Abstract
Background and Aims Many previous studies conclude that pre-zygotic barriers such as mechanical isolation account for most reproductive isolation between pairs of taxa. However, the inheritance and persistence of barriers such as these after the first generation of hybridization is rarely quantified, even though it is a vital consideration in understanding gene flow potential. There is an asymmetrical pre-zygotic mechanical barrier to hybridization between Eucalyptus nitens and Eucalyptus globulus, which completely prevents small-flowered E. nitens pollen from mating with large E. globulus flowers, while the reverse cross is possible. We aimed to determine the relative importance of pre- and post-zygotic barriers in preventing gene flow following secondary contact between E. nitens and E. globulus, including the inheritance of barriers in advanced-generation hybrids.
Methods Experimental crossing was used to produce outcrossed E. nitens, E. globulus and their F1, F2, BCg and BCn hybrids. The strength and inheritance of a suite of pre- and post-zygotic barriers were assessed, including 20-year survival, growth and reproductive capacity.
Key Results The mechanical barrier to hybridization was lost or greatly reduced in the F1 hybrid. In contrast, intrinsic post-zygotic barriers were strong and persistent. Line-cross analysis indicated that the outbreeding depression in the hybrids was best explained by epistatic loss.
Conclusions The removal of strong mechanical barriers between E. nitens and E. globulus allows F1 hybrids to act as a bridge for bi-directional gene flow between these species. However, strong and persistent post-zygotic barriers exist, meaning that wherever F1 hybridization does occur, intrinsic post-zygotic barriers will be responsible for most reproductive isolation in this system. This potential transient nature of mechanical barriers to zygote formation due to additive inheritance in hybrids appears under-appreciated, and highlights the often important role that intrinsic post-mating barriers play in maintaining species boundaries at zones of secondary contact.
Keywords: Reproductive isolation, speciation, hybridization, pre-zygotic barriers, post-zygotic barriers, exotic gene flow, Eucalyptus nitens, Eucalyptus globulus, experimental crossing, Tasmania
INTRODUCTION
Understanding reproductive barriers is central to unravelling the processes that cause speciation (Levin, 1978a; Noor and Feder, 2006; Abbott et al., 2013; Baack et al., 2015). These barriers control the potential for hybridization between diverging populations or taxa. Hybridization is common in nature and has wide-ranging effects on speciation (Abbott et al., 2013). For example, hybridization can facilitate gene flow so as to homogenize diverging populations and constrain speciation (Ellstrand and Elam, 1993; Coyne and Orr, 2004) or, conversely, it can generate unique adaptive phenotypes that drive species divergence (Rieseberg et al., 2003). Hybridization has also probably been historically important. Phylogeographic studies show that palaeoclimatic oscillations have led to repeated episodes of population divergence and isolation followed by secondary contact in numerous lineages (Hewitt, 2000), meaning that hybridization may have influenced the evolution of many extant species (Hewitt, 2001; Guo et al., 2015). The consequences of such hybridization largely depend on the degree of genetic divergence between taxa and the strength of intrinsic and extrinsic reproductive barriers that have developed in allopatry (Abbott et al., 2013; Seehausen et al., 2014). Therefore, in instances of secondary contact a key question is whether or not hybridization will facilitate gene flow and introgression between diverging taxa (Hewitt, 2001; Arnold and Martin, 2010). The specific barriers preventing gene flow in such situations are likely to be important for understanding speciation.
The combined effects of pre-zygotic and post-zygotic barriers, as well as the strength and inheritance of these barriers in first- and later-generation hybrids, will determine patterns of gene flow and speciation following secondary contact (Grant, 1993; Hewitt, 2001; Arnold and Martin, 2010). Within this context, one of the most widely debated aspects of the evolution of reproductive isolation is the relative importance of pre- versus post-zygotic barriers (Rice and Hostert, 1993; Schemske, 2000; Sobel et al., 2010). Some authors argue that once complete isolation is achieved through intrinsic post-zygotic barriers, speciation is likely to be permanent, whereas ‘complete’ pre-zygotic isolation can be quite easily lost, e.g. through range expansion (Hendry et al., 2009; Nosil et al., 2009; Moyle and Nakazato, 2010). Conversely, others point out that barriers act sequentially, so that pre-zygotic barriers are likely to cause the majority of isolation, and most empirical studies support this expectation (Ramsey et al., 2003; Lowry et al., 2008a; Schemske, 2000; Sobel et al., 2010; Sobel and Streisfeld, 2015). This debate is ongoing (Sobel and Streisfeld, 2015) and has so far focused largely on a few herbaceous groups, including Mimulus (Ramsey et al., 2003) and Aquilegia (Grant, 1992). These studies have been vital and often ground-breaking (Ramsey et al., 2003), but in their recent review of the topic Baack et al. (2015) called for an expansion of the organisms under study to include trees and other poorly represented groups, in order to ascertain the generality of the patterns that are emerging.
Eucalyptus is a large (∼800 species), taxonomically complex genus native to Australia and the islands to its north (Centre for Plant Biodiversity Research, 2006). Eucalypts are mainly trees that dominate Australian forests and woodlands, making them one of the most ecologically important plant groups in the southern hemisphere (Pryor and Johnson, 1981). Taxonomic complexity in the genus is often attributed to incomplete reproductive isolation between species that have come into secondary contact following climatically driven range expansion and contraction during the group’s ∼56-million-year history (Pryor and Johnson, 1981; Thornhill et al., 2015). While natural hybridization is frequently reported in the genus, strong barriers to hybridization do exist (Griffin et al., 1988). For example, hybridization is not possible between the ten major subgenera (Griffin et al., 1988), and even within subgenera phylogenetic relationships govern compatibility, with the likelihood of hybridization decreasing rapidly with increasing genetic distance between species (Larcombe et al., 2015).
Studies using manipulated hybridization have shown that both pre-zygotic and post-zygotic barriers are responsible for reproductive isolation between eucalypt species (Gore et al., 1990; Ellis et al., 1991; Lopez et al., 2000b; Barbour et al., 2006b; Dickinson et al., 2012a, b). In terms of post-zygotic mechanisms, karyotype number is consistent across the genus (n= 11; Grattapaglia et al., 2012) and there is no indication to date of major chromosomal rearrangements (e.g. large inversions or translocations; Rieseberg, 2001; Myburg et al., 2004; Hudson et al., 2012). However, there is evidence consistent with Dobzhansky–Muller type intrinsic incompatibilities (Dobzhansky and Dobzhansky, 1937; Muller, 1942), which are often expressed as reduced hybrid fitness at later ages, e.g. 4–14 years (Lopez et al., 2000b; Barbour et al., 2006a; Costa e Silva et al., 2012; Larcombe et al., 2014). Conversely, pre-zygotic barriers such as flowering time (Barbour et al., 2006b) and physical isolation (Barbour et al., 2005b) often represent strong isolating mechanisms in closely related species in the wild (Potts et al., 2003).
The importance of pre-zygotic barriers in eucalypt species is well illustrated by the case of unilateral (one-way) incompatibility between Eucalyptus globulus and E. nitens (Gore et al., 1990). While taxonomically these species have been considered closely related and grouped in the same series [Globulares (subgenus Symphyomyrtus, section Maidenaria); Nicolle, 2015], this is being increasingly questioned by phylogenetic analysis (McKinnon et al., 2008; Steane et al., 2011). Despite similarities in juvenile and adult foliage, the two species are morphologically distinct, with E. globulus having large single flowers and E. nitens having small flowers in umbels of seven (Fig. 1). There is overlap in flowering time where these species are grown together (Lopez et al., 2000a; Barbour et al., 2006b), and the flower size differences are likely to produce two pre-zygotic barriers. Firstly, the size differences are likely to result in distinct (although not completely independent) pollinator guilds in each species (Hingston et al., 2004a, b), causing a pre-mating barrier. Secondly, differences in style length lead to a unilateral post-mating barrier to F1 hybridization, where pollen–pistil size incompatibility prevents small-flowered E. nitens pollen from pollinating large E. globulus flowers, while the reverse cross is possible (Gore et al., 1990). It is also thought that reduced seed set in the reverse cross may be due to E. globulus pollen tubes overshooting the ovaries of E. nitens (Gore et al., 1990).
Fig. 1.
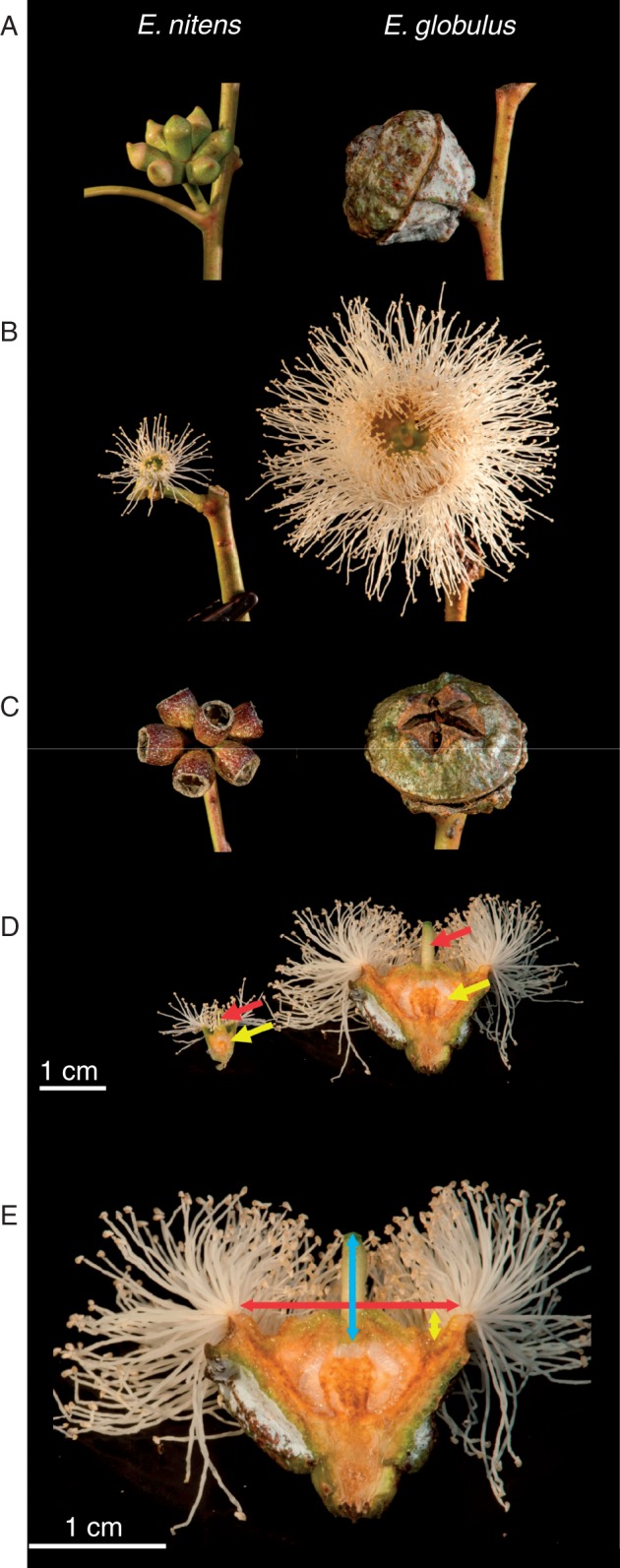
Flower and fruit morphology of E. nitens and E. globulus, showing: (A) a single umbel with seven small buds in E. nitens and one large bud in E. globulus; (B) a single open flower; (C) mature fruit, known as capsules (note that one flower has aborted in the E. nitens umbel, leaving six rather than seven capsules); (D) dissected single flowers showing the large differences in style length (red arrows) and ovary depth (yellow arrows); (E) flower morphology measurements including style length (blue), receptacle width (red) and disk depth (yellow). Note the change of scale in (E). Photographs by Robert Wiltshire.
