Abstract
Stimulator of interferon genes (STING) is a cellular sensor that controls cytosolic DNA-activated innate immune signaling. We have previously demonstrated that STING-deficient mice are resistant to carcinogen-induced skin cancer, similar to myeloid differentiation primary response gene 88 (MyD88) deficient mice, since the production of STING-dependent DNA-damage-induced proinflammatory cytokines, that likely require MyD88 signaling to exert their growth-promoting activity, are prevented. In contrast, MyD88-deficient mice are sensitive to colitis-associated cancer (CAC), since selected cytokines generated following DNA-damage also activate repair pathways, which can help prevent tumor development. Here, we demonstrate that STING signaling facilitates wound repair processes and that analogous to MyD88-deficient mice, STING-deficient mice (SKO) are prone to CAC induced by DNA-damaging agents. SKO mice harboring tumors exhibited low levels of tumor-suppressive interleukin-22 binding protein (IL-22BP) compared to normal mice, a cytokine considered critical for preventing colon-related cancer. Our data indicate that STING constitutes a critical component of the host early response to intestinal damage and is essential for invigorating tissue repair pathways that may help prevent tumorigenesis.
INTRODUCTION
The innate immune system provides the first line of defense against pathogen infection though can also influence pathways than can control tumorigenesis.1,2 For example, it is known that the cellular adapter MyD88 (myeloid differentiation primary response gene 88) that facilitates Toll-like receptor and interleukin-1 receptor (IL-1R) signaling pathway in the innate immune response can regulate tumorigenesis through control of nuclear factor-κB (NF-κB) activation, cytokine secretion and inflammation.2 Mice lacking MyD88 are resistant to carcinogen (7,12-dimethylbenz(a)anthracene (DMBA)) induced skin cancer since MyD88-controlled cytokine and growth factor production, which augments skin tumorigenesis is averted.3 Of note is that the mechanisms underlining carcinogen-driven cytokine production largely remain unknown.
Stimulator of interferon genes (STING) is a cellular protein that is critical for activating the production of various cytokines such as type I interferon in response to the detection of microbial double-stranded DNA (dsDNA) or cyclic dinucleotides (CDNs).4–6 In addition, self-DNA is also capable of activating STING-dependent signaling and this pathway has now been shown to play a key role in the cause of a variety of autoinflammatory diseases.7,8 Recently, it has been further reported that STING-dependent cytokine production can be induced by DNA-damaging agents, including DMBA. It was observed that DMBA caused nucleosome release into the cytosol to trigger STING activity.9 STING was found to control the expression of cytokines requiring MyD88 signaling for the extended production of proinflammatory cytokines such as tumor necrosis factor α.9 Thus, STING-deficient mice were observed to be resistant to DMBA-aggravated skin carcinogenesis, similar to MyD88-deficient mice.9
However, MyD88-deficient mice are known to be susceptible to colitis-associated carcinogenesis (CAC) induced by drugs such as azoxymethane (AOM) and dextran sulfate sodium (DSS).10 AOM is the metabolite of 1,2-dimethylhydrazine (DMH) and is converted to methylazoxymethanol, which mediates O-methylguanine formation to trigger DNA damage responses.11 A single injection of AOM into mice, followed by administration of the inflammatory agent DSS via drinking water induces almost 100% colon cancer. In this situation, MyD88 exerts a protective effect in part by facilitating the production of interleukin-18 (IL-18), in epithelial cells, which downregulates dendritic cell production of the interleukin-22 (IL-22) binding protein (IL-22BP).12 IL-22BP suppresses the function of IL-22 which is produced from innate lymphoid cells in response to cellular/tissue damage and which potently stimulates the proliferation of intestinal epithelial cells.
