Abstract
Background
As the population grows older, the incidence and prevalence of conditions which lead to a predisposition for poor wound healing also increases. Ultimately, this increase in non-healing wounds has led to significant morbidity and mortality with subsequent huge economic ramifications. Therefore, understanding specific molecular mechanisms underlying aberrant wound healing is of great importance. It has, and will continue to be the leading pathway to the discovery of therapeutic targets as well as diagnostic molecular biomarkers. Biomarkers may help identify and stratify subsets of non-healing patients for whom biomarker-guided approaches may aid in healing.
Methods
A series of literature searches were performed using Medline, PubMed, Cochrane Library, and Internet searches.
Results
Currently, biomarkers are being identified using biomaterials sourced locally, from human wounds and/or systemically using systematic high-throughput “omics” modalities (genomic, proteomic, lipidomic, metabolomic analysis). In this review we highlight the current status of clinically applicable biomarkers and propose multiple steps in validation and implementation spectrum including those measured in tissue specimens e.g. β-catenin and c-myc, wound fluid e.g. MMP’s and interleukins, swabs e.g. wound microbiota and serum e.g. procalcitonin and MMP’s.
Conclusions
Identification of numerous potential biomarkers utilizing different avenues of sample collection and molecular approaches is currently underway. A focus on simplicity, and consistent implementation of these biomarkers as well as an emphasis on efficacious follow-up therapeutics is necessary for transition of this technology to clinically feasible point-of-care applications.
Introduction
Wound healing is a multifaceted process governed by sequential, yet overlapping phases including: hemostasis, inflammation, proliferation and remodeling. This process is executed through communication between the various local skin compartments, supporting extracellular matrix (ECM) and systemic contributors. Under physiological conditions in a healthy individual the process of cutaneous epidermal repair is highly efficient; however, when this process stalls tissue fails to regain structural and functional integrity resulting in chronic wounds. In the context of an increasing elderly population with a myriad of progressively prevalent disease states such as diabetes, vascular diseases, and obesity, clinicians, particularly those in surgical specialties and physicians treating healing disorders daily encounter chronic non-healing wounds including diabetic foot ulcers (DFUs), venous leg ulcers (VLUs) and pressure ulcers (PUs).
It is estimated that the number of people with chronic wounds in the US is over 6.5 million and continues to increase, as population ages and incidence of diseases predisposing to poor wound healing escalate1,2. The economic impact of the cost of care for such wounds is significant, with projections in the billions in the US alone3. In addition to morbidity, chronic wounds pose significant mortality, with a 5 year rate for DFUs and ischemic ulcers being higher than commonly encountered cancers such as breast and prostate4–6. Furthermore, in cases in which amputation is necessary treatment approach the 5-year mortality rate is nearly 50%6.
Despite an obvious alarming clinical need and extensive studies conducted in recent years, basic science and clinical research targeted at understanding and improving delayed healing is still lagging due to multifactorial reasons. Patients with chronic wounds usually consist of elderly people with multiple comorbid diseases in addition to poly-pharmacologic effects that make patient-to-patient variability a significant challenge. This variability further underscores the need for large-scale studies and personalized approaches. Furthermore, such complexity of the disease presents a challenge in development of suitable pre-clinical animal models to study chronic wounds and potential treatments, further limiting translation from pre-clinical trials to clinic7.
Tools that can be used to better direct therapy, predict healing outcomes, monitor disease progression and measure response to treatment are in great need. Here we focus on the pathophysiology of chronic wounds and describe how knowledge gained is utilized in a journey of discovery of molecular biomarkers that may, in the near future, allow for personalized, efficacious and cost effective treatment.
Biology of Wound Healing
Physiologic wound healing initiated after tissue injury jumpstarts a signaling network between different cell types and compartments of the skin. Upon injury, hemostasis results in the formation of a fibrin clot whose constituents serve both as a scaffold and a source of growth factors and chemokines that recruit a gambit of cells, including inflammatory cells, to migrate into the wound bed8–10. Changes in the expression of numerous adhesion molecules on endothelial cells allow for the recruitment and extravasation of neutrophils and macrophages from the circulation to clear potential infection. These inflammatory cells release growth factors and cytokines including interleukins (IL1, IL6), tumor necrosis factor-α (TNFα), platelet-derived growth factor (PDGF), and fibroblast growth factor-2 (FGF2)2. Fibroblasts then proliferate and lay down provisional matrix over which keratinocytes migrate from the wound margins. In response to injury, keratinocytes at the wound edge release cytokines to communicate that the barrier has been compromised2. Together with simultaneous rearrangement of integrins and cytoskeletal components and the expression of proteases such as matrix metalloproteinases (MMPs), keratinocytes are able clear a path for migration along the interface between the fibrin clot and the underlying dermis. This process deemed re-epithelization is followed by keratinocyte proliferation at the wound margin thus reestablishing stratified epidermis11. The integrity of the epithelial barrier is then restored and the remodeling of the underlying matrix resumes.
Aberrant execution of normal repair mechanisms can result in healing impairment and development of chronic wounds. Chronic wounds are defined by inadequate progress through a timely sequence of anatomic and functional restoration12. They can broadly be categorized as fitting into one of 3 major etiologic groups: vascular insufficiency (e.g. VLUs), diabetes mellitus (e.g. DFUs) and local-pressure effects (e.g. PUs). Additionally, various systemic factors can impair healing2. However, at the heart of non-healing wounds are shared characteristics irrespective of distinct wound etiologies: presence of prolonged and sub-optimal inflammation and concurrent infection, deregulation of proteases, reduced growth factor activity, stem cell dysfunction, and cellular senescence13–15.
Prolonged sub-optimal inflammation in the wound microenvironment, elevated levels of MMPs and reduced levels of their inhibitors, all contribute to inhibition of cell migration and healing13,16–18. Perpetuating this inflammatory state is polymicrobial infection of the wound; often with microbes capable of forming biofilms that together sustain the influx of proinflammatory cells while respectively hampering the “host response” to infection17,19–22. Impaired angiogenesis and vasculogenesis, through aberrant regulation and cleavage of growth factors, and their receptors leads to insufficient oxygenation and inadequate delivery of nutrients to the wound23,24. Epidermal stem cell dysfunction due to constant cycling and subsequent depletion together with deregulated chemokines necessary to recruit bone marrow and endothelial progenitors leads to inadequate healing11,25. Furthermore, prematurely senescent fibroblasts and keratinocytes secrete elevated levels of MMPs, antiangiogenic factors and ILs26, leading to de-regulation of their target genes subsequently contributing to a delay in healing. Any number of these de-regulated molecules and cellular processes can be potentially utilized for diagnostic purposes and monitoring of healing progression.
