Abstract
Background and Aims Mite domatia are small structures on the underside of plant leaves that provide homes for predacious or fungivorous mites. In turn, mites inhabiting domatia defend the plant by consuming leaf herbivores and pathogens, which can result in a domatia-mediated, plant–mite defence mutualism. Several recent studies have suggested that plants receive enhanced benefits when they provide a foliar food source, such as sugars secreted from extrafloral nectaries, to mite mutualists alongside mite domatia. However, the effect of foliar sugar on reducing leaf pathogen load via domatia-inhabiting mites has not been directly investigated.
Methods To fill this gap, the links between foliar sugar addition, domatia-inhabiting mite abundance, and pathogen load were experimentally evaluated in wild grape. Furthermore, because the proposed combined benefits of providing food and housing have been hypothesized to select for the evolutionary correlation of extrafloral nectaries and domatia across plant lineages, a literature survey aimed at determining the overlap of mite domatia and extrafloral nectaries across plant groups was also conducted.
Key Results It was found that leaves with artificial addition of foliar sugar had 58–80 % more mites than leaves without foliar sugar addition, and that higher mite abundances translated to reduced powdery mildew (Erysiphe necator) loads on leaves. It was found that mite domatia and extrafloral nectaries occur non-randomly in the same clades across Eudicots. Genera with both traits are reported to highlight candidate lineages for future studies.
Conclusions Together, the results demonstrate that foliar sugar can indeed enhance the efficacy of domatia-mediated plant–mite mutualisms, and suggest that this synergism has the potential to influence the co-distribution of foliar nectar and mite domatia across plants.
Keywords: Mite domatia, acarodomatia, extrafloral nectar, foliar nectar, defence mutualism, indirect defence, powdery mildew, grape, Vitis, Erysiphe necator
INTRODUCTION
Mite domatia are specialized plant traits that can house predacious or fungivorous mites on leaves in return for protection against natural enemies, such as pathogenic fungi and small herbivores (Lundström, 1887; O’Dowd and Willson, 1991; Romero and Benson, 2005). They are taxonomically widespread, have evolved many times across the plant tree of life, and occur in economically important crop plants such as cherry, coffee, and avocado (Brouwer and Clifford, 1990). Because their only known function is to defend plants via the retention of mutualistic mite bodyguards, mite domatia can serve as a model trait for understanding the evolution and ecology of mutualistic defence. However, while mite domatia frequently house predacious or fungivorous mites, they can also contain a myriad of other small arthropods, including plant parasites, and studies testing for impacts of domatia-inhabiting mites on plant enemies have produced mixed results (Walter et al., 1995; Walter, 1996). Because of the context dependency of these interactions, clarifying how mite domatia interact with other plant traits to reduce plant damage, as well as what other phenotypes impact the evolutionary distribution of domatia across plants, is of interest for disentangling what factors govern mutualism gain, loss and maintenance over ecological contexts and evolutionary time.
One widespread plant trait hypothesized to impact the selective benefit of mite domatia is extrafloral nectar: a sugary substance secreted from non-floral plant glands (called extrafloral nectaries) that attracts and feeds beneficial arthropods (Koptur, 1992). Although extrafloral nectar has typically been studied for its role in attracting ant bodyguards to plants (Bentley, 1977; Heil, 2008), several recent studies have suggested that extrafloral nectar may also enhance plant–mite defence mutualisms by facilitating larger standing populations of mites on leaves (Walter et al., 1995; Walter, 1996; van Rijn and Tanigoshi, 1999; Weber et al., 2012). In work on the castor oil plant Ricinus communis, predacious mites were observed visiting extrafloral nectaries, and the presence of extrafloral nectar was experimentally demonstrated to increase population growth of predacious mites (Walter, 1996; van Rijn and Tanigoshi, 1999). Walter et al. (1995) found that experimentally excising extrafloral nectaries from leaves reduced the number of domatia-dwelling fungivorous mites. Finally, in a common garden of phylogenetically diverse species of Viburnum, the full factorial experimental manipulation of both domatia and extrafloral nectaries revealed that extrafloral nectaries on the lamina increase the abundance of fungus-eating mites inhabiting domatia (Weber et al., 2012). In this last study, the observed additive effect of housing (domatia) and food (extrafloral nectar) rewards on mite abundance was hypothesized to have driven, in part, the macro-evolutionary correlation of extrafloral nectaries and domatia across the Viburnum genus.