We have previously demonstrated that the cellular protein STING facilitates cytosolic DNA-triggered innate immune signaling pathways, independent of Toll-like receptor 9 or the DNA sensor absent in melanoma 2 (AIM II).13 In humans, STING is a 348 amino acid endoplasmic reticulum-associated molecule predominantly expressed in epithelial cells as well as cells of the hematopoietic lineage, that has been shown to play a key role in triggering innate immune signaling pathways in response to infection by viruses such as herpes simplex virus 1, and even bacteria.4 STING has also been shown to be responsible for triggering vascular and pulmonary syndrome, self-DNA-induced inflammatory diseases such as Aicardi–Goutieres syndrome and perhaps forms of severe systemic lupus erythematosus.8,14–16 STING may associate with dsDNA species directly and is highly activated by CDNs (cyclic di-GMP-AMP[cGAMP-c(G(2′,5′)pA(3′,5′)p)]) generated by certain bacteria or by cytosolic dsDNA triggering the activation of a synthase, referred to as cGAS (cyclic GMP-AMP synthase, C6orf150, Mab-21 domain-containing protein).6
Given that STING appears to play a pivotal role in controlling a variety of inflammation-driven events and may control MyD88-dependent carcinogen-induced skin cancer, we wished to address the role of STING in inflammation-aggravated intestinal tumorigenesis. Using the AOM/DSS model, we observed that similar to MyD88, STING-deficient mice (SKO) are sensitive to CAC indicating a protective role for STING in tumorigenesis. Our data indicate that STING may be a key sensor that promotes the elimination of damaged intestinal epithelial cells.
RESULTS AND DISCUSSION
Activation of STING-dependent genes by AOM
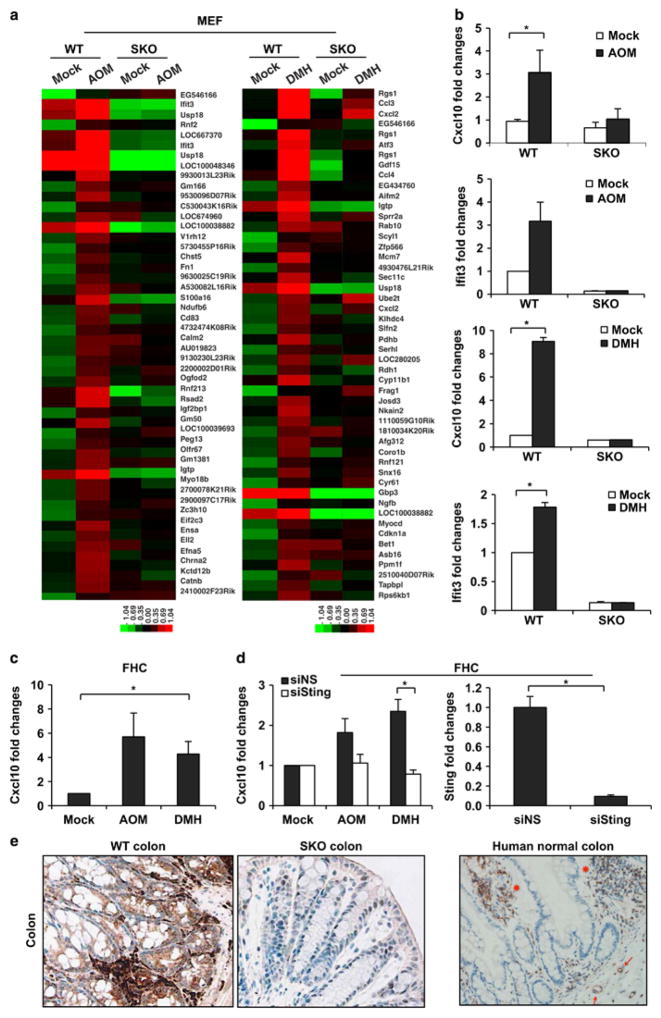
Given that chronic inflammation is known to aggravate colon cancer and that STING has been shown to influence inflammatory responses, especially those invoked by cytosolic self or pathogen-related DNA, we examined the role of STING in the control of inflammatory CAC.1,2,15 Toward these objectives, we utilized the AOM/DSS model and analyzed the effects of AOM and precursor DMH on STING signaling. Principally, wild-type (WT) or STING-deficient (SKO) murine embryonic fibroblasts (MEFs) were treated in vitro with DMH or metabolite AOM for 8 h, and microarray analysis performed to analyze the consequences to gene expression. This study indicated that AOM activated mRNA production of a wide array of innate immune-related genes in WT cells, including interferon-induced protein with tetratricopep-tide repeats 3 (IFIT3) and chemokine (C-X-C motif) ligand 2 (CXCL2) (Figure 1a and Supplementary Figure 1). However, there was a marked decrease in the production of the same genes in SKO MEFs indicating that AOM was indeed capable of activating the STING pathway (Figure 1a, left panel). A similar effect was observed following the treatment of cells with DMH (Figure 1a, right panel). STING-dependent gene expression was confirmed following RT–PCR analysis of mRNA representing Cxcl10 and IFIT3 (Figure 1b). We similarly treated normal human colon epithelial cells (FHCs) with AOM and observed a comparable induction of innate immune genes, controlled by STING, including Cxcl10 (Figure 1c). The production of Cxcl10 by AOM was similarly reduced in FHCs treated with RNAi to STING (Figure 1d). To determine the consequences of DMH/AOM treatment on STING’s ability to activate these key transcription factors, we carried out immunofluorescence analysis on MEFs and FHCs treated with these drugs. This study indicated that DMH/AOM could instigate the translocation of interferon regulatory factor 3/NF-κB in treated cells (Supplementary Figure 2). Thus, the DNA-damaging agent DMH/AOM can invoke STING-dependent signaling.
