Abstract
Background and Aims Mycoheterotrophy entails plants meeting all or a portion of their carbon (C) demands via symbiotic interactions with root-inhabiting mycorrhizal fungi. Ecophysiological traits of mycoheterotrophs, such as their C stable isotope abundances, strongly correlate with the degree of species’ dependency on fungal C gains relative to C gains via photosynthesis. Less explored is the relationship between plant evolutionary history and mycoheterotrophic plant ecophysiology. We hypothesized that the C and nitrogen (N) stable isotope compositions, and N concentrations of fully and partially mycoheterotrophic species differentiate them from autotrophs, and that plant family identity would be an additional and significant explanatory factor for differences in these traits among species. We focused on mycoheterotrophic species that associate with ectomycorrhizal fungi from plant families Ericaceae and Orchidaceae.
Methods Published and unpublished data were compiled on the N concentrations, C and N stable isotope abundances (δ13C and δ15N) of fully (n = 18) and partially (n = 22) mycoheterotrophic species from each plant family as well as corresponding autotrophic reference species (n = 156). These data were used to calculate site-independent C and N stable isotope enrichment factors (ε). Then we tested for differences in N concentration, 13C and 15N enrichment among plant families and trophic strategies.
Key Results We found that in addition to differentiating partially and fully mycoheterotrophic species from each other and from autotrophs, C and N stable isotope enrichment also differentiates plant species based on familial identity. Differences in N concentrations clustered at the plant family level rather than the degree of dependency on mycoheterotrophy.
Conclusions We posit that differences in stable isotope composition and N concentrations are related to plant family-specific physiological interactions with fungi and their environments.
Keywords: Carbon and nitrogen, Ericaceae, mycoheterotrophy, mixotrophy, mycorrhizal fungi, Orchidaceae, plant adaptations, stable isotopes
INTRODUCTION
The ability of plants to fix atmospheric carbon (C) and convert it into sugars through photosynthesis (autotrophy) sets this kingdom of organisms apart from others and is the key to the earth’s primary productivity. However, some plants have completely lost this ability and rely on alternative means of nutrition such as feeding off symbiotic relationships with mycorrhizal fungi. Some plants retain the ability to photosynthesize, but under certain conditions meet a portion of their C demands via associations with mycorrhizal fungi. Both of these intriguing plant adaptations fall under the category of mycoheterotrophy. Mycoheterotrophy entails plants meeting all or a portion of their C and nutrient demands via symbiotic mycorrhizal fungi (Merckx, 2013). The most striking examples of mycoheterotrophic plants are those that have completely lost the ability to photosynthesize, and their above-ground structures serve only for dispersal and reproduction (Merckx, 2013). Over approximately the last decade, the marriage of methods from plant ecophysiology and molecular ecology has led to new revelations about the population ecology of mycoheterotrophs (reviewed in Merckx et al., 2009; Selosse and Roy, 2009). However, fundamental questions remain on the ecology and evolution of this unique plant adaptation. Here we address a set of these questions – can nitrogen (N) concentrations and C and N stable isotope abundances of mycoheterotrophs distinguish distantly related plants that are dependent upon the same functional guild of mycorrhizal fungi?
Mycoheterotrophy has arisen independently in at least 17 plant families (Merckx, 2013), but Orchidaceae and Ericaceae species have received by far the most attention from researchers (Bidartondo, 2005; Hynson and Bruns, 2010; Dearnaley et al., 2012). In a Tansley Review from 1994, Jonathan Leake coined the term ‘myco-heterotrophy’ for plants that met their C and nutrient demands exclusively via fungi (Leake, 1994). On the heels of his review came a slew of new research that engaged recently developed tools from molecular biology such as Sanger DNA sequencing of environmental samples to identify the fungal partners of ericaceous and orchidaceous mycoheterotroph populations from temperate forests (Cullings et al., 1996; Taylor and Bruns, 1997; Bidartondo et al., 2000; McKendrick et al., 2000). These studies (among numerous others) revealed that many mycoheterotrophic taxa partnered with specific lineages of fungi that simultaneously formed ectomycorrhizal (EM) associations with trees that provide C to both the fungi and mycoheterotrophs. Relative to other plants that can partner with multiple EM fungi simultaneously, the apparent extreme specificity of the mycorrhizal interactions in ericaceous and orchidaceous mycoheterotrophic species stood out as an anomaly, and was likened to specialized host–parasite interactions (Smith and Read, 2008). This pattern of fungal specificity held for other mycoheterotrophic species that associated with different functional guilds of fungi such as arbuscular mycorrhizal (AM) fungi (Bidartondo et al., 2002) and saprotrophs (Ogura-Tsujita et al., 2009), leading researchers to believe that fungal specificity must be a requisite for the mycoheterotrophic lifestyle. However, more recent studies have shown that not all fully mycoheterotrophic species specialize on particular lineages of fungi. Instead, fungal specificity tends to lie at the level of functional guild (EM, AM or saprotrophic fungi), rather than fungal species identity (Hynson and Bruns, 2009; Roy et al., 2009).
In tandem with the research on mycoheterotrophs and their fungal ‘hosts’ was the work of Gebauer and Meyer (2003) and Trudell et al. (2003) on the ecophysiology of mycoheterotrophy. These research teams analysed the natural abundances of C and N stable isotopes from fully mycoheterotrophic species, leafy green orchids and other vegetation. Working on different sides of the globe, they independently came to the same conclusions that the stable isotope signatures of mycoheterotrophs were significantly enriched in the heavy isotopes of both carbon (13C) and nitrogen (15N) compared with surrounding autotrophic species and most similar to those of EM fruit bodies. The work of Gebauer and Meyer (2003) also detected a new isotopic pattern among some species of apparently autotrophic orchids. A selected number of green orchid species from their study sites in southern France and Bavaria had intermediate 13C enrichment values relative to fully mycoheterotrophic and autotrophic species. This finding was the first line of evidence for what is now known as partial mycoheterotrophy – a form of mixotrophy where a plant meets its C demands through both fungi and photosynthesis (Selosse and Roy, 2009). Additional lines of support for the existence of partial mycoheterotrophy in orchids came from the work of Bidartondo et al. (2004), Selosse et al. (2004), Julou et al. (2005) and Abadie et al. (2006) who found similar patterns of 13C enrichment among other species of green orchids and also found that these orchids partnered with a diversity of EM fungi shared with surrounding trees rather than orchid mycorrhizal fungi in the genera Tulasnella, Ceratobasidium or taxa in the order Sebacinales (grouped in the polyphyletic ‘rhizoctonias’). These results were later corroborated in ericaceous species in studies led by Zimmer et al. (2007) and Tedersoo et al. (2007).
To date, the most well-investigated groups of both partially (PMH) and fully mycoheterotrophic (FMH) plants remain orchidaceous and ericaceous species that partner with EM fungi (Hynson and Bruns, 2010). However, there are a rising number of studies that have examined the stable isotope profiles of FMH species that partner with AM (Merckx et al., 2010; Courty et al., 2011) and saprotrophic fungi (Ogura-Tsujita et al., 2009; Martos et al., 2009; Dearnaley and Bougoure, 2010; Lee et al., 2015), but evidence of partial mycoheterotrophy among species that partner with these guilds remains sparse (Cameron and Bolin, 2010; Bolin et al., 2015). The combined results of these efforts provide evidence that the 13C and 15N enrichment of FMH species can be distinguished based on the guild of their fungal host (AM, EM or saprotroph; Hynson et al., 2013). Also, among full mycoheterotrophs there are often interspecific differences in their C and N stable isotope profiles, but these values are relatively consistent within a species across its geographical range. The total N concentration of full mycoheterotrophs also varies significantly from species to species (Hynson et al., 2013).
Authors have put forth numerous explanations for these patterns, but most agree that due to mycoheterotrophs’ dependency on fungi to meet all or a portion of their C and N demands, the identity(ies) of their fungal symbionts should influence their C and N stable isotope profiles and N concentrations (Gebauer and Meyer, 2003; Bidartondo et al., 2004; Zimmer et al., 2007; Tedersoo et al., 2007; Hynson et al., 2009, Liebel et al., 2010). This is because among genera (and sometimes species) of fungi there exists a wide range of soil nutrient mining and catabolic abilities (Gebauer and Taylor, 1999; Emmerton et al., 2001; Taylor et al., 2004; Pritsch and Garbaye, 2011). Differences among fungi in the processing of C from surrounding autotrophs and N from the soil should affect their stable isotope composition, and in turn mycoheterotroph stable isotope profiles closely mirror those of their host fungi (Taylor et al., 2003; Hobbie et al., 2005; Mayor et al., 2009). For example, if an FMH species is relatively depleted in the heavy isotope of N (15N) this could be due to this species associating with a specific fungus that is particularly adept at accessing 15N-depleted mineral N (Gebauer and Taylor, 1999). However, this does not explain differences in 15N enrichment between mycoheterotrophic taxa that specialize on closely related fungi from the same functional guild with putatively similar biochemistry. For instance, the two ericaceous FMH species Sarcodes sanguinea Torr. and Pterospora andromedea Nutt. often grow in sympatry and partner with the same or closely related EM fungi in the genus Rhizopogon, but have significantly different enrichment in 15N (Fig. 1B; Bidartondo and Bruns, 2002; Hynson et al., 2013). The opposite pattern can also be seen in the ericaceous FMH species Hypopitys monotropa Crantz and Monotropa uniflora L. that each specialize on distantly related lineages of EM fungi, but share overlapping C and N stable isotope profiles (Fig. 1A; Bidartondo and Bruns, 2002; Hynson et al., 2013). These findings all indicate that among mycoheterotrophs that partner with the same functional guild of fungi, there exists some form of plant, rather than fungal, control over the assimilation and processing of C and N.
Fig. 1.
