depression (standardised mean difference in depression score −0.41 (95% CI −0.64 to −0.18)). This estimate was associated with significant heterogeneity (I2=60%, P=0.0003) that was not sufficiently explained by subgroup analyses. There was also high risk of bias in the included trials limiting the study interpretation.
Conclusions
The overall quality of evidence is poor due to high risk of bias and inconsistency. However, considering that light therapy has minimal side-effects and our meta-analysis demonstrated that a significant proportion of patients achieved a clinically significant response, light therapy may be effective for patients with non-seasonal depression and can be a helpful additional therapeutic intervention for depression.
Declaration of interest
None.
Copyright and usage
© The Royal College of Psychiatrists 2016. This is an open access article distributed under the terms of the Creative Commons Non-Commercial, No Derivatives (CC BY-NC-ND) licence.
Depression is a common psychiatric disorder, affecting an estimated 350 million people worldwide.1 Depression is projected to become the second global leading cause of disability by the year 2020, trailing only heart disease.2 Severe depression can be fatal, as approximately 60% of the individuals who took their own life had a depressive disorder.3 Despite the wide availability of antidepressant treatments including medications and cognitive–behavioural therapy,4 many patients fail to respond or experience adverse events.4 Only 50–60% of patients will sufficiently respond to first-line treatments, and only 35–40% will experience a remission of symptoms during an 8-week trial of antidepressants.5,6 Therefore, alternative or adjunctive interventions are required to improve depressive symptoms.
Light therapy is an established non-pharmacological intervention used to treat seasonal affective disorder (SAD), a clinical subtype of mood disorder that consists of recurrent episodes of major depression occurring with a predominately seasonal pattern.7,8 The efficacy of light therapy for SAD has been investigated in over 70 clinical trials9 and further supported by several meta-analyses and clinical guidelines.10,11 The clinical potential of light therapy as a treatment for non-seasonal depressive disorders has been previously investigated.11–13 However, the results have failed to yield any firm conclusions, and a new systematic review is warranted to elucidate the application of light therapy as a non-pharmacological option for non-seasonal depression.
Light therapy involves daily exposure to artificial bright light for a set period of time, typically in the morning.8 The light is often delivered through a light box that is equipped with fluorescent tubes, a reflector and a diffusing screen; or using fluorescent ceiling units.8,14 The rationale for using light therapy in individuals with non-seasonal depression is that they often experience atypical symptoms that may be associated with circadian abnormalities, such as irregular sleep–wake patterns, altered social rhythms, diurnal mood swings and altered circadian patterns of hormones and core body temperature.14–16 Light therapy is thought to work through the eyes by activating the suprachiasmatic nucleus (SCN), the circadian pacemaker of the brain. This has been hypothesised as one mechanism by which bright light therapy positively affects mood, sleep, circadian rhythms and hypothalamic–pituitary–adrenal axis activity in patients with SAD.15–17 However, the precise mechanism of the effect of light therapy remains elusive.
A recent review has been published to investigate the efficacy of light therapy in both seasonal and non-seasonal depression.18 However, this review included only two trials for non-seasonal depression due to strict inclusion criteria, thus precluding an adequate assessment of the efficacy of light therapy in non-seasonal depression. A significant gap in summarising the literature has developed since the prior review by Even et al,12 who included 15 studies and did not conduct a meta-analysis. Seven trials have been conducted since the previous reviews,16,19–24 and the four largest trials to date are yet to be included for meta-analysis.16,24–26
The present systematic review aims to summarise the efficacy of light therapy in adults with non-seasonal depression by conducting a qualitative review of the literature, a standardised risk of bias assessment, and computing pooled summary estimates of light therapy's treatment effect. Secondary objectives of this review are to explore potential covariates that may modify the efficacy of light therapy and to summarise potential adverse effects.
Method
The following methodology was established a priori in an unpublished protocol that was registered with PROSPERO (registration number CRD42015017887) and is available on request from the authors. This review was written and conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).27
Study selection
The following electronic databases were searched from their inception until September 2015: MEDLINE®, EMBASE®, PsycINFO, The Cochrane Central Register of Controlled Trials (CENTRAL) and the Cumulative Index to Nursing and Allied Health Literature (CINAHL). A health science librarian (N.B.) and the first author (S.P.) collaborated to develop the search strategy, which is outlined in the Appendix. The reference lists from included articles and past reviews were searched by hand. In regards to grey literature, unpublished and ongoing trials were searched on clincaltrials.gov and from conference proceedings of the Society of Light Treatment and Biological Rhythms (SLTBR).
Inclusion criteria
The search was limited to human studies. Randomised controlled trials (RCTs) that compared light therapy as a stand-alone treatment to an inactive placebo/control group were included. RCTs that used light therapy as an adjunctive treatment (i.e. in combination with pharmacotherapy or psychotherapy) in comparison with the inactive placebo/control group were also included. Trials were included if they compared treatment effect using a standardised and validated depression rating tool. Participants included adults (≥18 years of age) who have non-seasonal depression according to the DSM, the Research Diagnostic Criteria (RCM), ICD or based on a validated psychiatric assessment tool. Individuals with major depressive disorder (MDD) (single episode or recurrent), persistent depressive disorder (dysthymia), bipolar disorder with depression and clinically significant non-seasonal depressive symptoms were included. All formats of light therapy with regard to the timing of administration, the brightness and duration of light exposure, and the selected lighting device were included. Non-randomised trials and quasi-randomised studies were excluded. If investigations enrolled the sample of same patient in multiple trials, only the largest trial was included.
Exclusion criteria
Individuals with a diagnosis of depression with a seasonal pattern, such as SAD or subsyndromal SAD, were excluded. Individuals with premenstrual dysphoric disorder (PMDD) were also excluded.
Data management
Citations were managed using RefWorks© (ProQuest LLC). Two authors independently screened titles and abstracts, and excluded studies that failed to meet at least one eligibility criteria. Full-text articles of the relevant citations were reviewed and included if they satisfied all eligibility criteria. Screening disagreements were resolved by consensus, and a third author was consulted if a resolution could not be reached.
Data extraction
Two authors extracted data using a pilot-tested data extraction form. The data were entered into the Cochrane Collaboration's RevMan Analysis Version 5.3 statistical software for meta-analysis and preption of graphical figures.
Risk of bias and quality assessment
Potential biasing factors of individual studies were assessed independently by two reviewers using the Cochrane Collaboration's Risk of Bias Tool.28 The following factors were adjudicated to have ‘high’, ‘low’ or ‘uncertain’ risk of bias: random sequence generation; allocation concealment; masking of participants, personnel and outcome assessors; incomplete outcome assessment (attrition); selective reporting and other potential biases. A funnel plot was generated to visually inspect publication bias. Outcome-specific rating of the overall quality of evidence was performed using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) system for systematic reviews and implemented using GRADE Pro GDT software (http://tech.cochrane.org/gradepro).
