Abstract
Objective
To determine the incidence and predictors of infective endocarditis in a population-based cohort of mitral valve prolapse(MVP) patients.
Patients and methods
We identified all adult Olmsted County residents with MVP diagnosed by echocardiography from January 1989 to December 1998 and cross-matched them with the Rochester Epidemiologic Project-identified Olmsted County cases of infective endocarditis(IE) from January 1986 to December 2006. We retrospectively analyzed and de-novo confirmed each IE case using the modified Duke criteria.
Results
There were 896 Olmsted County residents with echocardiographic MVP diagnosis, mean age 53±21 years, 565(63%) women. Mean follow-up was 11±5 years. The 15-year cohort-risk of IE after MVP diagnosis was 1.1±0.4%; incidence of 86.6[95% CI, 43.3–173.2]cases per 100,000 person-years; age- and sex-adjusted relative-risk of IE in MVP of 8.1[95% CI: 3.6–18.0] compared to the County general population(P<.001). There were no IE cases in patients without prior diagnosed mitral regurgitation. Conversely, IE incidence was higher in MVP patients with ≥moderate mitral regurgitation(289.5[108.7–771.2] cases per 100,000 person-years, P=.02 compared to <moderate regurgitation), and in patients with flail leaflets(715.5[178.9–2861.0] cases per 100,000 person-years, P=.02 compared to no flail leaflet)
Conclusions
The population-based incidence of IE in MVP adults is higher than previously reported in case-control tertiary care-center studies. MVP patients with ≥moderate mitral regurgitation or a flail leaflet are at notable risk of developing IE as compared to those without mitral regurgitation.
Introduction
Mitral valve prolapse(MVP) is a common valvular abnormality that affects approximately 2–3% of the general population1, and constitutes the primary cause of chronic organic/degenerative mitral regurgitation(MR) in developed countries, with a prevalence of 6 to 9% in patients older than 65 years2,3. MVP is characterized by billowing of one or both mitral leaflets at least 2 mm beyond the echocardiographic parasternal long-axis annular plane(Figure 1). Mitral leaflets can be further categorized as myxomatous or redundant when thickness of the leaflet is 5 mm or more4. Infective endocarditis(IE) is a serious condition associated to severe complications, including congestive heart failure, stroke, systemic emboli, abscess formation, and death in up to 25% of patients 5. Studies dating 20 to 30 years back have suggested that patients with MVP exhibit an increased risk of IE; particularly men, patients with thickened leaflets and patients with a systolic murmur 4,6–8, however, these risks have never been directly compared with the general population. Historically, the incidence of IE in the general population has been variably estimated to be between 1.6 to 11.6 cases per 100,000 person-years 9–12. More recently, population-based studies in Olmsted County, MN, have estimated the incidence of IE in the general population to be 5.0 to 7.9 cases per 100,000 person-years 13,14.
Figure 1.