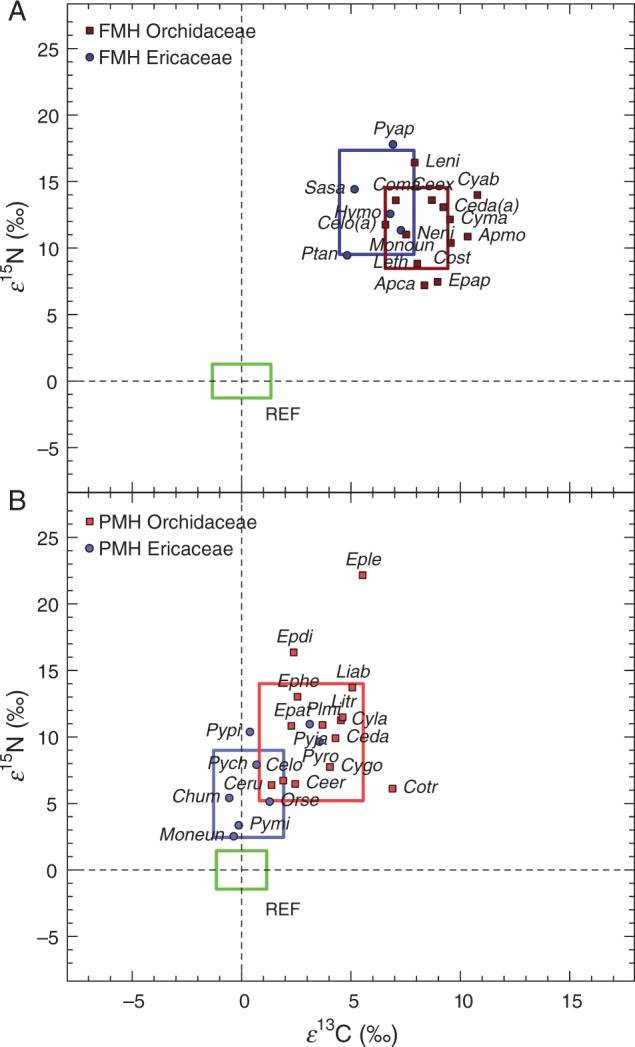
Mean enrichment factors (ε) for 13C and 15N of (A) fully mycoheterotrophic (FMH) Orchidaceae and Ericaceae and (B) partially mycoheterotrophic (PMH) Orchidaceae and Ericaceae associated with fungi forming ectomycorrhizas. Boxes represent one standard deviation of the mean ε values for the four significantly distinguished groups of (partially) mycoheterotrophic Orchidaceae and Ericaceae and for their respective photosynthetic reference plants (REF, n = 1433. Abbreviations of the species and numbers of replicates (n) are as follows: FMH Orchidaceae (n = 126): Apca, Aphyllorchis caudata; Apmo, A. montana; Ceda(a), Cephalanthera damasonium albino; Ceex, C. exigua; Celo(a), C. longifolia albino; Coma, Corallorhiza maculata; Cost, Corallorhiza striata; Cyab, Cymbidium aberrans; Cyma, C. macrorhizon; Epap, Epipogium aphyllum; Leni, Lecanorchis nigricans; Leth, Lecanorchis thalassica; Neni, Neottia nidus-avis; (REF, n = 628). FMH Ericaceae (n = 134): Hymo, Hypopitys monotropa; Monoun, Monotropa uniflora; Ptan, Pterospora andromedea; Pyap, Pyrola aphylla; Sasa, Sarcodes sanguinea; (REF, n = 403). PMH Orchidaceae (n = 189): Ceda, Cephalanthera damasonium; Ceer, C. erecta; Celo, C. longifolia; Ceru, C. rubra; Cotr, Corallorhiza trifida; Cygo, Cymbidium goeringii; Cyla, C. lancifolium; Epat, Epipactis atrorubens; Epdi, E. distans; Ephe, E. helleborine; Eple, E. leptochila; Liab, Limodorum abortivum; Litr, L. trabutianum; Plmi, Platanthera minor; (REF, n = 662). PMH Ericaceae (n = 606): Chum, Chimaphila umbellata; Moneun, Moneses uniflora; Orse, Orthilia secunda; Pych, Pyrola chlorantha; Pyja, P. japonica; Pymi, P. minor; Pypi, P. picta; Pyro, P. rotundifolia; (REF, n = 627).
With a critical mass of data now accumulated on both the identity and diversity of fungi that host species of orchidaceous and ericaceous mycoheterotrophs, we set out to test whether plant family identity is a significant predictor for N concentration and stable isotope abundances. To avoid the effects of fungal functional guild on mycoheterotroph stable isotope values and N concentration, we selected just those species that form symbioses with EM fungi. We compiled data from 22 published and unpublished studies on the stable isotope values and N concentration of FMH and PMH species in families Orchidaceae and Ericaceae. For our purposes, we considered full mycoheterotrophy to include achlorophyllous species known to share EM fungi with trees and that are enriched in both 13C and 15N relative to neighbouring autotrophs. We considered partial mycoheterotrophy to include leafy green species that associate with EM fungi shared with trees and that are enriched in 13C and 15N, or those only significantly enriched in 15N relative to surrounding autotrophs. Even though enrichment in both 13C and 15N provides the clearest indicator of partial mycoheterotrophy, we chose also to include those species only enriched in 15N because there is substantial variation in the 13C enrichment of EM fungi (Mayor et al., 2009), 13C enrichment in some partial mycoheterotrophs may turn out to be too small to be unequivocally identified (Selosse and Martos 2014; Stöckel et al., 2014; Gebauer et al., 2016). The 13C enrichment in fungal tissue, as well as in FMH plants, is always accompanied by enrichment in 15N. Thus, 15N enrichment in plants associated with EM fungi that are not significantly enriched in 13C serves as a substitute to identify organic matter (and thus C gain) from a fungal source. Hynson et al. (2013) called these plants ‘cryptic mycoheterotrophs’.
To make comparisons across plant populations, we used an isotope enrichment factor approach to normalize the data (Preiss and Gebauer, 2008). With these data we tested the hypotheses that: (1) C and N stable isotope abundances and N concentration would distinguish FMH Orchidaceae from FMH Ericaceae; (2) C and N stable isotope abundances and N concentration would distinguish PMH Orchidaceae from PHM Ericaceae; and (3) similar to previous population-level studies, C and N stable isotopes abundances would differentiate FMH and PMH plants from each other and autotrophic species across multiple populations.
MATERIALS AND METHODS
Data compilation
To test our hypotheses with an exhaustive data set, we conducted a traceable literature search (Koricheva and Gurevitch, 2014) using the web-based search engine Web of Science (Thomson Reuters, 2015) and the key words ‘mycoheterotroph*’ OR ‘myco-heterotroph*’ AND ‘stable isotope*’ on 3 February 2016 that returned 252 hits. Document types were restricted to articles, and duplicates were removed from the retrieved results, limiting the number of hits to 238. Only publications focused on full or partial mycoheterotrophs in the plant families Ericaceae and Orchidaceae with species partnering with EM fungi were included in our study. We analysed the full text of the resulting papers and included only those studies that performed sampling of neighbouring autotrophic reference plant samples together with target plant samples (FMH or PMH) in a suitable manor for enrichment factor calculations, i.e. a replicated plot-wise sampling of FMH or PMH target plants together with closely neighbouring autotrophic reference plants (Preiss and Gebauer, 2008). We identified 21 publications suitable for our study published between 2003 and 2015 and added one further so far unpublished data set (Table 1). We explicitly excluded from our data set investigations for which the sampling design did not allow calculation of enrichment factors (Trudell et al., 2003) or for which C and N isotope abundance was affected by experimental manipulations [shading and trenching (Hynson et al., 2012); fungicide application (Bellino et al., 2014); defoliation (Gonneau et al., 2014)], by investigation of chlorophyll concentration gradients (Stöckel et al., 2011) or by investigation of different developmental stages (Roy et al., 2013; Gonneau et al., 2014). We did include data from mutant achlorophyllous (albino) orchids that are fully mycoheterotrophic. Data on C and N stable isotope natural abundances as well as total N concentrations in leaf or stem tissues of FMH and PMH species in the plant families Ericaceae and Orchidaceae known to partner with EM fungi were either directly extracted from the original publications or were obtained by personal contact with the respective authors. Specifically, unpublished data on plant N concentrations from the investigations by Bidartondo et al. (2004), Zimmer et al. (2007, 2008), Hynson et al. (2009, 2015), Liebel et al. (2009), Motomura et al. (2010), Preiss et al. (2010), Yagame et al. (2012) and Johansson et al. (2015) were kindly made available by the authors. Unpublished data on N stable isotope abundance were supplied by Preiss et al. (2010). Furthermore, B. Burghardt and G. Gebauer provided unpublished C and N stable isotope abundance and N concentration data on Hypopitys monotropa and Epipactis leptochila (Godfrey) Godfrey.
Table 1.
