Abstract
Background and Aims Modularity is a ubiquitous and important structural property of ecological networks which describes the relative strengths of sets of interacting species and gives insights into the dynamics of ecological communities. However, this has rarely been studied in species-rich, tropical plant–pollinator networks. Working in a biodiversity hotspot in the Peruvian Andes we assessed the structure of quantitative plant–pollinator networks in nine valleys, quantifying modularity among networks, defining the topological roles of species and the influence of floral traits on specialization.
Methods A total of 90 transects were surveyed for plants and pollinators at different altitudes and across different life zones. Quantitative modularity (QuanBiMo) was used to detect modularity and six indices were used to quantify specialization.
Key Results All networks were highly structured, moderately specialized and significantly modular regardless of size. The strongest hubs were Baccharis plants, Apis mellifera, Bombus funebris and Diptera spp., which were the most ubiquitous and abundant species with the longest phenologies. Species strength showed a strong association with the modular structure of plant–pollinator networks. Hubs and connectors were the most centralized participants in the networks and were ranked highest (high generalization) when quantifying specialization with most indices. However, complementary specialization d' quantified hubs and connectors as moderately specialized. Specialization and topological roles of species were remarkably constant across some sites, but highly variable in others. Networks were dominated by ecologically and functionally generalist plant species with open access flowers which are closely related taxonomically with similar morphology and rewards. Plants associated with hummingbirds had the highest level of complementary specialization and exclusivity in modules (functional specialists) and the longest corollas.
Conclusions We have demonstrated that the topology of networks in this tropical montane environment was non-random and highly organized. Our findings underline that specialization indices convey different concepts of specialization and hence quantify different aspects, and that measuring specialization requires careful consideration of what defines a specialist.
Keywords: Asteraceae, Baccharis, floral traits, plant–pollinator networks, modularity, specialization, Apis mellifera, Bombus funebris, biodiversity hotspot, hummingbirds, topological roles, Peruvian Andes
INTRODUCTION
Ecological interactions between plants and their flower visitors are fundamental to the ongoing function of both natural and agricultural ecosystems (Klein et al., 2007; Ollerton et al., 2011). In the past decade network approaches have been developed that enable ecologists to probe these interactions in ever more detail, introducing many new indices to describe network topology, quantify the degree of specialization between partners, and assess network stability, robustness and ecosystem function (Memmot et al., 2004; Fortuna and Bascompte, 2006; Dormann et al., 2009).
Understanding the topology of ecological networks is fundamental when interpreting community and ecosystem responses to global change (Fortuna et al., 2010), and there is growing recognition of network structure, such as the distribution of strong and weak links and the presence of compartments or modules (Ings et al., 2009). Modularity is a ubiquitous and important structural property of ecological networks which describes the relationship between interacting species and gives insights into the dynamics of ecological communities. In modular networks subsets of species interact more frequently with each other than with species in other modules (Newman, 2004; Olesen et al., 2007).
The advent of sophisticated algorithms and indices for the analysis of quantitative networks also allows for comparisons of network-wide specialization and modularity among communities with differing species richness (Dormann and Strauss, 2014; Schleuning et al., 2014; Martín González et al., 2015). In addition to comparisons of modularity among entire communities, each species can be classified into different functional roles according to their position within and among modules (Olesen et al., 2007; Martín González et al., 2012). For instance, module hubs are highly connected generalist species linked to many species within their own module, while connectors are species linking several modules. Network hubs are generalist species, acting as both connectors and module hubs, and are thus important to the cohesiveness of both the network and its own module. Peripheral species are specialists, have few links and are linked almost exclusively to species within their module (Olesen et al., 2007; Martín González et al., 2012).
Modularity tends to prevail towards the tropics in areas of high contemporary precipitation (Dalsgaard et al., 2013; Schleuning et al., 2014). Specialization may also be expected in species-rich tropical communities, given that more feeding niches may become available and inter-specific competition may increase (e.g. Dalsgaard et al., 2011; but see Ollerton and Cranmer, 2002; Schleuning et al., 2012; Moles and Ollerton, 2016). However, although modularity may be regarded as a sign of interaction specialization, it does not necessarily involve highly specific links but rather a discrete partition of interactions among species in the network (Martín González et al., 2015). Ecological processes thought to shape network patterns and influence modularity include seasonal resource fluctuations, overlapping phenological schedules in highly seasonal climates, high productivity and resource diversity (Bosch et al., 2009; Martín González et al., 2012, 2015; Schleuning et al., 2012, 2014), and plant and animal traits (Donatti et al., 2011).
In this study we use a new method to detect modularity and to describe species’ roles across nine valleys in the Peruvian Andes, investigating modularity, topological roles of species and specialization of plant–pollinator communities. Specifically we addressed the following questions. (1) Network-level traits: how are the regional plant–pollinator networks structured in terms of interaction specialization and modularity? (2) Species-level traits: which species have important topological roles in the networks (i.e. network and module hubs), does their position change across valleys and are there similarities in module composition of widespread species among valleys? (3) Dominant species: do widespread plant and pollinator species share similar traits, and is there evidence of taxonomic and functional clustering across valleys? (4) Generalists and specialists: are network hubs generalists, widespread species and do peripheral species such as hummingbirds tend to be more specialized? Are species consistently generalized or specialized across valleys?
MATERIALS AND METHODS
Study sites, sampling design and species traits
The Vilcanota Highlands of south-eastern Perú contain a unique flora and fauna with high levels of diversity and endemism (Wege and Long, 1995; Stattersfield et al., 1998). A 10-year study of the flora of this region in several ecosystems and life zones (2700–4900 m), revealed 145 plant families, 450 genera and 871 species (Tupayachi, 2005). Despite being a biodiversity hotspot, no previous work has examined plant–pollinator networks in the region. Fieldwork was carried out in nine valleys of the Sacred Valley of the Incas, lying 60 km north of the city of Cusco. These valleys differ in their development from valley floor to snow level in terms of river volume, amplitude, width and human occupation. Therefore, the life zones are not uniform (Tupayachi, 2005). Surveys were conducted between the villages of Pisac, Ollantaytambo and Chillca, in the provinces of Calca and Urubamba, Department of Cusco. The study sites spanned an area approx. 60 km in length along the Urubamba river, from Huaran to the eastern limits of the Historical Sanctuary of Machu Picchu at Piscacucho, situated between 13°13′S, 72°2′W and 13°12′42″S, 72°21′41″W.
The vegetation is dominated by deciduous shrubs, abundant annual herbs, small trees, spiny shrubs and stunted Elfin forest. The canopy is generally not tall and is mostly subtropical humid montane, comprising approx. 10 % of the vegetation. Alnus acuminata (Betulaceae) has a restricted distribution, surviving only as a few individuals strewn in steep ravines and along water courses. Passiflora grows in Alnus stands but was too high up to include in surveys. Myrcianthes oreophylla (Myrtaceae) and Escallonia resinosa (Grossulariaceae) trees are small enough to survey at head height. Eucalyptus plantations were not present in transects and only the understorey of Polylepis (Rosaceae) forests was surveyed given that Polylepis is a wind-pollinated species. Anthropogenic pressures include livestock farming, agriculture, overgrazing, widespread planting of Eucalyptus and pine and the extraction of Polylepis wood by rural communities. A total of 390 honey-bee hives are owned within the Sacred Valley, with an average of ten hives per keeper (The Association of Beekeepers, Urubamba, Department of Cusco, pers. comm.).
Transects
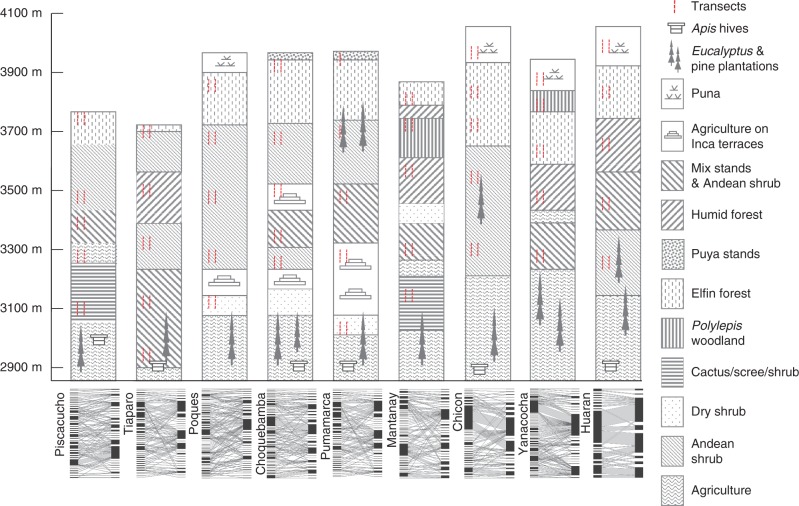
In each of the nine valleys we established ten transects covering a total altitudinal range of 1150 m. Each transect was subdivided at each altitude into two 500 × 3-m sampling areas, running parallel either side of established trails and were marked with ten points at intervals of 50 m.The topography of the mountain chain dictated where transects started and finished, and whether they were orientated horizontally across or vertically up the valleys. A total of 90 transects were surveyed once during the dry season, between April and October 2002, at five different altitudes and across different life zones (as defined by Holdridge, 1967) (Fig. 1). Sampling effort focused on one valley at a time, rather than spreading the effort across all sites due to the logistical constraints encountered in covering such a large sampling area. The order in which each transect was walked in each of the valleys was determined using random numbers (1–5), so that the timing of the transect surveys across valleys and elevations minimized biasing the results. Transects correspond approximately to the following life zones: subtropical montane thorn steppe (2700–3200 m; sampled between 3147 and 3235 m), subtropical montane dry forest (3000–3400 m, sampled at 3351–3424 m), subtropical humid montane forest (3500–3800 m, sampled at 3653–3746 m) and Polylepis forests (the majority of the approx. 30 species are classified as vulnerable; IUCN, 2010) (3700–4200 m, sampled at 3846–4003 m) (see Fig. 2 for plants and habitats). Surveys were undertaken between 0800 and 1700 h mostly under favourable conditions for a total of 90 h. Two observers slowly walked each 500-m transect belt (one surveying the left side of the trail and the other surveying the right side) for 60 min, recording only those visitors that while foraging for pollen and/or nectar made contact with either anthers or stigmas, i.e. potential pollinators. Those insects that could not be identified in the field were captured and deposited individually into labelled vials for later identification and/or assignment to morphospecies. Most bee and syrphid fly species were identified to species or genus; other groups were usually identified to family and assigned to morphospecies categories. Functional taxonomic groups of flower visitors (sensu Fenster et al., 2004; Ollerton et al., 2007) were identified as follows: Diptera were divided as Syrphidae, Tachinidae and all other Diptera; Hymenoptera were divided as all other solitary bees, Bombus spp., Vespidae and Apis. Voucher specimens of insects and plants are retained at the University of San Antonio Abad, Cusco, Perú. Hummingbirds were identified in the field using the field guide Birds of the High Andes (Fjeldså and Krabbe, 1990).