Despite evidence that extrafloral nectar can increase the abundance of domatia-dwelling mites, currently no research has directly evaluated whether this increase in mite abundance translates into a decreased pathogen or pest load for the plant, a key requirement if the interaction is to have a selective benefit. To fill this gap, we conducted an empirical test of the hypothesis that foliar sugar indirectly decreases leaf pathogen load by increasing the abundance of mutualistic mites inhabiting domatia. We use two species of wild grape, Vitis munsoniana and V. riparia (Vitaceae), and their naturally occurring mutualistic mite communities as study systems. Both grape species have domatia that are inhabited almost exclusively by mutualistic (fungivorous and predacious) mites rather than phytophagous mites (Walter and Denmark, 1991; Karban et al., 1995; O’Dowd and Willson, 1997). Furthermore, high levels of domatia-inhabiting mites have been demonstrated to reduce powdery mildew growth effectively in the riverbank grape V. riparia and in the cultivated grape V. vinifera, but these impacts were dependent upon sufficiently high abundances of mite mutualists on leaves, which are not always present in natural systems (Norton et al., 2000; English-Loeb et al., 2005).
We used experimental field manipulations to evaluate the hypothesis that foliar nectar enhances domatia-mediated mite–plant mutualisms. In particular, we asked the following two questions. (1) Is mutualistic mite abundance higher on leaves supplemented with foliar sugar compared with leaves without sugar? (2) If so, does this increase in domatia-inhabiting mites translate into a decreased load of powdery mildew (Erysiphe necator) on grape leaves? To assess the general co-occurrence of extrafloral nectar and leaf domatia across plant clades and to identify candidate clades for future study, we paired these experiments with a broad survey of the frequency with which extrafloral nectaries and mite domatia occur in the same groups across vascular plants.
MATERIALS AND METHODS
Experimental manipulations
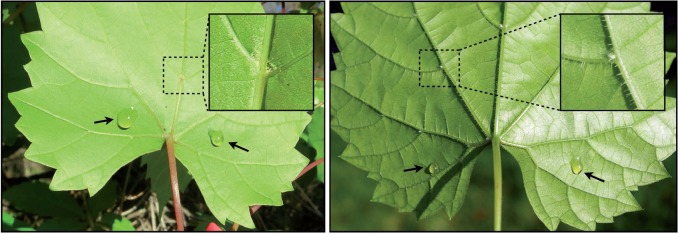
We simulated the presence of extrafloral nectar in field experiments on two species of wild grape: V. munsoniana and V. riparia. Neither species naturally possesses extrafloral nectar, but close relatives (members of the genus, Cissus) have extrafloral nectaries (Bentley, 1977; Baker et al., 1978; Schupp and Feener, 1991; Fiala and Linsenmair, 1995; Mexzón and Chinchilla, 1999; Díaz-Castelazo et al., 2004). Extrafloral nectar was simulated by applying two small drops (approx. 40–50 μL) of sugar solution to the abaxial surface of leaves (Fig. 1) daily between 0700 and 1000 h (hereafter referred to as the ‘foliar sugar’ treatment). Controls received the same amount of physical handling as treatment leaves, but no foliar sugar solution was applied. The sugar solution consisted of 60 % volume honey, 30 % volume water and 10 % volume artificial nectar (Songbird Essentials, Inc.: contains dextrose, sucrose, fructose and sodium salts). We chose this composition to mimic the high sugar concentration and complex composition (typically a mix of sugars and amino acids) common to extrafloral nectars (Bentley, 1977; Koptur, 1992).
Fig. 1.
Vitis munsoniana (left) and V. riparia (right) leaves with foliar sugar solution treatments (black arrows are pointing towards experimental sugar solution drops). The magnified portion shows examples of domatia occurring at vein axils. Domatia occur at many of the vein axils across the leaves.
Vitus munsoniana manipulation
Vitus munsoniana experiments were conducted at the Archbold Biological Station south of Lake Placid, Florida, USA, from 1 April 1 to 9 April 2014. A total of 40 leaves were haphazardly chosen from multiple grapevines and assigned to treatment (foliar sugar added, n = 20 leaves) or control (leaves handled but no foliar sugar added, n = 20 leaves). All leaves were mature (fully expanded), intact whole leaves of similar stages. After 9 d (to allow mite numbers to acclimate), leaves were destructively harvested, placed in moist paper towels, transported to the lab on ice, and surveyed for mites under a dissecting scope. Mites found on leaves were counted and scored according to morphospecies. Representatives from each morphospecies were mounted in Hoyers solution on microscope slides and identified to taxonomic family (Krantz and Walter, 2009). Because mites within families typically have a conserved diet range (i.e. mycophagous, predaceous, herbivorous) (Krantz and Walter, 2009), we used family as an indication of their interactions with the plant. For each leaf, we recorded the total number of domatia present, the number of domatia occupied by mites, the total abundance of mites per leaf and the relative abundance of all trophic guilds of mites (mutualistic vs. parasitic). Because larger leaves may have higher abundances of organisms simply due to their size, we controlled for differences in leaf size by recording leaf length measured as the distance from the base of the leaf to the leaf tip. While area is a more direct measure of size, several studies have shown that length is linearly related to total area in grape leaves (Elsner and Jubb, 1988; Blom and Tarara, 2007).