Biomarkers and Their Use
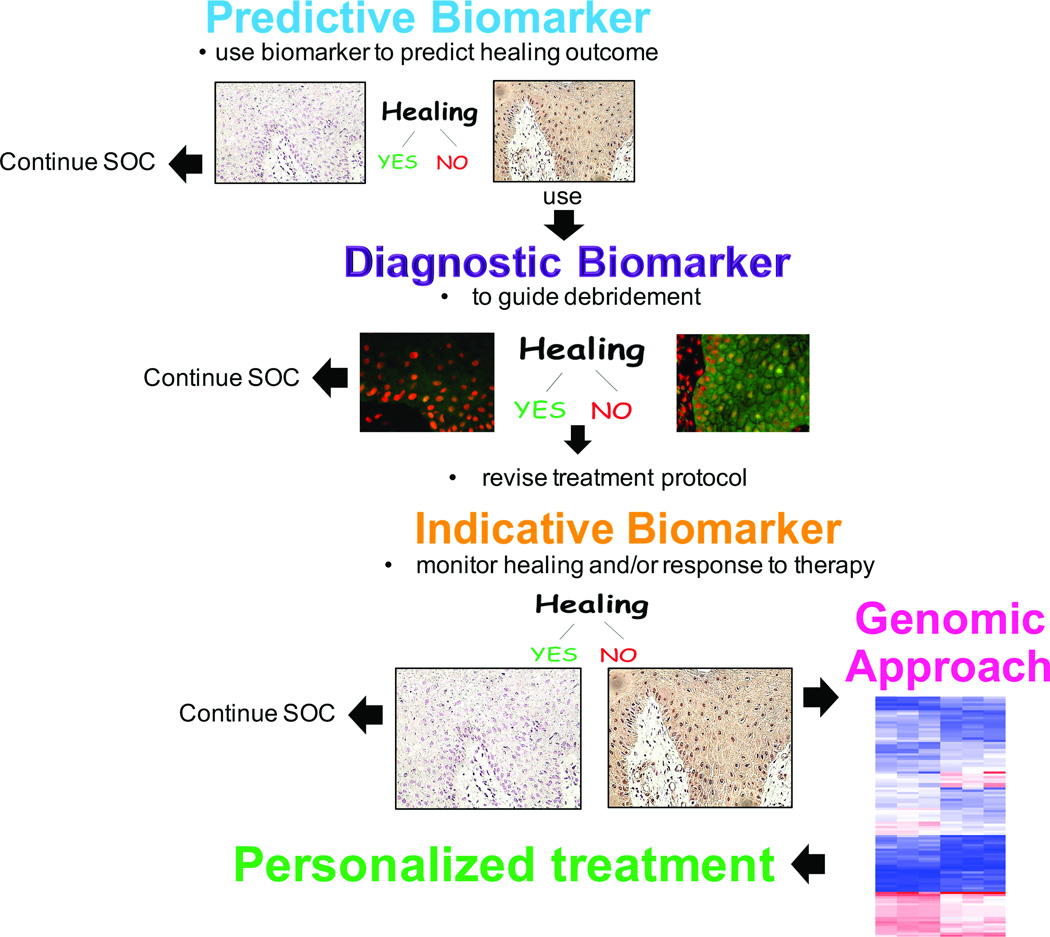
A biomarker is an objectively quantifiable substance that can be assessed as an indicator of a normal physiological or pathological process, or a pharmacologic response to a therapeutic intervention27. As such, although not mutually exclusive, biomarkers can be categorized based on the type of information they provide as predictive, diagnostic and indicative (Figure 1). Predictive biomarkers can be used to predict outcomes or provide likelihood of benefit from treatment. They can serve as a powerful tool in tailoring treatment modalities for specific subsets of patient populations. Diagnostic biomarkers can be utilized to identify the presence of a single or multiple factors that have the potential to influence the clinical outcome. An indicative biomarker can be utilized to monitor disease progression and/or response to therapy in real time.
Figure 1. The future.
The flow chart shows potential use of various types of biomarkers to personalize treatment approach in patients with chronic wounds.
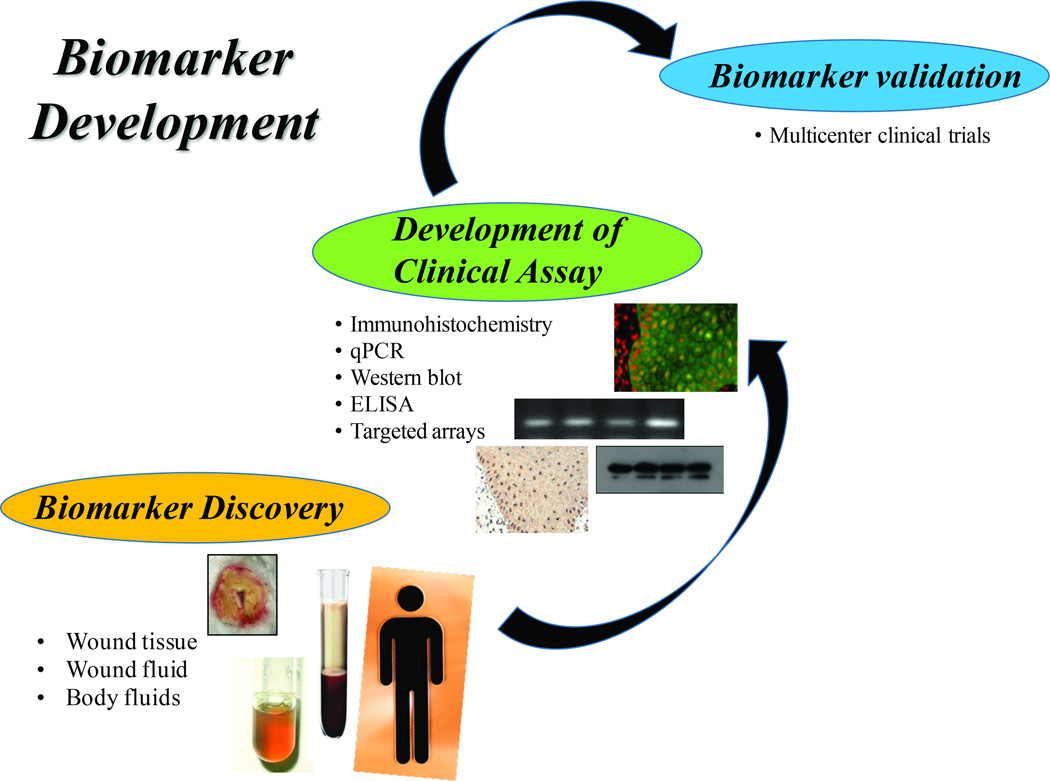
The development of biomarkers that are clinically applicable first involves the discovery process of finding molecules that inhibit or regulate healing followed by their validation in patients (Figure 2). Multiple high-throughput “omics” modalities using human wounds as a source of biomaterials are being employed to understand the mechanisms of impairment, thus facilitating the discovery of potential biomarkers11,13,17,28–31. It is hoped that such biomarkers will allow personalized assessment, provide predictions of patients’ healing status and insights into therapeutic approaches.
Figure 2. The long road of discovery and development of biomarkers.
Steps in development of biomarker start from biomarker discovery, development of specific assay to be used in clinic and a final biomarker validation in multicenter clinical trials before it becomes available for use as a guiding tool by physicians in clinics.
In order to assess the presence or follow dynamics of a biomarker during wound healing, specific molecular/cellular biology approaches are utilized to analyze biomaterials. Potential sources of biomaterials include wound fluid and swabs, tissue specimens and patient serum (Figure 2) each contributing different information. Wound fluid can be collected for protein and lipid analyses and for culturing of wound-associated inflammatory cells32–34. Wound swabs provide biomaterial that can be used for semi-quantitative and qualitative microbiology, global “omics” and selective proteomic evaluation, including specific proteases and their regulators. Histopathology, biomarker diagnostics, generation of primary cell cultures, quantitative and qualitative microbiology and profiling “omics” can all be ascertained using wound tissue specimens, highlighting the versatility of this type of biomaterial. On the other end of the biomarker spectrum, systemic markers of wound healing can be assessed using blood or serum from patients alone or in conjunction with the aforementioned sources. One can speculate that in the near future, change and/or presence of an associated biomarker will be used as one of the endpoints in clinical trials. However, evidence based data including extensive characterization and validation in multicenter clinical trials will likely be necessary, prior to transitioning to point-of-care.
Current Status of Biomarkers in Chronic Wounds
Tissue Biomarkers
Tissue specimens are an excellent source to obtain information regarding diagnosis, directing therapy and predicting outcomes, once molecular markers are identified and validated. Histology, in conjunction with different molecular markers, has long been established as a diagnostic approach for disease states such as vasculitis, kidney disease and transplant rejection as well as its most well-known application in cancer and pre-cancerous lesions35–37.