Fig. 1.
Schematic diagram representing the nine valleys surveyed in the Sacred Valley in terms of different habitats encountered along an elevational gradient from 2900 to 4100 m and their quantitative bipartite graphs. Pollinators are arranged on the left and plants on the right. The number of interactions is indicated by the width of the bars.
Fig. 2.
Plant species and habitats surveyed in the Sacred Valley: (A) Barnadesia horrida (Asteraceae); (B) Baccharis salicifolia (Asteraceae); (C) Passiflora tripartita var. mollissima (Passifloraceae); (D) Polylepis (Rosaceae) woodlands 3700–4200 m; (E) subtropical montane dry forest (3000–3400 m), characterized by steep rocky slopes with spiny shrubs such as Duranta mandonii (Verbenaceae) and many Puya sp.; (F) Lupinus mutabilis (Fabaceae); (G) Oreocallis grandiflora (Proteaceae). Photographs: (A, C, F, G) Stella Watts, (D, E) Jeff Ollerton, (B) Lynn Watson.
Body length for 5–10 insects captured on flowers was measured representing the main functional groups (see Table 5). Measurements of hummingbirds’ bills were taken from mist-net data collected in the field and from the literature. Corolla length for 10–20 flowers of each plant species was measured from the base of the calyx to the flower aperture using a digital calliper in the field. Plant species were identified using Gentry (1996) and with help from the staff from the Herbario Vargas, Universidad Nacional de San Antonio Abad del Cusco, Perú. Plants were assigned to floral traits and nectar was assessed following Ollerton and Watts (2000).
Table 5.
Summary of the main morphological traits of plants and flower visitors in the Sacred Valley
| Plant family/genera | Flower morphology | Accessibility to nectar and pollen | Flower orientation |
|---|---|---|---|
| Apocynaceae, Caryophyllaceae, Ranunculaceae, Rosaceae | Dish-shaped or bowl-shaped: actinomorphic (with several symmetry planes) 2–5 mm deep | Open-access flowers with exposed nectar and pollen, or pollen presented as pollinia. Nectar volume small | Upright or horizontal (0–90°) |
| Asteraceae: Bidens, Baccharis, Senecio, Ageratina, Aristeguietia | Open tube: actinomorphic characterized by a head of small ray and disc tubular flowers mostly 5–10 mm in length. Stamens and pistels exposed | Easy access to both pollen and nectar. Nectar volume small, concealed at the base of narrow tubes. Pollen exposed | Upright or horizontal (0–90°) |
| Fabaceae, Gentianaceae, Lamiaceae | Flag or gullet: bilaterally symmetrical, zygomorphous flowers 4–35 mm. Mechanically strong. Stamens and pistils exposed | Nectar concealed at the bottom of narrow or wide tubes. Nectar volume moderate and concentration high. Pollen exposed or absent | Horizontal (90°) |
| Verbenaceae, Passifloraceae, Melastomataceae, Bromeliaceae, Onagraceae | Tube: bilaterally symmetrical, zygomorphous flowers 5–135 mm in length. Some flowers mechanically strong. Stamens and pistels exposed | Nectar concealed in mostly deep narrow tubes. Pollen hidden or located anterior to the corolla, large amounts of nectar. Nectar concentration low | Horizontal to pendant (90–180°) |
| Pollinator functional group | Families/genera | Body/bill length | Resource |
| Diptera | Muscidae, Sphaeroceridae, Tachinidae, Sciariadae, Scianidae and Anthomyiidae | 4–10 mm | Mostly nectar |
| Syrphidae | Eristalis, Copestylum, Toxomerus, Platycheirus and Tuberculanostoma | >9 mm | Nectar and pollen |
| Trochilidae | Aglaeactis, Metallura, Colibri, Pterophanes, Oreotrochilus and Oreonympha | 13–32 mm | Nectar only; also nectar robbers |
| Hymenoptera: Apidae | Apis mellifera and several Bombus spp. | 10–16 mm; proboscis 6–10 mm | Pollen and nectar |
| Hymenoptera: Vespidae | Small to medium wasps | <10 mm | Pollen and nectar |
| Coleoptera | Chrysomelidae, Bruchidae, Curculionidae and Melyridae | 5–10 mm | Pollen and nectar |
| Lepidoptera | Hesperiidae and some small diurnal moths | 5–10 mm | Nectar |
| Hemiptera | All Lygaeus albornatus | >10 mm | Nectar |
Data analysis
Data represent interaction frequency matrices for nine valleys. Cell values indicate the frequency of interaction between species pairs, and cells with zeros indicate no interaction. For each of the nine valleys, matrices of interaction between P plant and A pollinator species were created by pooling data across the altitudinal gradient (1–5) and each matrix was then analysed separately. Additionally, we constructed the following two matrices. (1) Full matrix: a single plant–pollinator (A × P) network pooling all the data from nine valleys across the altitudinal gradient (110 plant and 143 pollinator species); and (2) reduced matrix: a single plant–pollinator (A × P) matrix (same as 1) but which excluded species with fewer than two interactions in at least two valleys. This exclusion reduced the total number of species to 26 plants and 39 pollinators. We used the R-package bipartite 2.03 (Dormann et al., 2009) to calculate all network indices. At the network level, we calculated complementary specialization H2′ and quantitative modularity (QuanBiMo: Dormann and Strauss, 2014). At the species level we used five measures to quantify specialization [species degree, weighted closeness, species strength, pollination service (PSI) and complementary specialization d′]. We then focused on three widespread abundant species across valleys, the honey bee (Apis mellifera; Apidae), a bumblebee (Bombus funebris; Apidae) and a hummingbird (Aglaeactis cupripennis; Trochilidae), to illustrate how the indices reflect the actual degree of specialization (niche partitioning between species), by contrasting observed visitations with expectations from a null model. These three species were selected because they were present in most valleys and at many altitudes so the sample sizes were sufficient. The measures of specialization chosen are suitable for comparisons across networks (Dormann, 2011). We chose these particular species because Apis mellifera is an introduced species reported in the literature to be a super generalist and hence likely to have a strong impact on network structure (Dupont et al., 2003). Similarly, some Bombus spp. are reported as generalists (see Dormann, 2011) and hummingbirds are predicted as specialists (Sonne et al., 2016). Thus, this presented an excellent opportunity to compare these predictions with our data. All statistical analyses were performed using R, version 3.1.0 (R Development Core Team, 2010). All means are given ±s.d. and medians are indicated as required.
Network-level metrics
Quantitative modularity (QuanBiMo) (Dormann and Strauss, 2014) computes modules in weighted, bipartite networks. This algorithm follows the approach of Clauset et al. (2008) based on a hierarchical representation of interaction frequencies and optimal allocation of species into modules. A module is defined by species having more interactions within the module than among modules, and thus modularity is the result of some degree of specialization in species interactions (Martín González et al., 2015). Modularity Q ranges from 0 for randomly configured networks to 1 for networks composed of perfect modules. We searched for the best organization of each network into modules in the best of five independent runs of the QuanBiMo algorithm following Schleuning et al. (2014). If no further improvement was recorded after 108 swaps, the run was terminated and the result interpreted as the optimum. QuanBiMo can be invoked recursively, searching for modules within modules (see Dormann and Strauss, 2014). Thus, to identify nested module structure at the highest level, we performed a separate modularity analysis focusing on hummingbirds using 106 steps. To determine whether hummingbirds and their plants were consistently ascribed to the same modules, we checked module identity by repeating the analysis 50 times and recorded the distribution of plants and hummingbirds across modules each time, following Gómez et al. (2013). To account for Q’s dependence on network size and sampling intensity (Dormann and Strauss, 2014) absolute values were corrected using null models based on the random placement of interactions observing the same marginal totals (Patefield, 1981). Corrected modularity Q was calculated as the difference between the value of the empirical network and the mean value obtained from 100 null models for QuanBiMo (Schleuning et al., 2014; Martín González et al., 2015).
To identify species with importance for modularity, c- and z-values were calculated for all species based on the number of links, where c refers to the even distribution of links within and across modules and z refers to the number of within-module interactions (Guimerà et al., 2005). Critical c- and z-values proposed by Olesen et al. (2007) were defined for binary networks and we thus adapted their approach by calculating weighted versions of z and c using species strength instead of species degree (sensu Bascompte et al., 2006). To objectively define thresholds we ran 100 null models for original networks and employed 95 % quantiles as critical c- and z-values.
Complementary specialization H2′ (Blüthgen et al., 2006) is a network-level index which measures the degree of complementary specialization (or exclusiveness) of the interactions at the level of the entire matrix. Specifically, it quantifies the deviation of observed interactions from those expected given the species’ abundances or interaction frequencies (measured as species’ marginal totals), so that the more exclusive the interactions, the larger is the H2′ value for the web. Complementary specialization H2′ ranges from 0 for the most generalized networks to 1 for a completely specialized network. As H2′ accounts for variability in the species’ total observation frequencies it can be used directly to make cross-network comparisons despite variation in total frequencies among communities (Blüthgen, 2010).
Species-level indices
Species’ degree (qualitative measure) (Jordano et al., 2003) is the number of species to which a species is linked. Degree is calculated based on a binary interaction matrix and thus describes specialization in a qualitative way. Specialists have lower degree than generalists.