Statistical analyses
Inter-reviewer agreement on study selection was quantified using a kappa statistic.29 Meta-analysis was implemented if the included studies were sufficiently homogeneous in terms of participants, interventions and outcomes to provide a plausible summary effect of light therapy.30 However, when establishing our eligibility criteria, we had intended to pool widely, and then utilise our a priori subgroup analyses to explain potential variability in our results. For continuous outcomes, pooled results were summarised using standardised mean differences (SMDs) and 95% confidence intervals (CIs). We used SMD because the included studies used various depression tools that could not be pooled on the same scale. For dichotomous outcomes, pooled results were summarised using relative risks (RRs) and 95% CIs. A significance level of 5% was used for all statistical tests.
All meta-analyses were carried out using a random-effects model.31 The I2 test statistic was used to assess heterogeneity. The I2 statistic quantifies the proportion of the variability in effect estimates that is caused by underlying differences in treatment effect between studies opposed to chance.32 Subgroup analyses were conducted to explain potential reasons for variability in the estimated effect beyond chance. Sensitivity analyses were employed to determine the impact of studies that included special population (e.g. pregnant women and older age) with depression on the pooled estimate.
The primary outcome for this review was the post-intervention depressive symptom scores following the administration of light therapy v. placebo/control, as measured by a standardised and validated psychometric depressive symptom scale. The large majority of studies did not report mean change scores or the standard deviation in change scores that would be required to use the average change in a depression score from baseline to trial end-point as the primary outcome. Therefore, post-intervention depression scores were used. A secondary outcome was the proportion of patients achieving a clinically significant response following light therapy as determined by a 50% reduction in depressive symptoms (e.g. ≥ 50% reduction on the Hamilton Rating Scale for Depression33 or other scales if used). An additional secondary outcome was to summarise the occurrence of adverse events among those receiving light therapy in comparison with those receiving placebo or standard care.
Subgroup analyses
A subgroup analysis was conducted based on the administration of light therapy as an adjunctive or as a stand-alone treatment. It was hypothesised that stand-alone light therapy would have a treatment effect favouring light therapy more than the studies that implemented light therapy as an adjunct.11
A subgroup analysis was conducted based on whether a dim light was used as a placebo/control or not. A hypothesised direction of effect was not specified beforehand.
A subgroup analysis was conducted for those trials that administered morning light therapy compared with those administering evening or mixed-timed light therapy (i.e. a subset of the intervention group was treated at different times of the day). Considering that the majority of evidence supports the efficacy of morning light therapy in treating SAD,14 it was hypothesised that studies administering morning light therapy would demonstrate a greater treatment effect.
To conduct a subgroup analysis based on depression severity, in-patient/out-patient status was used as a proxy. It was hypothesised that light therapy would have a greater treatment effect among out-patients, who presumably have milder depressive symptoms on average and less comorbidity.
It was expected that differing methodology and quality of the included trials would partially account for heterogeneity. To explain methodological heterogeneity, subgroup analyses were conducted using each Cochrane risk of bias item as a covariate. It was hypothesised that trials with high risk of bias would demonstrate a greater treatment effect of light therapy than trials with lower risk of bias.
Results
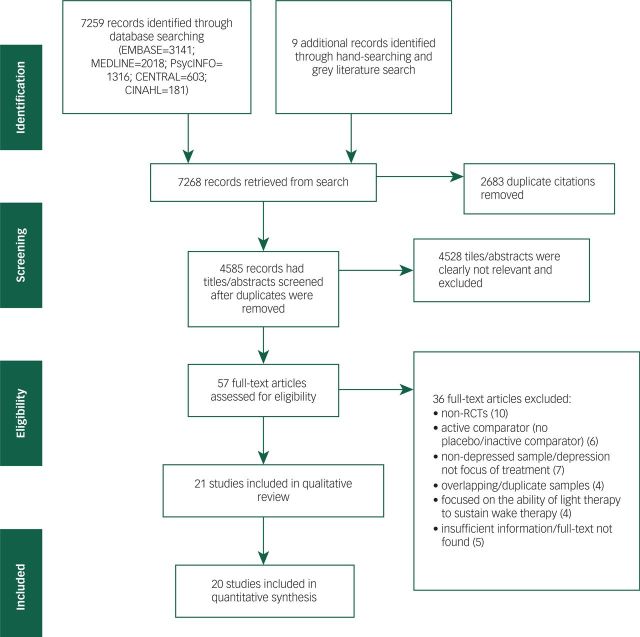
After screening 4585 unique citations, 21 trials were included in this review, including 894 participants (Fig. 1).16,19–26,34–45 The observed unweighted kappa for full-text screening between the two independent reviewers was 0.63 (95% CI 0.44–0.79), suggesting a ‘good’ level of agreement.46
Fig. 1. PRISMA flow chart of the study selection process. RCTs, randomised controlled trials.
Table 1 summarises individual study characteristics. The majority of participants were women as most studies included >50% women. The mean age of participants in each study varied from 32 to 84 years of age. Twenty studies were RCTs implementing a pllel design, and one study was a randomised crossover trial.35 In a recent trial by Özdemir et al,23 we included the 1-week depressive rating score for the treatment and control groups as the primary outcome because light therapy was only administered for 1 week of the 8-week study period. The brightness of light in the intervention groups varied from 400 lux (green light) to 10 000 lux (white/full-spectrum light). Red light is known to be the most biologically inactive frequency of visible light;15 therefore, 13 of 21 studies administered dim red light or dim light as a comptor. The remaining eight studies used alternative non-light-based placebo/control, as presented in Table 1.