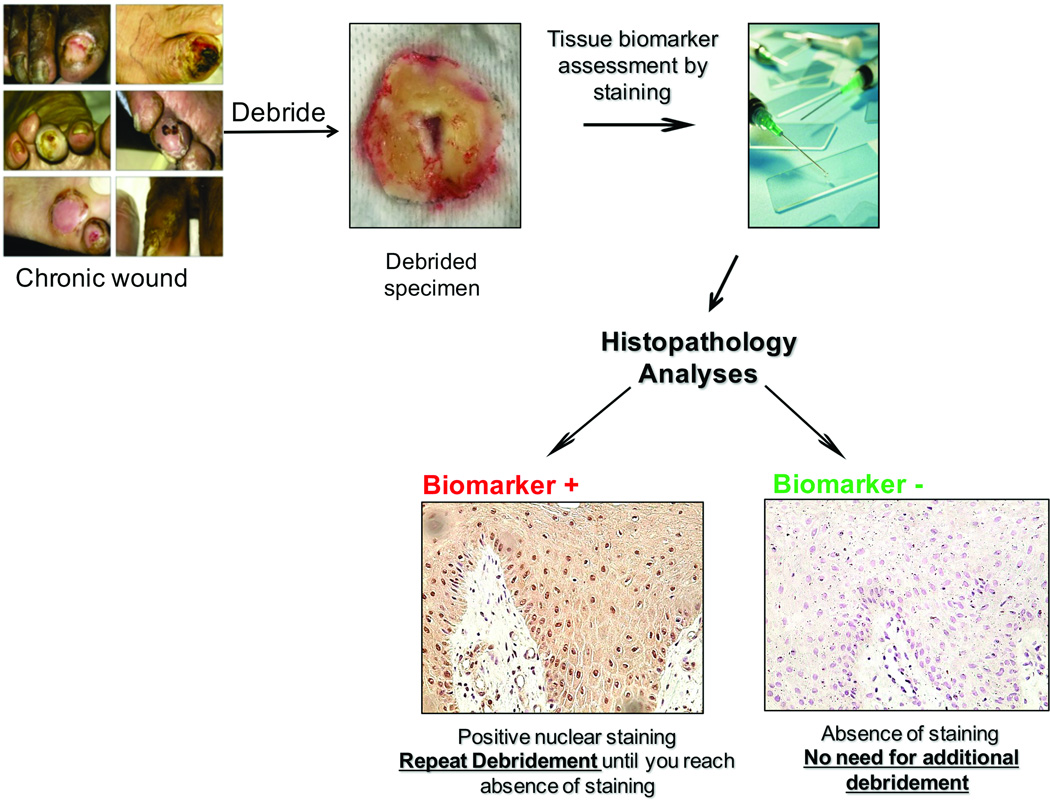
In the last decade, knowledge gained from patients’ tissue specimens revealed the first molecular markers associated with healing impairment. Tissue from the edge of non-healing wounds demonstrates hyperproliferative epidermis, various degrees of fibrosis and increased cellular infiltrate2,29. Our laboratory has described multiple tissue-based molecular markers downstream of the Wnt signaling pathway: nuclear β-catenin and c-myc, to be clinically associated with healing impairment29. The Wnt pathway is important for skin development and homeostasis in early stages of development during patterning and morphogenesis, contributing to the post-natal control of hair cycling, maintenance/control of stem cells and cellular fate in the epidermal compartments38,39. Intriguingly, studies in our laboratory have found activation of β-catenin and its downstream target, oncogene c-myc, in the non-healing edge of chronic wounds, resulting in thickened, hyperproliferative and parakeratotic epidermis indicating aberrant proliferation and inappropriate differentiation28. Nuclear presence of these biomarkers in tissue specimens is quantifiable by use of immunohistochemistry (Figure 1, 3), highlighting their clinical feasibility and utility as a tissue biomarker. Currently, these two biomarkers are being further evaluated and encouraging preliminary data from our laboratory suggest that these two biomarkers may potentially have significant predictive and diagnostic power and their clinical validation is undergoing.
Figure 3. Biomarker-based guidance to debridement.
Possible use of biomarkers as an indicator for a debridement margin e.g. how far wound care practitioner should debride a chronic wound.
Additional studies regarding molecular and cellular pathophysiology of chronic wounds may also serve as potential diagnostic molecular tools and they are summarized in Table 1. However, small sample sizes utilized in these studies may mitigate potential clinical implications and large multicenter prospective trials are clearly needed to confirm these data and allow transition to the point of care for clinical use. Additional challenges for tissue-based biomarkers originate from standardization of specimen retrieval40, logistics of service-based diagnostics (similar to pathology service) and incorporation of this approach into standard care and clinical trial protocols. Nevertheless, this approach may prove to be very valuable in early diagnostics that would facilitate more personalized treatment protocols.
Table 1.
Healing Impairment Associated Tissue and Wound Biomarkers
| Quantified Measure of Non-healing | Biomaterial/Method | Reference | |
|---|---|---|---|
| Tissue Markers: | |||
| ↑ β-catenin ↑ c-myc | ↑nuclear/cytoplasmic staining |
Biomaterial: debrided tissue Method: immunostaining (IHC/IF) |
14,28,29 |
|
↓ BMPR ↓ LRIG1 ↓ GATA3 ↓ IDR2,4 ↓ K15 |
↓ gene expression ↓ gene expression ↓ nuclear staining ↓membranous staining |
Biomaterial: debrided tissue Methods: microarray, q-PCR, IHC/IF |
14 |
| ↑ ADAM12 | ↑ gene expression ↑nuclear/cytoplasmic staining |
Biomaterial: debrided tissue Methods: microarray, q-PCR, IHC/IF |
96 |
| EGFR | ↑ cytoplasmic staining |
Biomaterial: debrided tissue Method: IHC/IF |
3 |
| ↓ cathelicidin | ↓ staining wound edge, wound bed infiltrate and exudate |
Biomaterial: debrided tissue Method: IHC/IF |
97 |
| tPA, uPA, PAI-1 | variable expression |
Biomaterial: debrided tissue Methods: mRNA in situ hybridization, IHC/IF |
98 |
| ↓ TGFβI,II,III ligands | ↓ gene expression ↓ staining |
Biomaterial: debrided tissue Methods: qPCR, IHC/IF |
99 |
| ↓ phospho-smad2 | ↓ nuclear staining |
Biomaterial: debrided tissue Methods: IHC/IF |
100 |
|
↑ miR-16, -20a, -21, -106a, -130a, -203 |
↑ expression |
Biomaterial: debrided tissue Methods: q-PCR, in situ hybridization |
80,101 |
| Wound Fluid Markers: | |||
|
↑ MMP1, 2, 3, 7, 8, 9, 10, 11, 13 |
↑ protein expression |
Biomaterial: collected using swabbing technique or wound vac Methods: ELISA; Point-of-care qualitative measurement device available in select countries for clinical use (WOUNDCHECK™) |
15,50–54,57,58, 70 |
| ↓ TIMP1 | ↓ protein expression |
Biomaterial: wound fluid Methods: ELISA, quantitative gelatin zymography |
56 |
| ↑ MMP9: TIMP1 | ↑ protein expression MMP9 ↓ protein expression TIMP1 |
Biomaterial: collected using porous, inert hydrophilic dextranomer beads applied to the wound Methods: ELISA, quantitative gelatin zymography |
55 |
| ↑ IL1 ↑ IL6 | ↑ protein expression |
Biomaterial: collected directly from wound bed Methods: ELISA |
69,70 |
|
↓albumin ↓ total protein |
< 20g/l ↓ protein expression |
Biomaterial: collected using transparent occlusive dressing Methods: Bayer Axon clinical chemistry analyzer |
70 |
Wound Fluid Biomarkers
The MMPs are metalloproteinase enzymes and their regulators, tissue inhibitor of matrix metalloproteinase (TIMPs), play important role in wound healing. Metalloproteinases are a group of endopeptidases that can be broadly categorized depending on their primary catalytic substrate: collagenases, gelatinases, stromelysins, matrilysins, and membrane-type MMPs41–46. MMPs mediate critical steps in almost every phase of the wound healing47–49. MMPs are required for a wound to heal properly at an appropriate level, in the correct location and for a precise duration of time. Thus, it is not surprising that chronically elevated levels of MMPs and reduced levels of TIMPS, or aberrations in their ratio have been correlated with non-healing in chronic wounds15,50–57,58, 59,60. Studies have shown that therapeutic interventions which lower MMP activity such as MMP absorbent collagen/oxidized regenerated cellulose dressings, promote healing of stalled wounds and that decreasing MMP-2 tissue levels are reflective of wound healing itself. These findings implicate MMPs as potential diagnostic, predictive and indicative biomarkers for wound healing61–64.