Eucalyptus nitens and E. globulus are today naturally allopatric species. However, E. nitens is the plantation species of choice on the island of Tasmania, where it is exotic and often grown within the range of native E. globulus (Barbour et al., 2006b). It has previously been suggested that the unilateral cross-incompatibility between the species removes the risk of exotic gene flow from E. nitens plantations to native E. globulus populations (Potts et al., 2003; Barbour et al., 2005a). Here we investigate this system in more detail to better understand the inheritance of reproductive barriers in hybrids and the likelihood of exotic gene flow from E. nitens plantations to native E. globulus populations. Firstly, using manipulated crossing, we assess the inheritance and strength of the unilateral barrier between E. globulus and E. nitens in first-generation (F1) hybrids. Secondly, we then measure germination, nursery performance and 20-year-old fitness (survival, growth and reproductive capacity) of the F1 and advanced-generation hybrids [F2 and backcross (BC) hybrids] in comparison with the pure species. Significant outbreeding depression has been reported in this hybrid system, which is likely to act as a post-zygotic barrier to hybridization (Volker et al., 2008; Costa e Silva et al., 2012). The overarching aim of this study was to assess the relative strengths of pre- and post-zygotic barriers in hybrids between these naturally allopatric species. This information should contribute to understanding the processes that maintain boundaries between sympatric/parapatric species in nature.
MATERIALS AND METHODS
A note on barrier terminology
In the speciation literature, the term ‘pre-zygotic’ is reserved for any barrier that prevents initial F1 hybridization between species (Campbell and Aldridge, 2006). However, this becomes somewhat restrictive when tracking inheritance and the effect of mechanical barriers to gene flow in later generation hybrids, as we do here. For example, style length is a pre-zygotic barrier between Eucalyptus globulus and Eucalyptus nitens, but when we quantify the strength of this same barrier/trait in the F1 it becomes a post-zygotic barrier, even though we measure barrier strength in terms of its ability to prevent hybrid zygote formation in the second generation. Therefore, we avoid the term ‘pre-zygotic’ and refer to such barriers as ‘mechanical barriers to zygote formation’, and refer to other post-zygotic barriers as ‘intrinsic post-zygotic barriers’ to distinguish them from post-zygotic mechanical barriers to zygote formation.
Flower morphology
Flower morphology was assessed on 20 individuals each of E. globulus, E. nitens and their F1 hybrid. From each tree, one to three flowers were dissected and measurements were made of the style length, receptacle width and disk depth, as shown in Fig. 1E.
Pollen viability and growth
Pollen was extracted and in vitro viability and growth were assessed on agar growth medium using the methods outlined in Gore et al. (1990). Pollen from 16 trees each of E. nitens, E. globulus and their F1 hybrid were tested in four replicates. In each replicate, pollen from each of the 48 trees was applied randomly to individual cells in 5×5 replidishes (two dishes per replicate). Incubation was undertaken at 25 °C for 24 h, after which the number of pollen grains that germinated and the number that failed to germinate were counted to determine relative viability, and the longest pollen tube in every cell was measured to quantify relative pollen tube length (Potts and Marsden-Smedley, 1989).
Crossing
A detailed explanation of the crossing and trial establishment is given by Costa e Silva et al. (2012) and is summarized here to make clear the design and scale of the experiment. Crossing was undertaken to produce fully outcrossed pure E. nitens and pure E. globulus, as well as four different interspecific cross types, including the F1 (E. nitens ♀ × E. globulus ♂), outcrossed F2s (unrelated F1 × F1), backcrosses to E. nitens (BCn) and backcrosses to E. globulus (BCg). The E. globulus grandparents were from the King Island (in Bass Strait) or Taranna provenances, which represent different races (Dutkowski and Potts, 1999), whereas all the E. nitens grandparents were from the Toorongo provenance on mainland Australia (Hamilton et al., 2011). Production of the F1s used as parents is described in Volker et al. (2008) and included a range of combinations from the same E. globulus and E. nitens provenances as those noted above. Parents were selected on the basis of the presence of flowers at age 3 years, accessibility and maximizing the diversity of grandparental and parental representation in each cross type from the available population. Within each cross type, crossing was random, except that related matings were avoided. For each tree used as a female, one single pair mating was generally undertaken with a single pollen parent from each of E. nitens, E. globulus and their F1 hybrid. The exception was the E. globulus trees, which were not crossed with E. nitens pollen due to the known unilateral barrier to hybridization (see Introduction). Following Hardner and Potts (1995), capsules were harvested ∼1 year after crossing and air-dried, and the number of viable (filled) seeds was counted. Seed set was measured in terms of the proportions of capsules set and viable seeds per capsule. These data were collected for each female × male cross combination (cross-level data) for assessing seed set, and the subsequent seedlots (family-level data) were used to assess post-dispersal fitness.
Fitness trials
In the first week of May 1995, germination was undertaken in the glasshouse with seedlots sown into individual trays that were randomly allocated to 20 blocks by cross type, with each cross type being evenly represented in each block. Seedlings were pricked out into individual pots ∼14 d after germination and maintained in their original family-level block design in the glasshouse until early September 1995. In September they were moved outside for hardening for 1 month. The number of days to first germination, germination success (number of germinates/number of seed sown) and nursery survival from pricking out to 6 months were recorded for every seedling to detect any evidence of early age barriers to hybrid success. Families from the successful crosses were planted in a field trial at Tyenna (42°43′22″ S, 146°39′43″ E) in southern Tasmania. The trial site was a wet ex-native-forest site with a relatively poor mudstone-derived soil type, and was located at an altitude of ∼300 m. The site was chosen on the basis of being as close as possible to intermediate between the two pure species and is just below the transitional altitude above which E. nitens normally replaces E. globulus in commercial plantings in Tasmania (Wardlaw, 2011). The site preparation included rip mounding, and the trial was established in October 1995.
The experimental layout was a randomized block design with seven replicates. Individual seedlings within a family from a given cross type were randomly allocated to replicates, and then planted as single-tree plots randomized within each replicate. For some families, the number of plants was insufficient to allow a complete distribution across the available number of replicates, and thus the missing positions within replicates were filled with individuals from other seedlots of the same cross type. The number of families at planting was 125, with a total of 919 individuals (ranging from 84 to 202 per cross type). Tree spacing was 2·5×3·0 m.
The field trial was assessed at 2, 3, 4, 6, 10, 14 and 20 years of age. Over-bark diameter at breast height (DBH) and survival data up to 14 years have been reported in Costa e Silva et al. (2012). We report the results from the 20-year-old assessment of survival (dead/alive) from planting, DBH on surviving trees and the reproductive output [flower buds and capsules (woody fruit)] of surviving trees. The numbers of flower buds and capsules were quantified separately using a logarithmic categorical scale, where 0 = 0, 1= 1–10, 2 = 11–100; 3 = 101–1000 and 4 ≥1000 umbels (of flower buds or capsules) per tree. These reproductive and survival data were used to calculate, for each cross type in each replicate (1) the proportion of surviving trees from planting and (2) the proportion of survivors that were reproductive (trees with either flower buds or capsules).
Data analysis
A series of generalized linear models were used to analyse the variation between cross types in the following dependent variables: capsule set, number of seeds per capsule, time to first germination, germination success (first germination and proportion of seeds germinated) and nursery survival. These raw data were at the cross or family levels, as described above. For all these models, the linear predictor incorporated a single independent variable, i.e. a factor for cross type fitted as a fixed effect. Variation in capsule set between the cross types was assessed by assuming a Poisson distribution for the dependent variable and using a log link function to relate it to the linear predictor. The average number of viable seeds per capsule was used to determine variation in cross success; however, because these data were highly overdispersed, the analysis assumed a negative binomial distribution and applied a log link function. The number of days to first germination was fitted by assuming a Gaussian distribution and using an identity link function, while the proportions of seed germination and nursery survival were both analysed under the assumption of a binomial distribution and applying a logit link function.
Variation in flower morphology and pollen traits between E. globulus, E. nitens and their F1 hybrid was also compared by using general linear models. Separate models were fitted for pollen viability, pollen tube length, style length, receptacle width and disk depth. Single-factor models fitting cross type as a fixed effect were used for style length, receptacle width and disk depth. In addition, a random term for individual pollen nested within cross type was fitted for pollen viability and pollen tube length only. In order to optimize the homogeneity of variance and normality of the residuals, style length and receptacle width were transformed using a log base 10, and pollen viability was arcsin-transformed. For the remaining dependent variables, no significant deviations from normality were detected.
For all of the above analyses, the Tukey–Kramer post hoc test was used to adjust for multiple comparisons and extract significant differences between cross-type means. Contrasts between hybrids and mid-parent values were also estimated and tested. These models and tests were implemented using the PROC GLIMMIX of SAS (version 9.3; SAS Institute).
For the field trial measurements at 20 years from planting, the analysis of cross-type differences and the estimation of composite genetic effects followed the methodology described by Costa e Silva et al. (2012). The analyses used trait observations at the individual tree level (for DBH) or at the replicate level (for capsule score, as well as for the percentages of survival and of alive trees that were reproductive). The Shapiro and Wilk (1965) test and the examination of quantile–quantile plots indicated no important deviations from normality for the residuals obtained under preliminary analyses of the trait observations.