Fully (FMH) and partially mycoheterotrophic (PMH) species of the plant families Ericaceae and Orchidaceae included in this investigation, their numbers of replicates for C and N stable isotope natural abundance (n ε13C = and n ε15N =) and total N concentration (n N conc. =) and the respective sources where the data were originally published
| Species | Type | nε13C= | nε15N= | nN conc.= | Publication |
|---|---|---|---|---|---|
| Family: Ericaceae | |||||
| Hypopitys monotropa | FMH | 38 | 38 | 31 | Tedersoo et al. (2007), Zimmer et al. (2007, 2008), Hynson et al. (2015), Johansson et al. (2015), B. Burghardt & G. Gebauer (unpubl. res.) |
| Monotropa uniflora | FMH | 8 | 8 | 8 | Ogura-Tsujita et al. (2009), Motomura et al. (2010) |
| Pterospora andromedea | FMH | 34 | 34 | 32 | Zimmer et al. (2007), Hynson et al. (2009) |
| Pyrola aphylla | FMH | 39 | 39 | 37 | Zimmer et al. (2007), Hynson et al. (2009) |
| Sarcodes sanguinea | FMH | 15 | 15 | 15 | Zimmer et al. (2007) |
| FMH Ericaceae | 134 | 134 | 123 | ||
| Chimaphila umbellata | PMH | 138 | 138 | 132 | Tedersoo et al. (2007), Zimmer et al. (2007) |
| Hynson et al. (2009), Johansson et al. (2015) | |||||
| Moneses uniflora | PMH | 99 | 99 | 99 | Hynson et al. (2015), Johansson et al. (2015) |
| Orthilia secunda | PMH | 140 | 140 | 134 | Tedersoo et al. (2007), Zimmer et al. (2007), Liebel et al. (2009), Johansson et al. (2015) |
| Pyrola chlorantha | PMH | 116 | 116 | 110 | Tedersoo et al. (2007), Zimmer et al. (2007), Johansson et al. (2015) |
| Pyrola japonica | PMH | 5 | 5 | 5 | Matsuda et al. (2012) |
| Pyrola minor | PMH | 48 | 48 | 48 | Zimmer et al. (2007), Liebel et al. (2009), Johansson et al. (2015) |
| Pyrola picta | PMH | 54 | 54 | 51 | Zimmer et al. (2007), Hynson et al. (2009) |
| Pyrola rotundifolia | PMH | 6 | 6 | 0 | Tedersoo et al. (2007) |
| PMH Ericaceae | 606 | 606 | 579 | ||
| Total Ericaceae | 740 | 740 | 702 | ||
| Family: Orchidaceae | |||||
| Aphyllorchis caudata | FMH | 3 | 3 | 3 | Roy et al. (2009) |
| Aphyllorchis montana | FMH | 4 | 4 | 4 | Roy et al. (2009) |
| Cephalanthera damasonium | FMH | 10 | 10 | 10 | Julou et al. (2005) |
| albino | |||||
| Cephalanthera exigua | FMH | 5 | 5 | 5 | Roy et al. (2009) |
| Cephalanthera longifolia | FMH | 9 | 9 | 9 | Abadie et al. (2006) |
| albino | |||||
| Corallorhiza maculata | FMH | 15 | 15 | 15 | Zimmer et al. (2007), Hynson et al. (2009) |
| Corallorhiza striata | FMH | 3 | 3 | 3 | Hynson et al. (2015) |
| Cymbidium aberrans | FMH | 3 | 3 | 3 | Motomura et al. (2010) |
| Cymbidium macrorhizon | FMH | 6 | 6 | 6 | Motomura et al. (2010) |
| Epipogium aphyllum | FMH | 8 | 8 | 8 | Liebel and Gebauer (2011), Hynson et al. (2015) |
| Lecanorchis nigricans | FMH | 3 | 3 | 3 | Motomura et al. (2010) |
| Lecanorchis thalassica | FMH | 5 | 5 | 5 | Lee et al. (2015) |
| Neottia nidus-avis | FMH | 52 | 52 | 38 | Gebauer and Meyer (2003), Bidartondo et al. (2004), Zimmer et al. (2007), |
| Zimmer et al. (2008), Liebel et al. (2010), Preiss et al. (2010), Stöckel et al. (2014) | |||||
| FMH Orchidaceae | 126 | 126 | 112 | ||
| Cephalanthera damasonium | PMH | 39 | 43 | 39 | Gebauer and Meyer (2003), Bidartondo et al. (2004) |
| Julou et al. (2005), Liebel et al. (2010), Preiss et al. (2010) | |||||
| Cephalanthera erecta | PMH | 3 | 3 | 3 | Motomura et al. (2010) |
| Cephalanthera longifolia | PMH | 42 | 42 | 42 | Abadie et al. (2006), Liebel et al. (2010), Johansson et al. (2015) |
| Cephalanthera rubra | PMH | 25 | 25 | 25 | Gebauer and Meyer (2003), Bidartondo et al. (2004), Preiss et al. (2010) |
| Corallorhiza trifida | PMH | 9 | 9 | 4 | Zimmer et al. (2008) |
| Cymbidium goeringii | PMH | 7 | 7 | 7 | Motomura et al. (2010) |
| Cymbidium lancifolium | PMH | 6 | 6 | 6 | Motomura et al. (2010) |
| Epipactis atrorubens | PMH | 11 | 11 | 11 | Gebauer and Meyer (2003), Bidartondo et al. (2004), Tedersoo et al. (2007) |
| Epipactis distans | PMH | 4 | 4 | 4 | Bidartondo et al. (2004) |
| Epipactis helleborine | PMH | 21 | 21 | 21 | Gebauer and Meyer (2003), Bidartondo et al. (2004), Abadie et al. (2006), |
| Liebel et al. (2010), Johansson et al. (2015) | |||||
| Epipactis leptochila | PMH | 4 | 4 | 4 | B. Burghardt and G. Gebauer (unpubl. res.) |
| Limodorum abortivum | PMH | 10 | 14 | 14 | Gebauer and Meyer (2003), Liebel et al. (2010) |
| Limodorum trabutianum | PMH | 5 | 5 | 5 | Liebel et al. (2010) |
| Platanthera minor | PMH | 3 | 3 | 3 | Yagame et al. (2012) |
| PMH Orchidaceae | 189 | 197 | 188 | ||
| Total Orchidaceae | 315 | 323 | 300 |
Thus, in total, we compiled C and N stable isotope abundance and N concentration data from 22 studies for a total of 18 FMH species, 22 PMH species and 156 of their neighbouring autotrophic reference species, of which 11 species were non-mycoheterotrophic Ericaceae (Table 1). We did not include any green orchids that partner with rhizoctonia fungi as references because all orchids are initially mycoheterotrophic in their germination stages. Data collection resulted in 260 data points for 13C and 15N abundances for full mycoheterotrophs, 795 data points for 13C and 803 data points for 15N abundances for partial mycoheterotrophs and 1433 data points for 13C and 1461 data points for 15N abundances for neighbouring autotrophic references (Figs 2 and 3). Nitrogen concentration data were only available for a reduced data set of 235 data points for full mycoheterotrophs, 767 for partial mycoheterotrophs and 1355 for autotrophic references (Figs 2 and 3). For non-mycoheterotrophic Ericaceae within the autotrophic reference species, 118 and 126 data points were available for 13C and 15N abundances, respectively, and 111 data points for N concentration data.
Fig. 2.
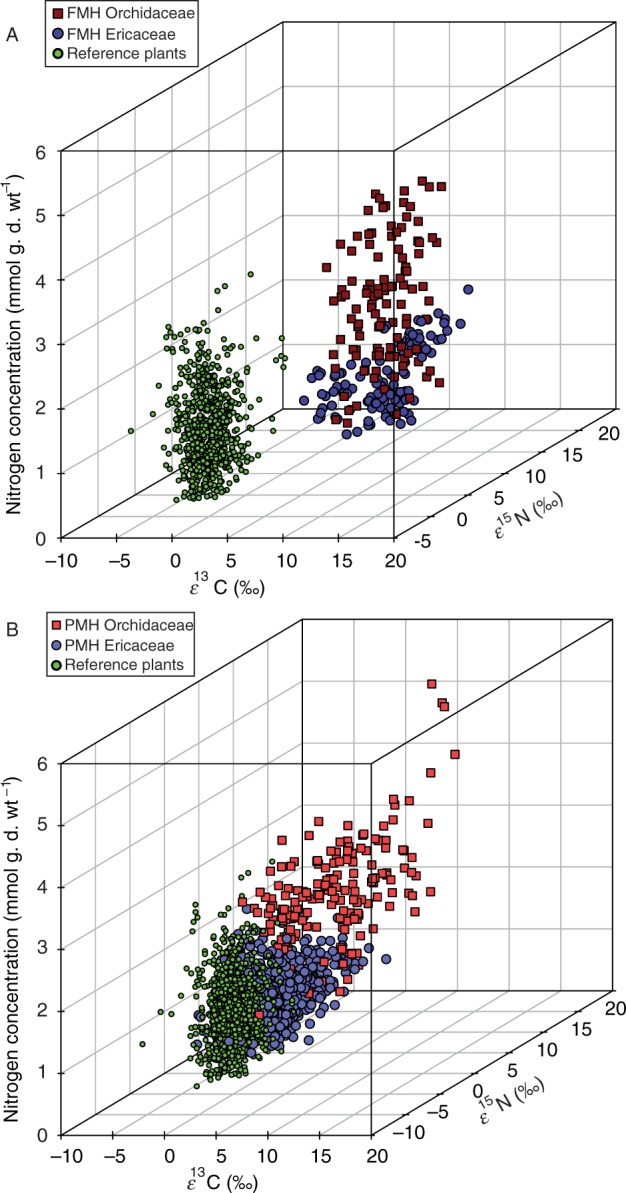
Single values for enrichment factors ε13C and ε15N and nitrogen concentrations (mmol g d. wt−1) of (A) fully mycoheterotrophic (FMH) Orchidaceae and Ericaceae and the respective photosynthetic reference plants (REF, n = 804) and (B) partially mycoheterotrophic (PMH) Orchidaceaeand Ericaceae associated with fungi forming ectomycorrhizas and the respective photosynthetic reference plants (REF, n = 1191).
Fig. 3.
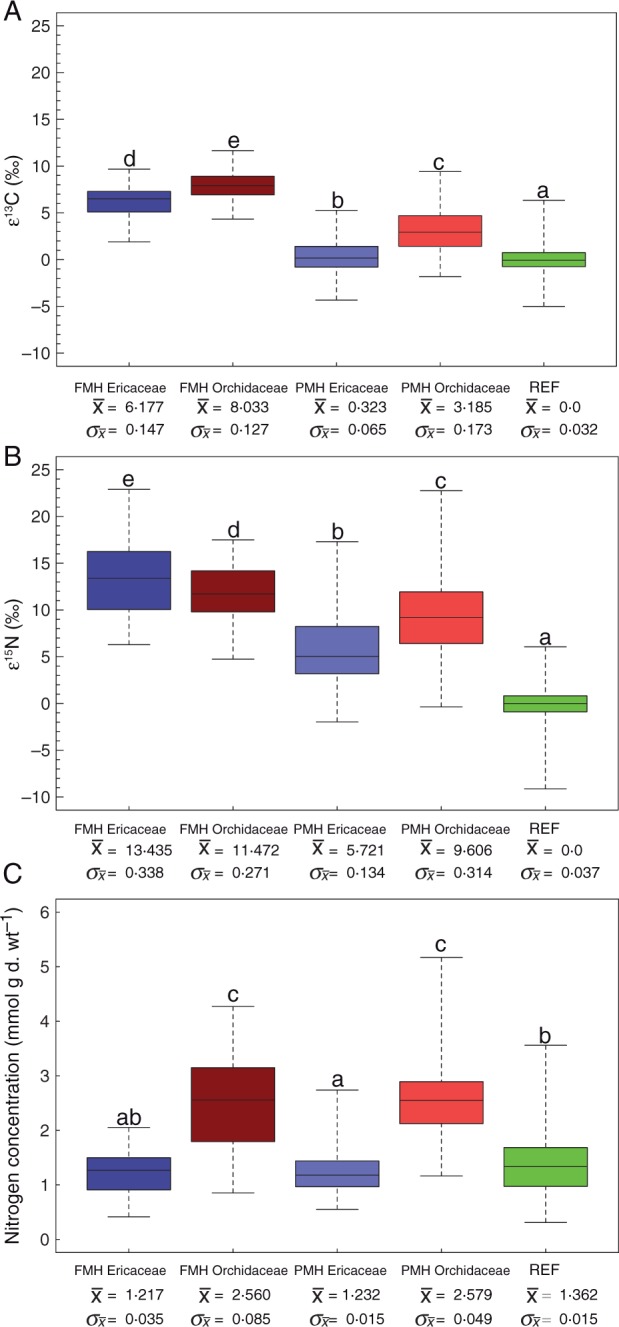
Box-and-whisker plots and summary statistics for the compiled data sets on FMH Orchidaceae and Ericaceae, PMH Orchidaceae and Ericaceae and autotrophic references in (A) enrichment factor ε13C, (B) enrichment factor ε15N and (C) nitrogen concentration. The box spans the first and third quartile, while the horizontal line in the box represents the median; whiskers extend to data extremes. Different letters indicate significant differences between the groups.