Table 1. Study characteristics.
| Study details | Light therapy (intervention) | Control | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Study (first author, year, country) | Diagnosis of depression and diagnostic criteria | Primary depression measure | Length of trial, days | Light therapy details and adjuncts | Number of participants (% female) | Mean age (s.d.) | Post-intervention score: mean (s.d.) | Light therapy details and adjuncts | Number of participants (% female) | Mean age (s.d.) | Post-intervention score: mean (s.d.) |
| Lam et al, 2015a (Canada)24 | MDD (DSM-IV-TR) Out-patients | MADRS | 56 | 10 000 lux 30min/day Morning + 20 mg fluoxetine hydrochloride/day | 29 (51.7%) | 38.9 (12.6) | 10.03 (8.06) | Inactive ion generator 30min/day Morning + 20 mg fluoxetine hydrochloride/day | 31 (71.0%) | 37.3 (11.2) | 17.94 (9.96) |
| Lam et al, 2015b (Canada)24 | MDD (DSM-IV-TR) Out-patients | MADRS | 56 | 10 000 lux 30min/day Morning | 32 (53.1%) | 35.1 (9.6) | 13.88 (11.11) | Inactive ion generator 30min/day Morning + placebo pill | 30 (73.3%) | 36.2 (11.5) | 19.50 (10.54) |
| Özdemir et al, 2015 (Turkey)23 | MDD (DSM-IV) In-patients | HRSD | 7 | 7000 lux 60min/day Morning + 75 mg Venlafaxine/day | 25 (56%) | 33.16 (7.94) | 21.52 (6.41) | No light treatment + 75 mg venlafaxine /day | 25 (52%) | 38.36 (11.84) | 24.56 (6.53) |
| Sit et al, 2014 (USA)19 | BD-I or BD-II with current MDE (SCID), stable-dosed antimanic drug therapy | SIGH-ADS | 42 | Broad-spectrum light 7000 lux 60min/day Afternoon | 23 (% NR) | Range: 18–75 | 10.4 (8.1) | Red light 50 lux 60 min/day Afternoon | 23 (% NR) | Range:18–75 | 17.4 (9.8) |
| Lieverse et al, 2011 (Netherlands)16 | MDD (DSM-IV) Out-patients | HRSD (original) | 21 | Pale blue light 7500 lux 60 min/day Morning | 40 (64%) | 69.67 (8.5) | 10.1 (6.1) | Red light 50 lux 60 min/day Morning | 44 (67%) | 69.00 (6.6) | 10.4 (6.3) |
| Wirz-Justice et al, 2011 (Switzerland)20 | MDD (antepartum onset) (DSM-IV) Out-patients | SIGH-SAD | 35 | White light 7000 lux 60 min/day Morning | 16 (100%) | 31.7 (4.7) | 12.3 (6.7) | Red light 70 lux 60 min/day Morning | 11 (100%) | 32.7 (5.4) | 15.6 (7.7) |
| Franchini et al, 2009 (Italy)21 | BD (MDE w/ psychotic Features (DSM-IV-TR) In-patients | HRSD (21-item) | 42 | Bright light 10 000 lux 30 min/day Morning + 300mg Fluvoxamine (after 1 week of titration) | 17 (41.2%) | 45.2 (14.9) | 2.79 (1.72) | No light treatment + 300 mg fluvoxamine (after 1 week of titration) | 10 (70%) | 54.0 (12.2) | 2.29 (3.15) |
| Corral et al, 2007 (Canada)22 | MDD (postpartum onset) (DSM-IV) Out-patients | SIGH-SAD | 42 | Bright light 10 000 lux 30 min/day Morning | 10 (100%) | 34.6 (4.0) | 14.9 (4.9) | Red light 600 lux 30 min/day Morning | 5 (100%) | 33.6 (2.1) | 15.4 (7.0) |
| Goel et al, 2005 (USA)36 | MDD, single episode, chronic duration = 2 years (DSM-IV) Out-patients | SIGH-SAD | 35 | Bright light 10 000 lux 60 min/day Morning | 10 (75%; total sample) | 43.7 (12.4; total sample) | 11.4 (8.6) | Low-density negative air ions (1.7 x 1011 ions/s) 60 min/day Morning | 10 (75%; total sample) | 47.3 (12.4; total sample) | 22.2 (7.7) |
| Loving et al, 2005 (USA)26 | GDS score = 11 (indicating probably major depression) Out-patients (Home-Based Trial) | GDS | 35 | Bright white light 8500 lux Morning (n=13) Mid-Day (n=15) Evening (n=13) | 41 (58%; total sample) | 67.7 (5.45; total sample) | 14.39 (6.88) | Red light 10 lux Morning (n=15) Mid-Day (n=16) Evening (n=9) | 40 (58%; total sample) | 67.7 (5.45; total sample) | 15.28 (7.07) |
| Martiny et al, 2005 (Denmark)25 | MDD (DSM-IV) Out-patients | HRSD (17 item) | 35 | Bright white light 10 000 lux 60 min/day Morning +50 mg sertraline daily | 48 (70.8%) | 43.1 (15.8) | 9.0 (4.4) | Red light 50 lux 30 min/day Morning + 50 mg sertraline daily | 54 (66.7%) | 45.9 (16.1) | 11.6 (4.3) |
| McEnany & Lee, 2005 (USA)44 | MDD (DSM-IV) Out-patients | BDI (13-item) | 26 | Bright light 2500 lux 60 min/day (visor not light box) Morning | 16 (100%) | NR | 14.1 (5.1) | Circadian adaptation glasses (filters out light) 60 min/day Night | 13 (100%) | NR | 22.49 (2.7) |
| Epperson et al, 2004 (USA)34 | MDD (antepartum onset) (DSM-IV) Out-patients | SIGH-SAD | 35 | Bright light 7000 lux 60 min/day Morning | 5 (100%) | 34.2 (3.96) | 13.4 (11.1) | Dim light 500 lux 60 min/day | 5 (100%) | 34.0 (1.58) | 11.3 (10.1) |
| Tsai et al, 2004 (Taiwan)43 | MDD or depressive disorders (DSM-IV) In-patients | GDS | 5 | Bright light 5000 lux 50 min/day Morning | 30 (40%) | 75.3 (7.4) | 13.2 (3.5) | NR | 30 (50%) | 74.6 (5.7) | 16.6 (4.7) |
| Benedetti et al, 2003 (Italy)42 | MDD and BD (without psychotic features) (DSM-IV) In-patients | HRSD | 28 | Green light 400 lux Morning + 40 mg citalopram daily | 18 (83.3%) | 53.0 (10.3) | 7.39 (7.72) | Deactivated negative ion generator Morning + 40 mg citalopram daily | 12 (75%) | 56.2 (12.3) | 13.08 (8.30) |
| Loving et al, 2002 (USA)45 | MDD (DSM-IV) Out-patients | HRSD | 7 | Bright white light 10 000 lux 30 min/day Morning | 7 (84.6%; total sample) | 44 (26–56; total sample) | 17.43 (11.44) | Dim red light 100 lux 30 min/day | 6 (84.6%; total sample) | 44 (26–56; total sample) | 15 (8.1) |
| Prasko et al, 2002 (Czech Republic)41 | Recurrent MDD (DSM-III-R) In-patients | HRSD (21-item) | 21 | Bright light 5000 lux 120 min/day +150 mg imipramine/day Morning | 11 (72.7%) | 41.0 (9.3) | 17.0 (11.2) | Dim red light 500 lux 120 min/day +150 mg Imipramine day | 9 (66.7%) | 43.2 (10.9) | 13.0 (7.9) |
| Sumaya et al, 2001 (USA) *Crossover trial35 | Score of 11-20 on GDS-institutionalised older adults | GDS | 5 | Bright light 10 000 lux 30 min/day Morning | 10 (60%) | 83.8 (9.56) | 11.3 (0.74) | Dim light 300 lux 30 min/day | 10 (60%) | 83.8 (9.56) | 15.4 (0.86) |
| Yamada et al, 1995 (Japan)40 | MDD (n=17) BD (n=10) with depression (DSM-III-R) In-patients | HRSD (21 item) | 7 | Bright light 2500 lux 120 min/day Morning (n=12) Evening (n=15) | 18 | 47.6 (total sample) | 11.37 (6.37) | Dim light 500 lux 120 min/day | 9 | 47.6 (total sample) | 19.33 (6.11) |