For the estimation of cross-type means and testing the significance of mean comparisons, a linear mixed model was fitted to the data, by using the cross-type factor as a fixed effect and replicates as a random effect (a random term for an interaction involving replicates and cross types could be fitted for DBH but was not statistically significant, and thus it was dropped from the model). In addition, under the individual tree model applied for DBH, a numerator relationship matrix (constructed from a pedigree file linking the progenies in the trial to their parents and grandparents; e.g. Henderson, 1984) could be used to account for the genetic covariance between the trial trees, thus (by creating ties among cross types) helping to increase the accuracy of their means and associated standard errors. Least-squares means (LSMs) were estimated for each cross type (in accordance with the linear mixed modelling procedure) and specific contrasts were undertaken to compare the F1 and F2 LSMs to the LSMs of the parental species populations (hereafter termed ‘mid-parent value’). Statistical tests (t- or Wald F-tests) were carried out to test the significance of pairwise differences between cross-type LSMs or the significance of the observed deviations (i.e. heterosis or outbreeding depression) in the F1 and F2, with the computation of the denominator degrees of freedom being based on the approximation proposed by Kenward and Roger (1997).
Composite genetic effects were estimated on the basis of the two-locus genetic model proposed by Hill (1982) to evaluate the genetic architecture underlying phenotypic differentiation among populations for a trait. Under Hill’s model for line-cross analysis (Hill, 1982), the F2 is used as the reference population relative to which the following composite genetic effects are derived (from contrasts made between source genes): an additive effect α1 (i.e. the net difference between the additive effects of the genes in the two source populations); a dominance effect δ1 (i.e. the extent to which the genes in one source population tend on average to be dominant over those in the other source population); and epistatic effects (i.e. the net directional epistasis due to interactions between genes from the two source populations), which include additive × additive (α2), additive × dominance (α1δ1) and dominance × dominance (δ2) interaction terms [see also Lynch and Walsh, 1998 (Chapter 9); Fenster et al., 1997]. Under the linear mixed model defined by Costa e Silva et al. (2012), the vector of fixed effects included additive and non-additive composite genetic effects fitted as covariates, followed by a cross-type class variable that represented a lack-of-fit term used for testing hypotheses about the adequacy of the genetic model via a Wald-type F statistic. In this context, an additive-dominance model (i.e. fitting the α1 and δ1 parameters only) was found to be suitable as a base model in order to test for more complex genetic models involving epistatic terms. Two-locus (i.e. digenic) epistatic interaction parameters were then added sequentially to the base model, and genetic models involving different combinations of composite effects were compared for their adequacy on the basis of the significance of the lack-of-fit term. The sequence of the genetic models fitted is given in Supplementary Data Table S1. In addition, calculated values and associated significance probabilities for a χ2-test statistic, computed under the joint-scaling test procedure described by Lynch and Walsh (1998, pages 216–217), are also given for comparison, with both of these testing approaches resulting in similar conclusions in terms of model adequacy to explain the data (Table S1).
For all the analyses involving field trial measurements, the heterogeneity of residual variances amongst cross types was explicitly incorporated in the linear mixed model (Costa e Silva et al., 2012) and variance parameters were estimated by restricted maximum likelihood. All of the model parameters and their associated approximate standard errors were estimated by using the ASReml software (Gilmour et al., 2014).
Reproductive isolation (RI) between E. nitens and E. globulus was calculated following Sobel and Chen (2014) using the formula RI5 = 1 – relative fitness (where relative fitness is absolute fitness of hybrids/absolute fitness of pure crosses). This produces a metric of barrier strength, which ranges from −1 (complete heterospecific mating) to 1 (complete reproductive isolation), with the intermediate value 0 corresponding to random mating. We calculated RI5 for: (1) the post-mating pre-dispersal barrier seed set, which assesses reproductive barriers operating between pollination and seed maturity, and so includes a pre-zygotic component; and (2) all significant post-mating post-dispersal barriers, including 6-month nursery survival, 20-year field survival and reproductive capacity. Total post-mating isolation was calculated using the formula RItotal = 1 − (total pre-dispersal fitness × total post-dispersal fitness), with all total estimates generated by multiplicatively combining individual barrier estimates. All RI calculations were undertaken in both directions for both first- and second-generation (backcross) hybrids. An estimate of the total strength of post-mating barriers to the production of reproductively fit backcross hybrids was calculated for each species by using the formula RItotalBC = 1 − [(likelihood of F1 formation) × (total pre-dispersal BC fitness × total post-dispersal BC fitness)], where the likelihood of F1 formation = 1 − total post-mating isolation for the F1 generation.
RESULTS
Inheritance of F1 hybrid floral morphology and pollen viability and growth
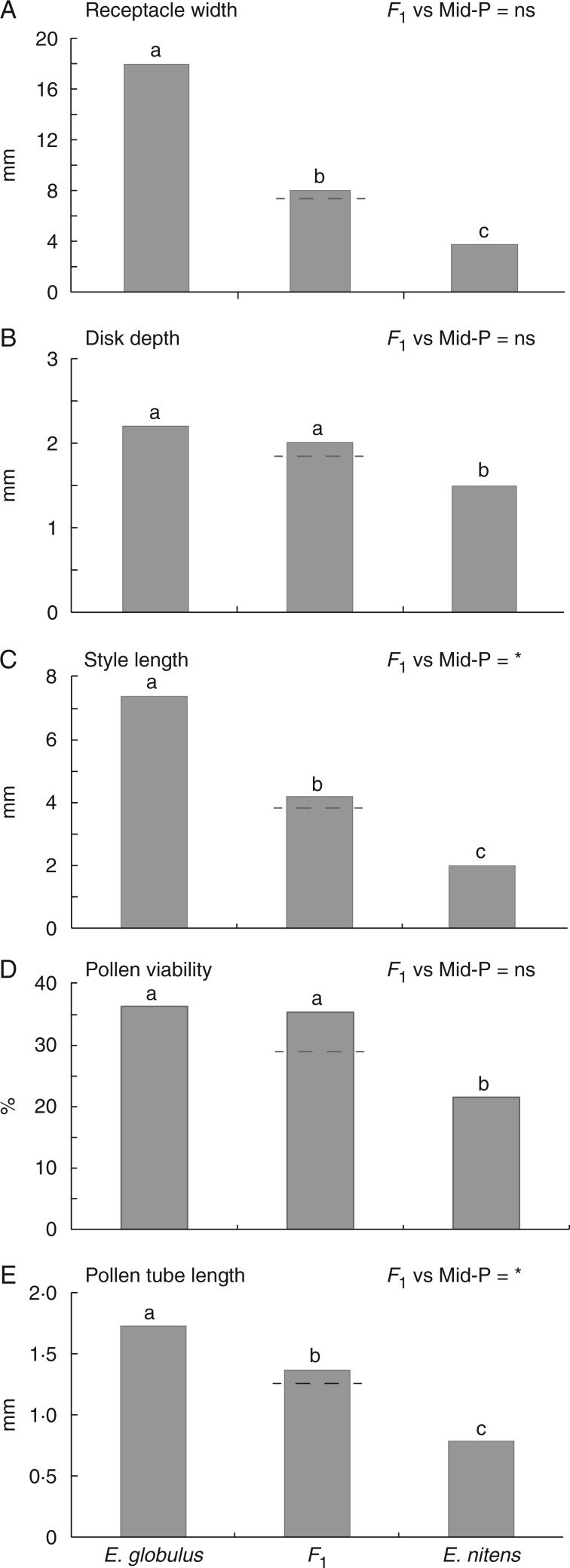
Floral characters that differentiate and contribute to reproductive isolation between E. globulus and E. nitens were inherited in a more or less intermediate manner in the F1 hybrids (Fig. 2). Intermediate inheritance was clearly evident in terms of receptacle width and disk depth (Fig. 2A, B). Despite marginal evidence for values above mid-parent values in style length and in vitro pollen tube growth in the F1 on the transformed scale, the traits were generally intermediate (Fig. 2C, E). In vitro pollen viability in the F1 was also more similar to E. globulus on the transformed scale, but did not differ significantly from the mid-parent value (Fig. 2D). Receptacle width and disk depth are both likely to be positively correlated with nectar production and therefore influence the class of pollinator (i.e. bird or insect) attracted to the flowers, whereas pollen viability, style length and pollen tube growth will affect post-mating cross success. Importantly we found no evidence of trait values below the mid-parent value in terms of floral morphology and pollen viability and growth, which could act as a mechanical barrier to hybridization in the F1. Additionally, bud number in the F1 hybrid inflorescence was more or less intermediate, with a modal number of 3 (for 20 observed trees), whereas in E. globulus and E. nitens the modal number of buds was 1 and 7, respectively (each with 20 observed trees).
Fig. 2.
Floral characteristics of E. globulus, E. nitens and their F1 hybrid, including mean receptacle width (A), disk depth (B), style length (C), in vitro pollen viability (D) and in vitro pollen tube length (E). The dashed line shows the mean mid-parent (Mid-P) value for comparison with the F1. The significance of specific contrasts comparing the F1 with the mid-parent value is shown: ***P < 0·001; **P < 0·01; *P < 0·05; ns (not significant) P > 0·05. Letters above the bars show Tukey contrasts, where different letters represent significant differences at the P < 0·001 level for receptacle width, style length and pollen tube length, and the P < 0·01 level for disk depth and pollen viability. Note that the statistical tests for style length and receptacle width were calculated on log (base 10)-transformed data, while pollen viability was arcsin-transformed, but the cross-type means and mid-parent values are presented for the back-transformed data. The other variables did not require transformation.
Barriers to seed set
The more or less intermediate inheritance of style length and pollen tube length in the F1 hybrids was sufficient to result in the loss of the unilateral mechanical barrier that prevents crossing between E. globulus (female parent) and E. nitens (pollen parent). The F1 pollen successfully pollinated E. globulus and E. nitens flowers, and E. globulus and E. nitens pollen successfully pollinated flowers of the F1 (Fig. 3A). There was no significant difference in capsule set among cross types [F(7,181) = 0·52, P = 0·815], but a highly significant difference in the number of viable seeds per capsule [F(7,76) = 4·21, P < 0·001]. This difference was mainly a female effect, reflecting the greater number of seeds per capsule in the larger E. globulus than the smaller E. nitens capsule. Seed set from the F1 hybrid females was intermediate and, whether tested as the mother or the pollen parent, the F1s were not significantly different from the mid-parent value on the transformed scales (P < 0·05). While there was no significant difference detected within the female cross types, the previously observed trend for reduced seed set following pollination of E. nitens flowers with E. globulus pollen was still evident (Fig. 3A).
Fig. 3.