Data treatment and statistical analysis
To enable comparisons of C and N stable isotope abundances across populations, between species and at the familiar level, we used an isotope enrichment factor approach to normalize the data. If isotope abundance data were published as δ values, normalized enrichment factors (ε) were calculated as ε = δS – δREF, where δS is a single value of an autotrophic, a PMH or FMH plant, and δREF is the mean value of all autotrophic reference plants by plot (Preiss and Gebauer, 2008). Some of the N concentration data were published as percentage nitrogen content (%N). To unify N concentration, these data were converted into millimoles of nitrogen per gram dry weight (mmol N g d.wt−1).
We tested for differences between the groups FMH Orchidaceae, FMH Ericaceae, PMH Orchidaceae, PMH Ericaceae and corresponding autotrophic reference plants’ isotopic enrichment factors (ε13C and ε15N) and N concentrations with non-parametric statistics due to non-normally distributed data using the Kruskal–Wallis H-test in combination with a post-hoc Mann–Whitney U-test for multiple comparisons. P-values were adjusted using the sequential Bonferroni correction (Holm, 1979). To account for different sample sizes in pairwise comparisons and to standardize for the magnitude of an observed effect we calculated Cohen’s d effect size with:
and
where is the group mean and σ the groups’ standard deviations (Cohen, 1988). Effect sizes >0·8 are considered as large (Cohen, 1992). Variance, vd, of the effect size d was calculated using:
where n is the groups’ sample size (Borenstein et al., 2009). The same statistical tools were used to test for differences in enrichment factors (ε13C and ε15N) and N concentrations between the groups FMH, PMH and autotrophic Ericaceae and the remaining reference plants.
We used non-metric multidimensional scaling (NMDS) to visualize the organization of samples in two-dimensional space graphically, whereas their spatial arrangement exactly represents the similarity between the objects. For this, the Bray–Curtis index was used to calculate distance matrices from ε13C, ε15N and N concentration data using the function ‘metaMDS’ with two dimensions and 100 permutations in the R package ‘vegan’ (Oksanen et al., 2015). Stress values were calculated to evaluate how well the configuration provides a representation of the distance matrices; generally, a stress value <0·05 provides an excellent representation in reduced dimensions. Fitted vectors were calculated to display the response variables ε13C, ε15N and N concentration in the ordination space and to indicate the differences between the groups in association with these variables (Fig. 4). Each arrow shows the direction of the increasing response variable while its length is proportional to the correlation (R2) between the variable and the ordination (Fig. 4, Oksanen et al., 2015).
Fig. 4.
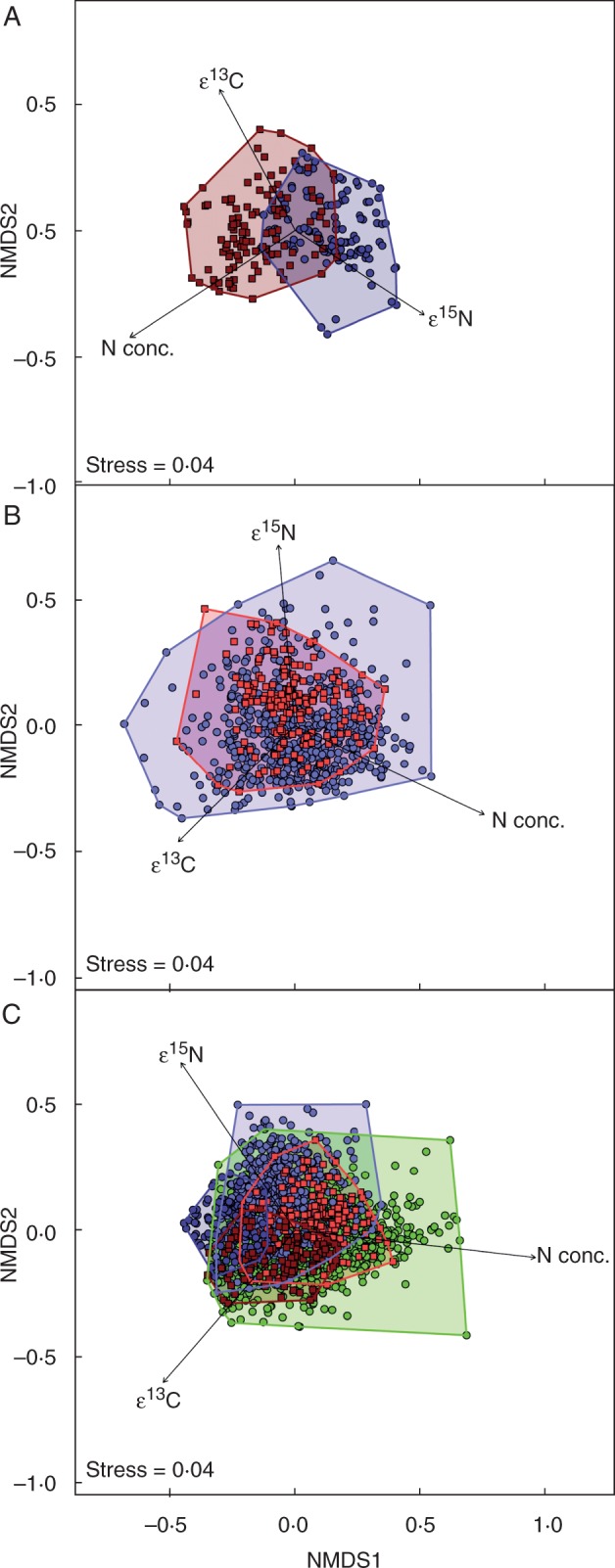
NMDS plots visualize Bray–Curtis dissimilarity matrices calculated from enrichment factors ε13C and ε15N and nitrogen concentration data in two-dimensional space. Fitted vectors display the response variables ε13C, ε15N and N concentration in the ordination space and indicate the differences between the groups in association with these variables. (A) FMH Ericaceae and Orchidaceae, stress = 0·04, 100 permutations; MANOVA R2 = 0·185, P < 0·001; (B) PMH Ericaceae and Orchidaceae, stress = 0·04, 100 permutations; MANOVA R2 = 0·22, P < 0·001; and (C) FMH Ericaceae, FMH Orchidaceae, PMH Ericaceae, PMH Orchidaceae and respective autotrophic references, stress = 0·04, 100 permutations; MANOVA R2 = 0·678, Padj < 0·001.
The function ‘adonis’ in the R package ‘vegan’ was used to perform a permutational MANOVA (multivariate analysis of variance) to test for significance of differences between group means using the aforementioned calculated distance matrices (Anderson, 2001). P-values from multiple pairwise comparisons were adjusted (Padj) using the sequential Bonferroni correction. For statistical analyses, we used the software environment R [version 3.1.2 (Pumpkin Helmet); R Development Core Team, 2014)] supported by the add-on packages ‘coin’ (version 1.0-24; Hothorn et al., 2006, 2008a), ‘multcomp’ (version 1.3-8; Hothorn et al., 2008b), ‘scattergrid’ (version 1.0; Gassem, 2015), ‘scatterplot3d’ (version 0.3-35; Ligges and Mächler, 2003) and ‘vegan’ (version 2.2-1; Oksanen et al., 2015) with a significance level of α = 0·05.
RESULTS
Based on comparisons of FMH and PMH Orchidaceae and Ericaceae, significant patterns have emerged (Fig. 3; Supplementary Data Table S2). Differences among our groups in 13C enrichment (ε) found support for all three of our hypotheses (Fig. 3A; Table S2). In support of hypotheses (1) and (2), FMH and PMH Orchidaceae were on average significantly more enriched in 13C than FMH and PMH Ericaceae (Fig. 3A; Table 2). In support of hypothesis (3) and as anticipated from previous plant population studies, autotrophic reference species were less enriched in 13C relative to PMH species from both families, which were less enriched in 13C than all FMH species (Fig. 3A; Table 2). A comparison of FMH and PMH Ericaceae with autotrophic Ericaceae from the reference plant group confirms these findings. Autotrophic Ericaceae were significantly depleted in 13C compared with FMH and PMH Ericeaceae and even more depleted in 13C than the remaining reference plants (Supplementary Data Fig. S1A; Table S1). Congruent with our non-parametric comparisons, effect sizes for ε13C among groups were high (Table 3), especially so for FMH Orchidaceae and Ericaceae relative to autotrophic references (d = 5·979 and 4·463, respectively). Interestingly, ε13C values of PMH Orchidaceae had a higher scatter than all other groups (Figs 2B and 3A) despite this variation in the data, P-values and effect sizes between this group, autotrophic references, FMH species and PMH Ericaceae were significant and high. The more tightly clustered ε13C values of PMH Ericaceae varied little based on effect size from references (d = 0·229), while being statistically distinguishable from references based on our non-parametric test (Padj < 0·001).
Table 2.
