Abstract
Background and Aims Putative processes related to floral diversification and its relation to speciation are still largely unaccounted for in the Melastomataceae. Leandra s.str. is one of the most diverse lineages of the Neotropical Miconieae and ranks among the ten most diverse groups in the Atlantic Forest. Here, we describe the floral diversity of this lineage in a continuous framework and address several questions related to floral evolution and putative developmental and environmental constraints in its morphology.
Methods The morphological data set includes individual size measurements and shape scores (from elliptical Fourier analysis) for hypanthia, petals, stamens and styles. We evaluate whether there is evidence of correlation among these floral structures, shifts and convergent patterns, and association of these traits with elevation.
Key Results Leandra s.str. flower structures present a strong phylogenetic signal and tend to be conserved among close relatives. The extremes in flower regimes seem to be quite distinct, but non-overlapping discrete flower types are not observed. Overall, the morphology of Leandra s.str. floral structures is correlated, and anther colour and inflorescence architecture correlate with flower structures. Additionally, the rates of species diversification and morphological evolution are correlated in most clades.
Conclusions Although some flower regimes tend to occur in different elevational ranges, no significant association is observed. The general idea that hypanthium–ovary fusion is associated with fruit types in the Melastomataceae does not hold for Leandra s.str., where, instead, hypanthium–ovary fusion seems to be associated with anther shape. The lowest rate of flower morphological change, when compared with species diversification rates, is observed in the clade that possesses the most specialized flowers in the group. While stuck on a single general pollination system, Leandra s.str. seems to be greatly wandering around it, given the flower diversity and convergent patterns observed in this group.
Keywords: Anther, buzz-pollination, floral diversification, floral morphospace, hypanthium, Leandra, Melastomataceae, morphometrics, neotropical, petal, pollination syndrome
INTRODUCTION
Comparative studies in the mega-diverse Melastomataceae are quite sparse and biased towards structures traditionally used in the classification of the family. Some studies have explored the seed morphology (Whiffin and Tomb, 1972; Martin and Michelangeli, 2009; Ocampo and Almeda, 2013; Ocampo et al., 2014) and trichomes (Wurdack, 1986), and there has been a comprehensive study focused on fruit trait evolution (Clausing et al., 2000). Surprisingly, flowers have been scarcely studied in a comparative framework, although some general surveys have been published; noteworthy are the classical study of stamen vasculature by Wilson (1950), studies on nectar-producing flowers (Stein and Tobe, 1989; Varassin et al., 2008; Kriebel and Zumbado, 2014) and one study evaluating the relationship of anther morphology and seed number (Brito et al., 2016). Most Melastomataceae are characterized by poricidal anthers and the flowers are usually hermaphroditic and actinomorphic, but variable degrees of zygomorphy are also observed due to the positioning of the stamens and the style (Renner, 1989).
Some descriptive studies have suggested that floral evolution has been extensive in the Melastomataceae, and that it has probably been the result of adaptation to different pollination systems (Almeda, 1977). This perspective was later challenged by Renner (1989), while presenting a comprehensive compilation of known pollinators in the Melastomataceae. Renner (1989) argued that, when compared with other families, the Melastomataceae show little floral diversification and are likely stuck on an adaptive peak. This scenario has been proposed given that the great majority of Melastomataceae are buzz-pollinated by bees, with pollen offered as reward (Renner, 1989). Despite the fact that most Melastomataceae share a single general pollination syndrome (buzz-pollination) and some floral structures, especially the corolla, are conserved, a great array of different floral morphologies is observed across the family. The androecium is particularly variable in the Melastomataceae, where a great diversity of shapes, sizes, colours, different degrees of connective elongation, the presence or absence of different appendices, heteromorphy, and pore size, number and position are observed (Renner, 1989). Additionally, putative processes related to flower diversification and its relation to speciation are still largely undocumented in Melastomataceae. Some studies have focused on the relatively few cases where there is a general pollination syndrome transition in the family (Stein and Tobe, 1989; Varassin et al., 2008; Brito et al., 2016). It has been suggested that shifts from buzz-pollination to vertebrate pollination or a much more generalist insect syndrome could be associated with changes in elevation (Stein and Tobe, 1989; Varassin et al., 2008), given that bee pollination services decline along an altitudinal gradient (Arroyo et al., 1982). Such shifts could be interpreted as a response to pollinator unpredictability in these high-elevation habitats, and some morphological adaptations have been observed in the anthers and other flower structures of these species (Varassin et al., 2008). Overall, changes in elevation could be associated with flower/pollinator shifts in several ways. For instance, changes in pollinator assemblages, preferences, behaviour and perception might occur along an altitudinal gradient (for a short review see Koski and Ashman, 2015). In addition to the decrease in bee dominance and diversity with increasing elevation, bee communities might display changes in traits such as body size along an altitudinal gradient (Hoiss et al., 2012).
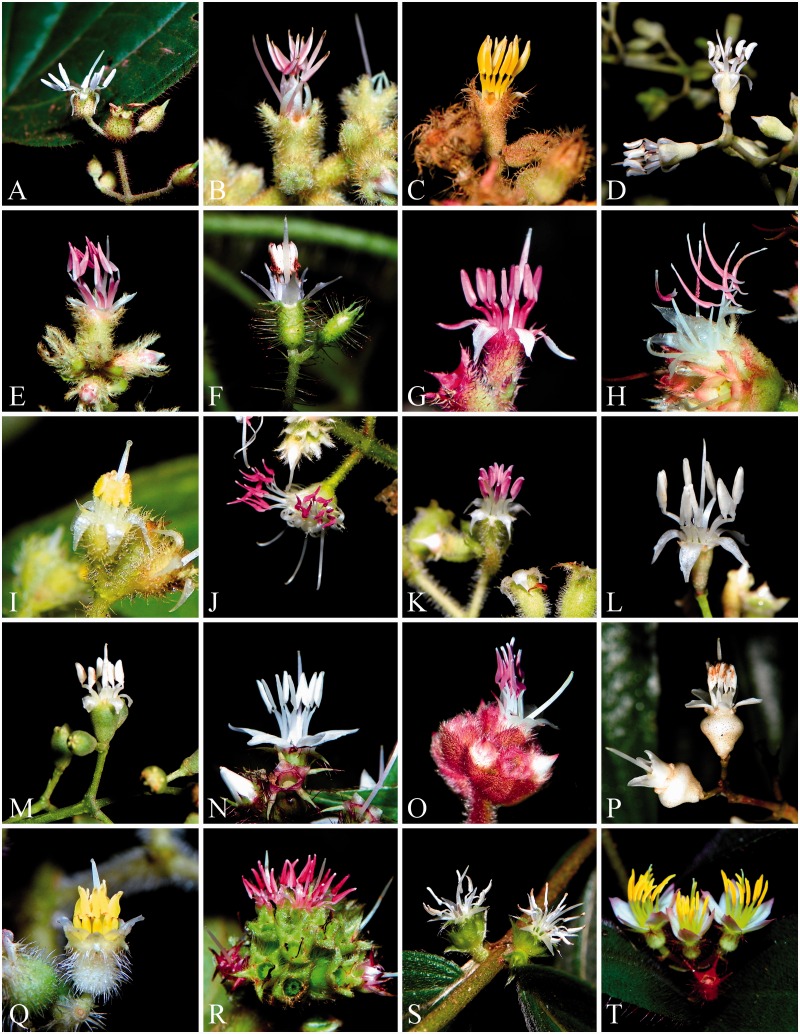
Leandra s.str. is part of the Neotropical Miconieae in the Melastomataceae and, with ∼200 species, it is one of the most diverse lineages in this tribe (Reginato and Michelangeli, 2016). The clade is almost exclusively restricted to eastern Brazil, with many individual species occurring as local endemics. Leandra s.str. ranks among the ten most diverse groups of plants in the Atlantic Forest (Stehmann et al., 2009), where most species are commonly found inside or at the edges of submontane or montane forests. Some species are also a conspicuous component of high-altitude vegetation (‘Campos de Altitude’), while others are found exclusively in the ‘Campos Rupestres’. The species are usually shrubs or occasionally treelets, and have berry fruits dispersed by birds. Leandra s.str. exhibits a great diversity of flowers (some examples are illustrated in Fig. 1), where different anther colours and shapes stand out. Additionally, ovary position ranges from totally inferior to fully superior, styles can be straight or sigmoid, surrounded by or opposite to the stamens, and hypanthium shape is highly variable. Some studies of reproductive biology in Melastomataceae included species of Leandra s.str. (Goldenberg and Shepherd, 1998; Goldenberg and Varassin, 2001), with an emphasis on their reproductive systems, but comparative morphological studies are lacking. Based on floral morphological observations, the species of Leandra s.str. are expected to be buzz-pollinated, but the pollinators are largely unknown. The only comprehensive summary of pollinators of Melastomataceae does not list any species from this clade (Renner, 1989). While very conspicuous morphological changes are observed in flowers of derived taxa in the Melastomataceae, which have departed from the buzz-pollination syndrome, such as in Brachyotum and Charianthus (Penneys and Judd, 2005; Varassin et al., 2008; Michelangeli et al., 2013), morphological changes on an exclusively buzz-pollinated lineage might be less obvious and have been overlooked in the family. The observed diversity of floral morphology and the presumed constant general pollination syndrome, along with a comprehensive phylogeny, make Leandra s.str. a good model to study floral evolution and its putative relationship to environmental and developmental constraints on the highly successful buzz-pollination system.