Complementary specialization d′ (Blüthgen et al., 2006) is a species-level specialization index related to complementary H2′, which estimates the complementarity of interactions based on the standardized Kullback–Lieber divergence (= relative entropy). As with H2′ for the entire web, the complementary d′ index determines the extent to which the interaction specialization of a focal species may differ from null-model expectations in which species interact with partners in proportion to their availability, again measured as species’ marginal totals (Blüthgen et al., 2006). It ranges from 0 (no specialization) and 1 (perfect specialization).
Species strength (Bascompte et al., 2006). The strength of a species is defined as the sum of dependences of the plants relying on an animal or the animals relying on a plant. It is a measure of the importance of this animal from the perspective of the plant set and vice versa. This measure is a quantitative extension of the species degree, which is the number of interactions per species in qualitative networks (Jordano et al., 2003). The higher the value, the more generalized a plant species is, because more pollinator species depend on it (and vice versa).
Pollination service index (PSI) (Dormann, 2011) estimates the importance of a pollinator for all plant species; it is hence an extension of the idea of species strength. Put simply, it measures the probability that intraspecific pollen is transferred to plant species i. This depends both on the proportion of visits a pollinator pays to species i and on the number of pollinators that visit i. For PSI, the importance of a pairwise interaction (for the plant) is calculated as: ‘dependence’ i on j multiplied by per-visit efficiency i visited by j, where per-visit efficiency i visited by j = (average proportion of visits to i by j in all visits by j)^β. It assumes that the order of plant species visited is random (no mixing, no constancy). To account for that not being true, β could be adjusted. We envisage a penalty for the fact that a pollinator has to make two (more or less successive) visits to the same plant species: the first to take the pollen up, the second to pollinate the next. Thus, using β = 2 as an exponent in step 1 would simulate that a pollinator deposits all pollen at every visit. In a sense, β = 2 represents a complete turnover of pollen on the pollinator from one visit to the next; only the pollen of the last-visited species is transferred. That is certainly a very strong penalization. At present we set the exponent to β = 1, because the step of controlling for ‘pollen purity’ is already a major improvement. It assumes, implicitly, that pollen is perfectly mixed on the pollinator and hence pollen deposited directly proportional to frequency of visits to the different plants. Also, the extent to which pollen gets mixed and/or lost during foraging flights is unknown, and hence the true exponent remains elusive. For a value of β = 0, PSI simplifies (and is equal) to species strength. At its maximum, PSI = 1, it shows that all pollen is delivered to one plant species that completely depends on the monolectic pollinator. At its minimum, PSI = 0, it indicates that a pollinator is irrelevant to all plant species. To any of the target species: accounting for the proportion of pollen actually delivered (due to floral constancy, irreversible pollen compaction, pollen viability, etc.) by a modifying exponent, β. A value of 1 (the default) makes pollen deposition proportional to the number of same-species visits, while a value of 2 would require the pollinator to have come from the same species the exact previous visit. We acknowledge that species will differ substantially in their β-value, and at present use PSI largely as an index of pollen-purity-at-visit.
Weighted closeness centrality measures the proximity of a node to all other nodes in the network (Freeman, 1979) based on path lengths to other nodes, and has been proposed as a measure of generalization in pollination networks by Martín González et al. (2010) as it measures the connectivity of the entire community. Thus, for each individual species we measure its connectivity to all other species in the community and then average all the individual connectivities to obtain a value that describes the entire community.
Weighted closeness centrality (Opsahl et al., 2010) calculates closeness, but based on weighted representation of the network. Low closeness scores indicate specialization and high closeness scores indicate nodes (pollinators) are more ‘central’, e.g. closer to all other species in the network.
As raw values for network indices may be affected by species frequencies and sampling intensities, network metrics were compared with an appropriate null model. We generated 1000 null models using the Patefield algorithm (Patefield, 1981) (method r2d implemented in the bipartite package of R), which generates null models with marginal totals identical to those of the observed matrix (see Blüthgen et al., 2008; Dormann et al., 2009). This null model redistributes interaction events among all the cells in the network randomly, while constraining the total number of interactions per species. It assumes that species interact randomly, without constraining the degree of specialization in a network.
Following Ollerton et al. (2007) we categorized the plants according to their level of functional and ecological specialization/generalization. ‘Functional’ refers to the number of functional groups (often higher taxonomic groups such as family) of pollinators which service a plant. ‘Ecological’ refers to the species richness of pollinators. Clearly for both of these categories there is a continuum between specialization and generalization: for the purposes of this analysis we define a functional specialist as one that is pollinated by only a single higher taxon (e.g. Trochilidae or Apidae); a strict ecological specialist is one that is pollinated by a single animal species.
RESULTS
A total of 1583 flower visits to 110 plant species from 143 animal species and morphospecies were recorded across all nine valleys (Table 1). When pooled across all sites we observed a total of 719 species–species links. Thirty-three plant families were recorded, of which Asteraceae (43 species) was the most frequently visited family, receiving 65 % of total visits, followed by Lamiaceae (10 %) and Myrtaceae (6 %). The highest diversity of flower visitors was on the dioecious flowers of Baccharis, receiving 29 % of all visits by a total of 73 pollinator species. The most frequent flower visitors belonged to the orders Diptera (48 %), Hymenoptera (33 %), Coleoptera (8 %), Trochilidae (6 %) and Lepidoptera (5 %). Apis mellifera dominated the bee fauna (26 %) while Vespidae comprised less than 1 % (see Supplementary Data Tables S3 and S4 for full species lists of plants and pollinators).
Table 1.
Total number of flower visitors for each functional group in each of the nine valleys starting from Huaran to the eastern limits of the Historical Sanctuary of Machu Picchu at Piscacucho, situated between situated between 13°13′S, 72°2′W and 13°12′42″S, 72°21′41″W)
| Apis | Lepidoptera | Bombus | Solitary bees | Diptera | Syrphidae | Tachinidae | Coleoptera | Trochilidae | Hemiptera | Vespidae | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Huaran | 40 | 0 | 3 | 0 | 4 | 8 | 1 | 0 | 26 | 0 | 0 |
| Yanacocha | 79 | 41 | 0 | 1 | 114 | 25 | 10 | 4 | 4 | 0 | 2 |
| Chicon | 104 | 4 | 5 | 1 | 26 | 20 | 4 | 10 | 33 | 0 | 0 |
| Mantanay | 47 | 5 | 20 | 2 | 12 | 32 | 21 | 5 | 19 | 0 | 0 |
| Pumamarca | 24 | 2 | 21 | 3 | 53 | 21 | 0 | 42 | 0 | 0 | 1 |
| Choquebamba | 7 | 0 | 19 | 1 | 84 | 24 | 3 | 14 | 1 | 15 | 3 |
| Poques | 29 | 5 | 10 | 0 | 50 | 43 | 4 | 37 | 0 | 0 | 3 |
| Tiaparo | 74 | 2 | 0 | 4 | 46 | 35 | 5 | 7 | 4 | 0 | 0 |
| Piscacucho | 24 | 14 | 4 | 0 | 22 | 61 | 14 | 16 | 0 | 0 | 0 |
| Total | 428 | 73 | 82 | 12 | 411 | 269 | 62 | 135 | 87 | 15 | 9 |
Network complimentary specialization (H2′) and modularity (Q)
All networks were significantly different from null models (P < 0·0001) (Supplementary Data Fig. S1), most of them being also moderately specialized (mean H2′ = 0·39 ± 0·10). Huaran was the most specialized site (H2′ = 0·58). All networks were more modular than expected from null models and showed very low variability in Q among runs (Table 2). Q was positively correlated with the number of modules detected at each site (Pearson’s correlation: t = 2·83; r = 0·53; P = 0·02). Q was negatively correlated with honey bee abundances across sites (Pearson’s correlation: t = −2·90; r = −0·73; P = 0·02) but not with H2′ (Pearson’s correlation: t = −0·73; r = 0·26; P = 0·48). Q and H2′ index values for the reduced matrix were similar to the other nine networks, suggesting that deleting species with fewer than two interactions in at least two valleys had little effect on index values (Table 2).
Table 2.
Network modularity and complimentary specialization H2′ for the nine valleys and the combined networks (full and reduced matrices– see Methods)
| Network | A | P | Network size | H2′ | No. of modules | Weighted Q | s.d. (w. Q) | PA | ΔQPA | Null model z score s.d. | P |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Huaran | 16 | 8 | 24 | 0·59 | 5 | 0·39 | 0·01 | 0·25 | 0·14 | 6·26 | << 0·001 |
| Yanacocha | 51 | 22 | 73 | 0·37 | 6 | 0·37 | 0·00 | 0·12 | 0·25 | 6·95 | << 0·001 |
| Chicon | 32 | 18 | 50 | 0·46 | 7 | 0·35 | 0·01 | 0·17 | 0·18 | 6·00 | << 0·001 |
| Mantanay | 34 | 24 | 58 | 0·39 | 7 | 0·50 | 0·00 | 0·26 | 0·24 | 9·22 | << 0·001 |
| Pumamarca | 36 | 26 | 62 | 0·40 | 10 | 0·48 | 0·00 | 0·31 | 0·17 | 6·03 | << 0·001 |
| Choquebamba | 43 | 25 | 68 | 0·43 | 10 | 0·55 | 0·00 | 0·21 | 0·34 | 6·19 | << 0·001 |
| Poques | 47 | 32 | 79 | 0·26 | 7 | 0·46 | 0·00 | 0·39 | 0·07 | 2·94 | < 0·01 |
| Tiaparo | 32 | 25 | 57 | 0·52 | 9 | 0·48 | 0·00 | 0·24 | 0·24 | 8·07 | << 0·001 |
| Piscacucho | 38 | 27 | 65 | 0·36 | 8 | 0·47 | 0·01 | 0·34 | 0·13 | 6·07 | << 0·001 |
| Reduced matrix | 39 | 26 | 65 | 0·27 | 5 | 0·30 | 0·00 | 0·13 | 0·17 | 22·52 | << 0·001 |
| Full | 143 | 110 | 253 | 0·31 | 9 | 0·31 | 0·00 | 0·10 | 0·21 | 5·46 | << 0·001 |
Modularity-related measures given are (1) by the number of detected modules, (2) by observed modularity Q with its standard deviation across five independent algorithm runs and (3) by the null-model corrected modularities using Patefield algorithm (null model PA) (ΔQPA), given by Q – mean QNULL for the respective null model.