| Holsboer-Trachsler et al, 1994 (Switzerland)39 | MDE (DSM-III-R) (n=12 first episode; n=23 recurrent; n=6 BD; n=1 dysthymia) In-patients | HRSD (17-item) | 42 | Bright light 5000 lux 120 min/day Evening Trimipramine 200 mg/day | 14 (64.3%) | 55.1 (10.7) | 14.07 (5.83) | No light treatment +200 mg/day trimipramine 200 mg/day | 14 (35.7%) | 50.6 (8.5) | 8.54 (8.53) |
| Kripke et al, 1992 (USA)38 | MDD or BD with depression (DSM-III) In-patients | 7 | Bright white light 2000–3000 lux 180 min/day Morning (n=12) Evening (n=39) | 25 (2.0%; total sample) | 48 (22–71; total sample) | 14.7 (5.4) | Dim red light 50 lux 180 min/day | 26 (2.0%; total sample) | 48 (22–71; total sample) | 14 (4.8) | |
| Mackert et al, 1991 (Germany)37 | MDD (research diagnostic criteria) In-patients | HRSD | 7 | Bright white light 2500 lux 120 min/day Morning | 22 (77.3%) | 51.5 (14.7) | 15.3 (5.0) | Dim red light 50 lux 120 min/day Morning | 20 (85%) | 57.2 (11.7) | 17.3 (6.2) |
BD, bipolar disorder; DSM, Diagnostic and Statistical Manual of Mental Disorders; GDS, Geriatric Depression Scale; HRSD, Hamilton Rating Scale for Depression; MADRS, Montgomery–Åsberg Depression Rating Scale; MDD, major depressive disorder; MDE, major depressive episode; NR, not reported; SIGH-SAD, Structured Interview Guide for the Hamilton Rating Scale for Depression, Seasonal Affective Disorders.
Adjunctive light therapy (fluoxetine + light therapy v. deactivated ion generator + fluoxetine).
stand-alone light therapy (light therapy + placebo pill v. deactivated ion generator + placebo pill).
Thirteen studies investigated the efficacy of light therapy among patients with MDD,16,20,22–25,34,36,37,41,43–45 including three studies of pregnant women with post-partum22 or antepartum onset.20,34 Four studies included patients with MDD or patients with bipolar disorder depression.38–40,42 One study investigated the efficacy of light therapy for treating patients with bipolar disorder depression,19 and another study recruited patients with bipolar disorder admitted to hospital because of a major depressive episode with psychotic features.21 Two studies recruited geriatric patients who were not clinically diagnosed with depression, but whose Geriatric Depression Scale (GDS) scores suggested moderate to severe levels of depressive symptoms.26,35
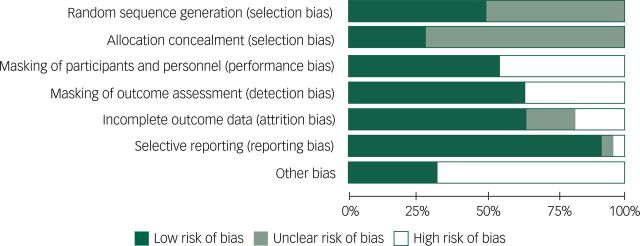
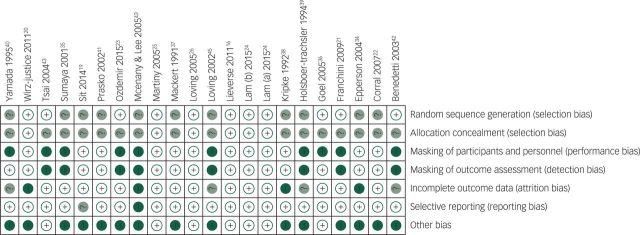
Risk of bias assessment
Figures 2 and 3 summarise the risk of bias assessments using the Cochrane Risk of Bias Tool. Most studies were judged to have ‘high’ or ‘unclear’ risk of bias for at least one essential methodological criterion. Approximately 75% of the randomised trials did not report the method of allocation concealment when randomising participants. Nearly 50% of the included trials failed to report the method of randomised sequence generation. Masking of outcome assessors was implemented by 13 of 21 studies, and only 11 of 21 studies clearly reported some form of masking of study participants. The majority of included studies did not state whether a ‘complete-case’ or ‘intention-to-treat’ analysis was conducted, and the number of individuals approached for enrolment was rarely reported. Although it was concluded that incomplete outcome data were of ‘low’ risk of bias in the majority of studies, without knowing the number of participants approached for enrolment, it is difficult to conclude whether final numbers included in analyses truly reflect attrition. ‘Other’ reasons for ‘high’ risk of bias included: reporting only total sample demographics as opposed to demographics specific to each trial arm26,44 and failing to present a power calculation. Furthermore, over 50% of the included studies were likely biased towards type II errors due to particularly small sample sizes of less than 20 people in each arm.
Fig. 2. Risk of bias graph: review authors’ judgements about each risk of bias item presented as percentages across all included studies.
Fig. 3. Risk of bias graph: review authors’ judgements about each risk of bias item for each included study.
+, low risk of bias; −, high risk of bias; ?, uncertain risk of bias.
Meta-analyses: effect of light therapy on depressive symptoms
One study was excluded from the meta-analysis because it was a randomised crossover design that used the same participants in the treatment and control arms.35 Including this study would violate the assumption of independence that assumes the effect sizes computed for each trial arm are independent of each other. Therefore, our analysis is applicable to pllel group study designs only, and the pooled summary estimate was computed using 20 studies and a collective sample of 881 individuals: 456 individuals who received light therapy and 425 individuals who received placebo/control. Lam et al24 randomised participants into four different groups to assess the efficacy of light therapy as a monotherapy (compared with a deactivated negative ion generator+placebo pill) and as an adjunctive therapy to fluoxetine (compared with a deactivated negative ion generator+fluoxetine). Therefore, we included two septe estimates of effect from Lam et al24 in our meta-analyses for their (a) adjunctive and (b) monotherapy comparisons. The forest plot (Fig. DS1) of the primary meta-analysis shows that the pooled post-trial SMD in depression scores was −0.41 (95% CI −0.64 to −0.18; P=0.0004), representing a small to moderate effect of light therapy in improving symptoms of depression in comparison with placebo/control treatment. However, this pooled summary estimate was associated with substantial heterogeneity as indicated by an I2 value of 60% (P=0.0003). Potential contributors to heterogeneity across the included studies were explored using subgroup analyses.