Fig. 1.
Examples of flowers of Leandra s.str. (clade name in parentheses). (A) Leandra adenothrix (Cerrado). (B) L. aurea (Carassanae). (C) L. australis (Carassanae). (D) L. barbinervis (Oxymeris). (E) L. carassana (Carassanae). (F) L. cardiophylla (Carassanae). (G) L. eichleri (Carassanae). (H) L. glazioviana (Leandraria). (I) L. hirtella (Oxymeris). (J) L. melastomoides (Leandraria). (K) L. purpureo-villosa (Oxymeris). (L) L. quinquedentata (Oxymeris). (M) L. quinquenodis (Oxymeris). (N) L. salicina (Capixabae). (O) L. sericea (Leandraria). (P) L. vesiculosa (Oxymeris). (Q) L. xanthostachya (Carassanae). (R) Ossaea congestiflora (Cerrado). (S) O. warmingiana (Cerrado). (T) Pleiochiton blepharodes (Pleiochiton).
Floral traits such as shape, size and colour, as well inflorescence architecture, are often under selection by different pollinators (Harder et al., 2004; Harder and Johnson, 2009). The coordinated functioning of pollination-related traits might enhance pollination, and selection therefore should favour stronger correlations between such characters (Stebbins, 1950; Berg, 1960; Brock and Weinig, 2007). We expect that, given the specialized pollination system found in the Leandra s.str. clade, correlation between flower structures will be observed. Additionally, given that some species are exclusively found at high or lower elevations, we hypothesize that altitudinal constraints in the distribution of pollinators would be reflected in the floral structures and regimes observed in Leandra s.str. We aimed to test these hypotheses by describing flower diversity in Leandra s.str. in a continuous and comparative framework. Other specific questions addressed in this study include whether flower morphology is conserved among close relatives and whether inflorescence architecture and anther colour are randomly distributed across different anther types. We then further discuss the putative significance of the observed patterns and differences in speciation/flower diversification rates across major clades of Leandra s.str.
MATERIALS AND METHODS
Phylogenetic hypothesis
The phylogenetic hypothesis presented by Reginato and Michelangeli (2016) was used to address the questions presented here. Information about voucher specimens included in the phylogenetic tree is available in Reginato and Michelangeli (2016). This phylogeny included 126 species of Leandra s.str. spanning the range of geographical distribution and morphological variation within the group. This number accounts for ∼60 % of the accepted species estimated for the clade. Overall, for most of the missing taxa the circumscriptions are not clear (i.e. species described in the 19th century and never the subject of a taxonomic review) and a morphologically close relative was sampled (Reginato and Michelangeli, 2016). The summary tree obtained by Reginato and Michelangeli (2016) was used for the analyses, but we pruned taxa for which flower material was not available (nine species), as well as all species included as outgroups. Clades are named following the informal scheme adopted in Reginato and Michelangeli (2016) and Fig. 3.
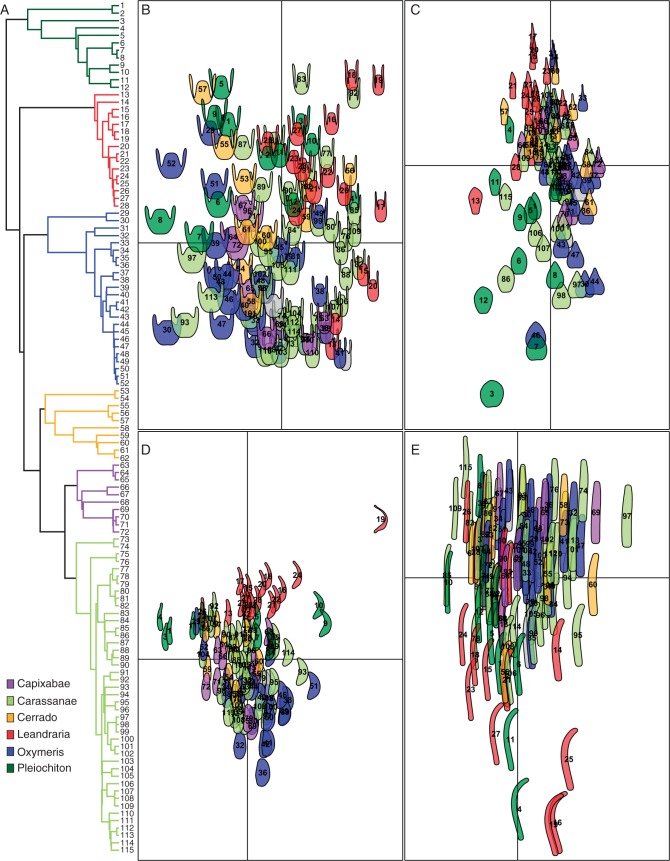
Fig. 3.
(A) Leandra s.str. phylogeny colour-coded by major clades. (B–E) Morphospaces of the flower structures from the elliptical Fourier analysis, first component is presented in the y axis and the second component in the x axis. (B) Hypanthia. (C) Petals. (D) Anthers. E. Styles. The morphospace colour scheme and labels (numbers) are shown in panel (A).
Morphological data
Flowers were obtained from herbarium specimens or fresh material fixed in the field in 50 % ethanol. The flowers were dissected and digitally imaged with a Nikon SMZ1500 stereoscope equipped with a Nikon DXM1200F camera. Floral traits were gathered for 117 species (one flower per species); voucher information and measurements are available in Supplementary Data Table S1. Most species of Leandra s.str. have isomorphic stamens, but some slightly heteromorphic stamens are also observed. In these cases, the larger stamens were used for the analyses. Measurements of the flower structures were taken from the images in the Fiji platform (Schindelin et al., 2012) and shape variables were retrieved by elliptical Fourier analysis. For this analysis, each structure was isolated and binarized in GIMP 2.8 (http://www.gimp.org) (Supplementary Data Figs S1–S4). The binary images were then read and processed in R (R Core Team, 2014) using the package Momocs (Bonhomme et al., 2014). Elliptical Fourier descriptors were calculated and summarized by a principal components analysis using the same R package. For each structure, the first two principal components were taken as continuous variables of shape diversity and included in further analyses. Additionally, two discrete characters were coded as follows: anther colour (white, 0; yellow, 1; pink, 3) and flowers in glomerules (absent, 0; present, 1).