The role of individual species and functional groups in the network structure
The roles of functional groups and plant families in network structure across valleys are presented in Table 3. Hymenopterans and plants from the family Asteraceae played the most important topological roles (i.e. were network hubs, module hubs and connectors) across networks. The majority of species were peripheral (83 %), with most of their links within their own module (Table 3, Figs 3 and 4). Species strength was positively related to weighted measures of c- and z-values, particularly z-values (z-values: r = 0·48, P < 0·000001; c-values: r = 0·05, P < 0·00001). Thus, species with high species strength have many interactions within their own module. By contrast, for c-values, where c refers to the even distribution of links within and across modules, although significant, the correlation was very weak. Only 29 pollinator species (20 %) and 19 plant species (17 %) exceeded the threshold for c- and z-values to be considered hubs or connectors. The strongest network and module hubs were Baccharis plants, Apis mellifera, Bombus funebris and Diptera spp., the most ubiquitous and abundant species with the longest phenologies, found at all altitudes, present in most valleys and covering several life zones (Supplementary Data Tables S1–S4). Just three plants, Baccharis salicifolia, Baccharis buxifolia and Jungia rugosa (Asteraceae), and two flower visitors, the honey bee Apis mellifera and Syrphidae sp.2, exceeded both thresholds in eight valleys, and were thus network hubs (Tables S1 and S2). Connectors were both plant and insect/bird species in approximately equal proportions. Introduced honey bees were hubs in 60 % of networks, or acted as module hubs, i.e. species with many interactions within their own module (low c, high z), or connector species, i.e. linking several modules (high c, low z) in the remaining networks (see Table S1). The bumblebee Bombus funebris was a module hub and connector in two networks. Syrphids (Diptera) were consistently connectors, while Lepidoptera, Coleoptera and Trochilidae were mostly peripheral. These functional groups had c- and z-values close to zero and were specialists, i.e. they had only a few links and almost always only to species within their module. Lepidoptera, Coleoptera and Trochilidae were observed quite frequently across most valleys and at most altitudes (Table 1, Table S4). Across networks, the majority of interactions aggregated around two hub and two plant connector species belonging to the family Asteraceae (78 %) (Fig. 4 and Tables S2 and S3 and Figs S6 and S7). As with pollinators, plants changed roles across networks.
Table 3.
The role of functional groups of pollinators and plant families in the nine networks
| Network hub | Module hub | Connector | Periphery | Valleys present | |
|---|---|---|---|---|---|
| Order | |||||
| Coleoptera | 0 | 1 | 3 | 25 | 8 |
| Diptera | 0 | 4 | 7 | 57 | 9 |
| Hemiptera | 0 | 1 | 0 | 0 | 1 |
| Hymenoptera | 1 | 3 | 3 | 16 | 9 |
| Lepidoptera | 0 | 1 | 1 | 13 | 7 |
| Syrphidae | 1 | 3 | 4 | 18 | 9 |
| Trochilidae | 0 | 1 | 1 | 7 | 6 |
| Family | |||||
| Apocynaceae | 0 | 0 | 1 | 0 | 2 |
| Asteraceae | 3 | 7 | 9 | 44 | 9 |
| Gentianaceae | 0 | 1 | 1 | 2 | 2 |
| Lamiaceae | 0 | 0 | 1 | 3 | 6 |
| Myrtaceae | 0 | 0 | 1 | 1 | 3 |
| Verbenaceae | 0 | 0 | 1 | 3 | 3 |
Numbers indicate the number of species per order. Species numbers do not add up to the total number of pollinator species (143) as some species acted as both network hubs, module hubs, connectors and periphery species depending on the site. Only those plant families with the most important topological roles are shown.
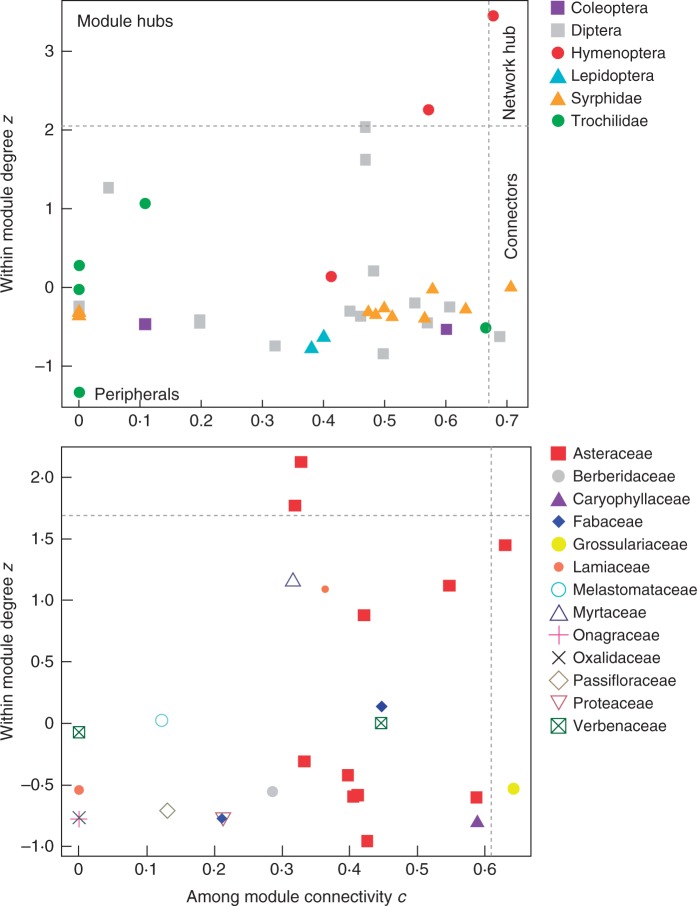
Fig. 3.
Scatterplot of species roles for the reduced matrix. The coefficients z and c refer to among-module connectivity and within-module degree, respectively. Dashed grey lines indicate 95 % quantiles from 100 null models and indicate the topographical space of network hubs (top right-hand rectangle, high z- and c-values), module hubs (top left-hand rectangle, high z- and low c-values), connectors (bottom right-hand rectangle, low z- and high c-values) and peripheral species (bottom left-hand rectangle, low z- and c-values). The top graph represents the role of functional groups of pollinator species, showing the presence of two bees in the role of module and network hubs, and two flies (one of them a syrphid) acting as connectors. For the purposes of this analysis, solitary bees and wasps are included within Hymenoptera and Tachinidae are included within Diptera. The bottom graph illustrates plant species, showing that the family Asteraceae has two module hubs and one connector species, the latter together with a Grossulariaceae species. No plant takes the role of network hub.
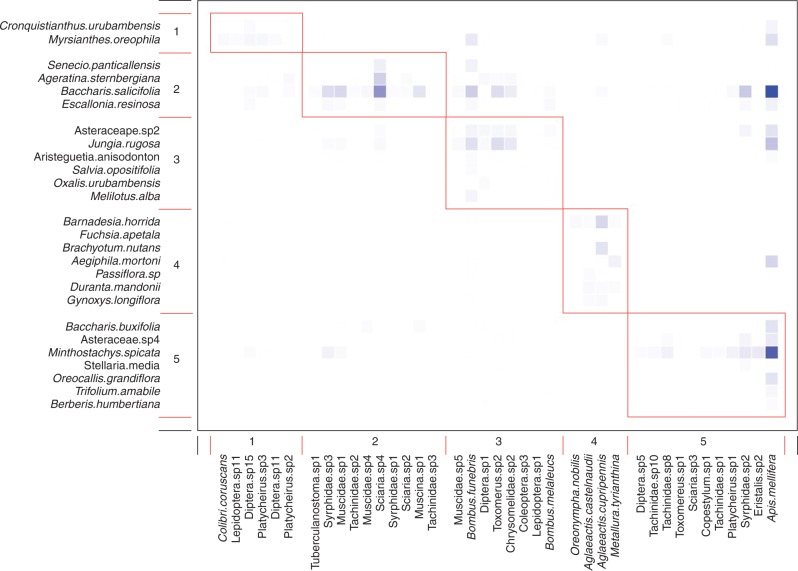
Fig. 4.
Reduced pooled matrix featuring five modules identified by QuanBiMo (with steps = 1e8; Q = 0·30; n = 5 independent runs). Species are sorted according to their modular affinity, plants as rows and pollinators as columns. Darker squares indicate more frequent interactions. Red boxes delineate the five modules and cells inside the boxes are the links within modules. As can be seen, Apis mellifera is clearly not randomly distributed over the five modules, thus linking modules 5, 4, 3, 2 and 1 (bottom to top right) into a coherent network. The dominant pollinator and flower type are as follows. Module 1: large syrphids, a large butterfly and a large long-billed hummingbird visiting open-access flowers; Module 2: small flies and syrphid flies visiting open-access Asteraceae flowers; Module 3: large bumblebees, large syrphids, large flies and beetles visiting open-access and flag/gullet flowers; Module 4: medium-sized hummingbirds with relatively short bills visiting long tubular flowers; Module 5: honey bees and mainly large flies, tachinid flies and syrphids visiting open-access and flag/gullet flowers. Asteraceae plants are as follows: Ageratina sternbergiana, Aristeguietia anisodonoton, Asteraceae sp.2, Asteraceae sp.4, Baccharis buxifolia, Baccharis salicifolia, Barnadesia horrida, Cronquistianthus urubambensis, Gynoxys longiflora and Senecio panticallensis.