Figure 1.
Activation of STING-dependent genes by AOM. (a) Gene array analysis of wild-type (WT) and STING-deficient (SKO) mouse embryonic fibroblasts (MEFs) treated with AOM at 0.14 mM for 8 h (left) and DMH at 1 mM for 8 h (right). MEFs were obtained from embryos from WT and SKO mice at embryonic 15 days by a standard procedure as described (Ishikawa 2008). Gene array analysis was examined by Illumina Sentrix BeadChip array (Mouse WG6 version 2). Highest variable genes are shown. Rows represent individual genes and columns represent individual samples. Pseudo-colors indicate transcript levels below (green), equal to (black) or above (red) the mean. Scale represents the intensity of gene expression (log2 scale ranges between – 2.4 and 2.4). (b) Quantitative real-time PCR analysis (qPCR) of Cxcl10 and Ifit3 in MEFs treated with AOM and DMH same as a. Total RNA was reverse transcribed using moloney murine leukemia virus (M-MLV) reverse transcriptase. qPCR was performed using TaqMan gene expression assay (Cxcl10: Mm00445235, Ifit3: Mm0170846). (c) qPCR analysis of Cxcl10 in human epithelial cell (FHC) treated with AOM and DMH at 1 mM for 24 h. (d) FHCs were transfected with STING or control siRNA for 72 h followed by AOM and DMH treatment same as c, and were then subjected to Cxcl10 mRNA expression (left). STING expression level after siRNA treatment was determined by qPCR (right). Data are representative of at least two independent experiments. Error bars indicate s.d. *P<0.05, Student’s t-test. (e) Immunohistochemistry staining of the colon tissue from WT and SKO mice (left) and human (right). All images were shown at original magnification, x200.
Loss of STING renders mice susceptible to CAC
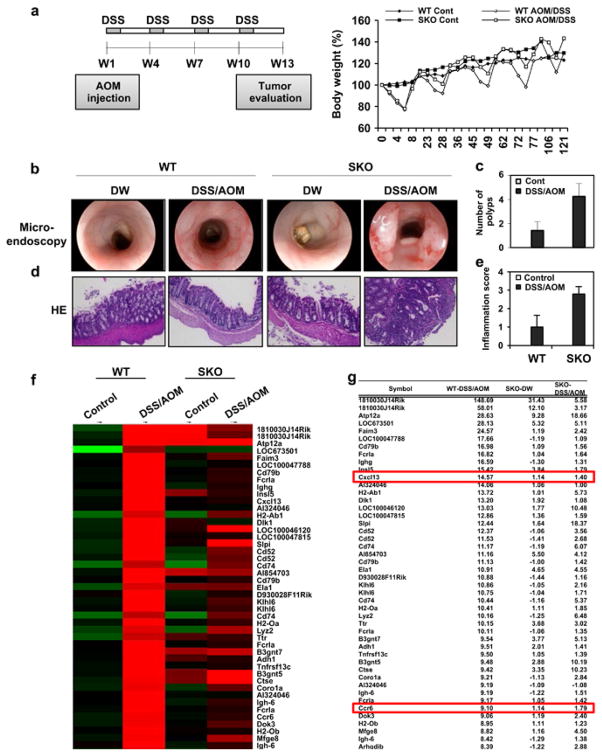
Our data indicate that DMH/AOM can activate STING in vitro. To examine the consequences of this in vivo, we treated mice once with AOM and subsequently orally with four treatments of DSS. Prior to this, we analyzed STING expression in the intestine by immunohistochemistry analysis. This study showed that STING was expressed in lamina propria cells as well as in endothelial and epithelial cells of the gastrointestinal tract (Figure 1e). After 13 weeks the mice were analyzed for tumor development in the colon. Surprisingly, we observed that SKO mice developed colonic tumors at a much higher frequency compared to WT mice (Figures 2a–c and Supplementary Figure S3). Indeed, 4/7 WT mice exhibited tumor formation compared to 7/7 SKO within the same time period (Figures 2b and c and Supplementary Figure S3a). Hematoxylin and eosin analysis confirmed that AOM/DSS-treated SKO mice exhibited significant inflammatory cell infiltration and development of adenocarcinoma in the colon, compared to similarly treated WT mice (Figures 2d and e and Supplementary Figure S3b). However, Microarray analysis indicated that tumors from WT mice exhibited higher levels of selected gene expression, such as Cxcl13 and Ccr6, compared to tumors retrieved from SKO mice, perhaps since loss of STING suppressed immunomodulatory transcriptional events (Figures 2f–h). Collectively, we postulate that STING may recognize damaged DNA and activate the production of cytokines that conceivably could promote tissue repair or stimulate the immune system to eradicate such cells. Thus, loss of STING may enable damaged cells to escape immune surveillance processes and progress more readily into tumors.