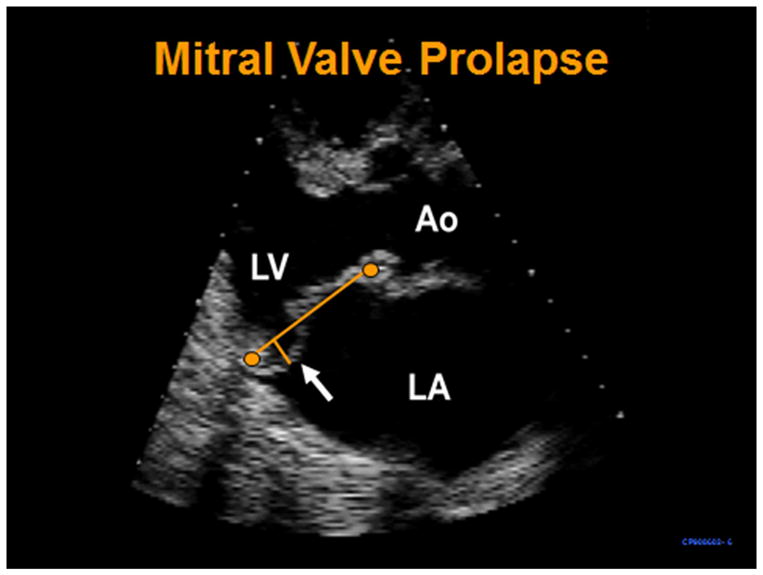
Transthoracic parasternal long-axis systolic frame shows prolapse of the mitral posterior leaflet (arrow) beyond the annular plane (long imaginary line traced between the anterior and posterior annulus). LV=left ventricle, Ao=aorta, LA=left atrium
Over the past three decades, some authors have published estimated incidence rates of IE in patients with MVP6,15–17. These studies concluded that patients with MVP have approximately 4 to 5 times greater risk to develop IE compared to the general population, and estimated an incidence of 14 IE cases per 100,000 MVP person-years15. However, these were case-control studies which identified MVP patients within IE cases referred to tertiary-care centers and results were compared to the MVP prevalence in non-IE control groups; a methodology potentially leading to significant bias. Moreover, these studies were performed at a time when MVP was overdiagnosed due to imprecise echocardiographic criteria(i.e., before 1988)18, and IE definitions were also based on older criteria. In light of a changing landscape in IE prevention strategies(i.e., peri-procedural antibiotic prophylaxis)19, determining the true burden and predictors of IE in MVP becomes critical in shaping future recommendations. Thus, we sought to determine the incidence and predictors of IE as defined by current modified Duke criteria in a population-based community-cohort with contemporary echocardiographic diagnosis of MVP, and compare to the general population.
Methods
The Rochester Epidemiology Project(REP) is a research database that links together nearly all medical records of residents of Olmsted County, MN for approved medical research20. REP allows access to all echocardiograms obtained in Olmsted County, and provides a unique opportunity to pursue population-based studies in a geographically defined area(i.e., Olmsted County). Thus, we identified all adult patients from our echocardiogram database; Olmsted County residents with MVP documented by echocardiography from January 1989 to December 1998(including all patients reported by Avierinos et al.21). A second REP-based electronic database was created that included all Olmsted County residents who were clinically diagnosed with IE from January 1986 to December 2006. We then cross-matched the two electronic databases and retrospectively analyzed and de-novo confirmed each IE case using the modified Duke Criteria22. Only patients with echocardiographically-diagnosed MVP prior to first presentation with IE were considered cases of MVP with IE. A comorbidity sum of diseases index was calculated at baseline echocardiogram23. The study was approved by the institutional review board.
Echocardiography
Mitral valve prolapse was defined as ≥2mm systolic displacement of one or both leaflets beyond the annular plane in the transthoracic parasternal long axis view1,18,24(Figure 1). Diagnosis of flail leaflet was based on failure of systolic leaflet coaptation25, with rapid systolic movement of the involved leaflet tip within the left atrium26. Mitral regurgitation was graded semiquantitatively 27 with use of supporting and specific echocardiographic parameters, including PISA quantification28 when available. The MVP cohort was divided into 3 mutually exclusive echocardiographic groups for analysis: 1) no mitral regurgitation(no MR); 2) trivial, mild, mild-moderate MR(<moderate MR); and 3) moderate, moderate-severe or severe MR(≥moderate MR). In addition, from the ≥moderate MR group, patients with a flail mitral leaflet were also analyzed separately for IE incidence calculation within them.
Statistical analysis
Continuous variables are expressed as mean ± SD for continuous variables and percentage for categorical variables. Paired t-test and Chi-square were used for comparison between continuous and categorical data, respectively. IE rates were determined with the Kaplan–Meier method. Association of baseline characteristics with the incidence of IE was analyzed with the Cox proportional-hazards method. The expected number of IE cases in the MVP cohort was estimated by applying the Olmsted County age- and gender-specific incidence rates of Tleyjeh et al.14. Event rates are summarized as events per 100,000 person years and 95% confidence intervals were calculated assuming that the event rate followed a Poisson distribution. The authors had full access to and take full responsibility for the integrity of the data.