Results from post-hoc pairwise comparisons between the groups FMH Orchidaceae, FMH Ericaceae, PMH Orchidaceae, PMH Ericaceae and autotrophic references with the non-parametric Mann–Whitney U-test after significant Kruskal–Wallis H-test (ε13C: H = 933·705, d.f. = 4, P < 0·001; ε15N: H = 1793·556, d.f. = 4, P < 0·001; N concentration: H = 574·618, d.f. = 4, P < 0·001)
| FMH Ericaceae |
FMH Orchidaceae |
PMH Ericaceae |
PMH Orchidaceae |
|||||
|---|---|---|---|---|---|---|---|---|
| U | Padj | U | Padj | U | Padj | U | Padj | |
| ε13C | ||||||||
| FMH Orchidaceae | 3409 | <0·001 | ||||||
| PMH Ericaceae | 79 505 | <0·001 | 75 590 | <0·001 | ||||
| PMH Orchidaceae | 21 339 | <0·001 | 22 553·5 | <0·001 | 17 892·5 | <0·001 | ||
| REF | 191 188 | <0·001 | 180 511 | < 0·001 | 479 972·5 | <0·001 | 242 162·5 | <0·001 |
| ε15N | ||||||||
| FMH Orchidaceae | 10 557·5 | <0·001 | ||||||
| PMH Ericaceae | 74 952 | <0·001 | 67 571 | <0·001 | ||||
| PMH Orchidaceae | 19 772 | <0·001 | 16 479 | <0·001 | 27 974 | <0·001 | ||
| REF | 195 774 | <0·001 | 184 069 | <0·001 | 849 267 | <0·001 | 285 895 | <0·001 |
| N concentration | ||||||||
| FMH Orchidaceae | 1245 | <0·001 | ||||||
| PMH Ericaceae | 36 479 | 1·0 | 59 398 | <0·001 | ||||
| PMH Orchidaceae | 700 | <0·001 | 10 458 | 1·0 | 3581 | <0·001 | ||
| REF | 72 306·5 | 0·1 | 131 120 | <0·001 | 334 105 | <0·001 | 234 909·5 | <0·001 |
P-values were adjusted using the sequential Bonferroni-correction.
Table 3.
Results of Cohen’s d effect size and variance vd calculations for ε13C, ε15N and N concentration in FMH Ericaceae, FMH Orchidaceae, PMH Ericaceae, PMH Orchidaceae and autotrophic references
| FMH Ericaceae |
FMH Orchidaceae |
PMH Ericaceae |
PMH Orchidaceae |
|||||
|---|---|---|---|---|---|---|---|---|
| d | vd | d | vd | d | vd | d | vd | |
| ε13C | ||||||||
| FMH Orchidaceae | 1·246 | 0·023 | ||||||
| PMH Ericaceae | 3·775 | 0·014 | 5·091 | 0·014 | ||||
| PMH Orchidaceae | 1·481 | 0·024 | 2·434 | 0·024 | 1·394 | 0·015 | ||
| REF | 4·463 | 0·012 | 5·979 | 0·012 | 0·229 | 0·004 | 1·652 | 0·014 |
| ε15N | ||||||||
| FMH Orchidaceae | 0·570 | 0·053 | ||||||
| PMH Ericaceae | 2·173 | 0·034 | 1·817 | 0·030 | ||||
| PMH Orchidaceae | 0·911 | 0·052 | 0·480 | 0·047 | 0·976 | 0·029 | ||
| REF | 4·711 | 0·029 | 4·866 | 0·025 | 2·281 | 0·006 | 2·851 | 0·024 |
| N concentration | ||||||||
| FMH Orchidaceae | 1·930 | 0·011 | ||||||
| PMH Ericaceae | 0·040 | 0·004 | 1·953 | 0·009 | ||||
| PMH Orchidaceae | 2·314 | 0·007 | 0·023 | 0·012 | 2·364 | 0·004 | ||
| REF | 0·321 | 0·004 | 1·598 | 0·092 | 0·304 | 0·001 | 1·875 | 0·004 |
From comparisons of 15N enrichment among our study groups, a similar pattern emerges where we found support for all three of our hypotheses (Fig. 3B; Table S2). However, in contrast to patterns of 13C enrichment, FMH Ericaceae were significantly more enriched in 15N relative to FMH Orchidacae (Padj = 0·005; Fig. 3B; Table 2). Similar to 13C, PMH Orchidaceae were significantly more enriched in 15N relative to PMH Ericaceae (Padj < 0·001; Fig. 3B; Table 2). All groups were significantly more enriched in 15N relative to references, and full mycoheterotrophs were significantly more enriched in 15N than partial mycoheterotrophs (Figs 2 and 3B; Table 2). Effect sizes among all groups were also high (Table 3), especially so for FMH Orchidacae and Ericaceae vs. references (d = 4·866 and 4·711, respectively). However, based on our non-parametric test, FMH Orchidaceae were significantly more enriched in 15N relative to PMH Orchidaceae (Padj < 0·001), effect size between these two groups was medium (d = 0·480). Autotrophic Ericaceae were significantly depleted in 15N compared with FMH and PMH Ericaceae and only slightly enriched in 15N compared with the remaining reference plants (Supplementary Data Fig. S1B; Table S1).
While N concentration data werenot available for all species for which we had stable isotope profiles (Fig. 3C; Table S2), we were still able to detect significant differences among groups. We found support for our first hypothesis where FMH Ericaceae had, on average, lower N concentrations relative to FMH Orchidaceae (Padj < 0·001; Fig. 3C; Table 2). We also found support for hypothesis (2) where PMH Orchidaceae had significantly higher N concentrations relative to PMH Ericaceae (Padj < 0·001; Fig. 3C; Table 2). However, we did not find significant differences between the N concentrations of FMH and PMH Ericaceae (Padj = 1·0) or FMH and PMH Orchidaceae (Padj = 1·0) (Fig. 3C; Table 2). The effect sizes for differences in N concentration lend further support to hypotheses (1) and (2) where comparisons between FMH Ericaceae and Orchidaceae and partial mycoheterotrophs in both families were high (Table 3). In general, mean N concentrations in FMH and PMH Orchidaceae were about twice as high as in reference plants (Fig. 3C; Table 2) while mean N concentrations in FMH and PMH Ericaceae were, on average, lower than in reference plants (Fig. 3C; Table 2). Furthermore, N concentrations of autotrophic Ericaceae among the reference plants which included both arbutoid and ericoid mycorrhizal species were lower than those found for FMH and PMH Ericaceae (Supplementary Data Fig. S1C; Table S1).
Ordination of a Bray–Curtis dissimilarity matrix calculated from ε13C, ε15N and N concentration data of FMH Orchidaceae and Ericaceae with NMDS supports hypothesis (1) as the groups are segregated in ordination space (Fig. 4A), and a MANOVA showed a significant effect of group on the ordination (R2 = 0·185, P = 0·001). Fitted vectors in the ordination of FMH Orchidaceae and Ericaceae were maximally correlated with N concentration (R2 = 0·821, P < 0·001), ε15N (R2 = 0·500, P < 0·001) and ε13C (R2 = 0·493, P < 0·001). NMDS for PMH Orchidaceae and Ericaceae (Fig. 4B) also supports hypothesis (2) as a MANOVA revealed a significant effect of group on the ordination (R2 = 0·220, P = 0·001). Here, fitted vectors in the ordination of PMH Orchidaceae and Ericaceae were maximally correlated with ε15N (R2 = 0·424, P < 0·001), N concentration (R2 = 0·313, P < 0·001) and ε13C (R2 = 0·261, P < 0·001). An ordination of a Bray–Curtis dissimilarity matrix calculated from ε13C, ε15N and N concentration data of FMH Orchidaceae and Ericaceae, PMH Orchidaceae and Ericaceae and autotrophic references with NMDS (Fig. 4C) supports hypothesis (3) through the distinct clustering of the groups (PMH, FMH and autotrophic species) in the ordination space, and a MANOVA showed a significant effect of group on the ordination (R2 = 0·678, Padj = 0·001). Here, fitted vectors in the ordination of FMH Orchidaceae and Ericaceae, PMH Orchidaceae and Ericaceae and autotrophic references were maximally correlated with N concentration (R2 = 0·442, P < 0·001), ε15N (R2 = 0·306, P < 0·001) and ε13C (R2 = 0·301, P < 0·001). Generally, the stress values of all ordinations provide an excellent representation in reduced dimensions (Fig. 4).
DISCUSSION
Overall patterns of nitrogen concentrations and stable isotope enrichment among partially and fully mycoheterotrophic plants and autotrophs
By assembling all available data sets of mycoheterotrophic species, we have confirmed that with an increasing dependency on fungal nutrition, there is a corresponding increase in N, and especially C isotope enrichment (Figs 1–3). Previous studies from plant populations have found similar patterns in isotope abundances where autotrophs, partial mycoheterotrophs and full mycoheterotrophs fit the theoretical principles of isotope enrichment along a food chain (Fry, 2006). However, we now find that this pattern holds across a much larger sample size assembled from study sites across the globe. Furthermore, by synthesizing the data from this extensive sampling, additional patterns have emerged. Despite depending upon the same functional guild of fungi, FMH and PMH Orchidaceae and Ericaceae associated with EM fungi behave isotopically dissimilarly. Also, the N concentration turns out to be an additional and critical factor to consider when examining the ecophysiology of putatively mycoheterotrophic taxa within these two plant families.
Drivers of nitrogen concentrations among plant families and trophic groups
Why are N concentrations so much higher in FMH and PMH orchids than in corresponding Ericaceae? One prediction might be that the physiology or substrate use of the EM fungi that partner with orchids differs from those that partner with ericaceous species (sensu Gebauer and Taylor, 1999; Taylor et al., 2003). This prediction is certainly worth investigating. However, the fact that there is substantial overlap in fungal partnerships among some species of mycoheterotrophic Orchidaceae and Ericaceae (e.g. Russulaceae spp. host both FMH orchids and ericaceous species), it seems to be the least likely explanatory factor. A potentially more important factor is differences in the anatomy and physiology of orchid vs. ericaceous mycorrhizae. When EM fungi colonize orchid protocorms or roots, they form intracellular pelotons (Burgeff, 1959). These pelotons are digested by the orchid, which probably uses the fungal biomass for its own growth (Bougoure et al., 2014). However, the relative flux of compounds from fungi to orchids via peloton digestion vs. active fungus–plant membrane transport is currently an unresolved question [see contradictory findings by Bougoure et al. (2014) and Kuga et al. (2014)]. Conversely, EM fungi associating with PMH or FMH Ericaceae form either monotropoid or arbutoid mycorrhizal structures (Smith and Read, 2008). While the exact functions of these structures are unknown (Smith and Read, 2008; Imhof et al., 2013, and references therein), ericaceous mycoheterotrophic species may rely more on active membrane transport of fungal compounds rather than mass flow, where the former probably represents a much more selective system. Because EM fungi have much higher N concentrations in their tissues than autotrophic plants from identical habitats (Gebauer and Dietrich, 1993; Gebauer and Taylor, 1999), high N concentration among mycoheterotrophic Orchidaceae may be largely due to differences in N transport mechanisms, where mass flow of N via digestion of fungal tissue would lead to an increased N concentration in orchids compared with other species (Tedersoo et al., 2007; Stöckel et al., 2014). However, to date, explicit tests of the relative contributions of fungal compounds by mass flow vs. active membrane transport to mycoheterorophic Orchidaceae or Ericaceae are mostly lacking.