Vitus riperia manipulation
We conducted a second experiment on a population of V. riparia in Ithaca, NY from 14 June to 6 July 2014. For this experiment, we manipulated a total of 40 leaves (20 = foliar sugar addition, 20 = control). Treatment leaves received two small drops (approx. 40–50 μL) of nectar solution on the abaxial surface daily between 0700 and 1000 h. Nine days after the start of the experiment, 20 leaves (ten control and ten treatment) were destructively sampled and surveyed for mutualistic mite abundance and domatia traits as described above. The remaining 20 leaves, however, were immediately inoculated with a laboratory-maintained powdery mildew (E. necator) population originating from V. vinifera leaves of cultivated grape in the NY Experimental Station in Geneva, NY. Erysiphe is a group of widespread and virulent plant pathogens, impacting many plant taxa globally. Erysiphe necator, the grapevine powdery mildew, is a major pathogen of Vitaceae species with devastating effects on both wild and cultivated populations. While highly virulent, E. necator can have a patchy distribution in both time and space. As such, we chose to inoculate with E. necator rather than use natural levels of the pathogen to ensure the presence of the disease on our experimental leaves. Two types of powdery mildew inoculation were applied to ensure high colonization of leaves with conidia. First, we depressed the lower, abaxial leaf surface of an infected V. vinifera leaf to the lower leaf surface of each experimental V. riparia leaf. Secondly, to ensure infection further, a suspension of E. necator conidia in distilled water was misted directly onto the abaxial leaf surface of each experimental leaf. We estimated the severity of mildew infection 8 d after inoculation by scoring the presence or absence of mycelia on the abaxial and adaxial surfaces at six, 1 cm diameter circles at standard locations across the leaf surface.
Statistical analyses
All analyses were carried out in R version 3.0.1 (R Development Core Team, 2015). The effects of the experimental addition of foliar sugar on mite abundance and/or domatia occupancy, and the relationship between mite abundance and powdery mildew growth (for the V. riparia experiment only), were assessed using generalized linear models using the glm function in R. Because response variables were count data, Poisson error distributions were utilized for all GLM analyses. One outlier was identified in the V. riparia data set using the Bonferroni outlier test in the car package (Fox and Weisberg, 2010) and subsequently removed. To disentangle the relevance of experimental treatment and leaf size for predicting response variables, we compared models based on Akaike’s information criterion (AIC) using the ‘dredge’ function in the ‘MuMln’ package.
Survey of co-occurrence of extrafloral nectaries in domatia-bearing clades
To determine how commonly extrafloral nectaries are known to occur in clades with mite domatia-bearing species, we cross-referenced the Annotated List of Domatia-Bearing Plants (Brouwer and Clifford, 1990; only includes acarodomatia, excludes ant domatia) with the World List of Plants with Extrafloral Nectaries (data as of 20 February 2015; Weber et al., 2015). Each entry in both lists was first corrected for taxonomic name changes, synonyms, and mis-spellings using the packages Taxize (Chamberlain and Szöcs, 2013) using the following pipeline. First, genus and species names were queried against The Plant List (www.ThePlantList.org) using the ‘TPL’ function in taxize. This function resolves genus and species names by replacing synonyms with accepted names, removing orthographical errors and assigning genera to families using their current placement according to the Angiosperm Phylogeny Group. We then assigned the resolved families to the correct taxonomic order according to the NCBI Taxonomy Database as of 6 June 2015 (http://www.ncbi.nlm.nih.gov/taxonomy) using the ‘tax_name’ function in taxize. Species that were not assigned to higher groups were hand checked for spelling errors and placed in the correct currently assigned clade according to Angiosperm Phylogeny Group classifications. Because many plants have not been scored for extrafloral nectary (Weber and Keeler, 2013) or mite domatia presence or absence at the species level, the corrected names and classifications were cross-referenced at the scale of family and genus. We visualized the distribution of groups reported to have both extrafloral nectaries and mite domatia across vascular plant groups using a rooted vascular plant mega-tree published by Zanne et al. (2013). We asked whether the presence of extrafloral nectaries and mite domatia was correlated across the plant family phylogeny using the phylogeny of Zanne et al. (2013), trimmed so that each family was represented by a single tip using the drop.tip function in the R package ape (Paradis et al., 2004). We tested for non-random patterns of overlap in extrafloral nectaries and mite domatia using Pagel’s discrete test (Pagel, 1994) implemented in the phytools package (Revell, 2012). Because both mite domatia and extrafloral nectaries have originated many times independently, this data set is not susceptible to issues associated with using Pagel’s discrete test single (or low) replication traits (Maddison and FitzJohn, 2014).