Subgroup analyses
The first subgroup analysis compared adjunctive light therapy with stand-alone light therapy (Fig. DS2). A test for interaction was in the plausible range to support a difference in the subgroup effect (χ2=2.46, P=0.12) albeit not statistically significant. The adjunct light therapy subgroup was associated with significant heterogeneity (I2=58%, P=0.008) and a non-significant overall SMD in depression scores of −0.25 (95% CI −0.53 to 0.03; P=0.08). The stand-alone light therapy subgroup was similarly associated with substantial heterogeneity (I2=60%, P =0.008); however, the stand-alone light therapy was shown to have a greater and significant treatment effect: −0.63 (95% CI −1.00 to −0.25, P=0.001).
The second subgroup analysis (Fig. DS3) compared light therapy administered in the morning compared with light therapy administered in the evening and/or at varying times of the day. A test for differences in effect between the aforementioned subgroups was not significant (χ2=1.52, P=0.22). The morning light therapy produced a greater treatment effect in reducing depressive symptoms than the light therapy given at other times of the day, with an SMD of −0.50 (95% CI −0.73 to −0.27, P<0.0001). However, substantial heterogeneity was seen (I2=48%, P=0.01). Evening or mixed-timed light therapy was associated with even greater heterogeneity (I2=74%, P=0.009) and a non-significant SMD of −0.08 (95% CI −0.70 to 0.53, P=0.79).
A third subgroup analysis examined the effect of light therapy in treating in-patient in comparison with out-patient settings. A test for differences in effect between the aforementioned subgroups was not significant (χ2=1.08, P=0.30). The effect of light therapy among out-patients was significant, producing an SMD in depression scores of −0.50 (95% CI −0.81 to −0.20, P=0.001) with significant heterogeneity (I2=58%, P=0.008). The effect of light therapy among in-patients was non-significant, an SMD in depression scores of −0.25 (95% CI −0.63 to 0.13, P=0.21), accompanied by substantial heterogeneity (I2=64%, P=0.004) (Fig. DS4).
The fourth subgroup analysis (Fig. DS5) compared those studies that used a form of placebo light with those that used a non-light-based control. Although there were no significant subgroup differences (χ2=1.80, P=0.18), those studies that compared light therapy with a placebo light (typically dim red light) demonstrated a smaller −0.27 (95% CI −0.51 to −0.04, P=0.02) treatment effect compared with those studies using a non-light-based comptor of −0.60 (95% CI −1.01 to −0.18, P=0.005). Studies that used light-based placebo were associated with non-significant heterogeneity (I2=35%, P=0.11) and those that used non-light-based placebo/control were associated with significant heterogeneity (I2=71%, P=0.005).
Secondary meta-analysis
Figure DS6 shows the results of a meta-analysis of 13 of 21 studies that reported the number of patients achieving a clinical response, defined as a 50% reduction in depressive symptoms, following a trial of light therapy or placebo light/control. Collectively, 172 (59.1%) individuals in the light therapy group and 104 (38.0%) individuals in the placebo/control group achieved a clinical response post-trial among a total of 565 participants with depression. The meta-analysis shows an RR of 0.67 (95% CI 0.54–0.82; P=0.0001, I2=21%, P=0.23), demonstrating a significant effect of light therapy in reducing depressive symptoms.
Sensitivity analyses
In the primary pooled analysis, removing the three studies that assessed light therapy in pregnant women with depression marginally affected the results.20,22,34 Similarly, removing the three studies assessing light therapy among geriatric patients only marginally affected the results.16,26,43
Adverse events
In total, 15 of 21 studies commented on adverse events associated with light therapy16,20–22,24–26,34,35,38,41–43 and only 5 of the 15 studies reported using a standardised method or tool to investigate differences in adverse events between the light therapy and control groups. Lam et al24 used the Adverse Events Scale and reported that the percentage of patients reporting at least one treatment-emergent adverse event was not significantly different between light therapy and control groups. Lieverse et al16 used masked interviews to assess adverse events on 28 different items using a 4-point rating scale. They reported that both light therapy and placebo were well tolerated and that both groups did not differ in their adverse event profile. Wirz-Justice et al20 monitored adverse effects with the self-reported Systematic Assessment for Treatment Emergent Effect (SAFETEE) questionnaire on a weekly basis and reported no clinically meaningful side-effects at any point in time. Furthermore, this study was conducted on patients with antepartum depression, and the authors reported no perinatal complications among all women in the sample. Martiny et al25 used the Udvalg for Kliniske Undersogelser (UKU) side-effect rating scale. They reported that nausea and diarrhoea increased equally in both the intervention and control groups, but headache and eye irritation increased more in the light therapy group. However, it was noted that side-effects were generally mild and were not interfering with patients’ overall performance. Last, Loving et al26 also used the SAFETEE questionnaire and found no significant differences in change scores between the light therapy and control group for all 94 items.
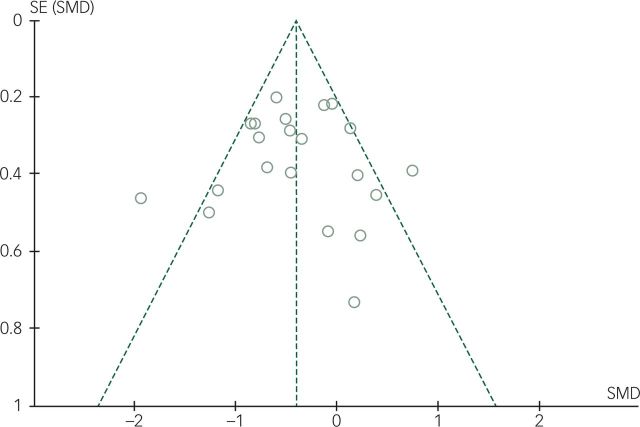
Publication bias
A funnel plot (Fig. 4) was generated using the 20 studies included in the meta-analysis. One study34 was small in size, with only five participants randomised to each arm, and two participants from the placebo arm had dropped out before the final outcome assessment. This study appears as an outlier in the bottom right quadrant of the plot. Excluding the aforementioned study, there appears to be a fair amount of symmetry about the funnel, suggesting little evidence of publication bias.