Elevation
A collection database for species of Leandra s.str. was compiled using herbarium records and online data available at the biodiversity portals speciesLink (http://splink.cria.org.br/) and GBIF (http://www.gbif.org). The taxonomy of the specimens in the database was updated and the data were filtered in several ways. Distributional outlier records for each species were flagged as ‘taxonomy suspicious’ and specimens with longitude and latitude of the centroid of the municipality or with up to two decimal places were flagged as ‘coordinates suspicious’; neither set was considered further. The elevations reported for the remaining collections were tabulated for each species. Additionally, elevational data for each species were extracted from the elevation layer of the Bioclim data set (Hijmans et al., 2005). The records were intersected to the elevation layer using the R package raster (Hijmans, 2013) and added to the other values, and the mean for all species was calculated.
Ancestral character estimation
Phylogenetic signal was calculated for all variables using Pagel’s λ parameter (Pagel, 1999) implemented in the R package phytools (Revell, 2012). The characters were mapped on the phylogeny of Leandra s.str. and some are presented in the results. Ancestral character estimation for the continuous characters was performed using the function contMap in the R package phytools (Revell, 2012). This function estimates the ancestral states in each node using maximum likelihood techniques and interpolates the states along the edges, following Felsenstein (1985).
Factor analysis
The flower variables were summarized using a factor analysis for mixed data (FAMD) implemented in the R package FactoMineR (Lê et al., 2008). FAMD is a principal components method of exploring data with both continuous and categorical variables. It can be seen roughly as a combination of principal components analysis (continuous variables) and multiple correspondence analysis (categorical variables). In FAMD the continuous variables are scaled to unit variance and the categorical variables are transformed into a disjunctive data table (crisp coding) and then scaled using the specific scaling of MCA (Lê et al., 2008).
Flower morphological shifts and convergent patterns
Flower morphological regimes were evaluated using the method proposed by Ingram and Mahler (2013) implemented in the R package surface. The analysis uses the Hansen model of stabilizing selection around multiple adaptive peaks (Butler and King, 2004) to infer a macroevolutionary adaptive landscape using trait data and a phylogenetic tree. Extensive information about the method is given by Ingram and Mahler (2013), but a summary is provided here. The analysis is based on two stepwise Akaike information criterion (AIC) routine phases. In the first, it adds regime shifts to a Hansen model, the change in corrected AIC (ΔAICc) of each possible shift placement is calculated, and an updated Hansen model is returned with one shift added. This process is iterated until the model stops improving beyond a threshold ΔAICc. In the second phase, beginning with a fitted Hansen model produced by the first phase, it tests pairwise collapses of regimes and identifies collapses that improve the fit (convergent regimes). The process is repeated until the model stops improving beyond the given AIC threshold. In this fashion, convergent and unique regime shifts can be identified. For our analyses, the first two components of the factor analysis were used as the flower trait data. Default thresholds were applied and the different regimes were interpreted as flower types. It was our expectation that a change in flower regime/type could be coupled with a change in pollination syndrome, but in the absence of detailed pollinator behaviour and/or assemblages for these species, we have opted to constraint our discussion to the morphology.
Character associations
To quantify the strength of relationships among continuous flower variables and between these variables and elevation, the pgls method (Freckleton et al., 2002) was implemented in the R package caper (Orme et al., 2013). This method fits a linear model while controlling for the non-independence between the samples resulting from the phylogenetic structure in the data (Freckleton et al., 2002). The structure of the phylogenetic signal was controlled by optimizing the parameter λ using maximum likelihood. The P values were corrected using the Holm–Bonferroni method for multiple comparisons (Holm, 1979). Additionally, some continuous variables of interest were tested for differences among the discrete variables. For instance, we wanted to test whether there is any difference between the length of the anthers in the different colour states or elevation and flower types. This test was performed using a phylogenetic analysis of variance (ANOVA) (Garland et al., 1993) in the package phytools (Revell, 2012), with the post hoc comparison option enabled.
Flower diversification and speciation rates
Speciation rates for the major clades of Leandra s.str. were estimated using the method-of-moments estimator for crown groups (Magallón and Sanderson, 2001), implemented in the R package laser (Rabosky and Schliep, 2013), and assuming an equal rate of extinction across clades (0). Similarly, the first principal component (PC1) of the factor analyses was used as a proxy of flower morphology and the rates of morphological diversification were estimated across the same clades. The PC1 evolution model under a Brownian motion process was estimated, and the variance of the Brownian motion model was taken as the diversification rate (Ackerly, 2009). Rates of morphological evolution were calculated as net change in variance of log-normal-transformed trait values and the analysis was performed using the R package geiger (Harmon et al., 2008).
RESULTS
Flower morphospace and phylogenetic signal
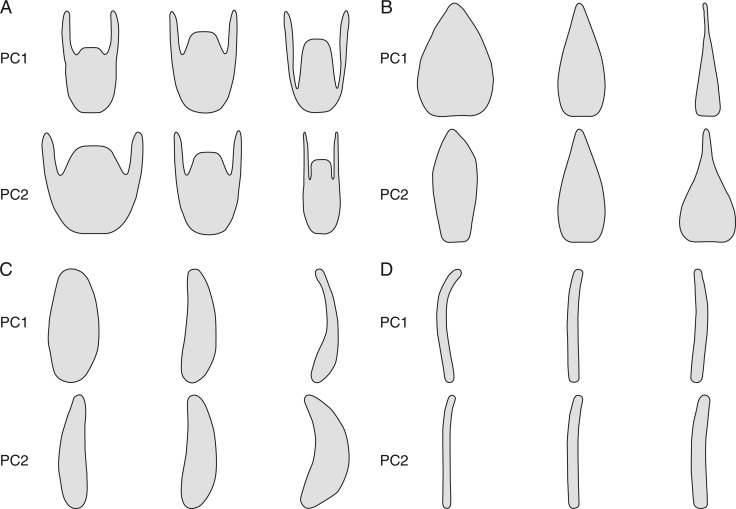
Elliptical Fourier analysis of flower structures in Leandra s.str. effectively captured most of the variation in the first components. The information summarized in the first three axes ranged from 87 % in the petals to 94 % in the styles, with 92 % in the anthers and 93 % in the hypanthia. In the hypanthia most of the variation related to the extent of ovary/hypanthium fusion (i.e. superior versus inferior ovary, PC1 58 % of the variation PC1 = 59 %) and whether they were narrowed versus wide (PC2 = 24 %). In the petals the first component also related to width (PC1 = 71 %) and the second component reflected whether the apex was acuminate or not (PC2 = 10 %). In the anthers the main variation was related to width and anther curvature was captured in the second component (from incurved to recurved, with straight anthers in the middle; PC2 = 28 %). For the styles the first component was related to the curvature at the apex (PC1 = 67 %) and also to width (PC2 = 16 %). The reconstruction of the shape variation in the first two axes for anthers, petals, hypanthia and styles is presented in Fig. 2.
Fig. 2.
First two components of the principal components analysis of elliptical Fourier descriptors of flower morphology in Leandra s.str. (A) Hypanthia. (B) Petals. (C) Anthers. (D) Styles.