Results
MVP Patient Population
In the 10-year period from 1989 to 1998, there were 896 Olmsted County residents with MVP diagnosed by echocardiography. Mean age at baseline echocardiogram was 53±21 years, 565(63%) were female. Left ventricular ejection fraction was 62±8%. Mean follow-up was 11±5 years. Of 896 echocardiograms, predominantly posterior leaflet prolapse was present in 273(31%) of patients, predominantly anterior prolapse in 204(23%), and bileaflet prolapse in 419(46%). Thirty-one(3.4%) had a flail mitral leaflet. Of 896 echocardiograms, 140(16%) patients had no MR, 588(65%) had <moderate MR, and 168(19%) had ≥moderate MR. The table presents baseline clinical and echocardiographic characteristics of patients stratified by MR group.
TABLE.
Baseline clinical and echocardiographic characteristics stratified by MR severity
| Variable | No MR n= 140 | <Moderate MR n= 588 | ≥Moderate MR n= 168 | P value |
|---|---|---|---|---|
| Age, years | 46±22 | 49 ± 20 | 70 ± 16 | <.001a |
| Sex, Female (%) | 68 | 67 | 47 | <.001b |
| Comorbidity index | 0.50±1.2 | 0.48±0.9 | 0.78±1.1 | <.002b |
| Atrial fibrillation (%) | 6 | 6 | 27 | <.001b |
| Left ventricular EF (%) | 61 ± 8 | 62 ± 7 | 61 ± 1 | >0.5 |
| Anterior leaflet n (%) | 29 (21) | 155(26) | 20 (12) | <.001c |
| Posterior leaflet n (%) | 38 (27) | 163(28) | 72 (43) | <.007b |
| Bileaflet n (%) | 73 (52) | 270(46) | 76 (45) | >0.20 |
Statistically significant between ≥moderate MR compared to the others. No MR vs. <moderate MR; P=.04.
Statistically significant between ≥moderate MR compared to the others, both <.001.
Only statistically significant between ≥moderate MR and <moderate MR
Infective Endocarditis Characteristics
During follow-up, of 896 MVP patients, 6 developed definite IE and 2 developed possible IE. Of these 8 patients, 5 were female and 3 were male(P=1.0). All cases involved a native mitral valve. Mean age at IE diagnosis was 62±22 years and mean time to IE from MVP diagnosis was 5±3 years. Recent dental work was documented for 2(25%) cases of IE, both without antibiotic prophylaxis. However, in both cases, dental work could not be established as definite source of infection. A source of infection was established in 2(25%) cases and included finger soft tissue abscess in one and deep wound infection s/p humeral head replacement in the other. There was no IE secondary to IV drug use. Identified pathogens included viridans group streptococci in 4(50%) patients, Staphylococcus aureus in 2(25%), Enterococcus sp. in 1 patient and Streptococcus pneumoniae 1 patient. Death or stroke did not occur in any IE patient. Emergent surgery was required in 2 cases. Recurrence of definite or possible IE occurred in 2(25%) of cases at 2 and 21 months after initial IE diagnosis.
Population-Based Rates of Infective Endocarditis
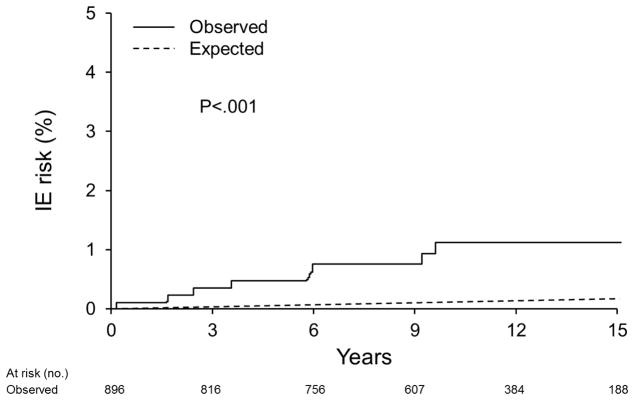
The 15-year cohort risk of IE after MVP echocardiographic diagnosis was 1.1±0.4%; incidence of 86.6[95% CI, 43.3–173.2]cases per 100,000 person-years(Figure 2). The general Olmsted County population incidence of IE has been reported at approximately 6.0 [95% CI, 4.9–7.2] cases per 100,000 person-years14. Thus, the age- and sex-adjusted relative-risk of IE in MVP is 8.1[95% CI: 3.6–18.0] compared to the County general population(P<.001)(Figure 2).