Fig. 4. Funnel plot showing publication bias.
Each dot on the funnel plot represents an individual study estimate included in the primary meta-analysis (Fig. DS1). The graph relates the precision of included studies to the magnitude of light therapy's treatment effect in each study. The y-axis displays the inverse of the standard error (precision), and the x axis displays the standardised mean difference (treatment effect). The absence of publication bias will result in a plot that resembles a symmetrical inverted funnel as outlined by the dashed lines. If there is publication bias, for example, because smaller studies without statistically significant effects are not published, the funnel plot will appear asymmetrical with a visible gap. SE, standard error; SMD, standardised mean difference.
Discussion
The present systematic review and meta-analysis sought to investigate the efficacy of light therapy in treating non-seasonal depressive disorders and symptoms in adults. We found that light therapy was associated with a small-to-moderate effect in reducing depressive symptoms as compared with placebo and control treatment. However, our primary pooled estimate was associated with substantial heterogeneity. Considering the criteria outlined by Sun et al47 to evaluate the credibility of subgroup analyses, none of our a priori hypothesised subgroup analyses revealed statistically significant differences in treatment effect that could sufficiently explain the existing variability between individual study estimates. Nonetheless, the subgroup analyses demonstrate that light therapy may be most effective when applied as a stand-alone treatment, when administered in the morning and among out-patients who presumably have less severe depressive symptoms and comorbidities than in-patients. Furthermore, it is evident that studies that did not use placebo light as a comptor partially inflated the overall pooled estimate as those studies demonstrated twice the reduction in depressive symptoms than studies administering placebo light to the control group. Adverse events previously reported to be associated with light therapy include eye strain, mild headache, nausea, agitation, hypomania, initial disruptions in sleep patterns, and some minor concerns of switching to mania8,15 were not of any significance in this review.
The majority of studies included in this review were small, which may have resulted in their individual point estimates being underpowered and prone to type II errors. The four largest studies made up 44.2% (389 of 881) of the participants in the primary pooled analysis,16,24–26 which included 20 studies in total. Notably, the aforementioned four studies were among the highest quality trials and were associated with the lowest risk of bias. High risk of bias was an issue among the majority of studies for at least one of the criteria of the Cochrane Risk of Bias Tool (Figs. 2 and 3). Masking and placebo effect remain a threat to the validity of the results of these trials. It is understandable, however, in these types of interventions, participants cannot be masked to light therapy or placebo light to the same degree that is possible with a drug trial because they will be aware of the colour and luminosity of the light. However, investigators often ‘masked’ participants to their study hypothesis.
Four previous systematic reviews have investigated the efficacy of light therapy for non-seasonal depression, including two qualitative reviews12,18 and two meta-analyses.11,13 The latest review by Mårtensson et al18 implemented strict inclusion criteria and included only two trials that investigate light therapy among patients with non-seasonal depression. Furthermore, they failed to include several trials that seem to meet their inclusion criteria.16,25 Even et al12 qualitatively reviewed 15 studies and contrastingly suggested that light therapy was the most efficacious as an adjunctive treatment to pharmacotherapy. Although several individual trials included in this review support light therapy as an adjunctive treatment,23–25 our results suggest that light therapy is most effective as a stand-alone treatment which is supported by the prior meta-analysis by Golden et al.11 Our meta-analysis is the largest to date because it included 20 trials in total.
We implemented the GRADE framework to evaluate the overall quality of evidence and to assess the confidence in our pooled estimates (Table 2; see supplementary Table DS1 for a summary of the findings). We concluded that the evidence supporting the overall efficacy of light therapy in reducing non-seasonal depressive symptoms is low. Among the greatest threats to the validity were high risk of bias among individual trials and inconsistency in study estimates as suggested by the high heterogeneity associated with the pooled estimate that could not be sufficiently explained in subgroup analyses. The overall quality of evidence in regards to the secondary dichotomous outcome of the proportion of individuals achieving a clinical response was considered to be moderate with minimal heterogeneity (I2=21%). Furthermore, the results revealed a beneficial effect of light therapy in reducing depressive symptoms (RR=0.67; 95% CI 0.54–0.82) among 565 patients (Fig. DS6).
Table 2. GRADE evidence table.
| Quality assessment | No. of patients | Effect | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Light therapy | Placebo/control | Relative (95% CI) | Absolute (95% CI) | Quality | Importance |
| Difference in depressive symptom scores SMD | ||||||||||||
| 20 | randomised trials | Serious1 | Serious2 | Not serious | Not serious | None | 456 | 425 | – | SMD 0.41 lower (0.64 lower to 0.18 lower) | ⊕⊕○○Low | Critical |
| Relative risk of achieving a clinical response defined as a 50% reduction in depressive symptoms | ||||||||||||
| 13 | Randomised trials | Serious3 | Not serious3 | Not Serious | Not Serious | None | 172/291(59.1%) | 104/274 (40.0%) | RR 0.67 (0.54–0.82) | 10 Fewer per 100 (from 6 fewer to 16 fewer) | ⊕⊕⊕○Moderate | Important |
SMD, standardised mean difference; RR, relative risk.
A large majority of trials had at least one criterion marked as 'high risk of bias' using the Cochrane Risk of Bias Tool. The majority of trials did not clearly report the methods of random sequence generation or allocation concealment. Masking was not conducted and/or reported for the majority of trials. Only 5 of 21 studies were considered low risk of bias (Lam et al,24 Lieverse et al,16 Wirz-Justice et al,20 Martiny et al25 and Loving et al26).
The pooled summary estimate was associated with substantial heterogeneity (I2=63%, P<0.001). Fifteen studies had a point estimate favouring light therapy; however, six studies had an estimate favouring placebo/control. Four a priori subgroup analyses were conducted (adjunct v. monotherapy; in-patients v. out-patients; morning v. evening/mixed time; placebo light v. white light). However, no subgroups were significantly different in treatment effect.
The majority of studies included in this meta-analysis had at least one high risk of bias item on the Cochrane Risk of Bias Tool. Furthermore, only 13 of 21 trials reported on this outcome and were included in the forest plot (Fig. DS6). The point estimate is associated with minimal heterogeneity (I2=21%, P=0.23) and 12 of 13 point estimates suggest a benefit of light therapy over placebo/control in reducing depressive symptoms.
SMD, standardised mean difference; RR, relative risk.