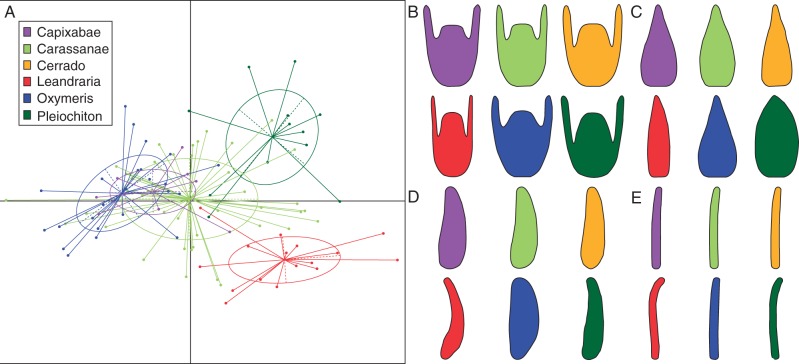
The morphospaces including the first two components of the four flower structures studied here are presented in Fig. 3, together with the Leandra s.str. phylogenetic hypothesis, where the major clades are colour–coded. The spaces of the structures do not present a strict differentiation among clades, with recurrent cases of species of different clades presenting the same shape. Although convergence seems pervasive, it can also be noted that members of the same clade tend to group together, indicating phylogenetic signal in the flower structures. Additionally, it is also evident that overlap among clades is extensive. Overall, the species of Leandraria and Pleiochiton seem to be more differentiated from the others, while Carassanae, Capixabae, Cerrado and Oxymeris usually overlap. Although these four clades overlap, Capixabae and Oxymeris form more cohesive groups in the morphospace, while Carassanae and Cerrado are usually found throughout the plot, indicating great variability within those groups. Remarkably, the anther morphospace seems to show the greatest phylogenetic structure (Fig. 3B), with Leandraria and Oxymeris exhibiting the two extremes of anther morphology in Leandra s.str. The mean shapes recovered in the first principal component of each structure in each clade are illustrated in Fig. 4B–E. In the anthers (Fig. 4B), Leandraria presented very subulate anthers, with Pleiochiton showing less pronounced subulate anthers, while Carassanae, Capixabae and Cerrado had intermediate anthers, and a more compact obovate anther was observed in Oxymeris. In the hypanthia, Leandraria and Pleiochiton showed a greater degree to which the ovaries were superior than the others. while the most inferior ovaries (degree) was found in Capixabae (Fig. 4C). Leandraria also presented the most tubular hypanthia (degree). The mean petal shapes of Carassanae, Capixabae, Cerrado and Oxymeris were very similar, while it was narrower in Leandraria and wider in Pleiochiton (Fig. 4D). The styles showed little variation, with most species of Leandraria and some Pleiochiton differentiated by the curved apex. Overall, the mean shape seemed to be a good representation for Leandraria, Pleiochiton and Capixabae. Nonetheless, in the groups with great variation, such as Carassanae and Cerrado, where extremes of variation were observed, the mean of the clade was very similar to the mean of the entire diversity.
Fig. 4.
(A) Factor analysis morphospace, first component is presented in the x axis and the second component in the y axis, 0.95 confidence ellipses by clade. (B–E) Mean shape (first component) of the four flower structures by clade. (B) Hypanthia. (C) Petals. (D) Anthers. E. Styles. Colours are explained in panel (A).
The factor analysis of all variables (including sizes, shape scores and discrete characters) accounted for 60 % of the variance in the first three dimensions (axis 1 = 38 %, axis 2 = 13 %). The contributions of each variable in the first three axes are presented in Table 1. The results of the factor analysis reinforced the patterns observed in the shape analyses of the individual structures. In Fig. 4A the first two axes are plotted, and show that Leandraria and Pleiochiton were morphologically more distinct from the others. The remaining clades overlapped extensively, with Oxymeris and Capixabae being more similar, while Cerrado and Carassanae were more widespread in the morphospace.
Table 1.
Factor analysis of variable contributions in the three first axes. The greatest contributions are in bold type
| Dimension |
|||
|---|---|---|---|
| 1 | 2 | 3 | |
| Anther shape PC1 | 10·33 | 4·28 | 5·11 |
| Anther shape PC2 | 0·03 | 0·24 | 34·80 |
| Hypanthium shape PC1 | 6·48 | 0·57 | 6·48 |
| Hypanthium shape PC2 | 2·56 | 8·47 | 2·22 |
| Petal shape PC1 | 0·28 | 25·68 | 0·02 |
| Petal shape PC2 | 6·09 | 0·12 | 8·20 |
| Style shape PC1 | 4·75 | 2·98 | 0·87 |
| Style shape PC2 | 4·77 | 0·18 | 7·26 |
| Anther length | 12·40 | 1·00 | 0·30 |
| Filament length | 12·93 | 0·21 | 0·03 |
| Hypanthium height | 9·53 | 1·50 | 9·79 |
| Hypanthium width | 4·18 | 11·30 | 14·80 |
| Petal length | 9·40 | 0·64 | 0·20 |
| Petal width | 5·21 | 18·08 | 0·02 |
| Anther colour | 5·20 | 13·62 | 8·72 |
| Glomerules | 5·85 | 11·12 | 1·19 |
Phylogenetic signal estimates for all variables, including the first three axes of the factor analysis, are provided in Supplementary Data Table S2. All variables presented some phylogenetic signal, with the factor analysis axes showing the greatest values. Among the other variables, the first axis of hypanthium shape, the filament length and the first axis of anther shape presented the highest signals. The only variables that did not show a significant phylogenetic signal were the second axis of the style shape and hypanthium width.
Flower morphological shifts and convergent patterns
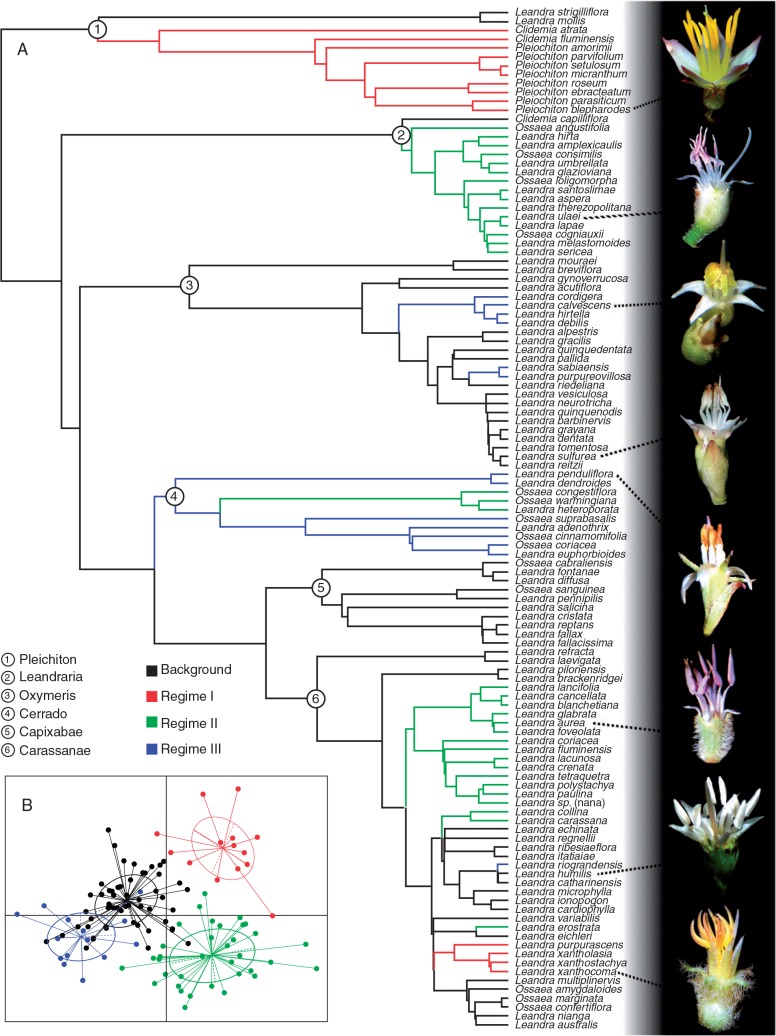
The flower morphological regimes recovered for Leandra s.str. are illustrated in Fig. 5A, along with some flower examples of different regimes and the morphospace colour-coded by the recovered regimes (Fig. 5B). The background regime, which included most of the species under analysis, is depicted in black and regime shifts are shown in colour; the same colour on different clades corresponds to convergent regimes. Eleven different shifts were identified, corresponding to three different regimes (plus the background): two convergent shifts to regime I, five to regime II and four to regime III. Interestingly, there were no reversals to the background regime and there was only one case in which the regime shift did not occur from the background regime; in the Cerrado clade there was a shift from regime III to II. The background regime was characterized by mean values of the variables, with its samples positioned towards the centre of the morphospace, while the other regimes were departures from the mean sizes and shapes. Figure 5B shows that regimes I and II were differentiated from regime III and background in the first axis, while the second axis differentiated regimes I from II and, to a lesser extent, the background from regime III. In the first axis, the main contributions were from filament and anther length and the first component of anther shape (Table 1); thus, regimes I and II were mainly differentiated from the others by the bigger stamens and more subulate anthers. In the second axis, the main contributions were from the first component of petal shape, petal width and anther colour, where regime I and II were mainly differentiated by wider petals and yellow anthers while narrower petals and predominantly pink anthers were found in regime II. The background and regime III overlapped extensively, although some separation was observed in both axes due to smaller and less subulate anthers in regime III.