RESULTS
Experimental manipulations
Mites found on leaves almost exclusively belonged to the largely mutualistic (mycophagous or predatory) family Tydeidae, but also included a small proportion of predatory Phytoseiidae mites, and one velvet mite Trombidiopidea (on V. munsoniana). All mites found on leaves during the study were thus treated as mutualistic (no herbivorous mites were encountered).
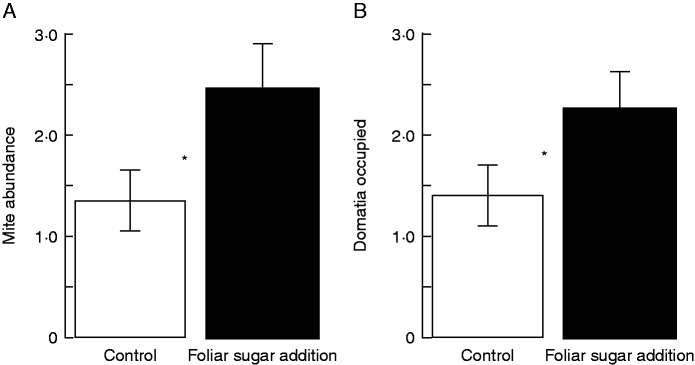
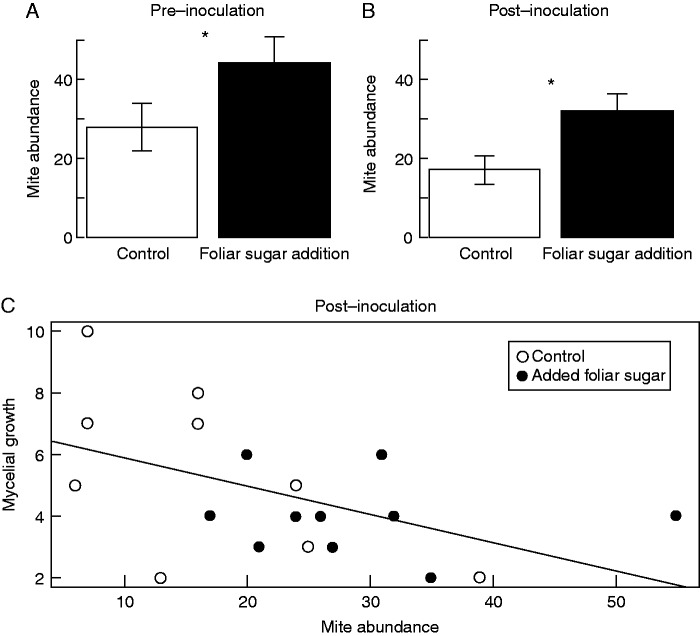
The total abundance of mutualistic mites found on leaves increased with the presence of foliar sugar in all experiments. For V. munsoniana, leaves with foliar sugar experimentally added had approx 80 % more mites than control leaves (z1,40 = 2·49, P = 0·013; Fig. 2A), and foliar sugar increased the number of domatia occupied by mites by 38 % (z1,40 = 1·97, P = 0·048; Fig. 2B). For V. riparia, foliar sugar addition increased mite abundance by approx. 58 % and approx. 69 %, respectively, for counts conducted before (z1,18 = 5·99, P < 0·001; Fig. 3A) and after mildew inoculation (z1,18 = 6·5, P < 0·001; Fig. 3B). For V. munsonia, mite abundance and the number of domatia occupied by mites were both best explained by foliar sugar presence (Table 1): alternative models containing leaf size (δ-AICc ≥1·6), and the size by sugar addition interaction term (δ-AICc ≥3·9), had substantially worse fit. In V. riparia, mite abundance was best explained by a model that included both foliar sugar treatment and leaf length (δ-AICc ≥9·27; Table 2). Finally, in V. riparia powdery mildew inoculation experiments, increased mite abundance was correlated with a decrease in the number of powdery mildew colonies found on V. riparia leaves (z1,18 = –2·171, P = 0·03; Fig. 3C).
Fig. 2.
The effect of foliar sugar solution addition on mite abundance and the number of domatia occupied by mites in V. munsoniana. For control leaves (white) and leaves with experimentally added foliar sugar solution (black), the mean (± s.e.) is shown for mite abundance (A) and the number of domatia occupied by mites (B). An asterisk indicates significance <0·05.
Fig. 3.
The effect of foliar sugar addition on mite abundance, and the relationship between mite abundance and powdery mildew colony abundance, on V. riparia. For control leaves (white) and leaves with foliar sugar experimentally added (black), the mean (± s.e.) is shown for (A) mite abundance before powdery mildew inoculation, (B) mite abundance 8 d after powdery mildew inoculation and (C) the relationship between the number of mites on each leaf and the number of powdery mildew colonies observed. An asterisk indicate significance <0·05.