Figure 2.
Kaplan-Meier curve depicts the observed IE risk for the entire MVP cohort versus expected IE risk in the general population
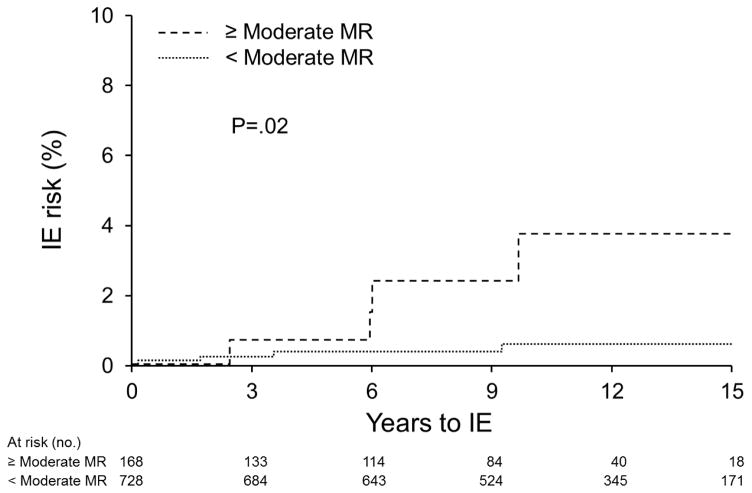
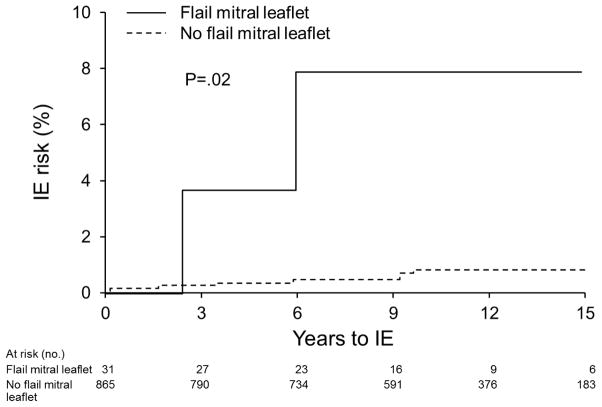
Univariate predictors of IE included ≥ moderate MR(Hazard Ratio = 5.43[95% CI, 1.28–23.05], P=.02)(Figure 3), and a flail mitral leaflet(HR = 11.05[1.62–48.07], P=.02)(Figure 4). Age, gender, predominant prolapsing mitral leaflet, leaflet thickening, hypertension, coronary disease, congestive heart failure, aortic regurgitation, and atrial fibrillation were not predictors(all P>.05).
Figure 3.
Kaplan-Meier curves depict the IE risk in the MVP group with <moderate MR(including no MR, trivial, mild and mild-moderate) versus MVP with ≥ moderate MR
Figure 4.
Kaplan-Meier curves depict the IE risk in MVP group with flail leaflet(n=31) versus MVP with no flail leaflet(regardless of MR severity).