Fig. 5.
(A) Flower regimes recovered for Leandra s.str. The background regime is shown in black. Convergent regimes have the same colour. On the right are shown some flower examples of different regimes. (B) Morphospace of the two axes used for the regime analysis. The colours of the 0.95 confidence ellipses represent the different regimes, as indicated above panel (B).
Ancestral character estimation and associations
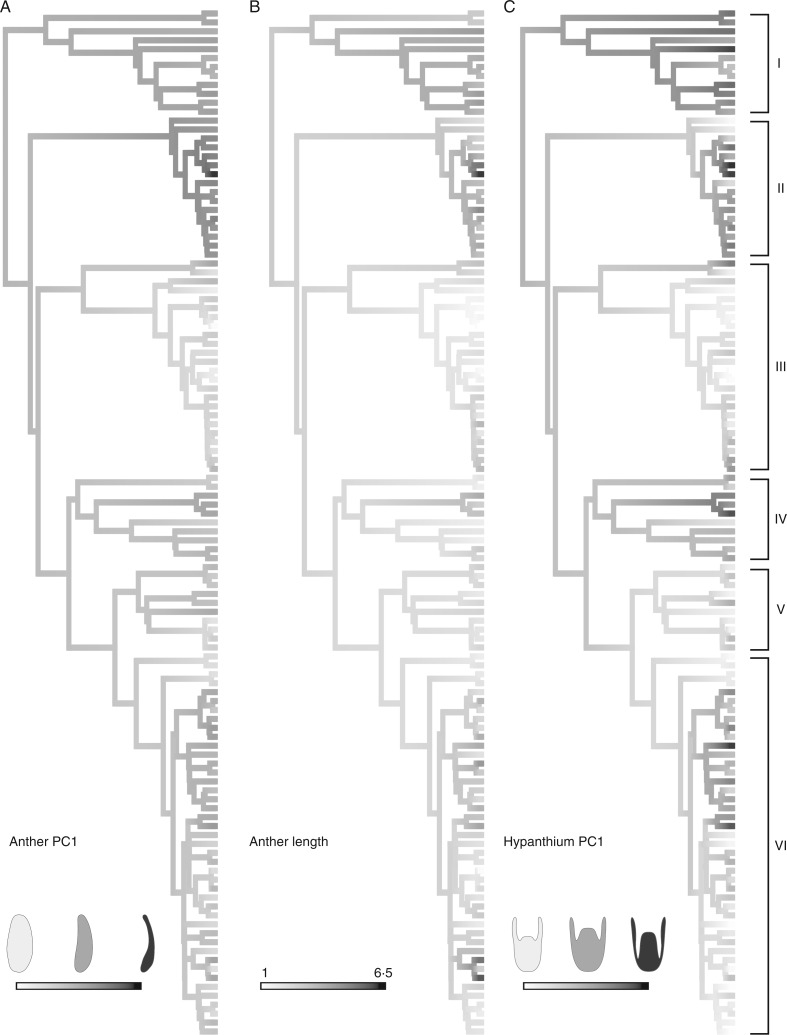
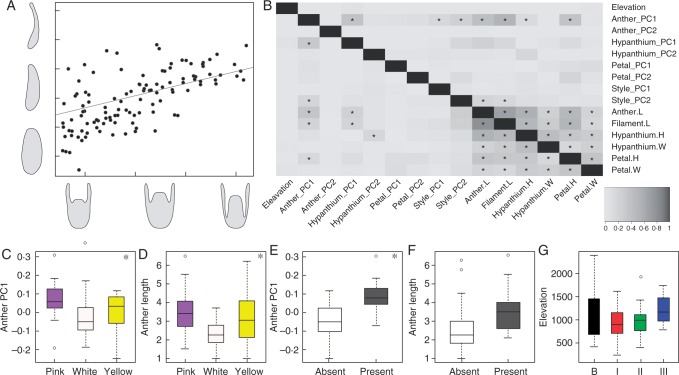
The ancestral reconstruction of the first component of anther shape, anther length and the first component of hypanthium shape is illustrated in Fig. 6. The three graphs show a very similar pattern, with recurrent changes across clades. The ancestral state estimated for Leandra s.str. seemed to be a slightly subulate median-sized anther with a semi-inferior ovary; although close to the mean, the ancestral states seemed more similar to the states observed in Pleiochiton and Leandraria clades. The extensive match observed in these reconstructions points to a scenario where these variables are correlated. This was tested and confirmed by the pgls analyses; the correlogram including all variables is presented in Fig. 7B. In this graph it is possible to note that the sizes of all structures were strongly correlated; thus, in the bigger flowers, bigger stamens, petals and hypanthia were observed. Some shapes seemed to be correlated with size, as in the first components of anthers and hypanthia and the second components of hypanthia and petals, where tubular hypanthia were associated with larger flowers, while smaller petals were also more acuminate (thus, larger petals tended to have rounded to obtuse apices). Nonetheless, allometry did not seem to account for the variation in most of the flower structures. Additionally, some shapes seemed to be correlated, as evidenced by the first component of anthers, hypanthia and styles, where the styles with a curved apex correlated with more subulate anthers.
Fig. 6.
Ancestral estimation of flower characters in Leandra s.str. (A) First component of anther shape. (B) Anther length. (C) First component of hypanthium shape. The estimated values in the phylogeny (shades of grey) are indicated at the bottom. Clade labels: I, Pleiochiton; II, Leandraria; III, Oxymeris; IV, Cerrado; V, Capixabae; VI, Carassanae.
Fig. 7.
(A) pgls model of the first component of hypanthium shape and the first component of anther shape. (B) Correlogram of flower structures and elevation (pgls). Asterisks indicate significant correlations; grey shades reflect the R2 values, indicated in the box at the bottom right of the panel. H, height; W, width; L, length. (C–G) Box plots of variables and groups. Asterisks indicate significance in the phylogenetic ANOVA test. (C) First component of anther shape by anther colour. (D) Anther length by anther colour. (E) First component of anther shape by presence/absence of glomerules. (F) Anther length by presence/absence of glomerules. (G) Elevation by flower regimes. Left to right: background (B), regimes I, II and III.
Differences in size and shape across the different anther colours are illustrated in Fig. 7C, D and Table 2. The graphs in Fig. 7C, D show a similar pattern, in which pink anthers were more subulate and bigger, white anthers were compact and smaller, while yellow anthers presented intermediate values and greater variability. Yellow anthers were not significantly different from the other colours, but the differences among white and pink anthers were significant. Additionally, differences in anther size and shape were compared with inflorescence architecture (Fig. 7E, F), the results indicating that species with glomerulate inflorescences had significantly more subulate anthers. The difference regarding size was not significant, although it was nearly so at 0·05, with lax inflorescences presenting smaller anthers.
Table 2.