Differences in the life history strategies of orchids and ericaceous species may also contribute to explaining differences in their N concentrations. The majority of PMH EM-associated orchid species are deciduous, while PMH Ericaceae are evergreen and sclerophyllous. In general, evergreen sclerophyllous plant tissues tend to have lower N concentrations than deciduous tissues (Gebauer et al., 1988). In our analyses, many more deciduous species than evergreen species served as reference plants, so mean N concentrations in PMH Ericaceae significantly lower than in reference plants may be explained by these morphological differences. Despite their perennial nature as geophytes that is more similar to orchids, the maintenance of low N concentrations in FMH Ericaceae points towards plant evolutionary history rather than trophic strategy as a determinant of N concentrations. However, N concentrations in FMH and PMH Orchidaceae twice as high as in reference plants cannot be explained exclusively by their perennial nature or evolutionary history.
Drivers of carbon and nitrogen stable isotope enrichment among plant families and trophic groups
Carbon isotope abundance in plant bulk tissues is mainly driven by three factors: the type of photosynthetic pathway (C3, C4 or CAM), stomatal regulation and origin of the carbon source. Since no CAM orchids are included in our data set, all investigated target plants (Orchidaceae and Ericaceae) are either C3 or are non-photosynthetic full mycoheterotrophs. Thus, differences in 13C discrimination among photosynthetic pathways can be ruled out as a driver for the differences in carbon isotope abundance patterns observed here. A decrease in stomatal conductance of C3 plants shifts their carbon isotope abundances towards 13C enrichment (Farquhar et al., 1989). Thus, one might assume that low stomatal conductance is a factor contributing to the overall 13C enrichment of PMH and FMH plants as well as the differences observed between the plant families Orchidaceae and Ericaceae. However, the patterns observed here of 13C depletion in evergreen sclerophyllous PMH Ericaceae relative to deciduous PMH Orchidaceae do not fit with the general tendency of sclerophyllous plants towards lower stomatal conductance and therefore greater 13C enrichment (Larcher, 2003). Furthermore, for non-photosynthetic FMH albino individuals of the orchid Cephalanthera damasonium, a significantly higher stomatal conductance and simultaneously higher 13C enrichment than in PMH individuals has been found (Julou et al., 2005; Roy et al., 2013). Consequently, systematic differences in stomatal conductance are also unlikely to be responsible for the differences in 13C enrichment found for FMH and PMH plants of the Orchidaceae and Ericaceae. Thus, the origin of the carbon source remains as the most likely factor responsible for the general 13C enrichment of FMH and PHM orchids and ericaceous species in relation to their reference plants and each other.
For FMH plants, all of their carbon originates from the fungal source. Therefore, differences in the 13C enrichment of fungi that serve as carbon sources for FMH Orchidaceae and Ericaceae are probably responsible for their relative 13C enrichment. The greater 13C enrichment found on average in FMH Orchidaceae relative to Ericaceae may be due to differences in the biochemical make-up of tissues (sensu Gebauer and Schulze, 1991; Badeck et al., 2005; Cernusak et al., 2009) or, again, possibly due to greater relative fungal C contributions from the digestion of pelotons entailing little 13C discrimination, as opposed to active C transport which discriminates against 13C.
For PMH plants, the situation is more complex, because they are composed of C from two different origins, atmospheric CO2 gained through C3 photosynthesis and organic matter from the fungal source, and the ratios of these two sources can vary based on environmental factors. For example, light availability has been shown to be an important determinant for the 13C enrichment of some PMH orchids and at least one PMH ericaceous species (Preiss et al., 2010; Matsuda et al., 2012). These studies found that as light becomes more limiting, some partial mycoheterotrophs increase their dependency on 13C-enriched fungal C. So, if some of the PMH species included in this study were collected in different light environments, this could have led to significant differences in their ε13C values. Another example is the leafless, but still stem-chlorophyllous PMH orchid Corallorhiza trifida. This species is significantly more enriched in 13C relative to other PMH orchids, while it is less enriched in 15N (Fig. 1B). There has been some debate in the literature regarding the ability of C. trifida to gain significant amounts of carbon through photosynthesis (Zimmer et al., 2008; Cameron et al., 2009), so, while we include this species among the partial mycoheterotrophs, it may actually be more similar to FMH orchids.
Similar to FMH species, the identity of fungal symbionts associating with partial mycoheterotrophs and differences in their C substrate use cannot be completely ruled out as a possible additional factor affecting partial mycoheterotrophs' 13C enrichment. However, partial mycoheterotrophs studied thus far tend to associate with a diversity of EM fungi and there is substantial overlap in the fungal taxa known to partner with PMH Orchidaceae and Ericaceae. Until future studies determine whether all or a sub-set of these partners are responsible for mycoheterotrophic C gains, there are no grounds to assume that differences in fungal partner identities are leading to differences in 13C enrichment between partial mycoheterotrophs in these families.
Nitrogen isotope abundance in plant tissue integrates the isotopic composition of the various N sources utilized by a plant (Robinson, 2001). From our investigations 15N enrichment of EM-associated FMH and PMH Orchidaceae and Ericaceae in comparison with reference plants indicates the utilization of different N sources by FMH and PMH plants relative to autotrophic plants. Ectomycorrhizal fungi can be highly variable in their 15N enrichment (Taylor et al., 2003; Hobbie et al., 2005; Mayor et al., 2009), because of a wide range of soil nutrient mining and catabolic abilities among genera (and sometimes species) of fungi (Gebauer and Taylor, 1999; Emmerton et al., 2001; Taylor et al., 2004; Pritsch and Garbaye, 2011). Similar to differences in 13C enrichment, interactions with different fungal hosts that differ in their N acquisition strategies and differences in the physiology of the fungus–plant matter exchange may explain some, but not all, of the significant interfamilial and interspecific variations in 15N enrichment among FMH and PMH Ericaceae and Orchidaceae. However, future investigations of two outlier species among the PMH Orchidaceae are needed; Epipactis distans and E. leptochila are significantly more enriched in 15N than all other PMH species (Fig. 1B). Interestingly, these two species are exclusively associated with EM Ascomycetes (J.M.-I. Schiebold, unpubl. data). Future investigation should test whether above-average 15N enrichment among PMH Orchidaceae is related to the 15N enrichment of EM Ascomycetes. Furthermore, it remains unknown why species that partner with closely related fungi (e.g. Sarcodes sanguinea and Pterospora andromedea) and grow in sympatry exhibit such significant differences in 15N enrichment (Fig. 1B). These species provide a potentially fruitful study system for examining the ecology of mycoheterotrophy; specifically niche partitioning through differences in ecophysiological traits.
Future directions
Given that we have confirmed a general isotope food chain model for FMH and PMH species across a large data set and geographic sampling area, while also finding that there are significant differences among plant families that occupy the same trophic position, how should future studies progress? We suggest that future studies should focus on identifying the physiological mechanisms leading to differences between mycoheterotrophic orchids and ericaceous species that associate with similar guilds of fungi. Similarly, future investigations should attempt to identify mechanisms leading to interspecific differences in isotope enrichment within the same plant family and trophic groups. Mycoheterotrophic species that partner with similar fungi and grow in sympatry, but have disparate ε13C and ε15N values, provide ideal study systems. Furthermore, until we have a better understanding of why these familial differences exist, future population studies of putative partial mycoheterotrophs that use a mixing model approach to identify the degree of partial mycoheterotrophy should only use FMH species from the same family as the FMH end-member (e.g. Tedersoo et al., 2007).
Adding N concentration as an additional explanatory variable will aid future research in distinguishing differences among full and partial mycoheterotrophs and autotrophs. When N concentrations along with ε15N and ε13C values were incorporated into an ordination of a Bray–Curtis dissimilarity matrix with NMDS, MANOVA showed a significant effect of group on the ordination (Padj = 0·001) and these three factors combined explain approx. 68% of the variation in the data set (Fig. 4). Similar models that segregated FMH Orchidaceae from Ericaceae and did the same for partial mycoheterotrophs for each family found significant differences between plant families, but the three factors only explained about 19 and 22 % of the variation in the data sets, respectively (Fig. 4A, B). Therefore, future investigations should consider measurements of additional explanatory response variables. For instance, analysis of concentrations and stable isotope abundances of additional elements involved in organic matter exchange such as hydrogen, oxygen or sulphur, may prove informative for teasing apart the dependency of mycoheterotrophic plants on fungal-derived organic matter (Gebauer et al., 2016). Also, studies that identify the C and N compounds and transfer pathways among different types of mycoheterotrophic plants are urgently needed.
Finally, much of the intra- and interspecific variation in N concentrations, and 13C and 15N enrichment of partial mycoheterotrophs is probably due to the environment in which these plants are subsisting (Preiss et al., 2010; Hynson et al., 2012; Matsuda et al., 2012). So, measurements of stable isotope composition throughout the life cycle of individual plants and over time within adult plants could add valuable explanatory power to these models, as would data on light environment, leaf chlorophyll concentrations, and plant–water and plant–nutrient relations (Preiss et al., 2010; Stöckel et al., 2011; Hynson et al., 2012; Matsuda et al., 2012).
Conclusions
In summary, we have found that measurements of C and N stable isotope abundances are able to distinguish mycoheterotrophic Ericaceae from mycoheterotrophic Orchidaceae and confirmed that isotopic differences among partial and full mycoheterotrophs and autotrophs hold across plant populations and are geographically widespread. Furthermore, N concentration in tissues of Orchidaceae and Ericaceae turned out to be an additional and hitherto insufficiently considered factor differentiating these two plant families. Though different identities of fungal hosts cannot be ruled out as factors contributing to the differences in C and N isotopic composition and N concentration between FMH and PMH Orchidaceae and Ericaceae, family- or species-specific characteristics in the physiology of matter exchange between fungi and plants are considered as the most likely reasons underlying the observed differences.
SUPPLEMENTARY DATA
Supplementary data are available online at www.aob.oxfordjournals.org and consist of the following. Figure S1: box-and-whisker plots and summary statistics for the compiled data sets on FMH Ericaceae, PMH Ericaceae, autotrophic Ericaceae among the references and the remaining autotrophic references in enrichment factor ε13C, enrichment factor ε15N and nitrogen concentration. Table S1: (A) Results from post-hoc pairwise comparisons between the groups of FMH Ericaceae, PMH Ericaceae, autotrophic Ericaceae among the references and the remaining autotrophic references. (B) Results from effect-size calculations. Table S2: mean enrichment factors ε13C and ε15N, mean nitrogen concentration data, standard deviation and number of samples for each fully mycoheterotrophic and partially mycoheterotrophic Ericaceae and Orchidaceae species.