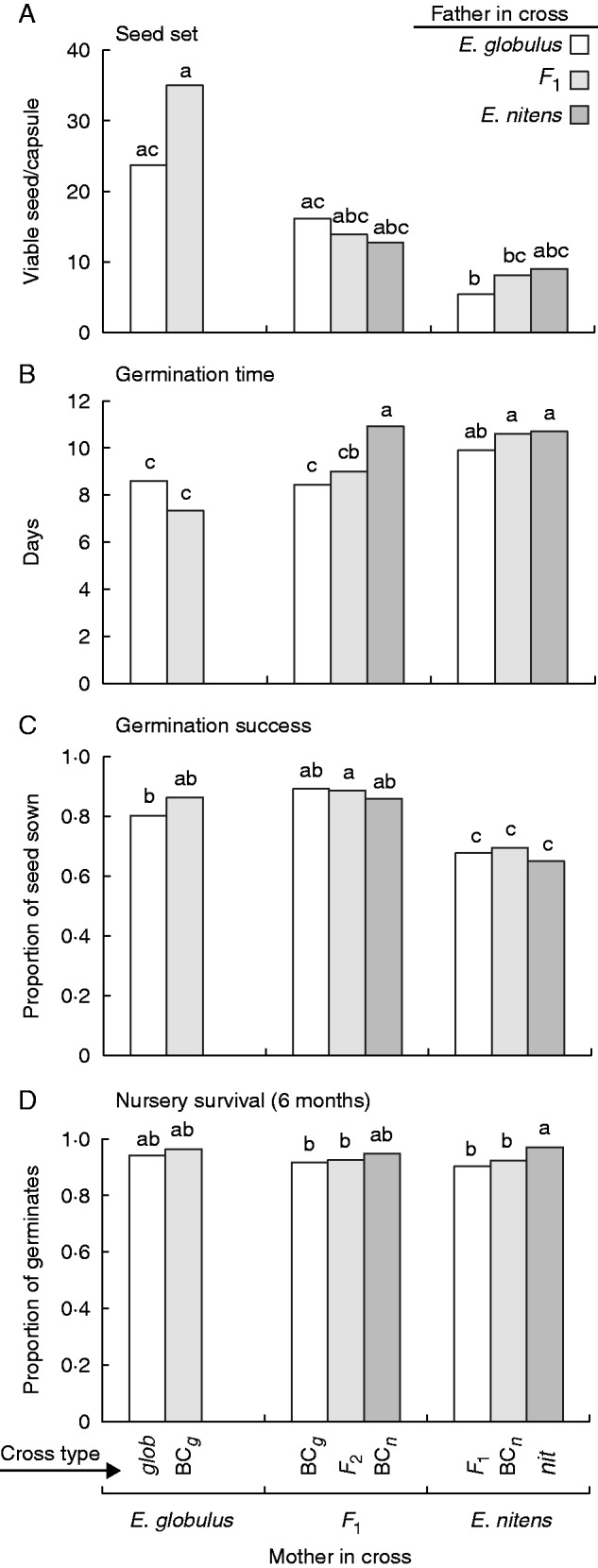
The effect of early-acting post-mating barriers to hybridization in all possible cross combinations between E. globulus (glob), E. nitens (nit) and their F1 hybrid. Barriers assessed were (A) seed set, (B) time to first germination, (C) germination success (number of germinates/number of seeds sown) and (D) survival of pot-grown germinates after 6 months. Cross types with different letters were significantly different at P < 0·05 based on the Tukey–Kramer adjustment for multiple comparisons. Note that the unilateral mechanical barrier to zygote formation between E. nitens (pollen) and E. globulus (flowers) was lost in the F1, i.e. crosses in both directions involving the F1 were successful. There was also no evidence of reduced intrinsic post-zygotic fitness in seed produced from hybrid mothers or fathers in these early life history stages.
Early post-dispersal barriers: 0–6 months of age
We found no evidence for barriers to hybridization in the early life-history stages. In terms of the time to first germination (days), germination success (number of germinates/number of seeds sown) and nursery survival after 6 months, the F1, F2 and BC hybrids showed no signs of lower than mid-parent fitness (Fig. 3B–D). In fact, there was possible evidence of heterosis. In the F1 mother, the BCg and the F2 were both significantly higher than the mid-parent value for germination success [F(1,132) = 40·28, P < 0·001 and F(1,132) = 14·66, P < 0·001, respectively; Fig. 3C], and the BCg germinated more quickly than the mid-parent rate [F(1,128) = 7·65, P = 0·007; Fig. 3B]. There was also one example of possible heterosis arising from the hybrid pollen, which was a marginally significant decrease in seed germination time in the F2 in comparison with the average of BCg and BCn in the F1 mother [F(1,128) = 4·65, P = 0·033; Fig. 3B]. However, it is not clear whether faster seed germination would have fitness benefits. Interestingly though, the earlier germination of E. globulus seed compared with E. nitens seed in the females was also evident when their pollen was crossed with the F1 female (Fig. 3B). This suggests that the difference in germination rate is not driven by a maternal effect, but probably has a nuclear genetic basis.
Later post-dispersal barriers: 1–20 years of age
Hybrid fitness.
In contrast to the nursery results, at 20 years post-planting there was clear evidence for reduced fitness in the hybrid classes. Survival, growth (DBH) and reproductive capacity were all significantly reduced in the F1, F2 and BC generations when compared with the pure E. globulus and E. nitens (Fig. 4). In all cases, F1 and F2 performance was significantly (P < 0·01) below the mid-parent value, with deviations ranging from 27·0 to 69·1 % of the mid-parent value (Table 1). Thus, not only was the poor hybrid fitness evident in survival, but the surviving hybrids had poorer growth, suggesting ongoing selection against the hybrid genotypes, as well as reduced reproductive capacity. Fewer than 30 % of the hybrids planted were reproductive at 20 years of age (F1, 16 %; F2, 10 %; BCg, 20 %; BCn, 29 %) compared with ≥ 60 % of the pure species (E. globulus, 61 %; E. nitens, 60 %). This equates to the integrated relative fitness of the hybrid cross types being between 0·1 and 0·6 of E. globulus (F1, 0·22; F2, 0·10; BCg, 0·46; BCn, 0·49) and E. nitens (F1, 0·27; F2, 0·11; BCg, 0·55; BCn, 0·59).
Fig. 4.
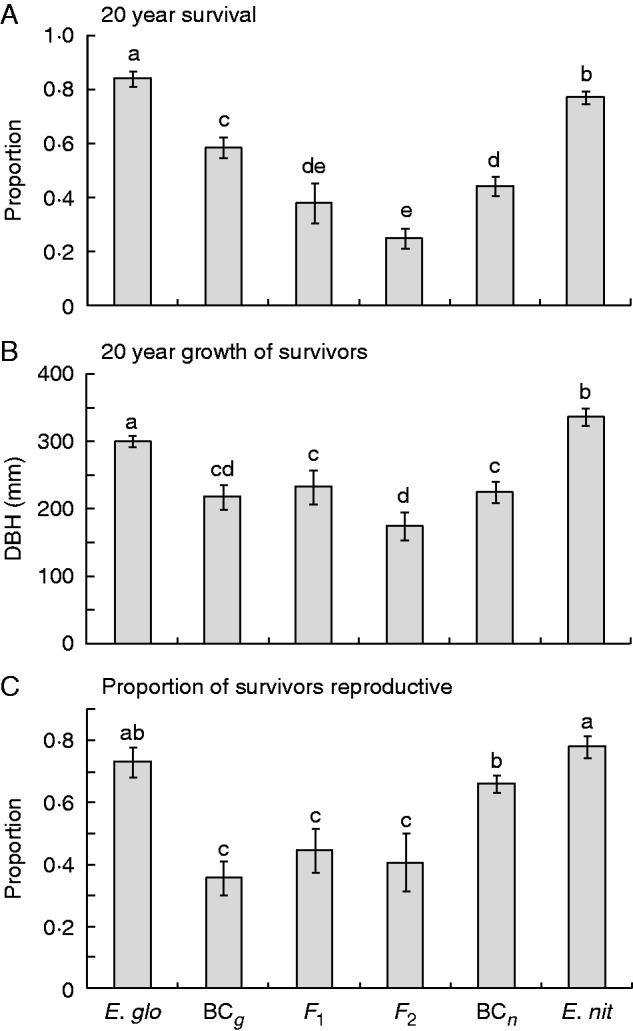
(A) Survival from planting, (B) DBH growth of survivors and (C) proportion of survivors that were reproductive in E. globulus (E. glo), E. nitens (E. nit) and their F1, F2 and backcross hybrid generations at 20 years from planting.
Table 1.
Observed outbreeding depression (± estimated standard errors) and corresponding significance probability in the F1 () and F2 () generations of E. globulus × E. nitens hybrids. Measured traits in the Tyenna trial at age 20 years from planting include percentage of tree survival, diameter at breast height (DBH) growth, percentage of alive trees that are reproductive, and capsule score for the survivors
| Percentage of tree survival (%) | DBH growth (cm) | Percentage of alive trees that are reproductive | Capsule score (score) | |
|---|---|---|---|---|
|
|
|
|
|
|
|
|
|
In parentheses, the H values are also expressed as a percentage of the mean of the parental populations (mid-parent value) to facilitate comparison of outbreeding depression across different traits [as well as for comparison with results at previous ages reported for DBH and tree survival by Costa e Silva et al. (2012)]. For all traits, both of the F1 and the F2 hybrid generation differed significantly (at the 5 % level) from either of the two parental populations (Fig. 4).
Composite genetic effects
Composite genetic effects contributing to species divergence were adequately predicted with the two-locus genetic models for growth and reproductive traits (Table 2) but not for survival (Table S1). Epistatic effects were the most important composite genetic effects for the DBH growth and reproduction of survivors (Table 2). This was particularly the case for the estimated additive × additive effects () for all of these measured components of fitness, although the estimated additive × dominance () component appeared to be also important for both of the reproduction traits. The estimation of reflects differences between parental and non-parental (hybrid) combinations of non-allelic gene pairs. Therefore, if is positive there is a net detrimental effect on hybrid fitness for the measured trait due to additive × additive epistatic interactions among genes from different parental populations (for a detailed explanation see Costa e Silva et al., 2012, page 254). The significant estimated additive effects () reflected the greater composite contribution of E. nitens genes to the DBH growth and reproduction of survivors. An adequate model could not be achieved for percentage of tree survival, as there was still a statistically significant lack of fit regardless of which digenic epistatic combinations were fitted (Table S1). This indicates that there was a significant discrepancy between the observed cross-type means and the corresponding estimates obtained under the fitted genetic model, which suggests that species differentiation for survival may involve significant contributions from higher-order epistatic interactions (i.e. involving more than pairs of loci). Although the results for composite genetic effects are not reported for survival, the estimates we have obtained at age 20 years showed a similar trend to previous results reported for this trait up to age 6 years (the age until which the genetic model seemed to fit the data) by Costa e Silva et al. (2012). Specifically, the magnitude (in absolute value) of unfavourable effects we have obtained at age 20 years was substantially (6-fold) higher than the beneficial effects (data not shown), which is in line with the observed tendency in previous results indicating that the difference between these two composite genetic parameters increased over time (Costa e Silva et al., 2012).
Table 2.