Phylogenetic ANOVA of anther size and shape (PC1) across the different anther colours and presence or absence of glomerulate inflorescences. Significant results are shown in bold type
| P value | |
|---|---|
| Anther PC1 | |
| Pink–white | 0·027 |
| Pink–yellow | 0·558 |
| White–yellow | 0·558 |
| Anther length | |
| Pink–white | 0·009 |
| Pink–yellow | 0·723 |
| White–yellow | 0·232 |
| Anther PC1 | |
| Glomerules present/absent | 0·001 |
| Anther length | |
| Glomerules present/absent | 0·133 |
| Elevation | |
| Background–regime I | 0·874 |
| Background–regime II | 0·873 |
| Background–regime III | 0·276 |
| Regime I–regime II | 0·968 |
| Regime I–regime III | 0·409 |
| Regime II–regime III | 0·379 |
We evaluated whether the flower structures correlated with elevation (Fig. 7B) and whether the different flower regimes represented differences in elevation (Fig. 7G). The results indicate that flower structures, sizes and shapes were not significantly correlated with elevation, and the mean differences in elevation across flower regimes were not significant. However, regimes I and II tended to occur at lower elevations than flower regime III, which was observed preferentially at higher elevations, while the background regime was found throughout the elevational range.
Flower diversification and speciation rates
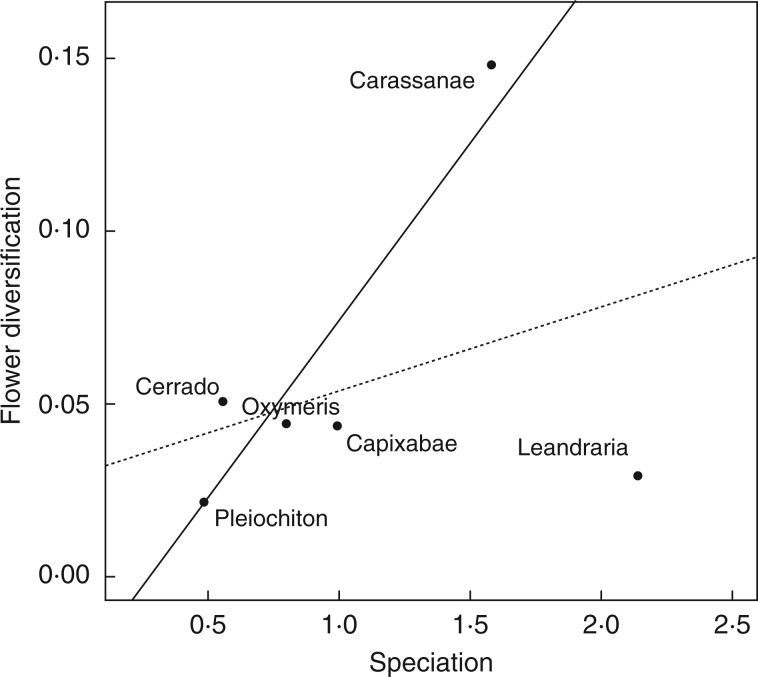
Estimated rates of speciation and flower morphological evolution of major clades of Leandra s.str. are presented in Fig. 8. The highest rates of flower morphological evolution were observed in the Carassanae and Cerrado clades, while speciation was highest in Leandraria and Carassanae. A significant relationship of speciation and flower diversification was not observed when all clades were compared (Pearson correlation coefficient = 0·32, P =0·53). Nonetheless, when the Leandraria clade was not taken into account (i.e. it was treated as an outlier), a significant relationship was observed (Pearson correlation coefficient = 0·91, P=0·03; Fig. 8).
Fig. 8.
Flower diversification and speciation rates in major clades of Leandra s.str. The dashed regression line model includes all clades (correlation coefficient = 0·32, P = 0·53). The solid regression line model does not include Leandraria (correlation coefficient = 0·91, P = 0·03).
DISCUSSION
Overall, in Leandra s.str. flowers are conserved among close relatives, with the Pleiochiton, Leandraria and Capixabae clades showing the greatest flower stasis, while the Carassanae clade shows the greatest number of shifts and different regimes. The same pattern is also observed in the morphospace when each clade is colour-coded (Fig. 4A), and in the phylogenetic signal estimates (Table S2). The regime analysis (Fig. 5) also confirms the observed overlap among clades in the morphospace, since no clade presents a unique flower regime. Morphological shifts in flowers are usually associated with pollinator transitions, and such morphological change is usually very conspicuous when the shift involves different pollination syndromes (bees to birds, wind, mammals, or to other insects; Stebbins, 1970). In Melastomataceae, this is evident in transitions from buzzing bees to hummingbirds, among others. For instance, several morphological changes are observed in floral traits of Brachyotum, a hummingbird-pollinated group derived from bee-pollinated ancestors (Michelangeli et al., 2013). Nonetheless, the extent of morphological change in transitions involving different buzzing bees remains unknown in the family. Despite the lack of published data regarding pollinators, given anther morphology and the absence of nectar, all species in Leandra s.str. are expected to be buzz-pollinated by pollen-collecting bees. The differences in size, shape and colour among the flower regimes in Leandra s.str. suggest that, if not exclusively pollinated by different species/groups of buzzing bees, at least differently sized buzzing bees would probably present different fits across the different flower regimes.
It has been suggested that Melastomataceae support Macior’s (1971) view of buzz-pollination as a very successful system that, once established, hinders the evolution of other pollination mechanisms (Renner, 1989). Additionally, it has been argued that Melastomataceae show little diversification in floral morphology and pollination strategies when compared with other families, and such lack of diversification could be interpreted as the result of being stuck on an adaptive peak (Renner, 1989). On the one hand, Leandra s.str. flower diversity seems to corroborate the success of buzz-pollination, since in our data set there is no evidence of any morphological change that would suggest a transition to a different general pollination system. On the other hand, a great array of diversity and convergent patterns is observed in the group. While stuck on a buzz-pollination system, Leandra s.str. seems to be greatly wandering on it, with different specific ways of exploiting this syndrome being observed.
As expected for groups with specialized pollination systems (Stebbins, 1950; Berg, 1960), in the flowers of Leandra s.str. most characters are shown to be correlated. The size of flower structures is strongly correlated, which would indicate that developmental and genetic architecture perhaps is constraining morphological evolution. However, the shapes of different flower structures seem more decoupled, whereby perhaps natural selection is overwhelming developmental and genetic constraints, allowing adaptive evolution to proceed (Armbruster et al., 1999 and references therein). There is strong correlation among the degree of hypanthium fusion (hypanthium PC1) and anther shape (anther PC1), whereby superior ovaries are correlated with subulate anthers (Fig. 7A). In Melastomataceae, hypanthium fusion is thought to be associated with fruit types, superior ovaries correlating with capsules and berries being associated with inferior ovaries (Clausing et al., 2000). As in all members of the tribe Miconieae, the fruits of Leandra s.str. are berries, but the full spectrum of hypanthium fusion is still observed in the group. In all Melastomataceae the stamens are inflexed while in bud, with the anthers accommodated between the style and walls of the hypanthium and ovary (examples in Supplementary Data Fig. S5). Thus, in Leandra s.str. this tight relationship of anther with ovary seems more likely a flower developmental constraint than an association with fruit type. Whether or not this is a general pattern remains to be investigated across the family. In parallel, the association of fruit type and ovary position still needs support from phylogenetic comparative studies.
Flowers are detected and discriminated by bees according to a combination of specific signals such as size, shape, odour and colour (Gumbert, 2000). Leandra s.str. shows an interesting variation in anther colour, whereby white, yellow and pink anthers are observed (Fig. 1), colours that are considered the most attractive to bees, along with blue and violet (Roubik, 1992). Our results indicate that this variation is not randomly distributed across different anther types, with pink anthers usually bigger and more subulate than the smaller and more compact white ones. Additionally, inflorescence traits can affect attraction and the incidence and consequences of joint visitation of flowers, influencing mating outcomes (Harder et al., 2004). Our results indicate that species with glomerulate inflorescences have significantly more subulate anthers, which also tend to be larger (near significance) in this type of inflorescence. Flower proximity increases the likelihood of flowers being visited by the same pollinator, with joint visitation allowing for correlation in the quality and quantity of pollen export and import (Harder et al., 2004). In general, the consequences of display architecture for pollinator attraction appear underexplored (Harder et al., 2004) and should be further studied in the Melastomataceae. The positive associations of anther morphology with colour and inflorescence architecture found here represent a first line of evidence that these traits might play a role in the pollination biology of Leandra s.str. More complex scenarios of both colour (i.e. UV spectrum, continuous colour measurements) and inflorescences (i.e. size, number of flowers, number of simultaneously opened flowers, or whether it is pendant or not, among others) should be further investigated.