Mitral Regurgitation as a Risk Factor for Infective Endocarditis in MVP
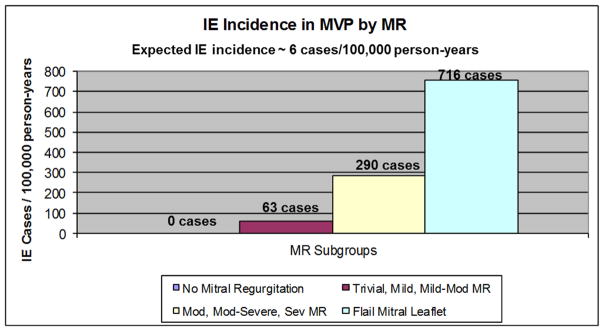
Of 140 patients with no mitral regurgitation, none developed IE. Of 588 patients with <moderate MR, 4 developed IE(incidence = 63.3 [95% CI: 23.8–168.7] cases per 100,000 person-years, 15-year rate= 0.77%±0.39%). Of 168 patients with ≥moderate MR, 4 developed IE(incidence of 289.5 [108.7–771.2] cases per 100,000 person-years, 15-year rate= 3.84%±1.95%, HR=5.43 [1.28–23.05], P=.02 as compared to <moderate MR). Of 31 patients with flail leaflet, 2 developed IE(incidence of (715.5[178.9–2861.0] cases per 100,000 person-years, 15-year rate= 8.22%±5.59%, HR=11.05 [1.62–48.07], P=.02 as compared to no flail leaflet)(Figures 4 and 5).
Figure 5.
Bar graph shows the incidences of IE per 100,000 person-years according to echocardiographic MR severity. Note that the flail group(n=31)is also shown separately
Discussion
To our knowledge, we report for the first time the population-based incidence of IE in patients with contemporary echocardiographic diagnosis of MVP, based on the largest geographically-defined MVP community-cohort with longest follow-up available for this purpose. Our study reports the incidence of IE in patients with echocardiographic MVP diagnosis to be approximately 87 cases per 100,000 person-years, which represents approximately 8 times the risk of IE in the general population. Prior studies reported wide variation in the estimated incremental relative risk of IE in MVP patients compared to non-MVP patients(2.9 to 8.2) 6,15–17. Our observed IE incidence is clearly higher than the previously case-control-estimated incidence of 14 cases per 100,000 person-years15. Similarly, pooled previous studies had estimated that 1 out of approximately 6000 MVP patients would develop IE per year6,15,17, while our study predicts the occurrence of IE in 1 out of approximately 1100 MVP patients per year, an important figure for MVP patients and their health care providers to recognize. This incidence underestimation from prior studies likely reflects overdiagnosis of MVP before 198818, resulting in an overestimated denominator of MVP patients at risk, and thus a lower incidence.
Interestingly, in a previous prospective study of 300 patients with MVP followed for a mean of 6 years29, 18(6%) developed IE, however, this inflated incidence likely resulted from all patients in this study being referred to a “tertiary center for further evaluation”29 and likely do not represent the natural history of MVP-related complications29.
The aforementioned case-control, tertiary-referral center studies also reported male gender and age to be associated with higher incidence of IE in MVP patients17. We did not find such associations in this large community cohort; development of IE was identical between genders and age at IE occurrence had a wide standard deviation suggesting significant variation in patient age at IE diagnosis. It is possible that previous case-control studies reflect a “sicker” tertiary-referral population where older men predominate. Notwithstanding, there was a predominance of women in our cohort which could have potentially attenuated an IE excess in men in our study. Indeed, population-based IE incidence in the general population has also been reported to be higher in men14.
Although IE is associated with significant mortality, there were no deaths from IE in our patient group. Significant complications of IE were also relatively uncommon. This could be the result of our community study-design while most prior studies were based on tertiary-referral “sicker” patient populations. Nonetheless, our study was not designed to examine complications of IE in MVP, as this task would require analysis of numerous cases of MVP-related IE.