Table 1.
Models explaining mite variables according to AICc selection for V. munsoniana manipulations
| Rank | Intercept | Leaf length | Foliar sugar | Leaf length × foliar sugar | d.f. | logLik | AICc | ΔAICc | AICw |
|---|---|---|---|---|---|---|---|---|---|
| Domatia occupied by mites | |||||||||
| 1 | 0·34 | + | 2 | –67·90 | 140·13 | 0·00 | 0·41 | ||
| 2 | –0·47 | 0·11 | + | 3 | –67·53 | 141·74 | 1·61 | 0·19 | |
| 3 | –0·72 | 0·17 | 2 | –68·78 | 141·88 | 1·75 | 0·17 | ||
| 4 | 0·60 | 1 | –69·90 | 141·91 | 1·78 | 0·17 | |||
| 5 | –0·26 | 0·08 | + | + | 4 | –67·49 | 144·12 | 3·99 | 0·06 |
| Mite abundance | |||||||||
| 1 | 0·30 | + | 2 | –70·98 | 146·28 | 0·00 | 0·55 | ||
| 2 | –0·44 | 0·10 | + | 3 | –70·66 | 147·99 | 1·71 | 0·23 | |
| 3 | –0·77 | 0·18 | 2 | –72·88 | 150·08 | 3·80 | 0·08 | ||
| 4 | –0·27 | 0·08 | + | + | 4 | –70·63 | 150·41 | 4·13 | 0·07 |
| 5 | 0·64 | 1 | –74·21 | 150·52 | 4·24 | 0·07 | |||
The ‘delta’ column shows the difference between a model’s AICc and that of the highest ranked model (rank = 1). The best fitting models (AICw >2) are in bold. Model coefficients are shown for leaf length and plus signs are shown for factor variables.
Table 2.
Models explaining mite and mildew variables according to AICc selection for V. riparia manipulations
| Rank | Intercept | Leaf length | Foliar sugar | Leaf length × Foliar sugar | d.f. | logLik | AICc | ΔAICc | AICw |
|---|---|---|---|---|---|---|---|---|---|
| Mite abundance pre-inoculation | |||||||||
| 1 | 3·92 | –0·05 | + | + | 4 | –131·38 | 273·43 | 0·00 | 1·00 |
| 2 | 3·33 | + | 2 | –141·54 | 287·78 | 14·35 | 0·00 | ||
| 3 | 3·19 | 0·01 | + | 3 | –141·16 | 289·82 | 16·38 | 0·00 | |
| 4 | 3·21 | 0·03 | 2 | –157·08 | 318·87 | 45·44 | 0·00 | ||
| 5 | 3·58 | 1 | –159·97 | 322·17 | 48·74 | 0·00 | |||
| Mite abundance post-inoculation | |||||||||
| 1 | 1·39 | 0·12 | + | + | 4 | –72·35 | 155·56 | 0·00 | 0·99 |
| 2 | 2·11 | 0·06 | + | 3 | –78·61 | 164·83 | 9·27 | 0·01 | |
| 3 | 2·24 | 0·07 | 2 | –88·50 | 181·75 | 26·19 | 0·00 | ||
| 4 | 2·83 | + | 2 | –88·61 | 181·98 | 26·42 | 0·00 | ||
| 5 | 3·14 | 1 | –103·11 | 208·46 | 52·90 | 0·00 | |||
The ‘delta’ column shows the difference between a model’s AICc and that of the highest ranked model (rank =1). The best fitting models (ΔAICc >2) are in bold. Model coefficients are shown for leaf length, and plus signs are shown for factor variables.