ACKNOWLEDGEMENTS
The authors would like to thank Iris Adam, Bastian Burghardt, Veronika Johansson, Katja Preiss, Marc-André Selosse and Leho Tedersoo for allowing us to include currently unpublished data for this analysis. This investigation contributes to the DFG project GE 565/7-2.
LITERATURE CITED
- Abadie JC, Püttsepp U, Gebauer G, Faccio A, Bonfante P, Selosse MA. 2006. Cephalanthera longifolia (Neottieae, Orchidaceae) is mixotrophic: a comparative study between green and nonphotosynthetic individuals. Canadian Journal of Botany – Revue Canadienne De Botanique 84: 1462–1477. [Google Scholar]
- Anderson MJ. 2001. A new method for non-parametric multivariate analysis of variance. Austral Ecology 26: 32–46. [Google Scholar]
- Badeck FW, Tcherkez G, Nogués S, Piel C, Ghashghaie J. 2005. Post-photosynthetic fractionation of stable carbon isotopes between plant organs– a widespread phenomenon. Rapid Communications in Mass Spectrometry 19: 1381–1391. [DOI] [PubMed] [Google Scholar]
- Bellino A, Alfani A, Selosse MA, Guerrieri R, Borghetti M, Baldantoni D. 2014. Nutritional regulation in mixotrophic plants: new insights fom Limodorum abortivum. Oecologia 175: 875–885. [DOI] [PubMed] [Google Scholar]
- Bougoure J, Ludwig M, Brundrett M, et al. 2014. High-resolution secondary ion mass spectrometry analysis of carbon dynamics in mycorrhizas formed by an obligately myco-heterotrophic orchid. Plant, Cell & Environment 37: 1223–1230. [DOI] [PubMed] [Google Scholar]
- Bidartondo MI. 2005. The evolutionary ecology of myco-heterotrophy. New Phytologist 167: 335–352. [DOI] [PubMed] [Google Scholar]
- Bidartondo MI, Bruns TD. 2002. Fine-level mycorrhizal specificity in the Monotropoideae (Ericaceae): specificity for fungal species groups. Molecular Ecology 11: 557–569. [DOI] [PubMed] [Google Scholar]
- Bidartondo MI, Kretzer AM, Pine EM, Bruns TD. 2000. High root concentration and uneven ectomycorrhizal diversity near Sarcodes sanguinea (Ericaceae): a cheater that stimulates its victims? American Journal of Botany 87: 1783–1788. [PubMed] [Google Scholar]
- Bidartondo MI, Redecker D, Hijri I, et al. 2002. Epiparasitic plants specialized on arbuscular mycorrhizal fungi. Nature 419: 389–392. [DOI] [PubMed] [Google Scholar]
- Bidartondo MI, Burghardt B, Gebauer G, Bruns TD, Read DJ. 2004. Changing partners in the dark: isotopic and molecular evidence of ectomycorrhizal liaisons between forest orchids and trees. Proceedings of the Royal Society B: Biological Sciences 271: 1799–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolin JF, Tennakoon KU, Majid MBA, Cameron DD. 2015. Isotopic evidence of partial mycoheterotrophy in Burmannia coelestis (Burmanniaceae). Plant Species Biology. doi:10.1111/1442–1984.12116 [Google Scholar]
- Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. 2009. Introduction to meta-analysis. Chichester, UK: Wiley, 21–32. [Google Scholar]
- Burgeff H. 1959. Mycorrhiza of orchids In: Withner K, ed. The orchids. New York: The Ronald Press Company, 361–395. [Google Scholar]
- Cameron DD, Bolin JF. 2010. Isotopic evidence of partial mycoheterotrophy in the Gentianaceae: Bartonia virginica and Obolaria virginica as case studies. American Journal of Botany 97: 1272–1277. [DOI] [PubMed] [Google Scholar]
- Cameron DD, Preiss K, Gebauer G, Read DJ. 2009. The chlorophyll-containing orchid Corallorhiza trifida derives little carbon through photosynthesis. New Phytologist 183: 358–364. [DOI] [PubMed] [Google Scholar]
- Cernusak LA, Tcherkez G, Keitel C, et al. 2009. Why are non-photosynthetic tissues generally C-13 enriched compared with leaves in C-3 plants? Review and synthesis of current hypotheses. Functional Plant Biology 36: 199–213. [DOI] [PubMed] [Google Scholar]
- Cohen J. 1992. A power primer. Psychological Bulletin 112: 155–159. [DOI] [PubMed] [Google Scholar]
- Cohen J. 1988. Statistical power analysis for the behavioral sciences, 2nd edn. Hillsdale, NJ: Lawrence Earlbaum Associates, 44. [Google Scholar]
- Courty PE, Walder F, Boller T, et al. 2011. Carbon and nitrogen metabolism in mycorrhizal networks and mycoheterotrophic plants of tropical forests: a stable isotope analysis. Plant Physiology 156: 952–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullings KW, Szaro TM, Bruns TD. 1996. Evolution of extreme specialization within a lineage of ectomycorrhizal epiparasites. Nature 379: 63–66. [Google Scholar]
- Dearnaley JDW, Bougoure JJ. 2010. Isotopic and molecular evidence for saprotrophic Marasmiaceae mycobionts in rhizomes of Gastrodia sesamoides. Fungal Ecology 3: 288–294. [Google Scholar]
- Dearnaley JDW, Martos F, Selosse MA. 2012. Orchid mycorrhizas: molecular ecology, physiology, evolution and conservation aspects In: Hock B, ed. Fungal associations, 2nd edn. Berlin: Springer, 207–230. [Google Scholar]
- Emmerton KS, Callaghan TV, Jones HE, Leake JR, Michelsen A, Read DJ. 2001. Assimilation and isotopic fractionation of nitrogen by mycorrhizal and nonmycorrhizal subarctic plants. New Phytologist 151: 513–524. [Google Scholar]
- Farquhar GD, Ehleringer JR, Hubick KT. 1989. Carbon isotope discrimination and photosynthesis. Annual Review of Plant Physiology and Plant Molecular Biology 40: 503–537. [Google Scholar]
- Fry B. 2006. Stable iotope ecology. New York: Springer. [Google Scholar]
- Gassem A. 2015. Scattergrid: add yz- and xz-grid to scatterplot3d. R package version 1.0.
- Gebauer G, Dietrich P. 1993. Nitrogen isotope ratios in different compartments of a mixed stand of spruce, larch and beech trees and of understorey vegetation including fungi. Isotopenpraxis 29: 35–44. [Google Scholar]
- Gebauer G, Meyer M. 2003. 15N and 13C natural abundance of autotrophic and myco-heterotrophic orchids provides insight into nitrogen and carbon gain from fungal association. New Phytologist 160: 209–223. [DOI] [PubMed] [Google Scholar]
- Gebauer G, Schulze ED. 1991. Carbon and nitrogen isotope ratios in different compartments of a healthy and a declining Picea abies forest in the Fichtelgebirge, NE Bavaria. Oecologia 87: 198–207. [DOI] [PubMed] [Google Scholar]
- Gebauer G, Taylor AFS. 1999. 15N natural abundance in fruit bodies of different functional groups of fungi in relationship to substrate utilization. New Phytologist 142: 93–101. [Google Scholar]
- Gebauer G, Rehder H, Wollenweber B. 1988. Nitrate, nitrate reduction and organic nitrogen in plants from different ecological and taxonomic groups of Central Europe. Oecologia 75: 371–385. [DOI] [PubMed] [Google Scholar]
- Gebauer G, Preiss K, Gebauer AC. 2016. Partial mycoheterotrophy is more widespread among orchids than previously assumed. New Phytologist 211: 11–15. [DOI] [PubMed] [Google Scholar]
- Gonneau C, Jersáková J, de Tredern E, et al. 2014. Photosynthesis in perennial mixotrophic Epipactis spp. (Orchidaceae) contributes more to shoot and fruit biomass than to hypogeous survival. Journal of Ecology 102: 1183–1194. [Google Scholar]
- Hobbie EA, Jumpponen A, Trappe J. 2005. Foliar and fungal 15N:14N ratios reflect development of mycorrhizae and nitrogen supply during primary succession: testing analytical models. Oecologia 146: 258–268. [DOI] [PubMed] [Google Scholar]
- Holm S. 1979. A simple sequentially rejective multiple test procedure. Scandinavian Journal of Statistics 6: 65–70. [Google Scholar]
- Hothorn T, Hornik K, van de Wiel MA, Zeileis A. 2006. A lego system for conditional inference. American Statistician 60: 257–263. [Google Scholar]
- Hothorn T, Hornik K, van de Wiel MA, Zeileis A. 2008a. Implementing a class of permutation tests: the coin package. Journal of Statistical Software 28: 1–23. [Google Scholar]
- Hothorn T, Bretz F, Westfall P. 2008b. Simultaneous inference in general parametric models. Biometrical Journal 50: 346–363. [DOI] [PubMed] [Google Scholar]
- Hynson NA, Bruns TD. 2009. Evidence of a myco-heterotroph in the plant family Ericaceae that lacks mycorrhizal specificity. Proceedings of the Royal Society B: Biological Sciences 276: 4053–4059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynson NA, Bruns TD. 2010. Fungal hosts for mycoheterotrophic plants: a nonexclusive, but highly selective club. New Phytologist 185: 598–601. [DOI] [PubMed] [Google Scholar]
- Hynson NA, Preiss K, Gebauer G, Bruns TD. 2009. Isotopic evidence of full and partial myco-heterotrophy in the plant tribe Pyroleae (Ericaceae). New Phytologist 182: 719–726. [DOI] [PubMed] [Google Scholar]
- Hynson NA, Mambelli S, Amend AS, Dawson TE. 2012. Measuring carbon gains from fungal networks in understory plants from the tribe Pyroleae (Ericaceae): a field manipulation and stable isotope approach. Oecologia 169: 307–317. [DOI] [PubMed] [Google Scholar]
- Hynson NA, Madsen TP, Selosse MA, et al. 2013. The physiological ecology of mycoheterotrophic plants In: Merckx VSFT, ed. Mycoheterotrophy: the biology of plants living on fungi. New York: Springer, 297–342. [Google Scholar]
- Hynson NA, Bidartondo MI, Read DJ. 2015. Are there geographic mosaics of mycorrhizal specificity and partial mycoheterotrophy? A case study in Moneses uniflora (Ericaceae). New Phytologist 208: 1003–1007. [DOI] [PubMed] [Google Scholar]