High-risk subgroups
Astutely, in previous case series, investigators were able to determine the prevalence of MVP in patients with a history of a murmur before the development of IE, and found a significant association between the presence of a prior murmur and development of IE8,17,30. They estimated that 1 out of approximately 1900 MVP patients with a known murmur would develop IE per year17. Our study confirms this prior association but the incidences are significantly higher; while patients with no mitral regurgitation did not experience IE, the group with trivial, mild, and mild-moderate MR(<moderate MR) had a higher incidence of IE than the general population, but the rate was still relatively low at 63 cases per100,000 person-years. However, the incidence of IE greatly increased in the moderate, moderate-severe and severe MR(≥moderate MR), and flail mitral leaflet groups; IE incidences of 290 and 716 cases per100,000 person-years, respectively. Thus, our study predicts the occurrence of IE in 1 out of approximately 340 MVP patients with ≥moderate MR per year; or a 0.3% patient-year risk. This risk roughly doubles (approximately 0.6%)if a flail leaflet is present. These findings suggest that not only the presence, but more importantly the degree of MR in MVP plays a critical role in determining the risk of IE in patients with MVP. Our findings are congruent with the injury-thrombus-infection theory31 where mechanical and hemodynamic stress(i.e., MR) cause valvular endothelial erosion leading to formation of sterile microthrombi nidi where bacterial adhesion and colonization may occur during transient bacteremia, resulting in clinical and pathologic endocarditis. Our study strongly suggests that the mechanical stress imposed by MVP without regurgitation is not sufficient to result in clinical IE.
The 2007 endocarditis prevention guidelines based recommendations for peri-procedural antibiotic prophylaxis on the presence of underlying cardiac conditions with the highest risk of adverse outcome from IE, but also on the risk of acquisition of IE19; MVP patients with ≥moderate MR or flail leaflets represent the highest spectrum of IE risk for this cardiac condition.
Strengths and Limitations
Despite the large patient number of our cohort and long-term follow-up, the absolute number of IE cases does not allow analysis of independent predictors of IE but only univariate ones. Although our study design is superior to the case-control tertiary-referral-based prior studies, it is a retrospective analysis with its inherent limitations. However, we confirmed each IE case de-novo by modified Duke criteria, and the highly morbid nature of IE makes overlooking IE cases highly unlikely. Although an attractive proposition, attempting to analyze and shed light on the complex issue of source-of-infection in IE, is beyond the scope of this investigation mostly due to limited absolute number of IE cases. Information on the spectrum of mitral valve pathology defined by echocardiography (i.e., fibroelastic deficiency versus myxomatous degeneration) was not available during the period studied. There may be differences in IE incidence between these valvular phenotypes which must be explored in prospective studies. A unique strength of our study was the ability to compare the MVP-related IE incidence to the general IE incidence within the same geographically-defined population; Olmsted County. Also, we included patients after the year 1988 when current echocardiographic MVP definition became practice. In addition, our IE case follow-up ended in 2006 to avoid including IE cases after the prevention guidelines changed in 2007, and thus, prevent potential bias in our incidence calculations. Finally, our study population was comprised of adult patients who are predominantly white and of Northern European descent and results could differ in other demographic settings.
Conclusion