Figure 2.
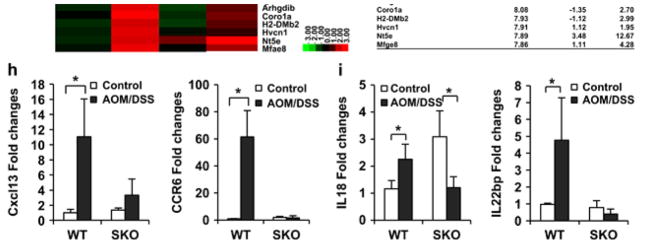
Loss of STING renders mice susceptible to CAC. (a) Schematic representation and body weight of AOM/DSS-induced colitis model. WT (n = 7) and SKO (n = 7) mice (B6;129 mix) were intravenously injected with AOM at a dose 10 mg/kg. After 1 day, mice were fed 5% DSS in drinking water for 7 days. This cycle was repeated four times. Normal drinking water was used for control group. At 91 days, micro-endoscopic procedure was performed in a blinded manner for counting number of polyps. Mice were killed at day 121 and the colon was resected, flushed with phosphate-buffered saline, fixed in formalin for histology or froze for RNA expression analysis. Representative photographs of macro-endoscopic colon tumors (b) and number of polyps (c). Hematoxylin and eosin staining (d) of WT (n = 7) and SKO (n = 7) mice either AOM/DSS treated or normal water treated and inflammation score (0: normal to 3: most severe; e). (f) Gene array analysis of normal tissue (control) and tumor tissue (DSS/AOM) from WT and SKO mice treated same as a. Rows represent individual genes and columns represent individual samples. Pseudo-colors indicate transcript levels below (green), equal to (black) or above (red) the mean. Scale represents the intensity of gene expression (log2 scale ranges between – 2.4 and 2.4). (g) Highest variable gene lists are shown. (h) qPCR of Cxcl13 and Ccr6 in normal tissue (control) and tumor tissue (DSS/AOM) from WT and SKO mice treated same as a. (i) qPCR analysis of IL-18 and IL-22BP (IL-18: Mm00434225, IL-22BP: Mm01192969) from normal tissue (control) and tumor tissue (DSS/AOM) from WT and SKO mice. Data are the mean of at least five mice. Error bars indicate s.d. *P<0.05, Student’s t-test.
Suppression of IL-22BP expression in STING-deficient mice
Although, we demonstrated that loss of STING facilitated colon cancer development, the tumor-suppressive mechanisms associated with STING activity remain to be clarified. It remained plausible that STING could exert direct tumor-suppressive, growth inhibitory or pro-apoptotic properties similar to tumor suppressor p53. Further, AOM treatment has been known to induce frequent Ras mutations, which in the context of loss of STING, could facilitate cellular transformation.17 Expression of oncogenic Ras in an environment where p53 function is lost renders normal murine cells, the ability to form foci in soft agar and to become tumorigenic in vivo.18 To evaluate the anti-oncogenic role of STING further, we transfected WT, SKO or p53-deficient MEFs, as positive controls, with Myc or activated Ras and monitored cellular transformation. MEFs lacking p53 were found to be readily transformed by the introduction of Myc or activated Ras (Supplementary Figure S4). However, MEFs lacking STING did not appear appreciably sensitive to transformation following overexpression of Myc or activated Ras. Thus, the absence of STING does not appear to exert an oncogenic stimulus, at least in vitro or to cooperate with Myc or Ras in the cellular transformation process.