Leandra s.str. are found preferentially at middle to high elevations in eastern Brazil. However some species are exclusively found at high or lower elevations, and we hypothesized that altitudinal constraints in the distribution of pollinators would be reflected in the flower structures and regimes observed in Leandra s.str. However, our results failed to show any significant association. Despite a tendency of some regimes to be more common in higher or lower elevations, the background regime is found throughout the whole elevational range (Fig. 7D). This relationship should be further investigated across a wider group, since a lack of significance in this kind of analysis might be due to few clades and/or not enough variation among them (Felsenstein, 1985).
A general relationship between rates of diversification and rates of morphological evolution may be expected due to adaptive radiation, whereby accelerated rates of speciation associated with divergence in ecologically relevant phenotypic traits are expected, or in cases where most evolutionary change occurs at speciation events (Adams et al., 2009 and references therein). Our results indicate that rates of species diversification and morphological evolution are correlated across most clades in Leandra s.str. While the greatest diversification rates observed in Carassanae seem to be compatible with speciation rates of the group, in the Leandraria clade a low rate of morphological change in flowers is observed when compared with the other clades. Conservative evolutionary change may arise from a range of processes, including the action of natural selection (Ackerly, 2009 and references therein). Interestingly, Leandraria seems to be unique among Leandra s.str. by presenting slightly zygomorphic flowers, due to positioning of the style opposite of the stamens (Fig. 1H, J, O; Reginato, 2016), a feature not quantified here. In general, bilaterally symmetrical (zygomorphic) flowers are thought to have evolved from a radially symmetrical (actinomorphic) form under selection favouring pollinator specificity (Neal et al., 1998). Leandraria is also the clade with the greatest degrees of anther tapering and curvature (Fig. 3B). It is possible that a correlation between styles opposite of elongated, curved anthers might be present in some of the more specialized flowers in the Melastomataceae. In a scenario where the style is positioned opposite to the stamens, the dorsal region of the bee is more likely to touch the stigma, and curved anthers might increase the odds of pollen being deposited in such regions. Changes from actinomophic to zygomorphic flowers are observed in other groups of Melastomataceae (Renner, 1989) and further studies may evaluate the generality of our considerations. Additionally, interesting prospects would include flower symmetry quantification using techniques such as 3D morphometrics (Van der Niet et al., 2010).
Conclusions
Leandra s.str. flowers have a strong phylogenetic signal and tend to be morphologically conserved among close relatives. Nonetheless, convergence is still observed across the group, while extreme flower regimes seem to be quite distinct and non-overlapping discrete flower types are not observed. Ultimately, shifts in floral morphology could imply concomitant pollinator transitions, but more natural history observations are necessary to confirm whether the different floral regimes observed in Leandra s.str. correspond to sub-syndromes or variants within the buzz-pollination system. Since different clades show differences in floral morphological evolution, with flowers more conserved in some groups than in others, such processes would likely be different across Leandra s.str. lineages. Interestingly, the lowest rate of flower morphological change, when compared with species diversification rates, is observed in the clade that possesses the most specialized flowers in the group, and the generality of these results should be further explored across the family. The general idea that hypanthium–ovary fusion is associated with fruit types in the Melastomataceae does not hold for Leandra s.str., where instead hypanthium–ovary fusion seems to be associated with anther shape. Additionally, anther colour and inflorescence architecture seem to be associated with flower structures and should be further investigated. Phylogenetic uncertainty is still pervasive in some regions of Leandra s.str. phylogeny, and a better picture of the relationships in the clade is desirable to further confirm the results presented here.
SUPPLEMENTARY DATA
Supplementary data are available online at www.aob.oxfordjournals.org and consist of the following. Figure S1: binary anther outlines used in the elliptical Fourier analysis. The generic name is abbreviated in the labels. Figure S2: binary hypanthium outlines used in the elliptical Fourier analysis. The generic name is abbreviated. Figure S3: binary petal outlines used in the elliptical Fourier analysis. The generic name is abbreviated in the labels. Figure S4: binary style outlines used in the elliptical Fourier analysis. The generic name is abbreviated in the labels. Figure S5: longitudinal sections of flower buds in Leandra s.str. showing the inflexed stamens. (A) Leandra cristata. (B) Leandra erostrata. (C) Leandra umbellata. Table S1: measurements, discrete coding and voucher information for the species included in the analysis. Table S2: phylogenetic signal (λ) and P value from the test of no phylogenetic signal.
ACKNOWLEDGEMENTS
We thank all collaborators of the PBI Miconieae who kindly provided samples and the two anonymous reviewers for their very helpful comments. This study was supported by the National Science Foundation through the PBI-Miconieae (DEB-0818399) and the Atlantic Forest Dimensions of Biodiversity (DEB-1343612) projects.
LITERATURE CITED
- Ackerly D. 2009. Conservatism and diversification of plant functional traits: evolutionary rates versus phylogenetic signal. Proceedings of the National Academy of Sciences of the USA 106: 19699–19706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams DC, Berns CM, Kozak KH, Wiens JJ. 2009. Are rates of species diversification correlated with rates of morphological evolution? Proceedings of the Royal Society B: Biological Sciences 276: 2729–2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeda F. 1977. Systematics of the neotropical genus Centradenia (Melastomataceae). Journal of the Arnold Arboretum 85: 73–108. [Google Scholar]
- Armbruster WS, Stilio VS, Tuxill JD, Flores C, Rrunk JV. 1999. Covariance and decoupling of floral and vegetative traits in nine neotropical plants: a re-evaluation of Berg's correlation pleiades concept. American Journal of Botany 86: 39–55. [PubMed] [Google Scholar]
- Arroyo MTK, Primack R, Armesto JJ. 1982. Community studies in pollination ecology in the high temperate Andes of central Chile. I. American Journal of Botany 69: 82–97. [Google Scholar]
- Berg RL. 1960. The ecological significance of correlation pleiades. Evolution 14: 171–180. [Google Scholar]
- Bonhomme V, Picq S, Gaucherel C, Claude J. 2014. Momocs: outline analysis using R. Journal of Statistical Software 56: 1–24. [Google Scholar]
- Brito VL, Fendrich TG, Smidt EC, Varassin IG, Goldenberg R. 2016. Shifts from specialized to generalized pollination systems in Miconieae (Melastomataceae) and their relation with anther morphology and seed number. Plant Biology 18: 585–593. [DOI] [PubMed] [Google Scholar]
- Brock MT, Weinig C. 2007. Plasticity and environment-specific covariances: and investigation of floral-vegetative and within flower correlations. Evolution 61: 2913–2924. [DOI] [PubMed] [Google Scholar]
- Butler MA, King AA. 2004. Phylogenetic comparative analysis: a modeling approach for adaptive evolution. American Naturalist 164: 683–695. [DOI] [PubMed] [Google Scholar]
- Clausing G, Meyer K, Renner SS. 2000. Correlations among fruit traits and evolution of different fruits within Melastomataceae. Botanical Journal of the Linnean Society 133: 303–326. [Google Scholar]
- Felsenstein J. 1985. Phylogenies and the comparative method. American Naturalist 125: 1–15. [DOI] [PubMed] [Google Scholar]
- Freckleton RP, Harvey PH, Pagel M. 2002. Phylogenetic analysis and comparative data: a test and review of evidence. American Naturalist 160: 712–726. [DOI] [PubMed] [Google Scholar]
- Garland T, Dickerman AW, Janis CM, Jones JA. 1993. Phylogenetic analysis of covariance by computer simulation. Systematic Biology 42: 265–292. [Google Scholar]
- Goldenberg R, Shepherd GJ. 1998. Studies on the reproductive biology of Melastomataceae in cerrado vegetation. Plant Systematics and Evolution 211: 13–29. [Google Scholar]
- Goldenberg R, Varassin IG. 2001. Sistemas reprodutivos de espécies de Melastomataceae da Serra do Japi, Jundiaí, São Paulo, Brasil. Revista Brasileira de Botânica 24: 283–288. [Google Scholar]
- Gumbert A. 2000. Color choices by bumble bees (Bombus terrestris): innate preferences and generalization after learning. Behavioral Ecology and Sociobiology 48: 36–43. [Google Scholar]
- Harder LD, Johnson SD. 2009. Darwin's beautiful contrivances: evolutionary and functional evidence for floral adaptation. New Phytologist 183: 530–545. [DOI] [PubMed] [Google Scholar]
- Harder LD, Jordan CY, Gross W, Routley MB. 2004. Beyond floricentrism: the pollination function of inflorescences. Plant Species Biology 19: 137–148. [Google Scholar]
- Harmon LJ, Weir JT, Brock CD, Glor RE, Challenger W. 2008. GEIGER: investigating evolutionary radiations. Bioinformatics 24: 129–131. [DOI] [PubMed] [Google Scholar]
- Hijmans RJ. 2013. raster: Geographic data analysis and modeling. R package version 2.1-49 http://CRAN.R-project.org/package=raster.
- Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. 2005. Very high resolution interpolated climate surfaces for global land areas. International Journal of Climatology 25: 1965–1978. [Google Scholar]
- Hoiss B, Krauss J, Potts SG, Roberts S, Steffan-Dewenter I. 2012. Altitude acts as an environmental filter on phylogenetic composition, traits and diversity in bee communities. Proceedings of the Royal Society of London B: Biological Sciences 279: 4447–4456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm S. 1979. A simple sequentially rejective multiple test procedure. Scandinavian Journal of Statistics 6: 65–70. [Google Scholar]
- Ingram T, Mahler DL. 2013. SURFACE: detecting convergent evolution from comparative data by fitting Ornstein-Uhlenbeck models with stepwise AIC. Methods in Ecology and Evolution 4: 416–425. [Google Scholar]
- Koski MH, Ashman TL. 2015. An altitudinal cline in UV floral pattern corresponds with a behavioral change of a generalist pollinator assemblage. Ecology 96: 3343–3353. [DOI] [PubMed] [Google Scholar]
- Kriebel R, Zumbado MA. 2014. New reports of generalist insect visitation to flowers of species of Miconia (Miconieae: Melastomataceae) and their evolutionary implications. Brittonia 66: 396–404. [Google Scholar]
- Lê S, Josse J, Husson F. 2008. FactoMineR: an R package for multivariate analysis. Journal of Statistical Software 25: 1–18. [Google Scholar]
- Macior LW. 1971. Co-evolution of plants and animals-systematic insights from plant-insect interactions. Taxon 20: 17–28. [Google Scholar]
- Magallón S, Sanderson MJ. 2001. Absolute diversification rates in angiosperm clades. Evolution 55: 1762–1780. [DOI] [PubMed] [Google Scholar]
- Martin CV, Michelangeli FA. 2009. Comparative seed morphology of Leandra (Miconieae, Melastomataceae). Brittonia 61: 175–188. [Google Scholar]
- Michelangeli FA, Guimaraes PJ, Penneys DS, Almeda F, Kriebel R. 2013. Phylogenetic relationships and distribution of New World Melastomeae (Melastomataceae). Botanical Journal of the Linnean Society 171: 38–60. [Google Scholar]
- Neal PR, Dafni A, Giurfa M. 1998. Floral symmetry and its role in plant-pollinator systems: terminology, distribution, and hypotheses. Annual Review of Ecology and Systematics 29: 345–373. [Google Scholar]
- Van der Niet T, Zollikofer CPE, de Leon MSP, Johnson SD, Linder HP. 2010. Three-dimensional geometric morphometrics for studying floral shape variation. Trends in Plant Science 15: 423–426. [DOI] [PubMed] [Google Scholar]
- Ocampo G, Almeda F. 2013. Seed diversity in the Miconieae (Melastomataceae): morphological characterization and phenetic relationships. Phytotaxa 80: 1–129. [Google Scholar]
- Ocampo G, Michelangeli FA, Almeda F. 2014. Seed diversity in the tribe Miconieae (Melastomataceae): taxonomic, systematic, and evolutionary implications. PLoS One 9: e100561. doi:10.1371/journal.pone.0100561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orme D, Freckleton R, Thomas G, et al. 2013. caper: Comparative Analyses of Phylogenetics and Evolution in R. R package version 0.5.2 http://CRAN.R-project.org/package=caper.
- Pagel M. 1999. Inferring the historical patterns of biological evolution. Nature 401: 877–884. [DOI] [PubMed] [Google Scholar]
- Penneys DS, Judd WS. 2005. A systematic revision and cladistic analysis of Charianthus (Melastomataceae) using morphological and molecular characters. Systematic Botany 30: 559–584. [Google Scholar]
- R Core Team. 2014. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; http://www.R-project.org/. [Google Scholar]
- Rabosky D, Schliep K. 2013. laser: Likelihood Analysis of Speciation/Extinction Rates from Phylogenies. R package version 2.4-1 http://CRAN.R-project.org/package=laser.
- Reginato M. 2016. Taxonomic revision of Leandra sect. Leandra (Melastomataceae, Miconieae). Phytotaxa 262: 1–97. [Google Scholar]
- Reginato M, Michelangeli FA. 2016. Untangling the phylogeny of Leandra s.str. (Melastomataceae, Miconieae). Molecular Phylogenetics and Evolution 96: 17–32. [DOI] [PubMed] [Google Scholar]
- Renner SS. 1989. A survey of reproductive biology in Neotropical Melastomataceae and Memecylaceae. Annals of the Missouri Botanical Garden 76: 496–518. [Google Scholar]
- Revell LJ. 2012. phytools: an R package for phylogenetic comparative biology (and other things). Methods in Ecology and Evolution 3: 217–223. [Google Scholar]
- Roubik DW. 1992. Ecology and natural history of tropical bees. New York: Cambridge University Press. [DOI] [PubMed] [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, et al. 2012. Fiji: an open-source platform for biological-image analysis. Nature Methods 9: 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stebbins GL. 1950. Variation and evolution in plants. New York: Columbia University Press. [Google Scholar]
- Stebbins GL. 1970. Adaptive radiation of reproductive characteristics in angiosperms. I. Pollination mechanisms. Annual Review of Ecology and Systematics 1: 307–326. [Google Scholar]
- Stehmann JR, Forzza R, Salino A, Sobral M, Costa DP, Kamino LHY. 2009. Plantas da Floresta Atlântica. Rio de Janeiro: Jardim Botânico do Rio de Janeiro. [Google Scholar]
- Stein BA, Tobe H. 1989. Floral nectaries in Melastomataceae and their systematic and evolutionary implications. Annals of the Missouri Botanical Garden 76: 519–531. [Google Scholar]
- Varassin IG, Penneys DS, Michelangeli FA. 2008. Comparative anatomy and morphology of nectar-producing Melastomataceae. Annals of Botany 102: 899–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiffin T, Tomb AS. 1972. The systematic significance of seed morphology in the neotropical capsular-fruited Melastomataceae. American Journal of Botany 59: 411–422. [Google Scholar]
- Wilson CL. 1950. Vasculation of the stamen in the Melastomataceae, with some phyletic implications. American Journal of Botany 37: 431–444. [Google Scholar]
- Wurdack JJ. 1986. Atlas of hairs for Neotropical Melastomataceae. Smithsonian Contributions to Botany 63: 1–80. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.