The population-based incidence of IE in MVP adults is higher than previously reported in case-control tertiary care-center studies. MVP patients with ≥moderate mitral regurgitation or a flail leaflet are at notable risk of developing IE compared to those without mitral regurgitation. These findings may influence future endocarditis prevention guidelines, and should prompt attention from the research community to study prevention and treatment strategies for IE in MVP patients with mitral regurgitation.
Abbreviations
- IE
infective endocarditis
- MVP
mitral valve prolapse
- MR
mitral regurgitation
- REP
rochester epidemiologic project
Footnotes
Author Financial Support and Disclosures: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Freed LA, Levy D, Levine RA, et al. Prevalence and clinical outcome of mitral-valve prolapse. N Engl J Med. 1999 Jul 1;341(1):1–7. doi: 10.1056/NEJM199907013410101. [DOI] [PubMed] [Google Scholar]
- 2.Michelena HI, Bichara VM, Margaryan E, et al. Progress in the treatment of severe mitral regurgitation. Rev Esp Cardiol. 2010 Jul;63(7):820–831. doi: 10.1016/s1885-5857(10)70167-5. [DOI] [PubMed] [Google Scholar]
- 3.Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet. 2006 Sep 16;368(9540):1005–1011. doi: 10.1016/S0140-6736(06)69208-8. [DOI] [PubMed] [Google Scholar]
- 4.Marks AR, Choong CY, Sanfilippo AJ, Ferre M, Weyman AE. Identification of high-risk and low-risk subgroups of patients with mitral-valve prolapse. N Engl J Med. 1989 Apr 20;320(16):1031–1036. doi: 10.1056/NEJM198904203201602. [DOI] [PubMed] [Google Scholar]
- 5.Mylonakis E, Calderwood SB. Infective endocarditis in adults. N Engl J Med. 2001 Nov 1;345(18):1318–1330. doi: 10.1056/NEJMra010082. [DOI] [PubMed] [Google Scholar]
- 6.Devereux RB, Hawkins I, Kramer-Fox R, et al. Complications of mitral valve prolapse. Disproportionate occurrence in men and older patients. Am J Med. 1986 Nov;81(5):751–758. doi: 10.1016/0002-9343(86)90339-6. [DOI] [PubMed] [Google Scholar]
- 7.Nishimura RA, McGoon MD, Shub C, Miller FA, Jr, Ilstrup DM, Tajik AJ. Echocardiographically documented mitral-valve prolapse. Long-term follow-up of 237 patients. N Engl J Med. 1985 Nov 21;313(21):1305–1309. doi: 10.1056/NEJM198511213132101. [DOI] [PubMed] [Google Scholar]
- 8.MacMahon SW, Hickey AJ, Wilcken DE, Wittes JT, Feneley MP, Hickie JB. Risk of infective endocarditis in mitral valve prolapse with and without precordial systolic murmurs. Am J Cardiol. 1987 Jan 1;59(1):105–108. doi: 10.1016/s0002-9149(87)80080-2. [DOI] [PubMed] [Google Scholar]
- 9.Smith RH, Radford DJ, Clark RA, Julian DG. Infective endocarditis: a survey of cases in the South-East region of Scotland, 1969–72. Thorax. 1976 Aug;31(4):373–379. doi: 10.1136/thx.31.4.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van der Meer JT, Thompson J, Valkenburg HA, Michel MF. Epidemiology of bacterial endocarditis in The Netherlands. I. Patient characteristics. Arch Intern Med. 1992 Sep;152(9):1863–1868. doi: 10.1001/archinte.152.9.1863. [DOI] [PubMed] [Google Scholar]
- 11.Berlin JA, Abrutyn E, Strom BL, et al. Incidence of infective endocarditis in the Delaware Valley, 1988–1990. Am J Cardiol. 1995 Nov 1;76(12):933–936. doi: 10.1016/s0002-9149(99)80264-1. [DOI] [PubMed] [Google Scholar]
- 12.Hogevik H, Olaison L, Andersson R, Lindberg J, Alestig K. Epidemiologic aspects of infective endocarditis in an urban population. A 5-year prospective study. Medicine (Baltimore) 1995 Nov;74(6):324–339. doi: 10.1097/00005792-199511000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Correa de Sa DD, Tleyjeh IM, Anavekar NS, et al. Epidemiological trends of infective endocarditis: a population-based study in Olmsted County, Minnesota. Mayo Clin Proc. 2010 May;85(5):422–426. doi: 10.4065/mcp.2009.0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tleyjeh IM, Steckelberg JM, Murad HS, et al. Temporal trends in infective endocarditis: a population-based study in Olmsted County, Minnesota. Jama. 2005 Jun 22;293(24):3022–3028. doi: 10.1001/jama.293.24.3022. [DOI] [PubMed] [Google Scholar]