However, it has been demonstrated that mice lacking certain cytokines such as IL-18, IL-22 or the innate immune adapter MyD88 are similarly susceptible to AOM/DSS-induced CAC.1,2,10,12 In this situation, MyD88 exerts a protective effect by facilitating the production of IL-18 through the IL-18R, which is required to inhibit IL-22BP.3,12,19–22 IL-22BP is necessary to suppress IL-22 function, which can promote the proliferation of intestinal epithelial cells following damage by carcinogens or inflammatory agents.12 Mice lacking IL-18 or IL-22BP are highly susceptible to CAC, similar to STING-deficient mice.12 To examine whether AOM/DSS treatment could affect IL-18 and IL-22BP expression in SKO mice, we analyzed IL-18 and IL-22BP levels in untreated normal mice (control) or tumor tissue taken from the AOM/DSS-treated WT or SKO mice. We observed that IL-18 expression was slightly upregulated in tumor tissue retrieved from WT mice treated with AOM/DSS (Figure 2i). We observed that IL-22BP expression levels were also elevated in the tumors of AOM/DSS-treated WT mice. In contrast, we noted the significantly reduced expression of IL-18 and IL-22BP in the tumors of SKO mice, when compared to WT mice (Figure 2i). The expression of IL-22 was seen to remain relatively unaffected (data not shown). It was surprising to note that IL-22BP levels were elevated in the presence of IL-18. However, it has been reported that the downregulation of IL-22BP can occur even in the absence of IL-18, indicating that alternate STING-induced cytokines may clearly contribute to the regulation of IL-22BP.12
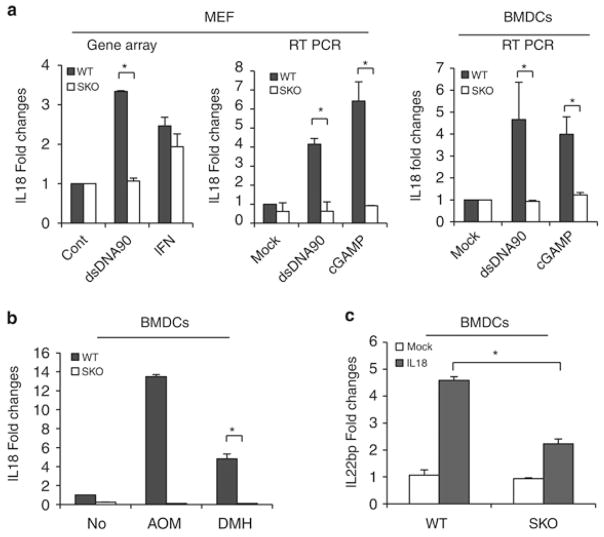
In addition, we noted from our microarray analyses that IL-18 levels were reduced in SKO MEFs treated with STING-activating dsDNA (dsDNA90 base pairs; Figure 3a, left panel). We therefore investigated the involvement of STING on the possible regulation of the IL-18/IL-22BP axis. First, we confirmed the influence of STING on IL-18 expression, since we additionally noted that the promoter of this cytokine is known to harbor numerous sites recognized by innate immune gene-activating transcription factors such as signal transducer and activator of transcription 1, NF-κB, IRF1 and IRF7 (Supplementary Figure S5). Our analysis indicated that IL-18, which is expressed in a wide variety of cell types, is a STING inducible gene, as determined following treatment of MEF cells and bone marrow-derived dendritic cells (BMDCs) with dsDNA or STING-activating CDNs (cGAMP; Figure 3a, middle and right panel). A similar study indicated that DMH/AOM could also trigger the production of IL-18 in dendritic cells, in a STING-dependent manner (Figure 3b). To further this study, we examined IL-22BP expression in BMDCs stimulated with IL-18 recombinant protein and observed the upregulation of IL-22BP in WT BMDCs compared to SKO BMDCs (Figure 3c and Supplementary Figure S6). These data are similar to Figure 2i showing that IL-18 and IL-22BP expression levels were also lower in the tumors of AOM/DSS-treated SKO mice compared to the tumors of WT mice (Figure 2i). These data suggest that STING may regulate IL-22BP expression by IL-18.