Quantitative assessments of MMPs as diagnostic biomarkers to predict wounds with poor healing potential are currently being tested in clinical trials65. In one study authors utilized wound fluid collected from PUs to assess MMP-9/MMP-1 ratio55. They show that the average MMP-9/MMP-1 ratio decreased significantly in healing wounds and that the ratio was significantly lower, at the time of collection, in wounds that healed over 36 days suggesting that MMP-9/MMP-1 ratio may serve as a predictive biomarker for PU healing55. Effect of topical negative pressure therapy was assessed by measurement of MMP9/TIMP-1 wound fluid ratio and MMP-9/TIMP-1 ratio was significantly lower after 10 days of treatment indicating biomarker utilization in the assessment of the effect of therapeutic modalities66. These findings have facilitated the development of technology that allows for point-of-care measurement of elevated protease activity (EPA) (including MMP-8 and MMP-9 levels) in the clinical setting using a swabbing technique67. Ideally, this point-of-care measurement would be used early to better direct care for those patients who would normally fail standard of care (SOC) therapy. It remains to be seen if patients identified to have EPA will benefit from employing protease reducing therapies at the initiation of treatment68. Currently, results are pending for two randomized controlled clinical trials evaluating the healing outcome of patients with DFUs or VLUs whose wounds were identified to have EPA. In the future it will also be important to more completely understand the subset of patients that show positive EPA and the underlying mechanisms leading to increased protease levels in order to develop molecular-based therapies.
Additionally, levels of multiple cytokines and growth factors have been found to be significantly higher in wound fluid of healing impaired VLUs69,70 and may also serve as prognostic biomarkers for healing outcomes and are summarized in Table 1.
Overall collection of wound biomaterials for diagnostic purposes shows great promise and is under clinical development. Ideally, each of the diagnostic biomarkers would be coupled with specific guidelines of each particular clinical protocol. For example, if a predictive biomarker at the patients’s initial visit shows that a wound is healing, one should continue using standard of care. If, however, the predictive biomarker indicates that the wound is non-healing one should implement a more aggressive treatment protocol (Figure 3). In spite of multiple challenges for clinical validation, one can envision their utilization and incorporation into standard clinical protocols in the near future. In the future, determination of specific targeted interventions that would benefit patients identified with such biomarkers is needed.
Systemic Biomarkers Associated with Wound Healing Impairment
It should come as no surprise that cross-talk between the body as a whole and local tissue impairment can be recognized on the molecular level. Therefore, along with local markers of non-healing, there have been studies showing the presence of systemic, healing-impairment-associated markers.
For instance, using serum from patients with acute combat wounds, systemic biomarkers indicative of non-healing have been identified71,72. In one such study patients were followed for 6 weeks and 24 cytokines and chemokines were quantified revealing an independent association between increased serum levels of IL-3 and IL-12p70 with heterotopic ossification and increased serum procalcitonin with failure to heal73. Another study evaluating serum levels of MMPs in acute combat wounds found elevated levels of MMP-2 and MMP-7 in patients who demonstrated impaired wound healing, defined as delayed wound closure >21 days past the injury or wound dehiscence71.
Recently, Thom et al. showed that the number of CD34+/CD45-dim stem/progenitor cells (SPC) in a patient’s serum and the cellular content of hypoxia-inducible factors can serve as predictors of healing outcome in DFU patients74. Results of this study show that more SPC entered the bloodstream in patients who healed than in those who did not74. One can further speculate how such approaches may be utilized to predict response to therapies, such as hyperbaric oxygen, or systemic cell therapies. In another study, plasma miRNA signatures in DFU patients displayed altered miRNA-200b or miRNA-191 levels compared to diabetic patients without ulcers. These miRNAs modulate cellular migration and angiogenesis via paracrine regulation of zonula occludens-1 to delay ulcer healing75 and may be used to predict the risk of DFU development (Table 2).
Table 2.
Systemic Biomarkers of Non-healing
| Quantified Measure | Biomaterial/Method | Reference |
|---|---|---|
| ↑ procalcitonin |
Biomaterial: serum Methods: Beadlyte1 Human 22-Plex Multi-Cytokine Detection System (Upstate/Millipore, Billerica, MA, USA) |
73 |
| ↑ MMP3 ↑ MMP2 |
Biomaterial: serum Methods Luminex multiplex system; (Upstate/Millipore, Billerica, MA, USA) |
71 |
| ↓ CD34+/CD45-dim circulating cells |
Biomaterial: plasma, debrided tissue Methods: Flow Cytometry, Immunohistochemistry |
74 |
| ↓ HIF1-2: HIF 3 |
Biomaterial: plasma, debrided tissue Methods: Flow Cytometry, Immunohistochemistry |
74 |
| ↓ miRNA-200b ↓miRNA -191 |
Biomaterial: plasma Methods: Plasma-derived microRNA (miRNA) array, qPCR |
75 |
Both blood and serum can easily be obtained from peripheral venous blood samples and are routinely collected for various clinical applications making serum biomarkers a feasible method for predicting healing outcomes if larger clinical trials validate biomarkers described above.
Wound Microbiota as Diagnostic Biomarkers
The human microbiome is the ecological community of commensal, symbiotic, and pathogenic microorganisms that quite literally share our body space. The skin serves as an important barrier for the body and is in direct contact with the outside world resulting in its colonization with a complex and dynamic microflora in a spatiotemporal manner76,77. Injury resulting in the compromise of the barrier leads to microbial influx and colonization of a wound; in response to which the host cells release anti-microbial peptides in an effort to maintain the bacterial load at a manageable level4,22,78–81. Chronic wounds, often harbor a high burden of polymicrobials prone to forming biofilms. The composition of biofilms differs according to wound etiology, location and clinical context but is dominated by Staphylococcus, Pseudomonas, and Corynebacterium generas including other anaerobic species78,82,83. Persistent polymicrobial infection in wounds has been suggested to contribute to poor healing by provoking a prolonged inflammatory phase secondary to sustained recruitment of pro-inflammatory cells20,84. Moreover, studies in animal models demonstrate that polymicrobial infection delays epithelialization19.
Although high bioburden is known to contribute to non-healing, it can be clinically challenging to identify wounds harboring problematic microbes due to lack of clinical signs of wound infection, formation of biofilms and culture methods that underrepresent many bacterial strains78,85,86. Historically, the gold standard for extracting this information has been through culture based methods, however, numerous studies using molecular techniques have now demonstrated that standard culture methods select for those microbes that survive best under the experimental laboratory conditions and do not reflect the true diversity of chronic wound flora especially with regard to anaerobic microbes76,85,87,88. When considering the impact of wound bioburden on healing outcomes three distinct facets of the wound microbiota need to be considered: total microbial load, microbial diversity, and the presence of pathogenic microbes, for most of which standard culture techniques are inadequate78,82,86,89,90. However, technological advances in DNA-sequencing now allow for the identification and quantification of microbial communities to such a degree that all of these facets can be addressed91.