Survey of occurrence of extrafloral nectaries and leaf domatia across plant groups
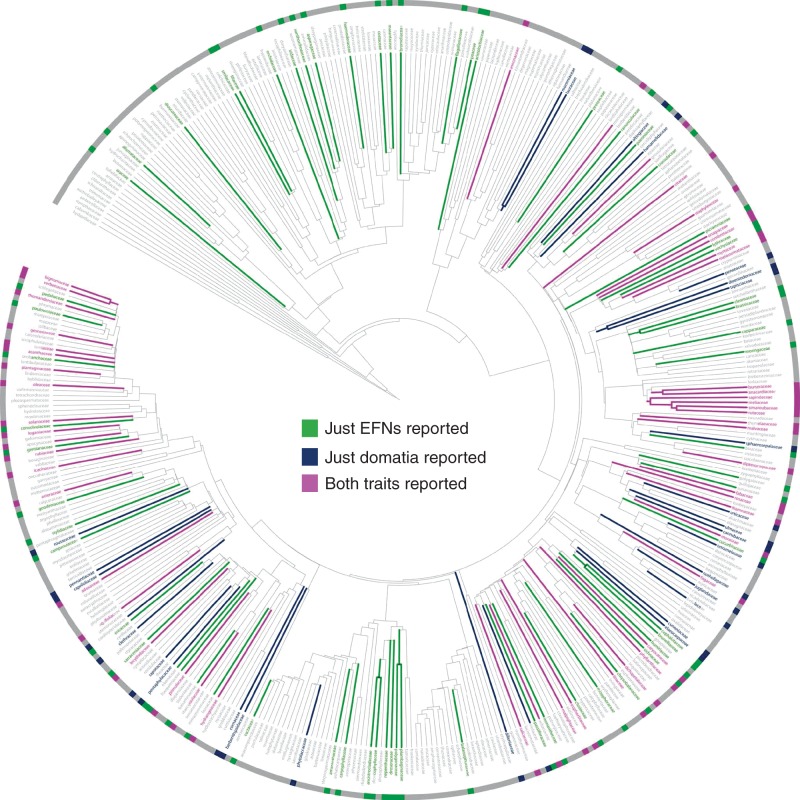
Cross-referencing the database of species with extrafloral nectaries (Weber et al., 2015) with the database of species with leaf domatia (Brouwer and Clifford, 1990), we found that approx. 61 % of the 87 plant families documented to contain species with mite domatia were also reported to have species with extrafloral nectaries. These 53 families contained 78 genera that were reported on both lists, approx. 45 % of which contained species reported to have both traits. Extrafloral nectaries and leaf domatia occurred together in the same plant families across the angiosperm phylogeny more than expected at random (Pagel’s discrete: likelihood ratio = 52·6, P < 0·0001). The plant groups that contain both extrafloral nectaries and leaf domatia were widely dispersed across the Eudicot portion of the plant phylogeny (Fig. 4), suggesting that they repeatedly evolved in the same plant clades over evolutionary history.
Fig. 4.
Extrafloral nectaries (EFNs) and domatia have evolved in multiple lineages across the angiosperm tree of life. A phylogeny of angiosperm families modified from Zanne et al. (2013) with families reported in the literature to have both extrafloral nectaries and domatia are in pink, and families reported to have either extrafloral nectaries or domatia are in green or blue, respectively.
DISCUSSION
Morphological structures on plant leaves can modify interactions between plants, plant enemies and the third trophic level. Despite the prevalence and well-recognized ecological importance of leaf traits that mediate these interactions, we know remarkably little about how different morphological leaf traits interact with one another to attract beneficial arthropods to plants or to retain them (Heil, 2008). Recent work has led to the hypothesis that extrafloral nectar, a widespread plant trait known for its role in attracting ant bodyguards, may also enhance mite–plant mutualisms by increasing the number of mites present in mite domatia (Walter et al., 1995; Walter, 1996; van Rijn and Tanigoshi, 1999; Weber et al., 2012). We found that experimentally adding foliar sugar to plant leaves increased the number of mutualistic mites inhabiting leaf domatia in two species of wild grape, Vitis munsoniana and V. riparia. Furthermore, in a simulated outbreak of powdery mildew on V. riparia leaves, this increased mite abundance negatively correlated with the extent of powdery mildew establishment on leaves. Our study is the first to demonstrate experimentally that foliar sugar can enhance the efficacy of plant–mite mutualisms by increasing the abundance of mutualistic mites inhabiting domatia, indirectly reducing pathogen load on plant leaves.
Our results revealed that the presence of foliar sugar solution facilitated larger standing populations of mutualistic mites on leaves, which in turn reduced subsequent plant infection and damage in a downstream outbreak. Because pathogen outbreaks can be strong selective agents in plants, the results of our study suggest that selection may favour leaf morphologies that attract and retain larger standing populations of mutualistic mites before outbreaks occur. In particular, it may be selectively beneficial for plants to have both domatia (as mite housing) and extrafloral nectar (as a non-fungal food alternative for mites) together on the same leaf. While adding traits to plants that naturally lack them is a powerful and long-practiced experimental approach to testing for potential trait effects, future studies evaluating the ecological impact of foliar nectar on plant–mite mutualisms should be conducted in systems that naturally possess extrafloral nectaries. These studies will directly address how extrafloral nectaries impact mite defence in the ecological and trait backgrounds in which they naturally occur. Additionally, one caveat of our study is that our experimental design did not allow us to distinguish which aspect of the foliar sugar solution impacted mite numbers. Follow-up studies that disentangle the relative contributions of leaf water, sugars and amino acids on mite populations will be fruitful.