Figure 3.
IL-18 controls IL-22BP in STING-dependent manner. (a) Fold changes from gene array analysis of IL-18 in WT and SKO MEFs administered with 4 ug/ml of dsDNA90 and IFNβ for 8 h (left). qPCR analysis of IL18 in WT and SKO MEFs, and bone marrow-derived dendritic cells (BMDCs) transfected with 4 μg/ml of dsDNA90 and cyclic-di-GMP-AMP (cGAMP) for 8 h (middle). (b) qPCR analysis of IL-18 in WT and SKO BMDCs with 1 mM of AOM and 1 mM of DMH for 8 h. (c) qPCR analysis of IL-22BP in WT and SKO BMDCs treated with 10 ng/ml of recombinant mouse IL-18 protein (MBL B002-5) for 24 h. Total RNA was reverse transcribed using M-MLV reverse transcriptase. qPCR was performed using TaqMan gene expression assay (IL-18: Mm00434225, IL-22BP: Mm01192969). Data are the mean of at least five mice. Error bars indicate s.d. *P<0.05, Student’s t-test.
Taken together, it is conceivable that DNA damage or the sensing of microbial ligands that may invade damaged colon tissue can trigger STING activity leading to the activation of inflammatory wound repair initiating cytokines as well as the indirect suppression of growth inhibitory IL-22BP. Our data thus indicate that similar to mice lacking MyD88, IL-18 or IL-22BP, STING-deficient mice are also prone to CAC induced by AOM/DSS.
Although, we demonstrate here a protective role for STING in the prevention of CAC induced by AOM/DSS carcinogenic treatment, these findings are in contrast to our previous data indicating that STING-deficient mice are resistant to DMBA-induced skin cancer.9 In this scenario, DNA-leaked from the nucleus of carcinogen damaged dermal-associated cells activates STING-dependent cytokine production, intrinsically, to attract infiltrating phagocytes. Such phagocytes engulf dying cells to activate extrinsic STING-dependent cytokine production, which further drives inflammatory processes that promote tumor development.9 This situation is reminiscent of similarly treated MyD88-deficient mice, which are also resistant to DMBA-aggravated skin cancer. Our data indicate that STING triggers the production of cytokines that bind to receptors requiring MyD88-dependent signaling for additional cytokine production, such as tumor necrosis factor α.3 Our data here conversely indicate that STING-driven cytokine production can protect against the development of other types of tumors, such as carcinogen-influenced colon cancer.23 This event may occur in part through STING’s ability to control the production of IL-22BP, although STING clearly controls the production of numerous other regulatory proteins and cytokines. Following tissue damage, for example by DSS, it has been reported that IL-22 is induced and manifests protective, wound-healing effects, including the promotion of tissue regeneration.1,2,10,12 However, if left uncontrolled, IL-22 can also endorse tumor development.12 IL-22 is therefore tightly regulated by secreted IL-22BP, which is expressed by CD11c+ dendritic cells.12 The importance of IL-22BP in controlling IL-22 has been emphasized through observing that IL-22BP-deficient mice are also susceptible to AOM/DSS-induced CAC, similar to STING-deficient mice.12 Nevertheless, IL-22 may have dual functions since mice lacking IL-22 have also been reported to exhibit enhanced inflammatory responses when treated repeatedly with DSS, plausibly because complete loss of IL-22 may cause a delay in intestinal repair, which in turn may actually aggravate inflammatory processes.1,2,10,12 The production of IL-22BP can be suppressed by IL-18, which is known to be induced early after DSS-induced intestinal damage. Accordingly, IL-18-deficient mice are also susceptible to colon cancer, presumably through chronic suppression of IL-22 activity, by unregulated IL-22BP, which may mimic the situation observed with IL-22-deficient mice.12 Nevertheless, the control of IL-22BP remains to be fully clarified since downregulation of IL-22BP has also been reported to occur in the absence of IL-18.12 In addition, it is known that loss of the Toll-like receptor and IL-1R/IL-18R adapter MyD88 also renders mice sensitive to CAC, in part due to loss of IL-18R signaling.20,21 Finally, susceptibility to AOM/DSS-induced CAC has been shown to be enhanced in mice lacking caspase-1, the adapter PYCARD (apoptosis-associated speck-like protein containing a caspase activation and recruitment domain; ASC) or nucleotide-binding domain, leucine rich repeat and pyrin domain-containing proteins 3 and 6 (NLRP3/6), presumably since Pro-IL-18 produced by epithelial cells or dendritic cells requires cleavage prior to secretion into an active form.24–26