The next-generation sequencing methods utilize the 16S ribosomal RNA (rRNA) gene to evaluate bacterial phylogeny and taxonomy91. Using this technology wound microbiota “footprints” can be generated and while most studies to date have focused on only generating footprints, a few studies have assessed the correlation of these footprints with clinical measures of wound healing17,85,92. Gardner at al85 found that DFU duration and depth positively correlated with relative abundance of anaerobic bacteria, while conversely finding a negative association with Staphylococcus abundance. In contrast, the most recent study focusing on DFU osteomyelitis identified Staphylococcus in all of the affected bone samples17. These findings highlight the potential of using wound microbiota footprints as biomarkers to predict the clinical outcome of wounds, although many other factors shown to influence wound microbiota must be taken into consideration. Therefore using personalized targeted topical anti-microbials, as already shown in retrospective studies, highlights the economic benefit of treating microbial infections identified with molecular techniques, claiming a 68% reduction in the total cost of care with better clinical outcomes as compared to SOC93. Sample collection with the use of a minimally invasive swabbing technique has been shown to provide similar representation of wound bioburden when compared to more invasive punch biopsies17,91,94,95. However, this approach does not come without challenges: technically questions remain about methods for sample collection as well as interpretation of data because molecular techniques do not discern between live and dead microbiome. Furthermore, longitudinal studies will be needed to properly stratify patients and extrapolate relationships between microbiome and healing outcomes. Although this technology is far from the bedside, looking forward, the future generation of microbiome-based biomarker profiles would help clinicians to better differentiate benign wound colonization from a pathogenic state of bioburden and guide better management and treatment91.
The future: “omics”- based diagnostics
Traditionally, genomics, proteomics, lipidomics, metalobomics, and other “omics” have been utilized for research purposes11,13,17,28–31. However, one can envision their use as personalized diagnostics. As computational-based analyses are being developed for large data sets, better known as “big data”, these personalized profiles may be coupled with other clinical diagnostics in electronic medical records to further identify specific disease pattern that can be followed by a specific, more customized, treatment plan. Although currently prohibitively expensive, technology developments are underway to decrease the costs of these analyses, making this approach feasible in the future.
Closing Remarks
Although the clinical field is desperate for better diagnostic tools, their development is slow. At the core of identifying clinically relevant biomarkers is the motivation for early recognition of patients that would both fail SOC therapy before starting treatment and would benefit from early therapeutic interventions tailored to their specific healing prognosis, ultimately resulting in improved and more cost effective outcomes. Many promising biomarkers utilizing different avenues of sample collection are currently in development. The transition to point-of-care clinically applicable technology will necessitate a focus on simplicity, economy and consistent implementation of these biomarkers as well as an emphasis on efficacious follow-up therapeutics.
Acknowledgments
This work is supported by National Institutes of Health NR013881(MT-C) and DK086364 (MT-C) and University of Miami SAC Award SAC 2013–19 (MT-C).
Footnotes
Authors declare no conflict of interest.
REFRENCES
- 1.Sen CK, Gordillo GM, Roy S, et al. Human skin wounds: a major and snowballing threat to public health and the economy. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2009 Nov-Dec;17(6):763–771. doi: 10.1111/j.1524-475X.2009.00543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eming SA, Martin P, Tomic-Canic M. Wound repair and regeneration: mechanisms, signaling, and translation. Sci Transl Med. 2014 Dec 3;6(265):265sr266. doi: 10.1126/scitranslmed.3009337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brem H, Stojadinovic O, Diegelmann RF, et al. Molecular markers in patients with chronic wounds to guide surgical debridement. Mol Med. 2007 Jan-Feb;13(1–2):30–39. doi: 10.2119/2006-00054.Brem. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armstrong DG, Wrobel J, Robbins JM. Guest Editorial: are diabetes-related wounds and amputations worse than cancer? Int Wound J. 2007 Dec;4(4):286–287. doi: 10.1111/j.1742-481X.2007.00392.x. [DOI] [PubMed] [Google Scholar]
- 5.Aulivola B, Hile CN, Hamdan AD, et al. Major lower extremity amputation: outcome of a modern series. Arch Surg. 2004 Apr;139(4):395–399. doi: 10.1001/archsurg.139.4.395. discussion 399. [DOI] [PubMed] [Google Scholar]
- 6.Sargen MR, Hoffstad O, Margolis DJ. Geographic variation in Medicare spending and mortality for diabetic patients with foot ulcers and amputations. J Diabetes Complications. 2013 Mar-Apr;27(2):128–133. doi: 10.1016/j.jdiacomp.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Richmond NA, Lamel SA, Davidson JM, et al. US-National Institutes of Health-funded research for cutaneous wounds in 2012. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2013 Nov-Dec;21(6):789–792. doi: 10.1111/wrr.12099. [DOI] [PubMed] [Google Scholar]
- 8.Uccioli L, Izzo V, Meloni M, Vainieri E, Ruotolo V, Giurato L. Non-healing foot ulcers in diabetic patients: general and local interfering conditions and management options with advanced wound dressings. J Wound Care. 2015 Apr;24(4 Suppl):35–42. doi: 10.12968/jowc.2015.24.Sup4b.35. [DOI] [PubMed] [Google Scholar]
- 9.Martin P. Wound healing--aiming for perfect skin regeneration. Science. 1997 Apr 4;276(5309):75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- 10.Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999 Sep 2;341(10):738–746. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 11.Pastar I, Stojadinovic O, Yin NC, et al. Epithelialization in Wound Healing: A Comprehensive Review. Advances in wound care. 2014 Jul 1;3(7):445–464. doi: 10.1089/wound.2013.0473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lazarus GS, Cooper DM, Knighton DR, et al. Definitions and guidelines for assessment of wounds and evaluation of healing. Arch Dermatol. 1994 Apr;130(4):489–493. [PubMed] [Google Scholar]
- 13.Eming SA, Koch M, Krieger A, et al. Differential proteomic analysis distinguishes tissue repair biomarker signatures in wound exudates obtained from normal healing and chronic wounds. J Proteome Res. 2010 Sep 3;9(9):4758–4766. doi: 10.1021/pr100456d. [DOI] [PubMed] [Google Scholar]
- 14.Stojadinovic O, Pastar I, Nusbaum AG, Vukelic S, Krzyzanowska A, Tomic-Canic M. Deregulation of epidermal stem cell niche contributes to pathogenesis of nonhealing venous ulcers. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2014 Mar-Apr;22(2):220–227. doi: 10.1111/wrr.12142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yager DR, Zhang LY, Liang HX, Diegelmann RF, Cohen IK. Wound fluids from human pressure ulcers contain elevated matrix metalloproteinase levels and activity compared to surgical wound fluids. The Journal of investigative dermatology. 1996 Nov;107(5):743–748. doi: 10.1111/1523-1747.ep12365637. [DOI] [PubMed] [Google Scholar]