Strengths and limitations
This review was conducted systematically with an a priori design that involved a qualitative and quantitative summary of the efficacy of light therapy in treating non-seasonal depression. A relatively large number of studies were included in a pooled summary estimate. However, the clinical interpretability of the pooled estimate is limited by opting to standardise the mean differences due to varying measurement tools among studies. Furthermore, only five studies conducted a standardised assessment of adverse events (using different methods) precluding a quantitative assessment of this secondary outcome. Depression scores at the end of each trial were used as the primary outcome in the meta-analyses as opposed to an average change in score from baseline given that the majority of studies did not report mean change scores and standard deviations. This is a limitation because the final depression scores are affected by between-participant differences at the baseline that may not have been perfectly balanced by randomisation. However, change scores require measuring levels of depression on two different occasions which may decrease precision due to measurement error.46
This review suggests that there is low quality evidence (e.g. due to high risk of bias, inconsistency in individual effect estimates, heterogeneous trial designs) to support light therapy's efficacy in treating non-seasonal depressive symptoms among adults. However, considering that light therapy is a non-pharmacological option with minimal side-effects, and that our pooled estimate demonstrated a statistically significant small to moderate treatment effect in reducing depressive symptoms, light therapy may be a useful option for patients with non-seasonal depression. The primary outcome was reinforced by secondary data demonstrating that a significantly greater proportion of patients with depression achieved at least 50% reduction in their depressive symptoms when using light therapy compared with placebo/control condition. Patients who are non-responsive or ineligible for pharmacotherapy may benefit from stand-alone light therapy. The safety profile may be of particular interest for women with depression in the perinatal period where medications may be inappropriate or ineffective.
Appendix
MEDLINE search strategy (January 2015)
depressive disorder.mp. or exp Depressive Disorder/
exp Depression/ or depression.mp.
depress*.mp.
bipolar depression.mp. or exp Bipolar Depression/
nonseasonal.mp.
non-seasonal.mp.
exp mood disorders/
dysthym*.mp.
((mood or affective*) adj3 disorder*).mp.
(bipolar or manic or mania or hypomani*.tw.
1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10
phototherap*.mp. or exp Phototherapy/
photo therap*.mp.
light therap*.mp.
light treatment*.mp.
bright light*.mp.
light.ti.
(blue adj4 light*).mp.
(light box or golite).mp.
12 or 14 or 15 or 16 or 17 or 18 or 19
11 and 20
animals/not humans/
21 not 22.
Funding
Z.S. is supported by the Canadian Institute for Health Research (CIHR) (Randomised Controlled Trials: Mentoring, code number 201303MTP-303860-182743). L.T. is the CIHR mentor on this award. She is also supported by the Brain and Behavior Research Foundation Young Investigator (Grant no. 19058). The funding bodies have no role in the design, collection, analysis or interpretation or reporting of data.
References
- 1.World Health Organzation (WHO). Depression Fact Sheet. WHO, 2015 (http://www.who.int/mediacentre/factsheets/fs369/en/).
- 2.Lopez AD, Murray CJ. The Global Burden of Disease: A Comprehensive Assessment of Mortality and Disability from Diseases, Injuries, and Risk Factors in 1990 and Projected to 2020. Harvard School of Public Health, 1996. [Google Scholar]
- 3.Cavanagh JT, Carson AJ, Sharpe M, Lawrie SM. Psychological autopsy studies of suicide: a systematic review. Psychol Med 2003; 33: 395–405. [DOI] [PubMed] [Google Scholar]
- 4.American Psychological Association. Practice Guideline for the Treatment of Patients with Major Depressive Disorder. APA, 2010. [Google Scholar]
- 5.Connolly KR, Thase ME. If at first you don't succeed. Drugs 2011; 71: 43–64. [DOI] [PubMed] [Google Scholar]
- 6.Fava M. Diagnosis and definition of treatment-resistant depression. Biol Psychiatry 2003; 53: 649–59. [DOI] [PubMed] [Google Scholar]
- 7.Rosenthal NE, Sack DA, Gillin JC, Lewy AJ, Goodwin FK, Davenport Y, et al. Seasonal affective disorder: a description of the syndrome and preliminary findings with light therapy. Arch Gen Psychiatry 1984; 41: 72–80. [DOI] [PubMed] [Google Scholar]
- 8.Terman M, Terman JS. Light therapy for seasonal and nonseasonal depression: efficacy, protocol, safety and side effects. CNS Spectr 2005; 10: 647–63. [DOI] [PubMed] [Google Scholar]
- 9.Lam R, Levitt A, Levitan R, Enns M, Morehouse R, Michalak E, et al. The Can-SAD study: a randomized controlled trial of the effectiveness of light therapy and fluoxetine in patients with winter seasonal affective disorder. Am J Psychiatry 2006; 163: 805–12. [DOI] [PubMed] [Google Scholar]
- 10.Lee T, Chan C. Dose-response relationship of phototherapy for seasonal affective disorder: a meta-analysis. Acta Psychiatr Scand 1999; 99: 315–23. [DOI] [PubMed] [Google Scholar]
- 11.Golden RN, Gaynes BN, Ekstrom RD, Hamer RM, Jacobsen FM, Suppes T, et al. The efficacy of light therapy in the treatment of mood disorders: a review and meta-analysis of the evidence. Am J Psychiatry 2005; 162: 656–62. [DOI] [PubMed] [Google Scholar]
- 12.Even C, Schröder CM, Friedman S, Rouillon F. Efficacy of light therapy in nonseasonal depression: a systematic review. J Affect Disord 2008; 108: 11–23. [DOI] [PubMed] [Google Scholar]
- 13.Tuunainen A, Kripke DF, Endo T. Light therapy for non-seasonal depression. Cochrane Database Syst Rev 2004; 2: CD004050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pail G, Huf W, Pjrek E, Winkler D, Willeit M, Praschak-Rieder N, et al. Bright-light therapy in the treatment of mood disorders. Neuropsychobiology 2010; 64: 152–62. [DOI] [PubMed] [Google Scholar]
- 15.Oldham MA, Ciraulo DA. Bright light therapy for depression: review of its effects on chronobiology and the autonomic nervous system. Chronobiol Int 2014; 31: 305–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lieverse R, Van Someren EJ, Nielen MM, Uitdehaag BM, Smit JH, Hoogendijk WJ. Bright light treatment in elderly patients with non-seasonal major depressive disorder: a randomized placebo-controlled trial. Arch Gen Psychiatry 2011; 68: 61–70. [DOI] [PubMed] [Google Scholar]