Our data here indicate that IL-18 and a variety of other cytokines and chemokines are inducible by dsDNA or CDN’s, or by AOM/DMH in a STING-dependent manner. Similar to the situation with IL-22, we propose that intestinal damage triggers STING activity (as a consequence of DNA damage or even from microbial ligands such as secreted CDNs or genomic DNA). This results in the downregulation of IL-22BP, which would enable IL-22 to promote tissue repair. However, similar to the situation with IL-22, long-term loss of STING may delay wound repair, facilitate microbial invasion and chronic inflammation, which would actually aggravate tumorigenesis.1,2,12 In addition, loss of STING may enable damaged cells to escape antitumor immunosurveillance.2 It was noted that IL-18 expression was not totally ablated in tumors from SKO mice, presumably since the expression of this cytokine could be induced by other pathways.12 Despite this, IL-22BP levels remained low in SKO mice evidently demonstrating the importance of STING in IL-22BP regulation. Collectively, our data indicate that STING plays a key role in controlling intestinal tissue damage and CAC in part through regulating IL-22BP’s suppression of IL-22. Our data also provide a mechanistic explanation for carcinogenic-induced immunomodulatory effects that can influence tumorigenesis.
Supplementary Material
Acknowledgments
We thank Dr Masayuki Fukata for micro-endoscopy analysis; Ms Delia Gutman and Ms Auristela Rivera for mice breeding; Dr Tianli Xia for helping with confocal analysis; Dr Biju Issac of the Sylvester Comprehensive Cancer Center Bioinformatics Core Facility for Gene expression array analysis; Dr Phillp Ruiz and Ms Dayami Hernandez for helping with immunohistochemistry.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supplementary Information accompanies this paper on the Oncogene website (http://www.nature.com/onc)
References
- 1.Goldszmid RS, Trinchieri G. The price of immunity. Nat Immunol. 2012;13:932–938. doi: 10.1038/ni.2422. [DOI] [PubMed] [Google Scholar]
- 2.Nowarski R, Gagliani N, Huber S, Flavell RA. Innate immune cells in inflammation and cancer. Cancer Immunol Res. 2013;1:77–84. doi: 10.1158/2326-6066.CIR-13-0081. [DOI] [PubMed] [Google Scholar]
- 3.Cataisson C, Salcedo R, Hakim S, Moffitt BA, Wright L, Yi M, et al. IL-1R-MyD88 signaling in keratinocyte transformation and carcinogenesis. J Exp Med. 2012;209:1689–1702. doi: 10.1084/jem.20101355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ishikawa H, Ma Z, Barber GN. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature. 2009;461:788–792. doi: 10.1038/nature08476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burdette DL, Monroe KM, Sotelo-Troha K, Iwig JS, Eckert B, Hyodo M, et al. STING is a direct innate immune sensor of cyclic di-GMP. Nature. 2011;478:515–518. doi: 10.1038/nature10429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cai X, Chiu YH, Chen ZJ. The cGAS-cGAMP-STING pathway of cytosolic DNA sensing and signaling. Mol Cell. 2014;54:289–296. doi: 10.1016/j.molcel.2014.03.040. [DOI] [PubMed] [Google Scholar]
- 7.Ahn J, Ruiz P, Barber GN. Intrinsic self-DNA triggers inflammatory disease dependent on STING. J Immunol. 2014;193:4634–4642. doi: 10.4049/jimmunol.1401337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gall A, Treuting P, Elkon KB, Loo YM, Gale M, Jr, Barber GN, et al. Autoimmunity initiates in nonhematopoietic cells and progresses via lymphocytes in an interferon-dependent autoimmune disease. Immunity. 2012;36:120–131. doi: 10.1016/j.immuni.2011.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahn J, Xia T, Konno H, Konno K, Ruiz P, Barber GN. Inflammation-driven carcinogenesis is mediated through STING. Nat Commun. 