- 16.Loots MA, Lamme EN, Zeegelaar J, Mekkes JR, Bos JD, Middelkoop E. Differences in cellular infiltrate and extracellular matrix of chronic diabetic and venous ulcers versus acute wounds. The Journal of investigative dermatology. 1998 Nov;111(5):850–857. doi: 10.1046/j.1523-1747.1998.00381.x. [DOI] [PubMed] [Google Scholar]
- 17.van Asten SA, La Fontaine J, Peters EJ, Bhavan K, Kim PJ, Lavery LA. The microbiome of diabetic foot osteomyelitis. European journal of clinical microbiology & infectious diseases : official publication of the European Society of Clinical Microbiology. 2016 Feb;35(2):293–298. doi: 10.1007/s10096-015-2544-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tarnuzzer RW, Schultz GS. Biochemical analysis of acute and chronic wound environments. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 1996 Jul-Sep;4(3):321–325. doi: 10.1046/j.1524-475X.1996.40307.x. [DOI] [PubMed] [Google Scholar]
- 19.Pastar I, Nusbaum AG, Gil J, et al. Interactions of methicillin resistant Staphylococcus aureus USA300 and Pseudomonas aeruginosa in polymicrobial wound infection. PloS one. 2013;8(2):e56846. doi: 10.1371/journal.pone.0056846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frank DN, Wysocki A, Specht-Glick DD, et al. Microbial diversity in chronic open wounds. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2009 Mar-Apr;17(2):163–172. doi: 10.1111/j.1524-475X.2009.00472.x. [DOI] [PubMed] [Google Scholar]
- 21.Roche ED, Renick PJ, Tetens SP, Ramsay SJ, Daniels EQ, Carson DL. Increasing the presence of biofilm and healing delay in a porcine model of MRSA-infected wounds. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2012 Jul-Aug;20(4):537–543. doi: 10.1111/j.1524-475X.2012.00808.x. [DOI] [PubMed] [Google Scholar]
- 22.Schroder JM. The role of keratinocytes in defense against infection. Current opinion in infectious diseases. 2010 Apr;23(2):106–110. doi: 10.1097/QCO.0b013e328335b004. [DOI] [PubMed] [Google Scholar]
- 23.Lauer G, Sollberg S, Cole M, et al. Expression and proteolysis of vascular endothelial growth factor is increased in chronic wounds. The Journal of investigative dermatology. 2000 Jul;115(1):12–18. doi: 10.1046/j.1523-1747.2000.00036.x. [DOI] [PubMed] [Google Scholar]
- 24.Edsberg LE, Wyffels JT, Brogan MS, Fries KM. Analysis of the proteomic profile of chronic pressure ulcers. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2012 May-Jun;20(3):378–401. doi: 10.1111/j.1524-475X.2012.00791.x. [DOI] [PubMed] [Google Scholar]
- 25.Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M. Growth factors and cytokines in wound healing. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2008 Sep-Oct;16(5):585–601. doi: 10.1111/j.1524-475X.2008.00410.x. [DOI] [PubMed] [Google Scholar]
- 26.Jun JI, Lau LF. The matricellular protein CCN1 induces fibroblast senescence and restricts fibrosis in cutaneous wound healing. Nature cell biology. 2010 Jul;12(7):676–685. doi: 10.1038/ncb2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Biomarkers Definitions Working G. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther. 2001 Mar;69(3):89–95. doi: 10.1067/mcp.2001.113989. [DOI] [PubMed] [Google Scholar]
- 28.Stojadinovic O, Pastar I, Vukelic S, et al. Deregulation of keratinocyte differentiation and activation: a hallmark of venous ulcers. J Cell Mol Med. 2008 Dec;12(6B):2675–2690. doi: 10.1111/j.1582-4934.2008.00321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stojadinovic O, Brem H, Vouthounis C, et al. Molecular pathogenesis of chronic wounds: the role of beta-catenin and c-myc in the inhibition of epithelialization and wound healing. Am J Pathol. 2005 Jul;167(1):59–69. doi: 10.1016/s0002-9440(10)62953-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krisp C, Jacobsen F, McKay MJ, Molloy MP, Steinstraesser L, Wolters DA. Proteome analysis reveals antiangiogenic environments in chronic wounds of diabetes mellitus type 2 patients. Proteomics. 2013 Sep;13(17):2670–2681. doi: 10.1002/pmic.201200502. [DOI] [PubMed] [Google Scholar]
- 31.Taverna D, Nanney LB, Pollins AC, Sindona G, Caprioli R. Multiplexed molecular descriptors of pressure ulcers defined by imaging mass spectrometry. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2011 Nov;19(6):734–744. doi: 10.1111/j.1524-475X.2011.00738.x. [DOI] [PubMed] [Google Scholar]
- 32.Brem H, Golinko MS, Stojadinovic O, et al. Primary cultured fibroblasts derived from patients with chronic wounds: a methodology to produce human cell lines and test putative growth factor therapy such as GMCSF. Journal of translational medicine. 2008;6:75. doi: 10.1186/1479-5876-6-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maione AG, Brudno Y, Stojadinovic O, et al. Three-dimensional human tissue models that incorporate diabetic foot ulcer-derived fibroblasts mimic in vivo features of chronic wounds. Tissue engineering. Part C, Methods. 2015 May;21(5):499–508. doi: 10.1089/ten.tec.2014.0414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shabbir A, Cox A, Rodriguez-Menocal L, Salgado M, Van Badiavas E. Mesenchymal Stem Cell Exosomes Induce Proliferation and Migration of Normal and Chronic Wound Fibroblasts, and Enhance Angiogenesis In Vitro. Stem cells and development. 2015 Jul 15;24(14):1635–1647. doi: 10.1089/scd.2014.0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kallenberg CG. Pathogenesis and treatment of ANCA-associated vasculitides. Clinical and experimental rheumatology. 2015 Jul-Aug;33(4 Suppl 92):S11–S14. [PubMed] [Google Scholar]
- 36.Schwarzenbach H, Pantel K. Circulating DNA as biomarker in breast cancer. Breast cancer research : BCR. 2015;17(1):136. doi: 10.1186/s13058-015-0645-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Young J, Schiffer CA. Genetic Biomarkers in Acute Myeloid Leukemia. Expert Rev Hematol. 2012;5(4):395–407. doi: 10.1586/ehm.12.32. [DOI] [PubMed] [Google Scholar]
- 38.Amini-Nik S, Cambridge E, Yu W, et al. beta-Catenin-regulated myeloid cell adhesion and migration determine wound healing. The Journal of clinical investigation. 2014 Jun;124(6):2599–2610. doi: 10.1172/JCI62059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Poon R, Nik SA, Ahn J, Slade L, Alman BA. Beta-catenin and transforming growth factor beta have distinct roles regulating fibroblast cell motility and the induction of collagen lattice contraction. BMC cell biology. 2009;10:38. doi: 10.1186/1471-2121-10-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stojadinovic O, Landon JN, Gordon KA, et al. Quality assessment of tissue specimens for studies of diabetic foot ulcers. Experimental dermatology. 2013 Mar;22(3):216–218. doi: 10.1111/exd.12104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ra HJ, Parks WC. Control of matrix metalloproteinase catalytic activity. Matrix Biol. 2007 Oct;26(8):587–596. doi: 10.1016/j.matbio.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Armstrong DG, Jude EB. The role of matrix metalloproteinases in wound healing. J Am Podiatr Med Assoc. 2002 Jan;92(1):12–18. doi: 10.7547/87507315-92-1-12. [DOI] [PubMed] [Google Scholar]
- 43.Gill SE, Parks WC. Metalloproteinases and their inhibitors: regulators of wound healing. Int J Biochem Cell Biol. 2008;40(6–7):1334–1347. doi: 10.1016/j.biocel.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stechmiller JK, Kilpadi DV, Childress B, Schultz GS. Effect of Vacuum-Assisted Closure Therapy on the expression of cytokines and proteases in wound fluid of adults with pressure ulcers. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2006 May-Jun;14(3):371–374. doi: 10.1111/j.1743-6109.2006.00134.x. [DOI] [PubMed] [Google Scholar]