Module composition
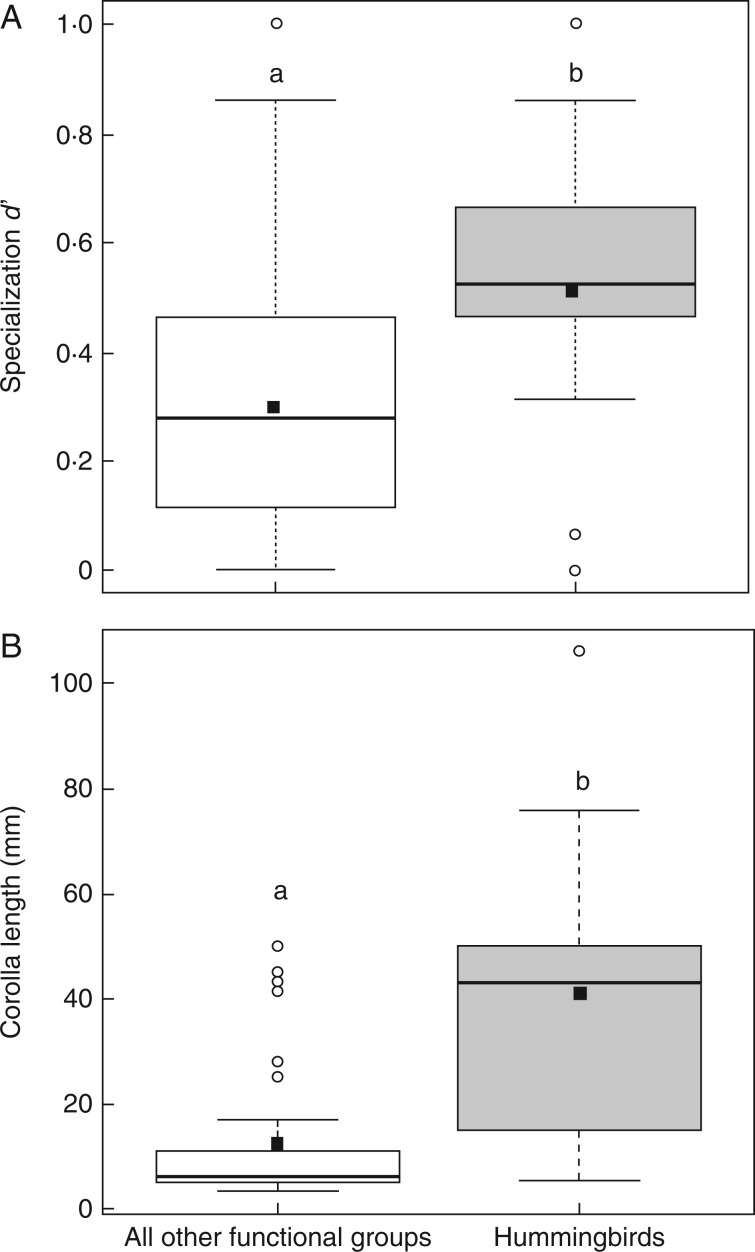
A total of 69 modules were detected when summing the number of modules recorded in each of the nine valleys (see Table 2). Seventy per cent of all those modules contained Diptera and 26 % of all modules were isolated species groups without any links to the remaining network (z-values = 0); of those, more than one-quarter were hummingbirds (see Figs 3 and 4). Complementary specialization d′ for hummingbirds was significantly higher than all other functional groups of flower visitors (Wilcoxon signed rank test with continuity correction v = 50, P < 0·01) (Fig. 5A). Likewise, corolla length of flowers visited by hummingbirds was significantly longer than flowers visited by all other functional groups of flower visitors (Wilcoxon signed rank test with continuity correction v = 273·5, P < 0·0001) (Fig. 5B). Seven modules were exclusively represented by hummingbird species and the plant species they interacted with across valleys. The module identity of hummingbirds and plants was 100 % consistent when the analysis was repeated across 50 independent algorithm runs (i.e. for each matrix, the same plants and hummingbirds were always members of the same module) (Table 4, Fig. 4 and Supplementary Data Fig. S7). Taxonomic and functional clustering in module composition was evident across sites. Modules consistently formed around similar hub plant and pollinator species mostly at the level of orders, but in some cases at the level of genus. Sets of interacting species which were repeatedly associated across valleys include: the hummingbird Aglaeactis cupripennis, which interacted with Barnadesia horrida (Asteraceae) in the same modules 75 % of the time; Apis mellifera, which interacted in the same modules with B. buxifolia and Minthostachys spicata (Lamiaceae) in 80 % of cases, and in the same modules as Myrsianthes oreophila (Myrtaceae) in 67 % of cases; and Bombus melaleucus (Apidae), which interacted in the same modules with Escallonia resinosa (Grossulariaceae) 75 % of the time (see Table 4, Fig. 4 and Supplementary Data Figs S6 and S7).
Fig. 5.
Complementary specialization d′ (A) and corolla length (B) for hummingbirds versus all other functional groups of flower visitors. Data are pooled across the five valleys, Huaran, Yanacocha, Chicon, Mantanay and Choquebamba, in which hummingbirds were observed. Box plots show the median (horizontal line) and ranges from the 25th and 75th percentiles, the solid square is the mean, and the tips of the whiskers indicate the fifth and 95th percentiles. Circles represent outliers. Different letters denote significant differences at P < 0·01.
Table 4.
Connection (c) and participation (z) values, complementary specialization d′ for hummingbirds and their plant species in six networks based on weighted strength from 100 null models, identifying species with important topological roles in the networks and how they change across valleys
| Valley | Hummingbird species | d′ | c | z | Network role | Module ascription | Frequency of belonging to each module | Plant species | d′ | c | z | Network role | Module ascription |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Huaran | Metallura tyrianthina | 0·31** | 0·47 | 0·15 | Connector | Module 1 | 1 | Aegiphila mortoni | 0·26* | 0·58 | −0·54 | Connector | Module 1 |
| Colibri coruscans | 0·06 NS | 0·00 | −0·34 | Periphery | Module 1 | 1 | |||||||
| Oreonympha nobilis | 0·51* | 0·00 | −0·71 | Periphery | Module 2 | 1 | Barnadesia horrida | 0·73*** | 0·26 | 0·71 | Periphery | Module 2 | |
| Aglaeactis cupripennis | 0·62*** | 0·06 | 1·14 | Periphery | Module 2 | 1 | Duranta mandonii | 0·74 NS | 0·00 | −0·71 | Periphery | Module 2 | |
| Aglaeactis castelnaudii | 1·00*** | 0·00 | 0·00 | Periphery | Module 5 | 1 | Fuchsia apetala | 0·70 NS | 0·00 | −0·70 | Periphery | Module 5 | |
| Passiflora tripartita | 0·86* | 0·00 | 0·70 | Periphery | Module 5 | ||||||||
| Yanacocha | Metallura tyrianthina | 1·00*** | 0·00 | 0·00 | Periphery | Module 6 | 1 | Barnadesia horrida | 0·74 NS | 0·00 | 0·71 | Periphery | Module 6 |
| Fuchsia apetala | 0·58 NS | 0·00 | −0·70 | Periphery | Module 6 | ||||||||
| Chicon‡ | Aglaeactis cupripennis | 0·66*** | 0·03 | 2·13 | Module hub | Module 2 | 1 | Gynoxys longiflora | 0·60*** | 0·27 | 0·63 | Periphery | Module 2 |
| Aglaeactis castelnaudii | 0·66* | 0·00 | −0·35 | Periphery | Module 2 | 1 | Brachyotum nutans | 0·78*** | 0·00 | 1·07 | Periphery | Module 2 | |
| Pterophanes cyanopterus | 0·45** | 0·05 | 0·24 | Periphery | Module 2 | 1 | Barnadesia horrida | 0·54* | 0·00 | −0·84 | Periphery | Module 2 | |
| Oreonympha nobilis | 0·52 | 0·00 | −0·76 | Periphery | Module 2 | 1 | Puya ferruginea | 0·77*** | 0·00 | 0·86 | Periphery | Module 2 | |
| Oretrochilus estella | 0·47 NS | 0·00 | −0·11 | Periphery | Module 2 | 1 | |||||||
| Mantanay | Metallura tyrianthina | 0·64* | 0·00 | −0·90 | Periphery | Module 5 | 1 | Passiflora tripartita | 0·39 NS | 0·16 | −0·23 | Periphery | Module 5 |
| Aglaeactis castelnaudii | 0·80*** | 0·00 | 1·08 | Periphery | Module 5 | 1 | Barnadesia horrida | 0·60*** | 0·14 | 1·02 | Periphery | Module 5 | |
| Aglaeactis cupripennis | 0·50*** | 0·13 | −0·18 | Periphery | Module 5 | 1 | Duranta mandonii | 0·73* | 0·00 | 0·49 | Periphery | Module 5 | |
| Module 5 | 1 | Siphocampylus actinothrix | 0·62 | 0·00 | −1·28 | Periphery | Module 5 | ||||||
| Choquebamba | Aglaeactis cupripennis | 1·00*** | 0·00 | 0·00 | Periphery | Module 8 | 1 | Brachyotum nutans | 1·00*** | 0·00 | 0·00 | Periphery | Module 8 |
| Reduced | Aglaeactis castelnaudii | 0·69*** | 0·00 | 0·28 | Periphery | Module 4 | 1 | Barnadesia horrida | 0·61* | 0·32 | 2·12 | Module hub | Module 4 |
| Metallura tyrianthina | 0·61*** | 0·00 | −0·02 | Periphery | Module 4 | 1 | Fuchsia apetala | 0·54* | 0·00 | −0·78 | Periphery | Module 4 | |
| Oreonympha nobilis | 0·52* | 0·00 | −1·33 | Periphery | Module 4 | 1 | Gynoxys longiflora | 0·42*** | 0·40 | −0·59 | Periphery | Module 4 | |
| Aglaeactis cupripennis | 0·66*** | 0·10 | 1·06 | Periphery | Module 4 | 1 | Passiflora tripartita | 0·26 NS | 0·13 | −0·70 | Periphery | Module 4 | |
| Duranta mandonii | 0·56*** | 0·00 | 0·07 | Periphery | Module 4 | ||||||||
| Brachyotum nutans | 0·55*** | 0·12 | 0·02 | Periphery | Module 4 | ||||||||
| Aegiphila mortoni | 0·25*** | 0·44 | 0·00 | Periphery | Module 4 |
The frequency of each hummingbird and plant belonging to each module when the modularity analysis is repeated 50 times. Module ascription was always the same for each plant and pollinator (100 % or 1·00) for each of the 50 runs. Values significantly different from1000 null models using the Patefield algorithm are as follows < 0·05*; < 0·01**; < 0·001***, NS = not significant. Marginal values are shown in italics.