2014;5:5166. doi: 10.1038/ncomms6166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salcedo R, Cataisson C, Hasan U, Yuspa SH, Trinchieri G. MyD88 and its divergent Toll in carcinogenesis. Trends Immunol. 2013;34:379–389. doi: 10.1016/j.it.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Robertis M, Massi E, Poeta ML, Carotti S, Morini S, Cecchetelli L, et al. The AOM/DSS murine model for the study of colon carcinogenesis: from pathways to diagnosis and therapy studies. J Carcinog. 2011;10:9. doi: 10.4103/1477-3163.78279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huber S, Gagliani N, Zenewicz LA, Huber FJ, Bosurgi L, Hu B, et al. IL-22BP is regulated by the inflammasome and modulates tumorigenesis in the intestine. Nature. 2012;491:259–263. doi: 10.1038/nature11535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ishikawa H, Barber GN. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature. 2008;455:674–678. doi: 10.1038/nature07317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu Y, Jesus AA, Marrero B, Yang D, Ramsey SE, Montealegre Sanchez GA, et al. Activated STING in a vascular and pulmonary syndrome. N Engl J Med. 2014;371:507–518. doi: 10.1056/NEJMoa1312625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahn J, Gutman D, Saijo S, Barber GN. STING manifests self DNA-dependent inflammatory disease. Proc Natl Acad Sci USA. 2012;109:19386–19391. doi: 10.1073/pnas.1215006109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Namjou B, Kothari PH, Kelly JA, Glenn SB, Ojwang JO, Adler A, et al. Evaluation of the TREX1 gene in a large multi-ancestral lupus cohort. Genes Immun. 2011;12:270–279. doi: 10.1038/gene.2010.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jacoby RF, Llor X, Teng BB, Davidson NO, Brasitus TA. Mutations in the K-ras oncogene induced by 1,2-dimethylhydrazine in preneoplastic and neoplastic rat colonic mucosa. J Clin Invest. 1991;87:624–630. doi: 10.1172/JCI115039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell. 1997;88:593–602. doi: 10.1016/s0092-8674(00)81902-9. [DOI] [PubMed] [Google Scholar]
- 19.Swann JB, Vesely MD, Silva A, Sharkey J, Akira S, Schreiber RD, et al. Demonstration of inflammation-induced cancer and cancer immuno-editing during primary tumorigenesis. Proc Natl Acad Sci USA. 2008;105:652–656. doi: 10.1073/pnas.0708594105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salcedo R, Worschech A, Cardone M, Jones Y, Gyulai Z, Dai RM, et al. MyD88-mediated signaling prevents development of adenocarcinomas of the colon: role of interleukin 18. J Exp Med. 2010;207:1625–1636. doi: 10.1084/jem.20100199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zenewicz LA, Yancopoulos GD, Valenzuela DM, Murphy AJ, Stevens S, Flavell RA. Innate and adaptive interleukin-22 protects mice from inflammatory bowel disease. Immunity. 2008;29:947–957. doi: 10.1016/j.immuni.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rakoff-Nahoum S, Medzhitov R. Regulation of spontaneous intestinal tumorigenesis through the adaptor protein MyD88. Science. 2007;317:124–127. doi: 10.1126/science.1140488. [DOI] [PubMed] [Google Scholar]
- 23.Zhu Q, Man SM, Gurung P, Liu Z, Vogel P, Lamkanfi M, et al. Cutting edge: STING mediates protection against colorectal tumorigenesis by governing the magnitude of intestinal inflammation. J Immunol. 2014;193:4779–4782. doi: 10.4049/jimmunol.1402051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fukata M, Vamadevan AS, Abreu MT. Toll-like receptors (TLRs) and Nod-like receptors (NLRs) in inflammatory disorders. Semin Immunol. 2009;21:242–253. doi: 10.1016/j.smim.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 25.Allen IC, TeKippe EM, Woodford RM, Uronis JM, Holl EK, Rogers AB, et al. The NLRP3 inflammasome functions as a negative regulator of tumorigenesis during colitis-associated cancer. J Exp Med. 2010;207:1045–1056. doi: 10.1084/jem.20100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elinav E, Strowig T, Kau AL, Henao-Mejia J, Thaiss CA, Booth CJ, et al. NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell. 2011;145:745–757. doi: 10.1016/j.cell.2011.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.