- 45.Simeon A, Monier F, Emonard H, et al. Expression and activation of matrix metalloproteinases in wounds: modulation by the tripeptide-copper complex glycyl-L-histidyl-L-lysine-Cu2+ The Journal of investigative dermatology. 1999 Jun;112(6):957–964. doi: 10.1046/j.1523-1747.1999.00606.x. [DOI] [PubMed] [Google Scholar]
- 46.Beidler SK, Douillet CD, Berndt DF, Keagy BA, Rich PB, Marston WA. Multiplexed analysis of matrix metalloproteinases in leg ulcer tissue of patients with chronic venous insufficiency before and after compression therapy. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2008 Sep-Oct;16(5):642–648. doi: 10.1111/j.1524-475X.2008.00415.x. [DOI] [PubMed] [Google Scholar]
- 47.Michopoulou A, Rousselle P. How do epidermal matrix metalloproteinases support re-epithelialization during skin healing? European journal of dermatology : EJD. 2015 Apr;25(Suppl 1):33–42. doi: 10.1684/ejd.2015.2553. [DOI] [PubMed] [Google Scholar]
- 48.Toriseva M, Laato M, Carpen O, et al. MMP-13 regulates growth of wound granulation tissue and modulates gene expression signatures involved in inflammation, proteolysis, and cell viability. PloS one. 2012;7(8):e42596. doi: 10.1371/journal.pone.0042596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rohani MG, McMahan RS, Razumova MV, et al. MMP-10 Regulates Collagenolytic Activity of Alternatively Activated Resident Macrophages. The Journal of investigative dermatology. 2015 Oct;135(10):2377–2384. doi: 10.1038/jid.2015.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wysocki AB, Staiano-Coico L, Grinnell F. Wound fluid from chronic leg ulcers contains elevated levels of metalloproteinases MMP-2 and MMP-9. The Journal of investigative dermatology. 1993 Jul;101(1):64–68. doi: 10.1111/1523-1747.ep12359590. [DOI] [PubMed] [Google Scholar]
- 51.Yager DR, Kulina RA, Gilman LA. Wound fluids: a window into the wound environment? Int J Low Extrem Wounds. 2007 Dec;6(4):262–272. doi: 10.1177/1534734607307035. [DOI] [PubMed] [Google Scholar]
- 52.Tarlton JF, Bailey AJ, Crawford E, Jones D, Moore K, Harding KD. Prognostic value of markers of collagen remodeling in venous ulcers. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 1999 Sep-Oct;7(5):347–355. doi: 10.1046/j.1524-475x.1999.00347.x. [DOI] [PubMed] [Google Scholar]
- 53.Wiegand C, Schonfelder U, Abel M, Ruth P, Kaatz M, Hipler UC. Protease and pro-inflammatory cytokine concentrations are elevated in chronic compared to acute wounds and can be modulated by collagen type I in vitro. Arch Dermatol Res. 2010 Aug;302(6):419–428. doi: 10.1007/s00403-009-1011-1. [DOI] [PubMed] [Google Scholar]
- 54.Wysocki AB, Kusakabe AO, Chang S, Tuan TL. Temporal expression of urokinase plasminogen activator, plasminogen activator inhibitor and gelatinase-B in chronic wound fluid switches from a chronic to acute wound profile with progression to healing. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 1999 May-Jun;7(3):154–165. doi: 10.1046/j.1524-475x.1999.00154.x. [DOI] [PubMed] [Google Scholar]
- 55.Ladwig GP, Robson MC, Liu R, Kuhn MA, Muir DF, Schultz GS. Ratios of activated matrix metalloproteinase-9 to tissue inhibitor of matrix metalloproteinase-1 in wound fluids are inversely correlated with healing of pressure ulcers. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2002 Jan-Feb;10(1):26–37. doi: 10.1046/j.1524-475x.2002.10903.x. [DOI] [PubMed] [Google Scholar]
- 56.Bullen EC, Longaker MT, Updike DL, et al. Tissue inhibitor of metalloproteinases-1 is decreased and activated gelatinases are increased in chronic wounds. The Journal of investigative dermatology. 1995 Feb;104(2):236–240. doi: 10.1111/1523-1747.ep12612786. [DOI] [PubMed] [Google Scholar]
- 57.Lobmann R, Ambrosch A, Schultz G, Waldmann K, Schiweck S, Lehnert H. Expression of matrix-metalloproteinases and their inhibitors in the wounds of diabetic and non-diabetic patients. Diabetologia. 2002 Jul;45(7):1011–1016. doi: 10.1007/s00125-002-0868-8. [DOI] [PubMed] [Google Scholar]
- 58.Weckroth M, Vaheri A, Lauharanta J, Sorsa T, Konttinen YT. Matrix metalloproteinases, gelatinase and collagenase, in chronic leg ulcers. The Journal of investigative dermatology. 1996 May;106(5):1119–1124. doi: 10.1111/1523-1747.ep12340167. [DOI] [PubMed] [Google Scholar]
- 59.Rayment EA, Upton Z, Shooter GK. Increased matrix metalloproteinase-9 (MMP-9) activity observed in chronic wound fluid is related to the clinical severity of the ulcer. Br J Dermatol. 2008 May;158(5):951–961. doi: 10.1111/j.1365-2133.2008.08462.x. [DOI] [PubMed] [Google Scholar]
- 60.Liu Y, Min D, Bolton T, et al. Increased matrix metalloproteinase-9 predicts poor wound healing in diabetic foot ulcers. Diabetes Care. 2009 Jan;32(1):117–119. doi: 10.2337/dc08-0763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Veves A, Sheehan P, Pham HT. A randomized, controlled trial of Promogran (a collagen/oxidized regenerated cellulose dressing) vs standard treatment in the management of diabetic foot ulcers. Arch Surg. 2002 Jul;137(7):822–827. doi: 10.1001/archsurg.137.7.822. [DOI] [PubMed] [Google Scholar]
- 62.Cullen B. The role of oxidized regenerated cellulose/collagen in chronic wound repair. Part 2. Ostomy Wound Manage. 2002 Jun;48(6 Suppl):8–13. [PubMed] [Google Scholar]
- 63.Ulrich D, Smeets R, Unglaub F, Woltje M, Pallua N. Effect of oxidized regenerated cellulose/collagen matrix on proteases in wound exudate of patients with diabetic foot ulcers. J Wound Ostomy Continence Nurs. 2011 Sep-Oct;38(5):522–528. doi: 10.1097/WON.0b013e31822ad290. [DOI] [PubMed] [Google Scholar]
- 64.Karim RB, Brito BL, Dutrieux RP, Lassance FP, Hage JJ. MMP-2 assessment as an indicator of wound healing: A feasibility study. Adv Skin Wound Care. 2006 Jul-Aug;19(6):324–327. doi: 10.1097/00129334-200607000-00011. [DOI] [PubMed] [Google Scholar]
- 65.Gibson DJ, Schultz GS. Molecular Wound Assessments: Matrix Metalloproteinases. Advances in wound care. 2013 Feb;2(1):18–23. doi: 10.1089/wound.2011.0359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Moues CM, van Toorenenbergen AW, Heule F, Hop WC, Hovius SE. The role of topical negative pressure in wound repair: expression of biochemical markers in wound fluid during wound healing. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2008 Jul-Aug;16(4):488–494. doi: 10.1111/j.1524-475X.2008.00395.x. [DOI] [PubMed] [Google Scholar]
- 67.Serena TE. Development of a Novel Technique to Collect Proteases from Chronic Wounds. Advances in wound care. 2014 Dec 1;3(12):729–732. doi: 10.1089/wound.2013.0463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Norman G, Westby MJ, Stubbs N, Dumville JC, Cullum N. A 'test and treat' strategy for elevated wound protease activity for healing in venous leg ulcers. Cochrane Database Syst Rev. 2016;1:CD011753. doi: 10.1002/14651858.CD011753.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Trengove NJ, Bielefeldt-Ohmann H, Stacey MC. Mitogenic activity and cytokine levels in non-healing and healing chronic leg ulcers. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2000 Jan-Feb;8(1):13–25. doi: 10.1046/j.1524-475x.2000.00013.x. [DOI] [PubMed] [Google Scholar]
- 70.James TJ, Hughes MA, Cherry GW, Taylor RP. Simple biochemical markers to assess chronic wounds. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2000 Jul-Aug;8(4):264–269. doi: 10.1046/j.1524-475x.2000.00264.x. [DOI] [PubMed] [Google Scholar]
- 71.Utz ER, Elster EA, Tadaki DK, et al. Metalloproteinase expression is associated with traumatic wound failure. J Surg Res. 2010 Apr;159(2):633–639. doi: 10.1016/j.jss.2009.08.021. [DOI] [PubMed] [Google Scholar]