Estimates of composite genetic effects and their statistical significance for the percentage of survival and, for survivors, growth in diameter at breast height (DBH), percentage reproductive and capsule score, measured at age 20 years from planting
| Genetic effects | DBH growth (cm) | Percentage of alive trees that are reproductive (%) | Capsule score (0–4 scale) |
|---|---|---|---|
|
|
|
|
|
|
|
|
|
|
|
|
| * | * | * | |
| * |
|
|
Estimates of composite genetic effects pertain to: additive () and dominance () effects, and additive × additive (), dominance × dominance () and additive × dominance () epistatic effects.
Composite parameter estimates were based on the genetic model that was considered to be the most adequate to explain population differentiation (i.e. the most parsimonious genetic model that led to the largest reduction in statistical significance of the lack of fit, to a value not significant at the 10 % level; see Table S1). *Composite effect not included in the model selected to explain the genetic basis of population divergence (as indicated in Table S1).
Estimates of composite effects, their standard errors and significance tests using a Wald-type F statistic were obtained after removing the non-significant lack-of-fit term from the mixed model. Note that reflects the change in genotypic value associated with differences between the parental and non-parental combinations of non-allelic gene pairs. Therefore, a positive value for indicates reduced hybrid fitness [for a detailed explanation see Costa e Silva et al. (2012), 254 ].
Overall strength of reproductive barriers
The strength of reproductive isolation showed the clear loss of the strong pre-zygotic barrier in E. globulus, with the barrier to seed set being 1 for the F1 generation and −0·17 for the BC generation. In fact, there was effective random mating in terms of post-mating pre-dispersal barriers in the BC generation, with E. nitens showing a barrier of just 0·01. The post-mating post-dispersal barriers were generally stronger, with reproductive capacity being the strongest single barrier in both the F1 and BC generations (Table 3). With the exception of E. globulus in the F1, total post-dispersal barriers were much stronger than total pre-dispersal barriers. When assessing the total cumulative effect of all isolation stages, the barrier to the production of reproductively fit BC individuals (our integrated estimate of gene flow potential across both F1 and BC generations) was 0·98 for E. globulus and 0·99 for E. nitens.
Table 3.
Estimates of total post-mating reproductive isolation (RI5; Sobel and Chen, 2014) between E. globulus and E. nitens for both first- and second-generation hybrids relative to the pure species. Estimates are given for: post-mating pre-dispersal, which assesses mechanical barriers to zygote formation and early intrinsic post-zygotic barriers between pollination and seed maturity; post-mating post-dispersal, which assess early- and late-acting intrinsic post-zygotic barriers, from seed germination through reproductive capacity at 20 years of age (the proportion of survivors that are reproductive); total post-dispersal; and total post-mating isolation
|
F1 generation |
BC generation |
|||
|---|---|---|---|---|
| Isolation stage and barriers assessed | E. globulus | E. nitens | E. globulus | E. nitens |
| Post-mating, pre-dispersal | ||||
| Seed set (total pre-dispersal) | 1 | 0·39 | −0·17 | 0·01 |
| Post-mating, post-dispersal | ||||
| 6-month nursery survival | 0·04 | 0·07 | 0·08 | 0·28 |
| 20-year field survival | 0·56 | 0·53 | 0·30 | 0·43 |
| Reproductive capacity | 0·74 | 0·74 | 0·67 | 0·52 |
| Total post-dispersal | 0·89 | 0·88 | 0·79 | 0·81 |
| Total post-mating isolation | 1 | 0·93 | 0·75 | 0·81 |
DISCUSSION
Speciation can be thought of as a continuum between panmictic populations and reproductively isolated, irreversibly diverged taxa (species) (Seehausen et al., 2014). Consequently, the timing and importance of reproductive barriers along this continuum are central to understanding how speciation advances (Seehausen et al., 2014). The most recent dated molecular phylogeny puts the divergence of E. globulus and E. nitens at between 5 and 6 million years (Thornhill et al., 2015). Therefore, the present study illustrates a point along the speciation continuum between two well-diverged taxa, which have yet to achieve complete reproductive isolation. We made three major findings regarding the persistence, action and relative importance of reproductive barriers in this system. Firstly, we found that intermediate inheritance of floral traits means that strong mechanical barriers to zygote formation between E. nitens (pollen) and E. globulus (flowers) are lost after the first generation of hybridization. Secondly, there was no obvious gamete or early-acting post-zygotic barriers to hybridization between the species in terms of pollen viability, seed set, germination or nursery survival at 67 months of age. Thirdly, there were clear later age barriers to hybrid fitness in terms of survival, growth and reproductive capacity at 20 years of age, consistent with a model of epistatic loss [i.e. detrimental effects caused by epistatic incompatibilities and/or disruption of co-adapted complexes (Lynch and Walsh, 1998; Fenster et al., 1997)] in the hybrid classes.
Loss of mechanical barriers to zygote formation
Pre-zygotic barriers are often found to be responsible for most reproductive isolation between pairs of taxa. For example, in a meta-analysis, Lowry et al. (2008a) found that in 19 pairs of angiosperm species, pre-zygotic barriers were twice as strong on average as post-zygotic barriers. Nevertheless, these barriers are notoriously ‘leaky’ (Widmer et al., 2009) and F1s are regularly found in wild populations where congeneric species co-occur (Mallet, 2007). This is certainly the case in Eucalyptus (Pryor and Johnson, 1981), where F1 hybrids are often found in open-pollinated seed and established along species boundaries (Griffin et al., 1988; Potts et al., 2003). The key issue for speciation is the extent to which species integrity is maintained in the presence of low-level F1 hybridization (Lindtke et al., 2014; Christe et al., 2016). This will in part depend on whether these hybrids facilitate backcrossing and act as conduits for gene flow and introgression between the species. The current study shows that multiple mechanical barriers to zygote formation are weakened in F1 hybrids through the more or less additive inheritance of reproductive traits, providing a potential pathway for an escalation in gene flow. We show that the F1 phenotype increases the likelihood of pollen transfer, post-mating pollination success, and thus backcross hybridization (see discussion below), forming a ‘bridge’ for gene flow between the otherwise well-isolated parents (Grant, 1993). Although the concept of hybrids providing a bridge for gene flow is well established (Levin, 1978a; Rieseberg et al., 1993), as far as we can see the issue has received surprisingly little empirical attention (but see Grant, 1993; Broyles, 2002 Stoepler et al., 2012; Lindtke et al., 2014), despite the fact that this could at least partly explain introgression in the face of strong barriers to gene flow (Arnold, 1992; Yatabe et al., 2007; Christe et al., 2016), as is predicted by theory (Chan and Levin, 2005).
Pre-zygotic barriers between E. globulus and E. nitens are strong, and appear to be operating at both pre- and post-mating stages. The species often have overlapping flowering times where they co-occur (Lopez et al., 2000a; Barbour et al., 2006b). However, different pollinator guilds are likely to be a key premating barrier limiting interspecific pollination. It is estimated that the large E. globulus flowers produce around 100 times more nectar per day than the small E. nitens flowers (Hingston et al., 2004b). Consequently, outcrossing in E. globulus appears to be mostly due to birds rather than insects (Hingston et al., 2004b), while E. nitens is exclusively insect-pollinated (Hingston et al., 2004a). Therefore, the intermediate inheritance of traits associated with nectar production in the F1 (receptacle width and disk depth) is likely to reduce this barrier. Studies in wild systems have shown overlap between parental pollinator communities and hybrids leading to effective backcrossing (Campbell et al., 1998; Broyles, 2002; Stoepler et al., 2012). However, in the current study barriers may still be asymmetrical. Insect pollinators are likely to facilitate backcrossing with both species, whereas bird pollination would be biased toward backcrossing with E. globulus (Hingston et al., 2004a). It is unlikely that birds would discriminate against the hybrids as one of the principal bird pollinators of E. globulus, the endangered swift parrot (Hingston et al., 2004b), is also know to feed on E. ovata (Brereton et al., 2005), which has flowers of similar size and dimensions to the F1 here (Centre for Plant Biodiversity Research, 2006). This hypothesized pollinator-mediated breakdown of premating barriers in the F1 would see a transfer of the defence against introgression to post-mating mechanisms.
Mechanical size incompatibility between the pollen of one species and the style of another has long been recognized as a strong post-mating reproductive barrier in plants (Levin, 1978b; Grant, 1994), which in the present case is clearly asymmetrical (Gore et al., 1990). However, there are surprisingly few studies that quantify the potential loss of this barrier due to the inheritance of floral characteristics in F1 hybrids (Grant, 1993). Floral morphology can be inherited in a dominant manner (Rieseberg et al., 1993; Bradshaw et al., 1995), which would favour asymmetrical backcrossing. However, as in the present study, intermediate inheritance of floral traits is often reported (Grant, 1993; Bradshaw et al., 1995). Here we have found that very strong (asymmetrical) mechanical barriers to zygote formation between the pure species appear to have been lost due to the more or less intermediate inheritance in the F1, and this is likely to allow backcrossing in both directions. It is possible that competition between the shorter F1 and the longer E. globulus pollen tubes may still constitute some barrier to backcrossing towards E. globulus in the wild (Campbell et al., 2003). However, we suspect that any such barrier will be of minor importance in preventing gene flow. There is no evidence for pollen completion (between self and outcrossed pollen) in E. globulus or E. nitens (Pound et al., 2003a, b), and we found that F1 viability and pollen tube length both actually tended towards E. globulus values (Fig. 2D, E). Furthermore, there are likely to be few barriers in the reverse cross, where E. globulus pollinates the F1 flower. The demographic rarity of F1s (Mallet, 2005) also means that this cross (where the F1 acts as the mother) will probably be the most important for generating initial backcrosses to E. globulus. Thus, the intermediate inheritance of floral morphology is likely to result in a major reduction in the strength of asymmetrical mechanical isolation in the F1, allowing gene flow to occur in both directions.
In eucalypts, the mechanical style length barrier is not restricted to the E. nitens × E. globulus cross, but has been shown to extend to a range of other eucalypt species native to Tasmania (Larcombe et al., 2016). Such flower size variation is also thought to have contributed to patterns of reticulate evolution, whereby small-flowered Tasmanian eucalypt species appear to have been sinks for introgression from larger-flowered species (McKinnon et al., 2004a, b). Therefore, by revealing the ephemeral nature of this asymmetrical flower size barrier, our study provides a new perspective on the hybridization dynamics of this system, as well as an empirical example of the loss of mechanical barriers to zygote formation due to hybridization.