‡Module also comprised of Diptera sp.11 visiting B. horrida and Hymenoptera sp.5 visiting G. longiflora.
Morphological traits
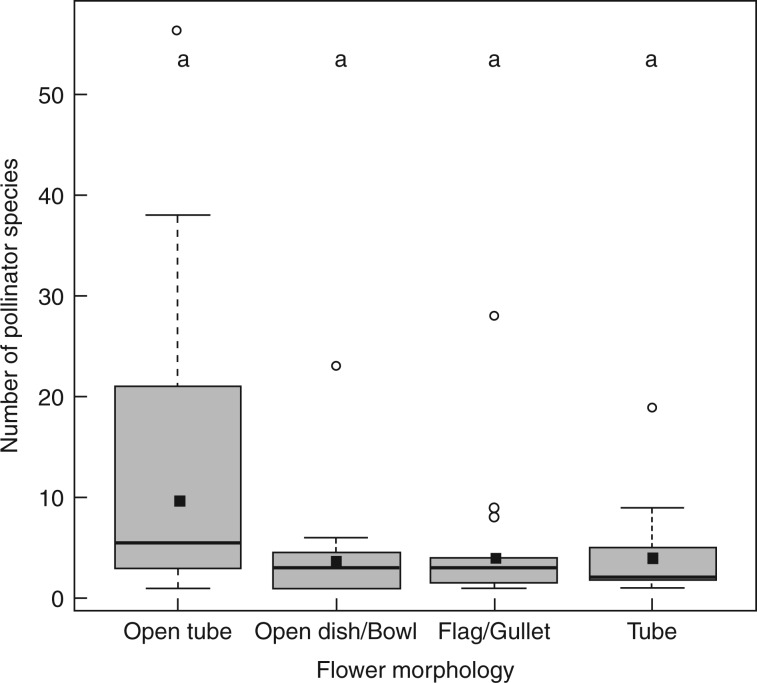
The relevant morphological traits of plant families and functional groups of pollinators are presented in Table 5. There was significant variation among groups for the median number of pollinator species visiting flowers with different morphologies (χ2 = 7·841, P < 0·05) with up to 57 species visiting plants with open tube morphology. However, a Bonferroni adjustment for the six comparisons rendered this finding non-significant (Fig. 6). Thus, bowl-shaped flowers or flowers with tubular-, flag- or gullet-shaped corollas were not visited by significantly more species than flowers with open-access tubular flowers. Hub, connector and peripheral insect flower visitors had short to medium mouthparts allowing easy access to both pollen and nectar to a wide range of corolla lengths. Peripheral, hub and connector hummingbirds had short to long bills (Table 4), which together with tongue maximal extension beyond bill tip (Watts et al., 2012) allowed legitimate and non-legitimate access to nectar from a wide range of corolla tube lengths (6 to > 100 mm) (Fig. 5B). The majority of hub and connector plants (Baccharis, Ageratina, Aristeguietia and Jungia) have numerous open tube flowers characterized by a head of small ray and disc flowers 5–10 mm in length. The stamens and pistels are exposed, which allows easy access to pollen, while the corolla tubes are short enough to allow access to the small amounts of nectar contained at the base. The remaining connectors had small white tubular flowers (5–6 mm), or open dish or open bowl flowers which permitted easy access to the reward for a wide variety of flower visitors (see Table 5, Fig. 2B for B. salicifolia and Table S2 for hub and connector plant species).
Fig. 6.
Number of pollinator species visiting plant species with open tube, open access, gullet/flag and tubular flowers. Data are pooled across all valleys. Box plots show the median (horizontal line) and ranges from the 25th and 75th percentiles, the solid square is the mean, and the tips of the whiskers indicate the fifth and 95th percentiles. Circles represent outliers. Bars with the same letters indicate no significant difference, P > 0·05 after Bonferroni adjustment.
Specialization indices and the role of individual species in the network structure
Network and module hub pollinators were ranked highly when quantifying species degree, species strength, weighted closeness and PSI (Table S1). The strongest network and module hubs were the most centralized participants in the networks (high ranking weighted closeness values indicating generalization). However, complementary specialization d′ quantified network hubs, module hubs and connectors as moderately specialized: d′flw. visitors = 0·42 ± 0·18; d′plants = 0·43 ± 0·16). Thus, in some cases, although network hubs such as Sciaria sp.4 yielded high species degree and weighted closeness values (high generalization), when measuring specialization in terms of exclusiveness of interactions complementary specialization d′ indicated a significant amount of specialization (see Table S1). PSI also yielded relatively high values and rankings, suggesting that network hubs and module hubs were potentially important pollinators for the plant in the networks. Similarly, the same high rankings were also found for network and module hub plants when calculating specialization indices (Table S2). The strongest connector plant species (species linking several modules) also yielded high rankings for specialization indices and were the most centralized participants in the networks (Jungia rugosa at Poques, M. spicata at Piscacucho and M. oreophila at Mantanay). The remaining connector species were still relatively central in the networks, but specialization index values and their rankings were lower than for hub species (Tables S1 and S2). Both plant and pollinator network hubs and module hubs were some of the most abundant in terms of visitation and their presence in transects.
Hubs and connectors were generally more abundant and widespread than peripherals, but not always (Tables S3 and S4). In some valleys, honey bees were peripherals, but were ranked highest in terms of visitation (Table 1). Similarly, B. salicifolia was the most visited plant in Pumamarca (46 visits), but was classified as a peripheral. Hummingbird complementary specialization d′ values indicated a relatively high level of specialization (d′flw. visitors = 0·61 ± 0. 23; d′plants = 0·60 ± 0·19). In 95 % of cases, d′flw. visitors values were significantly different from null models. Likewise, d′plants also yielded high values; in 74 % of cases values were significantly different from null models (Table 4). At Huaran, the most specialized hummingbird Aglaeactis castelnaudii interacted within its own module with the most specialized plants, whereas the most generalized hummingbird Metallura tyrianthina interacted with the most generalized plant Aegiphila mortoni (Verbenaceae). At Chicon, module 2 included the addition of Diptera sp.11 and Hymenoptera sp.5 visiting plants to collect pollen (Table 4, Fig. S6).
A summary of observed species-level specialization index values for the most relevant functional groups of pollinators is shown in Supplementary Data Table S5. Supplementary Data Figs S2–S4 show five specialization indices and the position of the observed values relative to the null models for three widespread abundant species across valleys: A. mellifera, B. funebris and A. cupripennis. These represent random realizations of a perfect generalist. Thus, when the observed value is within the histogram of null models, species are classified as generalist. Honey bees were moderately specialized, but this was not consistent across sites (i.e. Choquebamba and Poques, Fig. S2). The bumblebee B. funebris was the most generalist flower visitor; the observed values were consistently within the histogram of null models across most valleys (Fig. S3). Aglaeactis cupripennis was the most specialized; the observed values were consistently on one side of the histogram, indicating consistent specialization across valleys (Fig. S4). The indices and null model correction can be used to further highlight these irregularities. The raw data, the difference between observed and mean null model values, and z-scores for five specialization indices (degree, strength, PSI, weighted centrality and complementary specialization d′) are presented in Supplementary Data Fig. S5. The inconsistency for honeybees is reflected in the height of the summary box plots, for which d′ is very small for B. funebris (always a generalist) and considerably larger for A. mellifera (sometimes a generalist, sometimes a specialist).
DISCUSSION
In this work we investigated modularity, topological roles of species and specialization of plant–flower visitor networks in the tropical Peruvian Andes. Our results showed that all plant–pollinator networks were highly structured, deviating significantly from random species associations. For the network-wide complementary specialization index H2′, null models were unable to capture the observed structure of networks, suggesting a network property inexplicable merely from species abundances. Plant–flower visitor networks, especially those containing hummingbirds, showed moderate to high levels of specialization (or exclusiveness of interactions) and modularity. Modularity was higher in networks where A. mellifera numbers were generally lower, suggesting that in some sites subsets of species interact more frequently with each other than with species in other modules where honey bees are less dominant. All networks were significantly modular, regardless of size, which contrasts with reports that networks with <50 species were never modular (Olesen et al., 2007). This incongruence may result from a lack of detecting power of the algorithm used by previous studies at low network sizes (e.g. Guimerà et al., 2005; Olesen et al., 2007). On the other hand, the new QuanBiMo algorithm is more sensitive and also more specific than current binary algorithms (Dormann and Strauss, 2014).
On average, modularity in the nine valleys was neither high nor low and networks were only moderately specialized. Observed modules represent communities of pollinators and plants which were active in the same season. The networks were dominated by ecologically and functionally generalist plant species that are closely related taxonomically (e.g. Baccharis, Ageratina and Aristeguietia) with similar morphology and rewards. These plants exhibited high plasticity by changing their topological roles across sites and serving as either network hubs in some valleys, or switching to module hubs or connectors in other valleys (Table S2). Thus, our networks were structured mainly by hubs and connector plants and pollinators which were functionally and ecologically equivalent. Asteraceae plant hubs were ubiquitous and abundant in most valleys; they flowered throughout the season and were present at each altitude and most life zones. A similar pattern was also evident for the dominant pollinators such as A. mellifera, Syrphid sp.2, Sciaria sp.4 (Diptera) and B. funebris. Such pollinators have the ability to ‘fill the gap’ by changing topological roles; for example, where honey bees were less common (Pumamarca), bumblebees replaced them as module hubs. The weighted modularity analysis (which accounts for sampling bias with null-model corrections) also showed that modules comprised both plant hubs and flower visitor hubs, with more insects and hummingbirds than plants acting as hub or connector species. This is in contrast to other studies (Dupont and Olesen, 2008), where no insect species served as hubs and the majority of connectors were insects, or where all hubs were plant species (Bosch et al., 2009). Only 48 (19 %) of all species played a significant role in shaping network structure, while the majority of species were peripheral, in line with other studies (Olesen et al., 2007; but see Bosch et al., 2009). In each network, plant, insect and hummingbird species served as connectors in equal proportions, suggesting they play an important role in linking different modules or by gluing peripheral species together into modules. Across networks, most modules were dominated by dipterans and social bees, particularly introduced honey bees. Taxonomic and functional clustering was also evident across sites, with some plant species and functional groups of flower visitors repeatedly associated. This further supports the conclusion that the topology of networks is non-random and highly organized.