- 72.Hawksworth JS, Stojadinovic A, Gage FA, et al. Inflammatory biomarkers in combat wound healing. Annals of surgery. 2009 Dec;250(6):1002–1007. doi: 10.1097/sla.0b013e3181b248d9. [DOI] [PubMed] [Google Scholar]
- 73.Forsberg JA, Potter BK, Polfer EM, Safford SD, Elster EA. Do inflammatory markers portend heterotopic ossification and wound failure in combat wounds? Clin Orthop Relat Res. 2014 Sep;472(9):2845–2854. doi: 10.1007/s11999-014-3694-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Thom SR, Hampton M, Troiano MA, et al. Measurements of CD34+/CD45-dim Stem Cells Predict Healing of Diabetic Neuropathic Wounds. Diabetes. 2016 Feb;65(2):486–497. doi: 10.2337/db15-0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dangwal S, Stratmann B, Bang C, et al. Impairment of Wound Healing in Patients With Type 2 Diabetes Mellitus Influences Circulating MicroRNA Patterns via Inflammatory Cytokines. Arteriosclerosis, thrombosis, and vascular biology. 2015 Jun;35(6):1480–1488. doi: 10.1161/ATVBAHA.114.305048. [DOI] [PubMed] [Google Scholar]
- 76.Grice EA, Kong HH, Conlan S, et al. Topographical and temporal diversity of the human skin microbiome. Science. 2009 May 29;324(5931):1190–1192. doi: 10.1126/science.1171700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gao Z, Tseng CH, Pei Z, Blaser MJ. Molecular analysis of human forearm superficial skin bacterial biota. Proceedings of the National Academy of Sciences of the United States of America. 2007 Feb 20;104(8):2927–2932. doi: 10.1073/pnas.0607077104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Misic AM, Gardner SE, Grice EA. The Wound Microbiome: Modern Approaches to Examining the Role of Microorganisms in Impaired Chronic Wound Healing. Advances in wound care. 2014 Jul 1;3(7):502–510. doi: 10.1089/wound.2012.0397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nizet V, Ohtake T, Lauth X, et al. Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature. 2001 Nov 22;414(6862):454–457. doi: 10.1038/35106587. [DOI] [PubMed] [Google Scholar]
- 80.Pastar I, Khan AA, Stojadinovic O, et al. Induction of specific microRNAs inhibits cutaneous wound healing. The Journal of biological chemistry. 2012 Aug 24;287(35):29324–29335. doi: 10.1074/jbc.M112.382135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zeeuwen PL, Boekhorst J, van den Bogaard EH, et al. Microbiome dynamics of human epidermis following skin barrier disruption. Genome Biol. 2012;13(11):R101. doi: 10.1186/gb-2012-13-11-r101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Robson MC, Mannari RJ, Smith PD, Payne WG. Maintenance of wound bacterial balance. Am J Surg. 1999 Nov;178(5):399–402. doi: 10.1016/s0002-9610(99)00208-1. [DOI] [PubMed] [Google Scholar]
- 83.Redel H, Gao Z, Li H, et al. Quantitation and composition of cutaneous microbiota in diabetic and nondiabetic men. J Infect Dis. 2013 Apr;207(7):1105–1114. doi: 10.1093/infdis/jit005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bowling FL, Jude EB, Boulton AJ. MRSA and diabetic foot wounds: contaminating or infecting organisms? Curr Diab Rep. 2009 Dec;9(6):440–444. doi: 10.1007/s11892-009-0072-z. [DOI] [PubMed] [Google Scholar]
- 85.Gardner SE, Hillis SL, Heilmann K, Segre JA, Grice EA. The neuropathic diabetic foot ulcer microbiome is associated with clinical factors. Diabetes. 2013 Mar;62(3):923–930. doi: 10.2337/db12-0771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bowler PG, Duerden BI, Armstrong DG. Wound microbiology and associated approaches to wound management. Clin Microbiol Rev. 2001 Apr;14(2):244–269. doi: 10.1128/CMR.14.2.244-269.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Han A, Zenilman JM, Melendez JH, et al. The importance of a multifaceted approach to characterizing the microbial flora of chronic wounds. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2011 Sep-Oct;19(5):532–541. doi: 10.1111/j.1524-475X.2011.00720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rhoads DD, Wolcott RD, Sun Y, Dowd SE. Comparison of culture and molecular identification of bacteria in chronic wounds. Int J Mol Sci. 2012;13(3):2535–2550. doi: 10.3390/ijms13032535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gardner SE, Haleem A, Jao YL, et al. Cultures of diabetic foot ulcers without clinical signs of infection do not predict outcomes. Diabetes Care. 2014 Oct;37(10):2693–2701. doi: 10.2337/dc14-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bowler PG, Davies BJ. The microbiology of infected and noninfected leg ulcers. Int J Dermatol. 1999 Aug;38(8):573–578. doi: 10.1046/j.1365-4362.1999.00738.x. [DOI] [PubMed] [Google Scholar]
- 91.Hodkinson BP, Grice EA. Next-Generation Sequencing: A Review of Technologies and Tools for Wound Microbiome Research. Advances in wound care. 2015 Jan 1;4(1):50–58. doi: 10.1089/wound.2014.0542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dowd SE, Sun Y, Secor PR, et al. Survey of bacterial diversity in chronic wounds using pyrosequencing, DGGE, and full ribosome shotgun sequencing. BMC Microbiol. 2008;8:43. doi: 10.1186/1471-2180-8-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wolcott R. Economic aspects of biofilm-based wound care in diabetic foot ulcers. J Wound Care. 2015 May;24(5):189–190. 192–184. doi: 10.12968/jowc.2015.24.5.189. [DOI] [PubMed] [Google Scholar]
- 94.Kuczynski J, Lauber CL, Walters WA, et al. Experimental and analytical tools for studying the human microbiome. Nat Rev Genet. 2012 Jan;13(1):47–58. doi: 10.1038/nrg3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gardner SE, Frantz RA, Saltzman CL, Hillis SL, Park H, Scherubel M. Diagnostic validity of three swab techniques for identifying chronic wound infection. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2006 Sep-Oct;14(5):548–557. doi: 10.1111/j.1743-6109.2006.00162.x. [DOI] [PubMed] [Google Scholar]
- 96.Harsha A, Stojadinovic O, Brem H, et al. ADAM12: a potential target for the treatment of chronic wounds. J Mol Med (Berl) 2008 Aug;86(8):961–969. doi: 10.1007/s00109-008-0353-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Heilborn JD, Nilsson MF, Kratz G, et al. The cathelicidin anti-microbial peptide LL-37 is involved in re-epithelialization of human skin wounds and is lacking in chronic ulcer epithelium. The Journal of investigative dermatology. 2003 Mar;120(3):379–389. doi: 10.1046/j.1523-1747.2003.12069.x. [DOI] [PubMed] [Google Scholar]
- 98.Weckroth M, Vaheri A, Virolainen S, Saarialho-Kere U, Jahkola T, Siren V. Epithelial tissue-type plasminogen activator expression, unlike that of urokinase, its receptor, and plasminogen activator inhibitor-1, is increased in chronic venous ulcers. Br J Dermatol. 2004 Dec;151(6):1189–1196. doi: 10.1111/j.1365-2133.2004.06261.x. [DOI] [PubMed] [Google Scholar]
- 99.Cowin AJ, Hatzirodos N, Holding CA, et al. Effect of healing on the expression of transforming growth factor beta(s) and their receptors in chronic venous leg ulcers. The Journal of investigative dermatology. 2001 Nov;117(5):1282–1289. doi: 10.1046/j.0022-202x.2001.01501.x. [DOI] [PubMed] [Google Scholar]
- 100.Pastar I, Stojadinovic O, Krzyzanowska A, et al. Attenuation of the transforming growth factor beta-signaling pathway in chronic venous ulcers. Mol Med. 2010 Mar;16(3–4):92–101. doi: 10.2119/molmed.2009.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liu J, Xu Y, Shu B, et al. Quantification of the differential expression levels of microRNA-203 in different degrees of diabetic foot. International journal of clinical and experimental pathology. 2015;8(10):13416–13420. [PMC free article] [PubMed] [Google Scholar]