Absence of pollen inviability and early-acting post-zygotic barriers
We found no evidence of reduced viability of the F1 pollen. Post-mating hybrid incompatibilities are often detected as a reduction in F1 pollen viability (Ramsey et al., 2003; Archibald et al., 2005; Scopece et al., 2008). This is likely to be associated with differences in chromosomal architecture (chromosomal translocations, fusions, fissions and inversions) between the parental species that disrupt normal chromosome pairing in the F1, resulting in the production of F1 gametes that lack genes needed for normal functioning (King, 1995; Rieseberg, 2001). Such rearrangements have a more pronounced effect on F1 fertility in plants than in animals because more genes are expressed in pollen than in sperm, and seem to be more evident in male than female gametes due to complex interactions between the pollen and maternal tissues (Rieseberg, 2001; Scopece et al., 2008). Therefore, in the present study, the high fitness of F1 pollen argues against the existence of major chromosomal rearrangements in E. globulus and E. nitens. This is consistent with E. globulus and E. nitens having the same chromosome count (n = 11; Grattapaglia et al., 2012) and with mapping and genomic studies that indicate high genome synteny and co-linearity in Eucalyptus, even in phylogenetically wider crosses between E. grandis and E. globulus, which occur in different taxonomic sections (Myburg et al., 2004; Hudson et al., 2012).
We found no evidence of early-age intrinsic post-zygotic barriers operating from seed germination up to 6 months of age in the nursery. This is consistent with results from a previous study of F1 hybridization involving E. globulus (crossed with E. ovata), which found no evidence of early age reduction in hybrid survival or growth (Lopez et al., 2000b). However, early-acting post-zygotic barriers have been reported in other eucalypts (Potts and Dungey, 2004), including at the embryonic stage (Sedgley and Granger, 1996; Dickinson et al., 2012b), and it is possible that we have underestimated these barriers in the current study. We assessed early-age hybrid fitness in the benign nursery environment, and this probably underestimates early age selection, which has been shown to act against eucalypt hybrids in nature (Barbour et al., 2006a). Additionally, while not scored in the present study, previous work has shown that E. nitens × E. globulus F1s have higher frequencies of abnormal phenotypes than the parental species (Potts and Dungey, 2004). The frequency of such abnormal F1 phenotypes is higher still in phylogenetically wider eucalypt crosses (Potts and Dungey, 2004), and they are probably the first signal of the severe hybrid breakdown evident in later stages of the life cycle and later generations.
Strong later-acting post-zygotic barriers
There is little doubt that strong post-zygotic barriers to gene flow operate in F1 hybrids in this system, which will to some extent counteract the more ephemeral mechanical barriers to zygote formation between the species. These post-zygotic barriers appear to be endogenous, because previous studies have shown that the reduced hybrid vigour occurs across multiple sites (Tibbits, 2000; Volker et al., 2008; Costa e Silva et al., 2012), which is in stark contrast to the heterosis observed in inter-provenance crosses in E. globulus (Costa e Silva et al., 2014). While some F1 hybrids did survive in the current study, reproductive fitness was significantly reduced in subsequent generations, meaning that the combined effect of multiple post-zygotic barriers results in a marked reduction in Darwinian fitness of the hybrids. As with previous results obtained for survival and growth (Costa e Silva et al., 2012), our line-cross analysis argues that this reduction of reproductive capacity in the survivors is a reflection of epistatic loss, and thus outbreeding depression. This model implies a mode of evolution that is consistent with Dobzhansky–Muller incompatibilities (Lynch and Walsh, 1998). In fact, if ecological differences alone were operating on hybrid fitness (i.e. genetic incompatibilities were not affecting fitness), then the F1 hybrids might be expected to perform better than the parents in an intermediate habitat (Abbott et al., 2013). Therefore, the poor F1 performance found here on an intermediate trial site may be further evidence of Dobzhansky–Muller-type incompatibilities (see also the discussion in Costa e Silva et al., 2012, page 261). However, it is extremely difficult to distinguish between the effects of chromosomal rearrangements on hybrid inviability from those of genes/Dobzhansky–Muller-type processors (Rieseberg, 2001). Indeed, in Eucalyptus a dynamic picture of genome evolution is emerging, particularly with respect to gene duplications and transposable elements (Myburg et al., 2014).
Although we cannot dismiss the possibility that small chromosomal rearrangements contributed to the outbreeding depression observed in this study [including differences in gene duplication, and position (Myburg et al., 2014)], we believe it is unlikely that large karyotype differences are driving the effect. Firstly, as discussed above, we found no evidence of F1 pollen inviability, as would be expected if large chromosomal rearrangements existed (Rieseberg, 2001). Secondly, a related expectation is that reduced hybrid vigour (growth and survival) associated with large chromosomal rearrangements is unlikely to be expressed until the F2 generation (King, 1995). This is because underdominance caused by differences in chromosome arrangement in the F1 are masked by heterozygosity (King, 1995). Contrary to this, we found a large reduction in the fitness of both the F1 and the F2 generation, which is more consistent with a model of epistatic loss due to many small genetic changes (Seehausen et al., 2014). Thirdly, this is the latest in a series of studies – using both modelling of hybrid compatibility (Costa e Silva et al., 2012; Larcombe et al., 2015) and genomic mapping approaches (Hudson et al., 2012; Myburg et al., 2004) – that suggest that post-zygotic reproductive isolation in Eucalyptus results from the build-up of co-adapted complexes within species and small genetic changes between the genomes of diverging taxa. The significant lack of fit in models for survival in the current study indicates that the disruption of co-adapted complexes may extend beyond pairwise epistatic interactions, and, in line with previous studies (see Fig. 1 in Costa e Silva et al. 2012), the magnitude and complexity of these deleterious effects appear to increase with age.
Persistence and relative importance of reproductive barriers
There is no doubt that in many systems pre-zygotic barriers account for more reproductive isolation than post-zygotic barriers in the first generation. For example, in sympatric situations pollinator fidelity accounted for 97 % of the total reproductive isolation between Mimulus lewisii and Mimulus cardinalis (Ramsey et al., 2003). Similar findings have been reported from other Mimulus species (Martin and Willis, 2007; Lowry et al., 2008b) and a range of other taxa (Grant, 1994; Grant and Grant, 1964; Kay, 2006). Indeed, there is a strong mechanical pre-zygotic barrier between E. globulus (flowers) and E. nitens (pollen) in the first generation. However, the persistence of such mechanical barriers in later generations is rarely quantified, and if such barriers are easily lost in F1s, as we have shown here, then their contribution to reproductive isolation is greatly diminished wherever F1 hybridization can occur. There are a small number of observational (Grant, 1993) and empirical (Broyles, 2002; Stoepler et al., 2012) studies that investigate the effect of pollinator overlap between hybrids and pure species as a pathway to gene flow. Additionally, Field et al. (2010) discuss the potential effect of intermediate style length in F1 hybrids creating a bridge for gene flow between Eucalyptus aggregata and Eucalyptus rubida, but do not quantify the existence or loss of the barrier. Here we clearly documented the loss of mechanical barriers to zygote formation in E. globulus × E. nitens F1 hybrids. Consequently, intrinsic post-zygotic barriers become more important for preventing gene flow in this system. This underappreciated process highlights the probable importance of persistent post-zygotic barriers in maintaining species boundaries in zones of secondary contact. Interestingly, recent genomic analyses of naturally occurring hybrid zones in Populus came to the same conclusion about the importance of intrinsic post-zygotic barriers in maintaining species boundaries (Lindtke et al., 2014; Christe et al., 2016). Given the opposite trends found in many herbaceous systems (Lowry et al., 2008a), these early but consistent results coming from trees (including the current work) highlight the importance of studying organisms with diverse life history strategies.
Exotic gene flow from E. nitens in Tasmania
The loss of the strong mechanical barriers to zygote formation in E. globulus has practical implications for minimizing unwanted gene flow from E. nitens plantations in Tasmania. Because post-zygotic barriers are incomplete in this system, wherever F1 hybrids occur (from E. globulus pollen) between E. nitens plantations and native E. globulus populations there is a possibility of gene flow. In fact, because E. globulus is pollinated by both insects and birds, backcrossing towards E. globulus may be favoured, essentially reversing the asymmetry observed in compatibility from the first generation. Furthermore, style length has been used in management protocols to estimate the genetic risk posed by E. nitens to all other compatible Tasmanian eucalypt species (Roberts et al., 2009). Therefore, these protocols should recognize the transient nature of the style length barrier after the first generation. Nevertheless, style length is only one component of genetic risk assessment, which requires a detailed understanding of a range of other barriers (Potts et al., 2003).
Conclusion
We have presented a 20-year study that assesses reproductive isolation across multiple hybrid generations in a forest tree system. One salient feature of the study was strongly contrasting results in the 6-month compared with the 20-year assessments of hybrid fitness, demonstrating the value of such long-term datasets. We found that intermediate inheritance of floral morphology results in the loss of strong asymmetrical barriers to gene flow between E. globulus and E. nitens following the first generation of hybridization. This enables the F1 to act as a bridge for pollen/gene flow between the taxa. The bridging of mechanical barriers to zygote formation by F1 hybrids in models of reproductive isolation appears to be an underappreciated phenomenon. Although there were few early-acting post-zygotic barriers to hybrid fitness, 20-year-old survival, growth and reproductive capacity were strongly reduced in the hybrid classes including the F2 and BC generations, suggesting that intrinsic post-zygotic barriers are more persistent than mechanical barriers to zygote formation in this system. Our line-cross analysis indicated that this post-zygotic effect is likely to be a result of outbreeding depression due to epistatic loss in the hybrids. Therefore, where F1 hybridization is possible in areas of secondary contact between these species, it will be intrinsic post-zygotic barriers that contribute most meaningfully to the maintenance of species boundaries. This is in line with other recent studies in forest trees, but in contrast to most previous evidence from herbaceous systems.
SUPPLEMENTARY DATA
Supplementary data are available online at www.aob.oxfordjournal.org and consist of Table S1: results of testing hypothesis about the adequacy of alternative genetic models fitted sequentially to breast-height diameter (DBH) growth, percentage of tree survival, percentage of alive trees that are reproductive and capsule score, measured in the Tyenna trial at age 20 years from field planting
ACKNOWLEDGEMENTS
We thank the many people who assisted with nursery and field work (including Hugh Fitzgerald), Peter Volker for his initial hybridization work, which allowed the second-generation crossing to be undertaken, Robert Wiltshire for his exceptional photographs, and two anonymous reviewers of the manuscript for their helpful comments. We thank Norske Skog for provision of the trial site. Funding for crossing and trial establishment was provided by the CRC for Temperate Hardwood Forestry. Trial measurements were supported by the CRC for Sustainable Production Forestry as well as an Australian Research Council Linkage grant (LP0884001) partnered by the Southern Tree Breeding Association and seedEnergy Pty Ltd. The financial support given to João Costa e Silva by Fundação para a Ciência e a Tecnologia (Lisboa, Portugal), through the Programa Operacional Potencial Humano and the European Social Fund, is gratefully acknowledged. This, in combination with Australian Research Council Discovery grants (DP130104220 and DP160101650) to B.M.P., provided the opportunity to complete this long-term study.