By cross-listing the current list of taxa known to have extrafloral nectaries with the current list of taxa known to have mite domatia, we found that the two traits occur non-randomly in the same clades across Eudicots. While the co-occurrence of these traits at the gross level is a promising signal, several important caveats must be considered. First, ecological information on domatia inhabitancy was not considered when originally compiling the databases. This could lead to false-positive errors. For example, hydathodes or other non-sugar plant glands may be erroneously included as extrafloral nectaries, or structures may be assumed to be mite domatia when they are in reality primarily inhabited by ants (or other organisms). Secondly, a sampling bias could be present whereby larger clades are more likely to contain species with each trait by chance, even if the two traits do not actually co-occur in the same species within those clades. Formal studies of the co-occurrence and evolutionary correlation of these two common mutualistic traits at finer phylogenetic and morphological scales are needed to determine how frequently extrafloral nectaries and mite domatia are found together and correlate evolutionarily. To our knowledge, a detailed survey of extrafloral nectary and mite domatia co-occurrence has only been conducted at the genus level in the genus Viburnum, which revealed that the two traits were indeed positively evolutionarily correlated (Weber et al., 2012). We suggest that the 78 other genera that we report to contain both extrafloral nectaries and mite domatia (Supplementary Data Table S1) are promising starting points for other such studies.
Finally, because extrafloral nectaries and mite domatia are both common plant traits occurring in many agriculturally important crop species, the implications of their combined ecological benefits should be further investigated in light of agriculture. For example, the occurrence of these mutualistic traits in closely related species (Table S1) suggests that breeding or engineering for the co-expression of these traits within agriculturally relevant species that are vulnerable to mildew may be possible. Additionally, the effect of intercropping plants with extrafloral nectaries amongst mite domatia-bearing crops is worth further consideration, as the benefits of co-occurrence for these traits may extend to a community scale.
Conclusion
Many striking defence mutualisms occur on plants that reward their mutualists with several distinct beneficial traits (Heil, 2008; Weber et al., 2012). Here we tested for an interaction between two common plant traits that are independently known to mediate plant–arthropod defence mutualisms. By experimentally manipulating the presence of foliar sugar solution on leaves and documenting the effects of mutualist abundance and pathogen load, this study provides the previously missing link in testing the hypothesis that extrafloral nectaries can enhance plant–mite mutualisms by increasing mite abundance in domatia, indirectly decreasing pathogen load.
SUPPLEMENTARY DATA
Supplementary data are available online at www.aob.oxfordjournals.org and consist of Table S1: plant genera where both extrafloral nectaries and leaf domatia have been documented (not necessarily in the same species).
ACKNOWLEDGEMENTS
We thank Greg Loeb and David Gadoury for methodological advice and help with the grape–domatia–powdery mildew system; Harry Greene, Jed Sparks and the Cornell EEB graduate field course for facilitating the Florida manipulations; Nick Mason and Nick Fletcher for access to the New York field site; Sharon Strauss for advice on figures; and Anurag Agrawal for helpful discussion throughout the project.
LITERATURE CITED
- Baker HG, Opler PA, Baker I. 1978. A comparison of the amino acid complements of floral and extrafloral nectars. Botanical Gazette 139: 322–332. [Google Scholar]
- Bentley BL. 1977. Extrafloral nectaries and protection by pugnacious bodyguards. Annual Review of Ecology and Systematics 8: 407–427. [Google Scholar]
- Blom PE, Tarara JM. 2007. Rapid and nondestructive estimation of leaf area on field-grown Concord (Vitis labruscana) grapevines. American Journal of Enology and Viticulture 58: 393–397. [Google Scholar]
- Brouwer Y, Clifford H. 1990. An annotated list of domatia-bearing species. Notes from the Jodrell Laboratory 12: 1–33. [Google Scholar]
- Chamberlain SA, Szöcs E. 2013. taxize: taxonomic search and retrieval in R. F1000Research 2: 191. doi:10.12688/f1000research.2-191.v2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz-Castelazo C, Rico-Gray V, Oliveira PS, Cuautle M. 2004. Extrafloral nectary-mediated ant–plant interactions in the coastal vegetation of Veracruz, Mexico: richness, occurrence, seasonality, and ant foraging patterns. Ecoscience 11: 472–481. [Google Scholar]