The networks in the Sacred Valley were dominated by open-access flowers, which were visited by many small to medium-sized insects, with few morphological restrictions for the insects to access the reward. This is in accordance with findings of Kaiser-Bunbury et al. (2014), who also reported that flowers with a low complexity showed weak constraints in floral resource accessibility and interacted with most pollinator species. Moreover, some hummingbirds, bees and syrphids were still able to access such flowers by robbing nectar and pollen. The highest diversity of flower visitors was on the dioecious flowers of Baccharis, which is not surprising given that the genus has the richest galling fauna of the neotropics (Boldt and Robbins, 1990), and the highest diversity of visiting flies (Souza-Silva et al., 2001). The abundance of dipterans on Baccharis plants may not only signify the importance of the flowers in their diet, but also their importance as potential pollinators, and hence play an important role in ecosystem function (Souza-Silva et al., 2001). This suggests that species strength and specific dietary requirements of functional groups influence module structure in the Sacred Valley. Our networks were dominated by ecological and functional generalist plants, which were probably pollinated by whatever flower visitors were a suitable size and shape, and had appropriate behaviour.
Earlier binary modularity studies which implied that network hubs, module hubs and connectors are generalist species (e.g. Olesen et al., 2007) did not evaluate this using quantitative specialization indices and null models. This study is one of the few to measure the level of specialization for individual species with important topological roles within and across networks using quantitative data. We found that the strongest network hubs, module hubs and connectors were the most centralized participants in the networks and were ranked highest when quantifying specialization across the five different (species-level) specialization indices. Moreover, many of these species were consistently the most centralized participants across networks, suggesting a high level of generalization. Both plant and pollinator network hubs and module hubs were also the most abundant in terms of visitation and presence in transects. In contrast, however, network hubs, module hubs and connectors all showed a moderate degree of specialization (or exclusiveness) when measuring specialization using complementary specialization index d′, and a few species were highly specialized. This finding is in contrast to Olesen et al. (2007), who found that network hubs and connectors (i.e. species with both high c- and z scores) were super-generalists. These differences are likely to be attributed to the SA algorithm (see Guimerà et al., 2005; Olesen et al., 2007), which analyses each trophic level separately, and to the fact that in Olesen et al.’s study interactions are binary whereas in our study we use interaction strength. Finally, species strength is closely related to species abundance (Bascompte et al., 2007) and was positively related to weighted measures of within-module degree. This suggests that species strength and factors relating to abundance were the main determinants of the modular structure of plant–pollinator networks, in concordance with Schleuning et al. (2014). In contrast, the relationship between species strength and the even distribution of links across modules, although significantly positive, was weak, suggesting that links are not uniformly distributed among all the communities.
In the Sacred valley, specialization varied along a continuum between moderate generalization to moderate specialization, concurrent with other work (Waser et al., 1996; Johnson and Steiner, 2000). One interesting finding was how much the specialization of some species changed across sites, and how constant it remained in other species, a trend also evident in terms of the topological roles of plants and flower visitors. Across all seven sites where present, B. funebris was consistently a generalist flower visitor, but served as hub, connector or peripheral species. Degree is the number of plant links and is consistent with a strict definition of specialization, but it makes no use of the number of visits recorded for each interaction. Surprisingly, although honey bees recorded the highest number of links and visits of all flower visitors, when describing niche properties, they showed a moderate degree of complementary specialization (or exclusiveness of species interactions). These findings underline that specialization indices convey different concepts of specialization and hence quantify different aspects (Dormann, 2011). Hummingbirds and the plants they visited had the highest level of complementary specialization and exclusivity in modules (functional specialist). At the same time, the majority of plants visited and probably pollinated by hummingbirds (but see Watts et al., 2012) were usually visited by several species of hummingbirds and so in that sense could be considered as ecological generalists. Yet again, this highlights that measuring specialization requires careful consideration of what defines a specialist (Ollerton et al., 2007; Dormann, 2011).
The variability in specialization described above could be attributed to any of a number of factors including: a response of flower visitors to low plant diversity at some sites (Schleuning et al., 2012), community and geographical context of plant populations (Ollerton et al., 2007), spatio-temporal variation in pollinator abundance (Johnson and Steiner, 2000; Watts et al., 2013), variability in pollinator distribution and morphology (Newman et al., 2014), geographical phenotypic variation (Cosacov et al., 2014), or variation in flower visitors and floral and pollinator community composition (Kaiser-Bunbury et al., 2014). Finally, the changes in specialization across sites could also be explained by flower visitors switching to more rewarding plants throughout their activity periods.
A number of potential biases are important to highlight. As the pollinator assemblages studied were taxonomically very different in life histories, nesting preferences and behaviour, the transect census method undertaken may not have been appropriate to adequately characterize some of the taxa, particularly solitary bees and hummingbirds. For example, hummingbirds may have been under-represented in different samples because the composition and the relative abundance of hummingbird species is likely to be affected by their morphological–behavioural attributes, available resources, distributional/altitudinal limits or habitat affinities of a particular bird species and gradients in local climate (Borgella et al., 2001). Furthermore, hummingbirds were easily disturbed from foraging by observers walking transects and did not tend to visit many plants within the sampling area, but instead remained either on the periphery or in the canopy. However, most parts of the valleys did not have a high canopy, so we estimate that approx. 10 % of plant–hummingbird interactions were missed from the canopy in subtropical humid montane forests. These plants include Passiflora spp., which climbs up trees such as Alnus, Duranta spp., Fuchsia spp. and M. oreophila.
Micro-climatic differences among these valleys and changes in weather along the altitudinal gradient may have affected local distributions of butterfly species. Flower-visiting beetles can be inactive and infrequent visitors, whereas some small solitary bees are short-lived, have short flight ranges and are not easily detected (Gathmann and Tscharntke, 2002). For future work a number of alternative sampling designs might be incorporated in conjunction with the transect method to eliminate some of the potential biases such as data aggregation, one of which could have included fixed observation plots, which might also generate sufficient data to avoid pooling data.
In conclusion, during a single season snapshot in time, we have demonstrated that the topology of networks in this tropical montane environment was non-random and highly organized. Although we acknowledge that some taxa may have been under-represented in different samples and lacked sampling replication, the weighted modularity analysis (which accounts for sampling bias with null-model corrections) showed some remarkable consistency with many plant species and functional groups of flower visitors repeatedly associated. We used six different specialization indices to show that in the Sacred Valley, specialization varied along a continuum between moderate generalization to moderate specialization. Our findings also underline that specialization indices convey different concepts of specialization and hence quantify different aspects, and that measuring specialization requires careful consideration of what defines a specialist.
SUPPLEMENTARY DATA
Supplementary data are available online at www.aob.oxfordjournals.org and consist of the following. Figure S1: histograms for H2′ values for the analysis of each network. Figure S2: histograms of observed and null model specialization values of Apis mellifera, for the analysis of specialization shift. Figure S3: histograms of observed and null model specialization values of Bombus funebris for the analysis of specialization shift. Figure S4: histograms of observed and null model specialization values of Aglaeactis cupripennis for the analysis of specialization shift. Figure S5: histograms showing specialization index values for Bombus funebris, Apis mellifera and Aglaeactis cupripennis. Figure S6: Chicon featuring seven modules identified by QuanBiMo. Figure S7: Mantanay featuring seven modules identified by QuanBiMo. Table S1: connection (c) and participation (z) values and complementary specialization d′ for pollinators in ten networks based on weighted strength from 100 null models. Table S2: connection (c) and participation (z) values and complementary specialization d′ for plants in ten networks based on weighted strength from 100 null models. Table S3: full list of plant species surveyed in the Sacred Valley. Table S4: full list of pollinator species surveyed in the Sacred Valley. Table S5: summary of observed species-level specialization index values for the most relevant functional groups of pollinators.
ACKNOWLEDGEMENTS
We thank Federico and Fatty Argandoña, Carmen Aparicio, Carlos Calvo, Rossemeri Cuéllar, Rosa and Herbert Duran, Juan Flores, Marcela Moreno Herrera, David Huamàn Ovalle, Ramon Ipanaque, Karin Nuñez, Berioska Quispe Estrada, Mireya Natividad Raurau Quisiyupa, Javier Saldivar and Celia Zuñiga for field assistance, and the staff from Universidad Nacional de San Antonio Abad del Cusco, Perú. Permission to undertake fieldwork was granted by the Director of the National Institute of Natural Resources (INRENA) (permit numbers: 008799 and 0001982). This research was self-funded by S.W. and by grants from The British Ecological Society, Idea Wild, The Biodiversity Trust, The Anglo Peruvian Society and The Leslie Church Bursary Fund of the University of Northampton.