REFERENCES
- Abbott R, Albach D, Ansell S, et al. 2013. Hybridization and speciation. Journal of Evolutionary Biology 26: 229–246. [DOI] [PubMed] [Google Scholar]
- Archibald JK, Mort ME, Crawford DJ, Kelly JK. 2005. Life history affects the evolution of reproductive isolation among species of Coreopsis (Asteraceae). Evolution 59: 2362–2369. [PubMed] [Google Scholar]
- Arnold ML. 1992. Natural hybridisation as an evolutionary process. Annual Review of Ecology and Systematics 23: 237–261. [Google Scholar]
- Arnold ML, Martin NH. 2010. Hybrid fitness across time and habitats. Trends in Ecology & Evolution 25: 530–536. [DOI] [PubMed] [Google Scholar]
- Baack E, Melo MC, Rieseberg LH, Ortiz-Barrientos D. 2015. The origins of reproductive isolation in plants. New Phytologist 207: 968–984. [DOI] [PubMed] [Google Scholar]
- Barbour RC, Potts BM, Vaillancourt RE. 2005a. Gene flow between introduced and native Eucalyptus species: crossability of native Tasmanian species with exotic E. nitens. Australian Journal of Botany 53: 465–477. [Google Scholar]
- Barbour RC, Potts BM, Vaillancourt RE. 2005b. Pollen dispersal from exotic eucalypt plantations. Conservation Genetics 6: 253–257. [Google Scholar]
- Barbour RC, Potts BM, Vaillancourt RE. 2006a. Gene flow between introduced and native Eucalyptus species: early-age selection limits invasive capacity of exotic E. ovata x nitens F1 hybrids. Forest Ecology and Management 228: 206–214. [Google Scholar]
- Barbour RC, Potts BM, Vaillancourt RE, Tibbits WN. 2006b. Gene flow between introduced and native Eucalyptus species: flowering asynchrony as a barrier to F1 hybridisation between exotic E. nitens and native Tasmanian Symphyomyrtus species. Forest Ecology and Management 226: 9–21. [Google Scholar]
- Bradshaw H, Jr, Wilbert M, Otto K. 1995. Genetic mapping of floral traits associated with reproductive isolation in monkeyflowers (Mimulus). Nature 376: 31. [Google Scholar]
- Brereton R, Mallick SA, Kennedy SJ. 2005. Foraging preferences of swift parrots on Tasmanian blue-gum: tree size, flowering frequency and flowering intensity. Emu 104: 377–383. [Google Scholar]
- Broyles SB. 2002. Hybrid bridges to gene flow: a case study in milkweeds (Asclepias). Evolution 56: 1943–1953. [DOI] [PubMed] [Google Scholar]
- Campbell D, Alarcon R, Wu C. 2003. Reproductive isolation and hybrid pollen disadvantage in Ipomopsis. Journal of Evolutionary Biology 16: 536–540. [DOI] [PubMed] [Google Scholar]
- Campbell DR, Aldridge G. 2006. Floral biology of hybrid zones In: Harder L, Barrett S, eds. Ecology and evolution of flowers. Oxford: Oxford University Press, 326–345. [Google Scholar]
- Campbell DR, Waser NM, Wolf PG. 1998. Pollen transfer by natural hybrids and parental species in an Ipomopsis hybrid zone. Evolution 52: 1602–1611. [DOI] [PubMed] [Google Scholar]
- Centre for Plant Biodiversity Research. 2006. EUCLID: eucalypts of Australia, 3rd edn (DVD) Canberra: CSIRO Publishing. [Google Scholar]
- Chan K, Levin SA. 2005. Leaky prezygotic isolation and porous genomes: rapid introgression of maternally inherited DNA. Evolution 59: 720–729. [PubMed] [Google Scholar]
- Christe C, Stölting KN, Bresadola L, et al. 2016. Selection against recombinant hybrids maintains reproductive isolation in hybridizing Populus species despite F1 fertility and recurrent gene flow. Molecular Ecology 25: 2482–2498. [DOI] [PubMed] [Google Scholar]
- Costa e Silva J, Potts BM, Tilyard P. 2012. Epistasis causes outbreeding depression in eucalypt hybrids. Tree Genetics & Genomes 8: 249–265. [Google Scholar]
- Costa e Silva J, Potts BM, Lopez GA. 2014. Heterosis may result in selection favouring the products of long-distance pollen dispersal in Eucalyptus. PLoS One 9: e93811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyne JA, Orr AH. 2004. Speciation. Sunderland, MA: Sinauer Associates. [Google Scholar]
- Dickinson G, Wallace H, Lee D. 2012a. Reciprocal and advanced generation hybrids between Corymbia citriodora and C. torelliana: forestry breeding and the risk of gene flow. Annals of Forest Science 70: 1–10. [Google Scholar]
- Dickinson GR, Lee DJ, Wallace HM. 2012b. The influence of pre- and post-zygotic barriers on interspecific Corymbia hybridization. Annals of Botany 109: 1215–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobzhansky T, Dobzhansky TG. 1937. Genetics and the origin of species. New York: Columbia University Press. [Google Scholar]
- Dutkowski GW, Potts BM. 1999. Geographic patterns of genetic variation in Eucalyptus globulus ssp. globulus and a revised racial classification. Australian Journal of Botany 47: 237–263. [Google Scholar]
- Ellis MF, Sedgley M, Gardner JA. 1991. Interspecific pollen-pistil interaction in Eucalyptus L'Her. (Myrtaceae): the effect of taxonomic distance. Annals of Botany 68: 185–194. [Google Scholar]
- Ellstrand NC, Elam DR. 1993. Population genetic consequences of small population-size – implications for plant conservation. Annual Review of Ecology and Systematics 24: 217–242. [Google Scholar]
- Fenster CB, Galloway LF, Chao L. 1997. Epistasis and its consequences for the evolution of natural populations. Trends in Ecology & Evolution 12: 282–286. [DOI] [PubMed] [Google Scholar]
- Field D, Ayre D, Whelan R, Young A. 2010. Patterns of hybridization and asymmetrical gene flow in hybrid zones of the rare Eucalyptus aggregata and common E. rubida. Heredity 106: 841–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmour AR, Gogel BJ, Cullis BR, Welham SJ, Thompson R. 2014. ASReml user guide release 4.0. Hemel Hempstead, UK: VSN International. [Google Scholar]
- Gore PL, Potts BM, Volker PW, Megalos J. 1990. Unilateral cross-incompatibility in Eucalyptus: the case of hybridisation between E. globulus and E. nitens. Australian Journal of Botany 38: 383–394. [Google Scholar]
- Grant KA, Grant V. 1964. Mechanical isolation of Salvia apiana and Salvia mellifera (Labiatae). Evolution 18: 196–212. [Google Scholar]
- Grant V. 1992. Floral isolation between ornithophilous and sphingophilous species of Ipomopsis and Aquilegia. Proceedings of the National Academy of Sciences of the USA 89: 11828–11831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant V. 1993. Effects of hybridization and selection on floral isolation. Proceedings of the National Academy of Sciences of the USA 90: 990–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant V. 1994. Modes and origins of mechanical and ethological isolation in angiosperms. Proceedings of the National Academy of Sciences of the USA 91: 3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grattapaglia D, Vaillancourt RE, Shepherd M, et al. 2012. Progress in Myrtaceae genetics and genomics: Eucalyptus as the pivotal genus. Tree Genetics & Genomes 8: 463–508. [Google Scholar]
- Griffin AR, Burgess IP, Wolf L. 1988. Patterns of natural and manipulated hybridization in the genus Eucalyptus L'Herit.: a review. Australian Journal of Botany 36: 41–66. [Google Scholar]
- Guo YY, Luo YB, Liu ZJ, Wang XQ. 2015. Reticulate evolution and sea‐level fluctuations together drove species diversification of slipper orchids (Paphiopedilum) in South‐East Asia. Molecular Ecology 24: 2838–2855. [DOI] [PubMed] [Google Scholar]
- Hamilton MG, Dutkowski GW, Joyce KR, Potts BM. 2011. Meta-analysis of racial variation in Eucalyptus nitens and E. denticulata. New Zealand Journal of Forestry Science 41: 217–230. [Google Scholar]
- Hardner CM, Potts BM. 1995. Inbreeding depression and changes in variation after selfing Eucalyptus globulus subsp. globulus. Silvae Genetica 44: 46–54. [Google Scholar]
- Henderson CR. 1984. Applications of linear models in animal breeding. Guelph: University of Guelph. [Google Scholar]
- Hendry A, Bolnick D, Berner D, Peichel C. 2009. Along the speciation continuum in sticklebacks. Journal of Fish Biology 75: 2000–2036. [DOI] [PubMed] [Google Scholar]
- Hewitt G. 2000. The genetic legacy of the Quaternary ice ages. Nature 405: 907–913. [DOI] [PubMed] [Google Scholar]
- Hewitt GM. 2001. Speciation, hybrid zones and phylogeography - or seeing genes in space and time. Molecular Ecology 10: 537–549. [DOI] [PubMed] [Google Scholar]
- Hill WG. 1982. Dominance and epistasis as components of heterosis. Zeitschrift für Tierzüchtung und Züchtungsbiologie 99: 161–168. [Google Scholar]
- Hingston AB, McQuillan PB, Potts BM. 2004a. Pollinators in seed orchards of Eucalyptus nitens (Myrtaceae). Australian Journal of Botany 52: 209–222. [Google Scholar]
- Hingston AB, Potts BM, McQuillan PB. 2004b. The swift parrot Lathamus discolor (Psittacidae), social bees (Apidae) and native insects as pollinators of Eucalyptus globulus ssp. globulus (Myrtaceae). Australian Journal of Botany 52: 371–379. [Google Scholar]
- Hudson CJ, Kullan AR, Freeman JS, et al. 2012. High synteny and colinearity among Eucalyptus genomes revealed by high-density comparative genetic mapping. Tree Genetics & Genomes 8: 339–352. [Google Scholar]
- Kay KM. 2006. Reproductive isolation between two closely related hummingbird pollinated neotropical gingers. Evolution 60: 538–552. [PubMed] [Google Scholar]
- Kenward MG, Roger JH. 1997. Small sample inference for fixed effects from restricted maximum likelihood. Biometrics 53: 983–997. [PubMed] [Google Scholar]
- King M. 1995. Species evolution: the role of chromosome change. Cambridge: Cambridge University Press. [Google Scholar]
- Larcombe MJ, Barbour RC, Vaillancourt RE, Potts BM. 2014. Assessing the risk of exotic gene flow from Eucalyptus globulus plantations to native E. ovata forests. Forest Ecology and Management 312: 193–202. [Google Scholar]