- 17.Thalén BE, Mørkrid L, Kjellman B, Wetterberg L. Cortisol in light treatment of seasonal and non-seasonal depression: relationship between melatonin and Cortisol. Acta Psychiatr Scand 1997; 96: 385–94. [DOI] [PubMed] [Google Scholar]
- 18.Mårtensson B, Pettersson A, Berglund L, Ekselius L. Bright white light therapy in depression: a critical review of the evidence. J Affect Disord 2015; 182: 1–7. [DOI] [PubMed] [Google Scholar]
- 19.Sit D, McGowan J, Wiltrout C, Dills J, Weingarden J, Diler RS, et al. Light therapy for bipolar depression: a randomized, double-blind, parallel placebo-control trial. Neuropsychopharmacology 2014; 39: S56–67. [Google Scholar]
- 20.Wirz-Justice A, Bader A, Frisch U, Stieglitz R-D, Alder J, Bitzer J, et al. A randomized, double-blind, placebo-controlled study of light therapy for antepartum depression. J Clin Psychiatry 2011; 72: 986–93. [DOI] [PubMed] [Google Scholar]
- 21.Franchini L, Ballan S, Colombo C, Smeraldi E. Light therapy and fluvoxamine in the treatment of bipolar psychotic depression: a pilot study. Clin Neuropsychiatry 2009; 6: 166–74. [Google Scholar]
- 22.Corral M, Wardrop A, Zhang H, Grewal A, Patton S. Morning light therapy for postpartum depression. Arch Women's Ment Health 2007; 10: 221–4. [DOI] [PubMed] [Google Scholar]
- 23.Özdemir PG, Boysan M, Smolensky MH, Selvi Y, Aydin A, Yilmaz E. Comparison of venlafaxine alone versus venlafaxine plus bright light therapy combination for severe major depressive disorder. J Clin Psychiatry 2015; 76: 645–54. [DOI] [PubMed] [Google Scholar]
- 24.Lam RW, Levitt AJ, Levitan RD, Michalak EE, Morehouse R, Ramasubbu R, et al. Efficacy of bright light treatment, fluoxetine, and the combination in patients with nonseasonal major depressive disorder: a randomized clinical trial. JAMA Psychiatry 2016; 73: 56–63. [DOI] [PubMed] [Google Scholar]
- 25.Martiny K, Lunde M, Unden M, Dam H, Bech P. Adjunctive bright light in non-seasonal major depression: results from clinician-rated depression scales. Acta Psychiatrica Scandinavica 2005; 112: 117–25. [DOI] [PubMed] [Google Scholar]
- 26.Loving RT, Kripke DF, Elliott JA, Knickerbocker NC, Grandner MA. Bright light treatment of depression for older adults [ISRCTN55452501]. BMC Psychiatry 2005; 5: 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009; 151: 26–49. [DOI] [PubMed] [Google Scholar]
- 28.Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011; 343: d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Viera AJ, Garrett JM. Understanding interobserver agreement: the kappa statistic. Fam Med 2005; 37: 360–3. [PubMed] [Google Scholar]
- 30.Guyatt GH, Oxman AD, Kunz R, Atkins D, Brozek J, Vist G, et al. GRADE guidelines: 2. Framing the question and deciding on important outcomes. J Clin Epidemiol 2011; 64: 395–400. [DOI] [PubMed] [Google Scholar]
- 31.Guyatt G, Rennie D. Users’ Guides to the Medical Literature: A Manual for Evidence-based Clinical Practice. AMA Press, 2002. [Google Scholar]
- 32.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003; 327: 55–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23: 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Epperson CN, Terman M, Terman JS, Hanusa BH, Oren DA, Peindl KS, et al. Randomized clinical trial of bright light therapy for antepartum depression: preliminary findings. J Clin Psychiatry 2004; 65: 421–5. [DOI] [PubMed] [Google Scholar]
- 35.Sumaya IC, Rienzi BM, Deegan JF, Moss DE. Bright light treatment decreases depression in institutionalized older adults a placebo-controlled crossover study. J Gerontol A Biol Sci Med Sci 2001; 56: M356–60. [DOI] [PubMed] [Google Scholar]
- 36.Goel N, Terman M, Terman JS, Macchi MM, Stewart JW. Controlled trial of bright light and negative air ions for chronic depression. Psychol Med 2005; 35: 945–55. [DOI] [PubMed] [Google Scholar]
- 37.Mackert A, Volz H-P, Stieglitz R-D, Müller-Oerlinghausen B. Phototherapy in nonseasonal depression. Biol Psychiatry 1991; 30: 257–68. [DOI] [PubMed] [Google Scholar]
- 38.Kripke DF, Mullaney DJ, Klauber MR, Risch SC, Gillin JC. Controlled trial of bright light for nonseasonal major depressive disorders. Biol Psychiatry 1992; 31: 119–34. [DOI] [PubMed] [Google Scholar]
- 39.Holsboer-Trachsler E, Hemmeter U, Hatzinger M, Seifritz E, Gerhard U, Hobi V. Sleep deprivation and bright light as potential augmenters of antidepressant drug treatment – neurobiological and psychometric assessment of course. J Psychiatr Res 1994; 28: 381–99. [DOI] [PubMed] [Google Scholar]
- 40.Yamada N, Martin-Iverson MT, Daimon K, Tsujimoto T, Takahashi S. Clinical and chronobiological effects of light therapy on nonseasonal affective disorders. Biol Psychiatry 1995; 37: 866–73. [DOI] [PubMed] [Google Scholar]
- 41.Prasko J, Horacek J, Klaschka J, Kosova J, Ondrackova I, Sipek J. Bright light therapy and/or imipramine for inpatients with recurrent non-seasonal depression. Neuroendocrinol Lett 2002; 23: 109–13. [PubMed] [Google Scholar]
- 42.Benedetti F, Colombo C, Serretti A, Lorenzi C, Pontiggia A, Barbini B, et al. Antidepressant effects of light therapy combined with sleep deprivation are influenced by a functional polymorphism within the promoter of the serotonin transporter gene. Biol Psychiatry 2003; 54: 687–92. [DOI] [PubMed] [Google Scholar]
- 43.Tsai YF, Wong TK, Juang YY, Tsai HH. The effects of light therapy on depressed elders. Int J Geriatr Psychiatry 2004; 19: 545–8. [DOI] [PubMed] [Google Scholar]
- 44.McEnany GW, Lee KA. Effects of light therapy on sleep, mood, and temperature in women with nonseasonal major depression. Issues Ment Health Nurs 2005; 26: 781–94. [DOI] [PubMed] [Google Scholar]
- 45.Loving RT, Kripke DF, Shuchter SR. Bright light augments antidepressant effects of medication and wake therapy. Depress Anxiety 2002; 16: 1–3. [DOI] [PubMed] [Google Scholar]
- 46.Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Wiley Online Library, 2008. [Google Scholar]
- 47.Sun X, Briel M, Walter SD, Guyatt GH. Is a subgroup effect believable? Updating criteria to evaluate the credibility of subgroup analyses. BMJ 2010; 340: c117. [DOI] [PubMed] [Google Scholar]