- Elsner EA, Jubb GL. 1988. Leaf area estimation of Concord grape leaves from simple linear measurements. American Journal of Enology and Viticulture 39: 95–97. [Google Scholar]
- English-Loeb G, Norton AP, Gadoury D, Seem R, Wilcox W. 2005. Tri-trophic interactions among grapevines, a fungal pathogen, and a mycophagous mite. Ecological Applications 15: 1679–1688. [Google Scholar]
- Fiala B, Linsenmair KE. 1995. Distribution and abundance of plants with extrafloral nectaries in the woody flora of a lowland primary forest in Malaysia. Biodiversity and Conservation 4: 165–182. [Google Scholar]
- Fox J, Weisberg HS. 2010. An R companion to applied regression. Sage Publications. [Google Scholar]
- Heil M. 2008. Indirect defence via tritrophic interactions. New Phytologist 178: 41–61. [DOI] [PubMed] [Google Scholar]
- Karban R, English-Loeb G, Walker MA, Thaler J. 1995. Abundance of phytoseiid mites on Vitis species: effects of leaf hairs, domatia, prey abundance and plant phylogeny. Experimental and Applied Acarology 19: 189–197. [Google Scholar]
- Koptur S. 1992. Extrafloral nectary-mediated interactions between insects and plants In: Bernays E, ed. Insect–plant interactions. Boca Raton, FL: CRC Press, 81–129. [Google Scholar]
- Krantz GW, Walter DE. 2009. A manual of acarology. Texas Tech University, Lubbock, TX. [Google Scholar]
- Lundström AN. 1887. Pflanzenbiologische studien II. Die anpassungen der pflanzen an thiere, I. Von domatia. Nova Acta Regiae Societas Scientiartum Upsaliensis 13: 88. [Google Scholar]
- Maddison WP, FitzJohn RG. 2014. The unsolved challenge to phylogenetic correlation tests for categorical characters. Systematic Biology 64: 127–136. [DOI] [PubMed] [Google Scholar]
- Mexzón R, Chinchilla C. 1999. Plant species attractive to beneficial entomofauna in oil palm (Elaeis guineensis Jacq.) plantations in Costa Rica. ASD Oil Palm Papers 19: 1–22. [Google Scholar]
- Norton AP, English-Loeb G, Gadoury D, Seem RC. 2000. Mycophagous mites and foliar pathogens: leaf domatia mediate tritrophic interactions in grapes. Ecology 81: 490–499. [Google Scholar]
- O’Dowd DJ, Willson MF. 1991. Associations between mites and leaf domatia. Trends in Ecology and Evolution 6: 179–182. [DOI] [PubMed] [Google Scholar]
- O’Dowd DJ, Willson MF. 1997. Leaf domatia and the distribution and abundance of foliar mites in broadleaf deciduous forest in Wisconsin. American Midland Naturalist 137: 337–348. [Google Scholar]
- Pagel M. 1994. Detecting correlated evolution on phylogenies: a general method for the comparative analysis of discrete characters. In: Proceedings of the Royal Society B: Biological Sciences 255: 37–45. [Google Scholar]
- Paradis E, Claude J, Strimmer K. 2004. APE: Analyses of Phylogenetics and Evolution in R language. Bioinformatics, 20: 289–290. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. 2015. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- Revell LJ. 2012. phytools: an R package for phylogenetic comparative biology (and other things). Methods in Ecology and Evolution, 3: 217–223. [Google Scholar]
- Romero GQ, Benson WW. 2005. Biotic interactions of mites, plants and leaf domatia. Current Opinion in Plant Biology 8: 436–440. [DOI] [PubMed] [Google Scholar]
- Schupp EW, Feener D. 1991. Phylogeny, lifeform, and habitat dependence of ant-defended plants in a Panamanian forest In: Huxley CR, Cutler DF, eds. Ant–plant interactions. Oxford: Oxford University Press, 175–197. [Google Scholar]
- van Rijn PCJ, Tanigoshi LK. 1999. The contribution of extrafloral nectar to survival and reproduction of the predatory mite Iphiseius degenerans on Ricinus communis. Experimental and Applied Acarology 23: 281–296. [Google Scholar]
- Walter DE. 1996. Living on leaves: mites, tomenta, and leaf domatia. Annual Review of Entomology 41: 101–114. [DOI] [PubMed] [Google Scholar]
- Walter DE, Denmark HA. 1991. Use of leaf domatia on wild grape (Vitis munsoniana) by arthropods in central Florida. Florida Entomologist 74: 440–446. [Google Scholar]
- Walter DE, O’Dowd DJ, Lowman M, Nadkarni N. 1995. Life on the forest phylloplane: hairs, little houses, and myriad mites In: Lowman ND, Nadkarni NM, eds. Forest canopies. New York: Academic Press, 325–351. [Google Scholar]
- Weber MG, Keeler KH. 2013. The phylogenetic distribution of extrafloral necatries in plants. Annals of Botany, 111: 1251–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber MG, Clement WL, Donoghue MJ, Agrawal AA. 2012. Phylogenetic and experimental tests of interactions among mutualistic plant defense traits in Viburnum (Adoxaceae). American Naturalist 180: 450–463. [DOI] [PubMed] [Google Scholar]
- Weber MG, Porturas LD, Keeler KH. 2015. World list of plants with extrafloral nectaries. http://www.extrafloralnectaries.org. [Google Scholar]
- Zanne AE, Tank DC, Cornwell WK, et al. 2013. Three keys to the radiation of angiosperms into freezing environments. Nature 506: 89–92. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.