- Larcombe MJ, Holland B, Steane DA, et al. 2015. Patterns of reproductive isolation in Eucalyptus – a phylogenetic perspective. Molecular Biology and Evolution 32: 1833–1846. [DOI] [PubMed] [Google Scholar]
- Larcombe MJ, Barbour RC, Jones RC, Vaillancourt RE, Potts BM. 2016. Postmating barriers to hybridization between an island’s native eucalypts and an introduced congener. Tree Genetics & Genomes 12: 1–11. [Google Scholar]
- Levin DA. 1978a. Hybridization: an evolutionary perspective In: Jameson DL, ed. Benchmark papers in genetics. Stroudsberg, PA: Dowden, Hutchinson & Ross. [Google Scholar]
- Levin DA. 1978b. The origin of isolating mechanisms in plants. Evolutionary Biology 11: 185–313. [Google Scholar]
- Lindtke D, Gompert Z, Lexer C, Buerkle CA. 2014. Unexpected ancestry of Populus seedlings from a hybrid zone implies a large role for postzygotic selection in the maintenance of species. Molecular Ecology 23: 4316–4330. [DOI] [PubMed] [Google Scholar]
- Lopez GA, Potts BM, Gore PL. 2000a. The inheritance of flowering time in interspecific F1 hybrids of Eucalyptus In: Dungey HS, Dieters MJ, Nikles DG, eds. Hybrid breeding and genetics of forest trees. Proceedings of QFRI/CRC-SPF Symposium, 9–14th April 2000, Noosa, Queensland, Australia. Brisbane: Department of Primary Industries, 453–546. [Google Scholar]
- Lopez GA, Potts BM, Tilyard PA. 2000b. F1 hybrid inviability in Eucalyptus: the case of E. ovata x E. globulus. Heredity 85: 242–250. [DOI] [PubMed] [Google Scholar]
- Lowry DB, Modliszewski JL, Wright KM, Wu CA, Willis JH. 2008a. The strength and genetic basis of reproductive isolating barriers in flowering plants. Philosophical Transactions of the Royal Society B: Biological Sciences 363: 3009–3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry DB, Rockwood RC, Willis JH. 2008b. Ecological reproductive isolation of coast and inland races of Mimulus guttatus. Evolution 62: 2196–2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch M, Walsh B. 1998. Genetics and analysis of quantitative traits. Sunderland, MA: Sinauer Associates. [Google Scholar]
- Mallet J. 2005. Hybridization as an invasion of the genome. Trends in Ecology & Evolution 20: 229–237. [DOI] [PubMed] [Google Scholar]
- Mallet J. 2007. Hybrid speciation. Nature 446: 279–283. [DOI] [PubMed] [Google Scholar]
- Martin NH, Willis JH. 2007. Ecological divergence associated with mating system causes nearly complete reproductive isolation between sympatric Mimulus species. Evolution 61: 68–82. [DOI] [PubMed] [Google Scholar]
- McKinnon GE, Jordan GJ, Vaillancourt RE, Steane DA, Potts BM. 2004a. Glacial refugia and reticulate evolution: the case of the Tasmanian eucalypts. Philosophical Transactions of the Royal Society of London B: Biological Sciences 359: 275–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinnon GE, Vaillancourt RE, Steane DA, Potts BM. 2004b. The rare silver gum, Eucalyptus cordata, is leaving its trace in the organellar gene pool of Eucalyptus globulus. Molecular Ecology 13: 3751–3762. [DOI] [PubMed] [Google Scholar]
- McKinnon GE, Vaillancourt RE, Steane DA, Potts BM. 2008. An AFLP marker approach to lower-level systematics in Eucalyptus (Myrtaceae). American Journal of Botany 95: 368–380. [DOI] [PubMed] [Google Scholar]
- Moyle LC, Nakazato T. 2010. Hybrid incompatibility “snowballs” between Solanum species. Science 329: 1521–1523. [DOI] [PubMed] [Google Scholar]
- Muller H. 1942. Isolating mechanisms, evolution and temperature. Biological Symposia 6: 71–125. [Google Scholar]
- Myburg AA, Vogl C, Griffin AR, Sederoff RR, Whetten RW. 2004. Genetics of postzygotic isolation in Eucalyptus: whole-genome analysis of barriers to introgression in a wide interspecific cross of Eucalyptus grandis and E. globulus. Genetics 166: 1405–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myburg AA, Grattapaglia D, Tuskan GA, et al. 2014. The genome of Eucalyptus grandis. Nature 510: 356–362. [DOI] [PubMed] [Google Scholar]
- Nicolle D. 2015. Classification of the eucalypts (Angophora, Corymbia and Eucalyptus) Version 2 http://www.dn.com.au/Classification-Of-TheEucalypts.pdf.
- Noor MA, Feder JL. 2006. Speciation genetics: evolving approaches. Nature Reviews Genetics 7: 851–861. [DOI] [PubMed] [Google Scholar]
- Nosil P, Harmon LJ, Seehausen O. 2009. Ecological explanations for (incomplete) speciation. Trends in Ecology & Evolution 24: 145–156. [DOI] [PubMed] [Google Scholar]
- Potts BM, Dungey HS. 2004. Interspecific hybridization of Eucalyptus: key issues for breeders and geneticists. New Forests 27: 115–138. [Google Scholar]
- Potts BM, Marsden-Smedley JB. 1989. In vitro germination of Eucalyptus pollen: response to variation in boric acid and sucrose. Australian Journal of Botany 37: 429–441. [Google Scholar]
- Potts BM, Barbour RC, Hingston A, Vaillancourt RE. 2003. Turner Review No. 6: Genetic pollution of native eucalypt gene pools - identifying the risks. Australian Journal of Botany 51: 1–25. [Google Scholar]
- Pound LM, Patterson B, Wallwork MAB, Potts BM, Sedgley M. 2003a. Pollen competition does not affect the success of self-pollination in Eucalyptus globulus (Myrtaceae). Australian Journal of Botany 51: 189–195. [Google Scholar]
- Pound LM, Wallwork MAB, Potts BM, Sedgley M. 2003b. Pollen tube growth and early ovule development following self- and cross-pollination of Eucalyptus nitens. Sexual Plant Reproduction 16: 59–69. [Google Scholar]
- Pryor LD, Johnson LAS. 1981. Eucalyptus, the universal Australian. In: Keast A, ed. Ecological biogeography of Australia. The Hague: W. Junk, 499–536. [Google Scholar]
- Ramsey J, Bradshaw H, Schemske DW. 2003. Components of reproductive isolation between the monkeyflowers Mimulus lewisii and M. cardinalis (Phrymaceae). Evolution 57: 1520–1534. [DOI] [PubMed] [Google Scholar]
- Rice WR, Hostert EE. 1993. Laboratory experiments on speciation: what have we learned in 40 years? Evolution 47: 1637–1653. [DOI] [PubMed] [Google Scholar]
- Rieseberg LH. 2001. Chromosomal rearrangements and speciation. Trends in Ecology & Evolution 16: 351–358. [DOI] [PubMed] [Google Scholar]
- Rieseberg L, Ellstrand N, Arnold M. 1993. What can molecular and morphological markers tell us about plant hybridization? Critical Reviews in Plant Sciences 12: 213–241. [Google Scholar]
- Rieseberg LH, Raymond O, Rosenthal DM, et al. 2003. Major ecological transitions in wild sunflowers facilitated by hybridization. Science 301: 1211–1216. [DOI] [PubMed] [Google Scholar]
- Roberts N, Barbour RC, Duncan F. 2009. Management of gene flow from plantation eucalypt species Flora Technical Note 12. Hobart, Tasmania: Forest Practices Authority. [Google Scholar]
- Schemske DW. 2000. Understanding the origin of species. Evolution 54: 1069–1073. [Google Scholar]
- Scopece G, Widmer A, Cozzolino S. 2008. Evolution of postzygotic reproductive isolation in a guild of deceptive orchids. American Naturalist 171: 315–326. [DOI] [PubMed] [Google Scholar]
- Sedgley M, Granger L. 1996. Embryology of Eucalyptus spathulata and E. platypus (Myrtaceae) following selfing, crossing and reciprocal interspecific pollination. Australian Journal of Botany 44: 661–671. [Google Scholar]
- Seehausen O, Butlin RK, Keller I, et al. 2014. Genomics and the origin of species. Nature Reviews Genetics 15: 176–192. [DOI] [PubMed] [Google Scholar]
- Shapiro SS, Wilk MB. 1965. An analysis of variance test for normality (complete samples). Biometrika 52: 591–611. [Google Scholar]
- Sobel JM, Chen GF. 2014. Unification of methods for estimating the strength of reproductive isolation. Evolution 68: 1511–1522. [DOI] [PubMed] [Google Scholar]
- Sobel JM, Streisfeld MA. 2015. Strong premating reproductive isolation drives incipient speciation in Mimulus aurantiacus. Evolution 69: 447–461. [DOI] [PubMed] [Google Scholar]
- Sobel JM, Chen GF, Watt LR, Schemske DW. 2010. The biology of speciation. Evolution 64: 295–315. [DOI] [PubMed] [Google Scholar]
- Steane DA, Nicolle D, Sansaloni CP, et al. 2011. Population genetic analysis and phylogeny reconstruction in Eucalyptus (Myrtaceae) using high-throughput, genome-wide genotyping. Molecular Phylogenetics and Evolution 59: 206–224. [DOI] [PubMed] [Google Scholar]
- Stoepler TM, Edge A, Steel A, O’Quinn RL, Fishbein M. 2012. Differential pollinator effectiveness and importance in a milkweed (Asclepias, Apocynaceae) hybrid zone. American Journal of Botany 99: 448–458. [DOI] [PubMed] [Google Scholar]
- Thornhill AH, Ho SY, Külheim C, Crisp MD. 2015. Interpreting the modern distribution of Myrtaceae using a dated molecular phylogeny. Molecular Phylogenetics and Evolution 93: 29–43. [DOI] [PubMed] [Google Scholar]
- Tibbits WN. 2000. Evaluation of hybrids with Eucalyptus nitens In: Dungey HS, Dieters MJ, Nikles DG, eds. Hybrid breeding and genetics of forest trees. Proceedings of QFRI/CRC-SPF Symposium, 9–14th April 2000, Noosa, Queensland, Australia. Brisbane: Department of Primary Industries, 363–372. [Google Scholar]
- Volker PW, Potts BM, Borralho NMG. 2008. Genetic parameters of intra- and inter-specific hybrids of Eucalyptus globulus and E. nitens. Tree Genetics & Genomes 4: 445–460. [Google Scholar]
- Wardlaw T. 2011. A climate analysis of the current and potential future Eucalyptus nitens and E. globulus plantation estate on Tasmanian State forest. Tasforests 19: 17–27. [Google Scholar]
- Widmer A, Lexer C, Cozzolino S. 2009. Evolution of reproductive isolation in plants. Heredity 102: 31–38. [DOI] [PubMed] [Google Scholar]
- Yatabe Y, Kane NC, Scotti-Saintagne C, Rieseberg LH. 2007. Rampant gene exchange across a strong reproductive barrier between the annual sunflowers, Helianthus annuus and H. petiolaris. Genetics 175: 1883–1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.