LITERATURE CITED
- Bascompte J, Jordano P, Olesen JM. 2006. Asymmetric coevolutionary networks facilitate biodiversity maintenance. Science 312: 431–433. [DOI] [PubMed] [Google Scholar]
- Bascompte J, Jordano P. 2007. Plant–animal mutualistic networks: the architecture of biodiversity. Annual Review of Ecology, Evolution and Systematics 38: 567–593. [Google Scholar]
- Blüthgen N. 2010. Why network analysis is often disconnected from community ecology: a critique and an ecologist’s guide. Basic Applied Ecology 11: 185–195. [Google Scholar]
- Blüthgen N, Menzel F, Blüthgen N. 2006. Measuring specialization in species interaction networks. BMC Ecology 6: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blüthgen N, Fründ J, Vázquez DP, Menzel F. 2008. What do interaction network metrics tell us about specialization and biological traits? Ecology 89: 3387–3399. [DOI] [PubMed] [Google Scholar]
- Boldt PE, Robbins TO. 1990. Phytophagous and flower-visiting insect fauna of Baccharis salicifolia (Asteraceae) in the southwestern United States and northern Mexico. Environmental Entomology 19: 515–523. [Google Scholar]
- Borgella R, Jr, Snow AA, Gavin TA. 2001. Species richness and pollen loads of hummingbirds using forest fragments in southern Costa Rica. Biotropica 33: 90–109. [Google Scholar]
- Bosch J, Martín González AM, Rodrigo A, Navarro D. 2009. Plant–pollinator networks: adding the pollinator’s perspective. Ecology Letters 12: 409–419. [DOI] [PubMed] [Google Scholar]
- Clauset A, Moore C, Newman MEJ. 2008. Hierarchical structure and the prediction of missing links in networks. Nature 453: 98–101. [DOI] [PubMed] [Google Scholar]
- Cosacov A, Cocucci AA, Sérsic AN. 2014. Geographical differentiation in floral traits across the distribution range of the Patagonian oil-secreting Calceolaria polyrhiza: do pollinators matter? Annals of Botany 113: 251–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalsgaard B, Magård E, Fjeldså J, et al. 2011. Specialization in plant–hummingbird networks is associated with species richness, contemporary precipitation and Quaternary climate-change velocity. PLoS One 6: e25891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalsgaard B, Trøjelsgaard K, Martín González AM, et al. 2013. Historical climate-change influences modularity and nestedness of pollination networks. Ecography 36: 1331–1340. [Google Scholar]
- Donatti CI, Guimaraes PR, Galetti M, Pizo MA, Marquitti FMD, Dirzo R. 2011. Analysis of a hyper-diverse seed dispersal network: modularity and underlying mechanisms. Ecology Letters 14: 773–781. [DOI] [PubMed] [Google Scholar]
- Dormann CF. 2011. How to be a specialist? Quantifying specialization in pollination networks. Network Biology 1: 1–20. [Google Scholar]
- Dormann CF, Strauss R. 2014. A method for detecting modules in quantitative bipartite networks. Methods in Ecology and Evolution 5: 90–98. [Google Scholar]
- Dormann CF, Fründ J, Blüthgen N, Gruber B. 2009. Indices, graphs and null models: analysing bipartite ecological networks. The Open Ecology Journal 2: 7–24. [Google Scholar]
- Dupont YL, Olesen J. 2008. Ecological modules and roles of species in heathland plant–insect flower visitor networks. Journal of Animal Ecology 78: 346–353. [DOI] [PubMed] [Google Scholar]
- Dupont YL, Hansen DM, Olesen JM. 2003. Structure of a plant–flower-visitor network in the high-altitude sub-alpine desert of Tenerife, Canary Islands. Ecography 26: 301–310. [Google Scholar]
- Fenster CB, Armbruster WS, Wilson P, et al. 2004. Pollination syndromes and floral specialization. Annual Review of Ecology, Evolution and Systematics 35: 375–403. [Google Scholar]
- Fjeldså J, Krabbe N. 1990. Birds of the high Andes. Copenhagen: Apollo Books. [Google Scholar]
- Fortuna MA, Bascompte J. 2006. Habitat loss and structure of plant–animal mutualistic networks. Ecology Letters 9: 281–286. [DOI] [PubMed] [Google Scholar]
- Fortuna MA, Stouffer DB, Olesen JM, et al. 2010. Nestedness versus modularity in ecological networks: two sides of the same coin? Journal of Animal Ecology 79: 811–817. [DOI] [PubMed] [Google Scholar]
- Freeman LC. 1979. Centrality in social networks I: Conceptual clarification. Social Networks 1: 215–239. [Google Scholar]
- Gathmann A, Tscharntke T. 2002. Foraging ranges of solitary bees. Journal of Animal Ecology 71: 757–764. [Google Scholar]
- Gentry AH. 1996. A field guide to the families and genera of woody plants of northwest South America (Colombia, Ecuador, Perú) with supplementary notes on herbaceous taxa, 2nd edn. Chicago: University of Chicago Press. [Google Scholar]
- Gómez JM, Muñoz-Pajares AJ, Abdelaziz M, Lorite J, Perfectti F. 2013. Evolution of pollination niches and floral divergence in the generalist plant Erysimum mediohispanicum. Annals of Botany 113: 237–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimerà R, Mossa S, Turtschi A, Amaral LAN. 2005. The worldwide air transportation network: anomalous centrality, community structure, and cities global roles. In: Proceedings of the National Academy of Science USA 102: 7794–7799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdridge LR. 1967. Life zone ecology. Costa Rica: Tropical Science Centre. [Google Scholar]
- Ings TC, Montoya JM, Bascompte J, et al. 2009. Ecological networks – beyond food webs. Journal of Animal Ecology 78: 253–269. [DOI] [PubMed] [Google Scholar]
- IUCN. 2010. IUCN red list of threatened species, Version 2010.1. www.iucnredlist.org (last assessed 3 January 2016).
- Johnson SD, Steiner KE. 2000. Generalization versus specialization in plant pollination systems. Trends in Ecology and Evolution 15: 140–143. [DOI] [PubMed] [Google Scholar]
- Jordano P, Bascompte J, Olesen JM. 2003. Invariant properties in coevolutionary networks of plant–animal interactions. Ecology Letters 6: 69–81. [Google Scholar]
- Kaiser-Bunbury CN, Vázquez DP, Stang M, Ghazoul J. 2014. Determinants of the microstructure of plant–pollinator networks. Ecology 95: 3314–3324. [Google Scholar]
- Klein AM, Vaissière BE, Cane JH, et al. 2007. Importance of pollinators in changing landscapes for world crops. In: Proceedings of the Royal Society B 274: 303–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín González AM, Dalsgaard B, Olesen JM. 2010. Centrality measures and the importance of generalist species in pollination networks. Ecological Complexity 7: 36–43. [Google Scholar]
- Martín González AM, Allesina S, Rodrigo A, Bosch J. 2012. Drivers of compartmentalization in a Mediterranean pollination network. Oikos 121: 2001–2013. [Google Scholar]
- Martín González AM, Dalsgaard B, Nogués-Bravo D, et al. 2015. The macroecology of phylogenetically structured hummingbird-plant networks. Global Ecology and Biogeography 24: 1212–1224. [Google Scholar]
- Memmott J, Waser NM, Price MV. 2004. Tolerance of pollination networks to species extinctions. In: Proceedings of the Royal Society of London B 271: 2605–2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moles A, Ollerton J. 2016. Is the notion that species interactions are stronger and more specialized in the tropics a zombie idea? Biotropica 48: 141–145. [Google Scholar]
- Newman MEJ. 2004. Fast algorithm for detecting community structure in networks. Physical Review E 69: 066133. [DOI] [PubMed] [Google Scholar]
- Newman E, Manning J, Anderson B. 2014. Matching floral and pollinator traits through guild convergence and pollinator ecotype formation. Annals of Botany 113: 373–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olesen JM, Bascompte J, Dupont YL, Jordano P. 2007. The modularity of pollination networks. In: Proceedings of the National Academy of Sciences USA 104: 19891–19896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ollerton J, Cranmer L. 2002. Latitudinal trends in plant–pollinator interactions: are tropical plants more specialized? Oikos 98: 340–350. [Google Scholar]
- Ollerton J, Watts S. 2000. Phenotype space and floral typology: towards an objective assessment of pollination syndromes. Det Norske Videnskaps-Akademi. I. Matematisk-Naturvidenskapelige Klasse, Skrifter, Ny Serie 39: 149–159. [Google Scholar]
- Ollerton J, Killick A, Lamborn E, Watts S, Whitston M. 2007. Multiple meanings and modes: on the many ways to be a generalist flower. Taxon 56: 717–728. [Google Scholar]
- Ollerton J, Winfree R, Tarrant S. 2011. How many flowering plants are pollinated by animals? Oikos 120: 321–326. [Google Scholar]
- Opsahl T, Agneessens F, Skvoretz J. 2010. Node centrality in weighted networks: generalizing degree and shortest paths. Social Networks 32: 245–251. [Google Scholar]
- Patefield WM. 1981. Algorithm AS159. An efficient method of generating r x c tables with given row and column totals. Applied Statistics 30: 91–97. [Google Scholar]
- R Development Core Team 2010. R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; www.r-project.org. [Google Scholar]
- Schleuning M, Fründ J, Klein AM, et al. 2012. Specialization of mutualistic interaction networks decreases toward tropical latitudes. Current Biology 22: 1925–1931. [DOI] [PubMed] [Google Scholar]
- Schleuning M, Ingmann L, Strauß R, et al. 2014. Ecological, historical and evolutionary determinants of modularity in weighted seed-dispersal networks. Ecology Letters 17: 454–463. [DOI] [PubMed] [Google Scholar]
- Sonne J, Martín González AM, Maruyama PK, et al. 2016. High proportion of smaller ranged hummingbird species coincides with ecological specialization across the Americas. In: Proceedings of the Royal Society B 283: doi:10.1098/rspb.2015.2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza-Silva M, Fontenelle JCR, Martins RP. 2001. Seasonal abundance and species composition of flower-visiting flies. Neotropical Entomology 30: 351–358. [Google Scholar]
- Stattersfield JA, Crosby MJ, Long AJ, Wege DC. 1998. Endemic bird areas of the world: priorities for biodiversity conservation. Cambridge: Birdlife International. [Google Scholar]
- Tupayachi A. 2005. Flora de la Cordillera de Vilcanota. Arnaldoa 12: 126–144. [Google Scholar]
- Waser NM, Chittka L, Price MV, Williams NM, Ollerton J. 1996. Generalization in pollination systems, and why it matters. Ecology 77: 1043–1060. [Google Scholar]
- Watts S, Huamán Ovalle D, Moreno Herrera M, Ollerton J. 2012. Pollinator effectiveness of native and non-native flower visitors to an apparently generalist Andean shrub, Duranta mandonii (Verbenaceae). Plant Species Biology 27: 147–158. [Google Scholar]
- Watts S, Sapir Y, Segal B, Dafni A. 2013. The endangered Iris atropurpurea (Iridaceae) in Israel: honey bees, night-sheltering male bees and female solitary bees as pollinators. Annals of Botany 111: 395–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wege DC, Long AJ. 1995. Key areas for threatened birds in the Neotropics. Cambridge, UK: Birdlife International. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.