- 15.Hickey AJ, MacMahon SW, Wilcken DE. Mitral valve prolapse and bacterial endocarditis: when is antibiotic prophylaxis necessary? Am Heart J. 1985 Mar;109(3 Pt 1):431–435. doi: 10.1016/0002-8703(85)90543-5. [DOI] [PubMed] [Google Scholar]
- 16.Clemens JD, Horwitz RI, Jaffe CC, Feinstein AR, Stanton BF. A controlled evaluation of the risk of bacterial endocarditis in persons with mitral-valve prolapse. N Engl J Med. 1982 Sep 23;307(13):776–781. doi: 10.1056/NEJM198209233071302. [DOI] [PubMed] [Google Scholar]
- 17.MacMahon SW, Roberts JK, Kramer-Fox R, Zucker DM, Roberts RB, Devereux RB. Mitral valve prolapse and infective endocarditis. Am Heart J. 1987 May;113(5):1291–1298. doi: 10.1016/0002-8703(87)90957-4. [DOI] [PubMed] [Google Scholar]
- 18.Levine RA, Stathogiannis E, Newell JB, Harrigan P, Weyman AE. Reconsideration of echocardiographic standards for mitral valve prolapse: lack of association between leaflet displacement isolated to the apical four chamber view and independent echocardiographic evidence of abnormality. J Am Coll Cardiol. 1988 May;11(5):1010–1019. doi: 10.1016/s0735-1097(98)90059-6. [DOI] [PubMed] [Google Scholar]
- 19.Wilson W, Taubert KA, Gewitz M, et al. Prevention of infective endocarditis: guidelines from the American Heart Association: a guideline from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation. 2007 Oct 9;116(15):1736–1754. doi: 10.1161/CIRCULATIONAHA.106.183095. [DOI] [PubMed] [Google Scholar]
- 20.Rocca WA, Yawn BP, St Sauver JL, Grossardt BR, Melton LJ., 3rd History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clinic proceedings. 2012 Dec;87(12):1202–1213. doi: 10.1016/j.mayocp.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Avierinos JF, Gersh BJ, Melton LJ, 3rd, et al. Natural history of asymptomatic mitral valve prolapse in the community. Circulation. 2002 Sep 10;106(11):1355–1361. doi: 10.1161/01.cir.0000028933.34260.09. [DOI] [PubMed] [Google Scholar]
- 22.Li JS, Sexton DJ, Mick N, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis. 2000 Apr;30(4):633–638. doi: 10.1086/313753. [DOI] [PubMed] [Google Scholar]
- 23.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 24.Perloff JK, Child JS. Mitral valve prolapse. Evolution and refinement of diagnostic techniques. Circulation. 1989 Sep;80(3):710–711. doi: 10.1161/01.cir.80.3.710. [DOI] [PubMed] [Google Scholar]
- 25.Mintz GS, Kotler MN, Parry WR, Segal BL. Statistical comparison of M mode and two dimensional echocardiographic diagnosis of flail mitral leaflets. Am J Cardiol. 1980 Feb;45(2):253–259. doi: 10.1016/0002-9149(80)90643-8. [DOI] [PubMed] [Google Scholar]
- 26.Suri RM, Vanoverschelde JL, Grigioni F, et al. Association between early surgical intervention vs watchful waiting and outcomes for mitral regurgitation due to flail mitral valve leaflets. Jama. 2013 Aug 14;310(6):609–616. doi: 10.1001/jama.2013.8643. [DOI] [PubMed] [Google Scholar]
- 27.Helmcke F, Nanda NC, Hsiung MC, et al. Color Doppler assessment of mitral regurgitation with orthogonal planes. Circulation. 1987 Jan;75(1):175–183. doi: 10.1161/01.cir.75.1.175. [DOI] [PubMed] [Google Scholar]
- 28.Zoghbi WA, Enriquez-Sarano M, Foster E, et al. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr. 2003 Jul;16(7):777–802. doi: 10.1016/S0894-7317(03)00335-3. [DOI] [PubMed] [Google Scholar]
- 29.Duren DR, Becker AE, Dunning AJ. Long-term follow-up of idiopathic mitral valve prolapse in 300 patients: a prospective study. J Am Coll Cardiol. 1988 Jan;11(1):42–47. doi: 10.1016/0735-1097(88)90164-7. [DOI] [PubMed] [Google Scholar]
- 30.Corrigall D, Bolen J, Hancock EW, Popp RL. Mitral valve prolapse and infective endocarditis. Am J Med. 1977 Aug;63(2):215–222. doi: 10.1016/0002-9343(77)90235-2. [DOI] [PubMed] [Google Scholar]
- 31.Thiene G, Basso C. Pathology and pathogenesis of infective endocarditis in native heart valves. Cardiovasc Pathol. 2006 Sep-Oct;15(5):256–263. doi: 10.1016/j.carpath.2006.05.009. [DOI] [PubMed] [Google Scholar]