- Imhof S, Massicotte HB, Melville LH, Peterson RL. 2013. Subterranean morphology and mycorrhizal structures In: Merckx VSFT, ed. Mycoheterotrophy: the biology of plants living on fungi. New York: Springer, 157–214. [Google Scholar]
- Johansson VA, Mikusinska A, Ekblad A, Eriksson O. 2015. Partial mycoheterotrophy in Pyroleae: nitrogen and carbon stable isotope signatures during development from seedling to adult. Oecologia 177: 203–211. [DOI] [PubMed] [Google Scholar]
- Julou T, Burghardt B, Gebauer G, Berveiller D, Damesin C, Selosse MA. 2005. Mixotrophy in orchids: insights from a comparative study of green individuals and nonphotosynthetic individuals of Cephalanthera damasonium. New Phytologist 166: 639–653. [DOI] [PubMed] [Google Scholar]
- Koricheva J, Gurevitch J. 2014. Uses and misues of meta-analysis in plant ecology. Journal of Ecology 101: 828–844. [Google Scholar]
- Kuga Y, Sakamoto N, Yurimoto H. 2014. Stable isotope cellular imaging reveals that both live and degrading fungal pelotons transfer carbon and nitrogen to orchid protocorms. New Phytologist 202: 594–605. [DOI] [PubMed] [Google Scholar]
- Larcher W. 2003. Physiological plant ecology, 4th edn. Berlin: Springer. [Google Scholar]
- Leake JR. 1994. Tansley review No. 69. The biology of myco-heterotrophic (‘saprophytic’) plants. New Phytologist 127: 171–216. [DOI] [PubMed] [Google Scholar]
- Lee YI, Yang CK, Gebauer G. 2015. The importance of associations with saprotrophic non-Rhizoctonia fungi among fully mycoheterotrophic orchids is currently underestimated: novel evidence from subtropical Asia. Annals of Botany 116: 423–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebel HT, Gebauer G. 2011. Stable isotope signatures confirm carbon and nitrogen gain through ectomycorrhizas in the ghost orchid Epipogium aphyllum Swartz. Plant Biology 13: 270–275. [DOI] [PubMed] [Google Scholar]
- Liebel HT, Preiss K, Gebauer G. 2009. Partiell mykoheterotrofi hos norske vintergrønnarter – relevans for vernetiltak overfor truede arter. Blyttia 67: 138–143. [Google Scholar]
- Liebel HT, Bidartondo MI, Preiss K, et al. 2010. C and N stable isotope signatures reveal constraints to nutritional modes in orchids from the Mediterranean and Macaronesia. American Journal of Botany 97: 903–912. [DOI] [PubMed] [Google Scholar]
- Ligges U, Mächler M. 2003. Scatterplot3d – an R package for visualizing multivariate data. Journal of Statistical Software 8: 1–20. [Google Scholar]
- Martos F, Dulormne M, Pailler T, et al. 2009. Independent recruitment of saprotrophic fungi as mycorrhizal partners by tropical achlorophyllous orchids. New Phytologist 184: 668–681. [DOI] [PubMed] [Google Scholar]
- Matsuda Y, Shimizu S, Mori M, Ito SI, Selosse MA. 2012. Seasonal and environmental changes of mycorrhizal associations and heterotrophy levels in mixotrophic Pyrola japonica (Ericaceae) growing under different light environments. American Journal of Botany 99: 1177–1188. [DOI] [PubMed] [Google Scholar]
- Mayor JR, Schuur EAG, Henkel TW. 2009. Elucidating the nutritional dynamics of fungi using stable isotopes. Ecology Letters 12: 171–183. [DOI] [PubMed] [Google Scholar]
- McKendrick SL, Leake JR, Taylor DL, Read DJ. 2000. Symbiotic germination and development of myco-heterotrophic plants in nature: ontogeny of Corallorhiza trifida and characterization of its mycorrhizal fungi. New Phytologist 145: 523–537. [DOI] [PubMed] [Google Scholar]
- Merckx V, Bidartondo MI, Hynson NA. 2009. Myco-heterotrophy: when fungi host plants. Annals of Botany 104:1255–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merckx V, Stöckel M, Fleischmann A, Bruns TD, Gebauer G. 2010. 15N and 13C natural abundance of two mycoheterotrophic and a putative partially mycoheterotrophic species associated with arbuscular mycorrhizal fungi. New Phytologist 188: 590–596. [DOI] [PubMed] [Google Scholar]
- Merckx VSFT. 2013. Mycoheterotrophy: the biology of plants living on fungi. New York: Springer. [Google Scholar]
- Motomura H, Selosse MA, Martos F, Kagawa A, Yukawa T. 2010. Mycoheterotrophy evolved from mixotrophic ancestors: evidence in Cymbidium (Orchidaceae). Annals of Botany 106: 573–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogura-Tsujita Y, Gebauer G, Hashimoto T, Umata H, Yukawa T. 2009. Evidence for novel and specialized mycorrhizal parasitism: the orchid Gastrodia confusa gains carbon from saprotrophic Mycena. Proceedings of the Royal Society B: Biological Sciences 276: 761–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oksanen J, Blanchet FG, Kindt R, et al. 2015. Vegan: community ecology package R package version 2.2-1. http://CRAN.R-project.org/package=vegan.
- Preiss K, Gebauer G. 2008. A methodological approach to improve estimates of nutrient gains by partially myco-heterotrophic plants. Isotopes in Environmental and Health Studies 44: 393–401. [DOI] [PubMed] [Google Scholar]
- Preiss K, Adam IKU, Gebauer G. 2010. Irradiance governs exploitation of fungi: fine-tuning of carbon gain by two partially myco-heterotrophic orchids. Proceedings of the Royal Society B: Biological Sciences 277: 1333–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritsch K, Garbaye J. 2011. Enzyme secretion by ECM fungi and exploitation of mineral nutrients from soil organic matter. Annals of Forest Science 68: 25–32. [Google Scholar]
- R Development Core Team. 2014. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- Robinson D. 2001. δ15N as an integrator of the nitrogen cycle. Trends in Ecology and Evolution 16: 153–162. [DOI] [PubMed] [Google Scholar]
- Roy M, Watthana S, Stier A, Richard F, Vessabutr S, Selosse MA. 2009. Two mycoheterotrophic orchids from Thailand tropical dipterocarpacean forests associate with a broad diversity of ectomycorrhizal fungi. BMC Biology 7: 51. doi:10.1186/1741-7007-7-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy M, Gonneau C, Rocheteau A, et al. 2013. Why do mixotrophic plants stay green? A comparison between green and achlorophyllous orchid individuals in situ. Ecological Monographs 83: 95–117. [Google Scholar]
- Selosse MA, Martos F. 2014. Do chlorophyllous orchids heterotrophically use mycorrhizal fungal carbon? Trends in Plant Science 19: 683–685. [DOI] [PubMed] [Google Scholar]
- Selosse MA, Roy M. 2009. Green plants that feed on fungi: facts and questions about mixotrophy. Trends in Plant Science 14: 64–70. [DOI] [PubMed] [Google Scholar]
- Selosse MA, Faccio A, Scappaticci G, Bonfante P. 2004. Chlorophyllous and achlorophyllous specimens of Epipactis microphylla (Neottieae, Orchidaceae) are associated with ectomycorrhizal septomycetes, including truffles. Microbial Ecology 47: 416–426. [DOI] [PubMed] [Google Scholar]
- Smith SE, Read DJ. 2008. Mycorrhizal symbiosis, 3rd edn. London: Academic Press. [Google Scholar]
- Stöckel M, Meyer C, Gebauer G. 2011. The degree of mycoheterotrophic carbon gain in green, variegated and vegetative albino individuals of Cephalanthera damasonium is related to leaf chlorophyll concentrations. New Phytologist 189: 790–796. [DOI] [PubMed] [Google Scholar]
- Stöckel M, Těšitelová T, Jersáková J, Bidartondo MI, Gebauer G. 2014. Carbon and nitrogen gain during the growth of orchid seedlings in nature. New Phytologist 202: 606–615. [DOI] [PubMed] [Google Scholar]
- Taylor DL, Bruns TD. 1997. Independent, specialized invasions of the ectomycorrhizal mutualism by two non-photosynthetic orchids. Proceedings of the National Academy of Sciences, USA 94: 4510–4515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor AFS, Fransson PM, Högberg P, Högberg MN, Plamboeck AH. 2003. Species level patterns in C-13 and N-15 abundance of ectomycorrhizal and saprotrophic fungal sporocarps. New Phytologist 159: 757–774. [DOI] [PubMed] [Google Scholar]
- Taylor AFS, Gebauer G, Read DJ. 2004. Uptake of nitrogen and carbon from double-labelled (15N and 13C) glycine by mycorrhizal pine seedlings. New Phytologist 164: 383–388. [DOI] [PubMed] [Google Scholar]
- Tedersoo L, Pellet P, Kõljalg U, Selosse MA. 2007. Parallel evolutionary paths to mycoheterotrophy in understorey Ericaceae and Orchidaceae: ecological evidence for mixotrophy in Pyroleae. Oecologia 151: 206–217. [DOI] [PubMed] [Google Scholar]
- Thomson Reuters. 2015. Thomson Reuters Web of Science.
- Trudell SA, Rygiewicz PT, Edmonds RL. 2003. Nitrogen and carbon stable isotope abundances support the myco-heterotrophic nature and host-specificity of certain achlorophyllous plants. New Phytologist 160: 391–401. [DOI] [PubMed] [Google Scholar]
- Yagame T, Orihara T, Selosse MA, Yamato M, Iwase K. 2012. Mixotrophy of Platanthera minor, an orchid associated with ectomycorrhizal-forming Ceratobasidiaceae fungi. New Phytologist 193: 178–187. [DOI] [PubMed] [Google Scholar]
- Zimmer K, Hynson NA, Gebauer G, Allen EB, Allen MF, Read DJ. 2007. Wide geographical and ecological distribution of nitrogen and carbon gains from fungi in pyroloids and monotropoids (Ericaceae) and in orchids. New Phytologist 175: 166–175. [DOI] [PubMed] [Google Scholar]
- Zimmer K, Meyer C, Gebauer G. 2008. The ectomycorrhizal specialist orchid Corallorhiza trifida is a partial myco-heterotroph. New Phytologist 178: 395–400. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


