Abstract
Background and Aims Rhizomes are underground stems with meristematic tissues capable of generating shoots and roots. However, mechanisms controlling rhizome formation and growth are yet to be completely understood. The objectives of this study were to investigate whether rhizome development could be regulated by cytokinins (CKs) and gibberellic acids (GAs), and determine underlying mechanisms of regulation of rhizome formation and growth of tall fescue (Festuca arundinacea) by a CK or GA through proteomic and transcript analysis.
Methods A rhizomatous genotype of tall fescue (‘BR’) plants were treated with 6-benzylaminopurine (BAP, a synthetic cytokinin) or GA3 in hydroponic culture in growth chambers. Furthermore, comparative proteomic analysis of two-dimensional electrophoresis and mass spectrometry were performed to investigate proteins and associated metabolic pathways imparting increased rhizome number by BAP and rhizome elongation by GA3.
Key Results BAP stimulated rhizome formation while GA3 promoted rhizome elongation. Proteomic analysis identified 76 differentially expressed proteins (DEPs) due to BAP treatment and 37 DEPs due to GA3 treatment. Cytokinin-related genes and cell division-related genes were upregulated in the rhizome node by BAP and gibberellin-related and cell growth-related genes in the rhizome by GA3.
Conclusions Most of the BAP- or GA-responsive DEPs were involved in respiratory metabolism and amino acid metabolism. Transcription analysis demonstrated that genes involved in hormone metabolism, signalling pathways, cell division and cell-wall loosening were upregulated by BAP or GA3. The CK and GA promoted rhizome formation and growth, respectively, by activating metabolic pathways that supply energy and amino acids to support cell division and expansion during rhizome initiation and elongation in tall fescue.
Keywords: Rhizome, tall fescue, hormone, protein, transcription
INTRODUCTION
Rhizomes are underground stems that are composed of nodes, internodes and scale leaves, which grow horizontally from the rhizome nodes in crowns in grasses. Lateral and apical buds of the rhizome have meristematic tissues capable of generating adventitious roots and new shoots (Jernstedt and Bouton, 1985; Li and Beuselinck, 1996). Rhizomes serve as storage organs for carbohydrates, nutrients and water. Therefore, plants with extensive rhizomes are able to establish quickly and are competitive in acquiring water and nutrients as well as being persistent through prolonged periods of stress (Tao et al., 2001; Sacks et al., 2006; Zhou et al., 2014); furthermore, rhizomatous plants, especially perennial crops, have a great potential to preserve ecosystems in mountainous areas that are fragile due to serious soil erosion (Cox et al., 2002). Rhizomes are also desirable traits for breeding of forage and turfgrass, as they benefit plants by promoting rapid establishment and increasing their density, thus helping them to withstand traffic and tolerate biotic and abiotic stresses (De Battista and Bouton, 1990). Understanding mechanisms of promoting rhizome production and growth are of great significance for developing rhizomatous perennial grass species.
Recent research has begun to uncover the molecular mechanisms of rhizome development. For instance, 48 essential transcription factors, including the YABBY, NAM, TCP, TALE, AP2 and bHLH families, were identified that are specifically expressed or highly enriched in the rhizome tips and elongation zones of wild rice (Oryza longistaminata) (Hu et al., 2011; He et al., 2014; Zhang et al., 2014). Five candidate transcriptional protein complex factors (zinc finger family protein, ribosomal protein S11, nuclear RNA-binding protein, mitogen-activated protein kinase and 26S proteasome regulator) were detected and upregulated in the rhizome tips of wild sorghum (Sorghum halepense and Sorghum propinquum) (Jang et al., 2006). REVOLUTA, CLAVATA1 and SINAT5 were highly expressed in the rhizome buds of bamboo (Phyllostachys praecox) and contributed to rhizome bud formation and procambial development (Wang et al., 2009). In addition, energy metabolism-related genes and proteins were also in higher abundance in the rhizomes, including monosaccharide transporter and oligosaccharyl transferase in S. halepense (Jang et al., 2006); β-glucosidase, starch branching enzyme and trehalose 6-phosphate synthase in bamboo (Wang et al., 2009); and sucrose synthase, glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and alcohol dehydrogenase in perennial wild rice (Hu et al., 2011; He et al., 2014), horsetail (Equisetum hyemale) (Balbuena et al., 2012) and lotus (Nelumbo nucifera) roots (Cheng et al., 2013). Metabolite profiling showed that fructose, sucrose, glucose-1-phosphate and amino acids, including asparagine, glutamine and methionine, accumulated in the rhizome tissues (He et al., 2014). Some hormone-related genes and proteins were also identified, especially gibberellic acid (GA)-responsive cis-element motifs, including a pyrimidine box, TATCCA box and CAREs box, which were enriched in rhizome tips of S. halepense (Jang et al., 2006); downregulation of auxin response factor 8 and auxin efflux carrier 3 and upregulation of GA 2-β-dioxygenase and GA-regulated proteins were detected in rhizome tips of O. longistaminata (Hu et al., 2011); a cytokinin responsive cis element (TATTAG) was also present in higher abundance in S. propinquum (Zhang et al., 2014). He et al. (2014) also reported that 24 unigenes of cytokinins (CKs) were enriched in wild rice rhizomes, and a higher CK content was detected in bamboo rhizomes before transfer to the bamboo shoot (Hu et al., 1995).
Cytokinins are important regulators and mediate many aspects of plant growth and development, including cell division, axillary initiation and outgrowth (Choi and Hwang, 2007). Cytokinin derivatives (isopentenyladenine, trans-zeatin and cis-zeatin) are primarily synthesized in roots by isopentenyltransferases (IPTs), riboside 5′-monophosphate phosphoribohydralase (lonely guy, LOG) and cytochrome P450 monooxygenases (CYP735A1/A2) and degraded by cytokinin dehydrogenases (CKXs). The CK signalling pathway is involved in the two-component phosphorelay pathway, including transmembrane CK receptor histidine kinases (HKs), histidine phosphotransfer proteins (HPs) and type-B response regulators (RRs) which switch on a subset of primary CK-response genes (Ha et al., 2012). Rice mutants with impaired expression or overexpression of CK metabolism or signalling pathway genes showed an abnormal shoot apical meristem, inflorescence meristem and root system (Ashikari et al., 2005; Kurakawa et al., 2007; Gao et al., 2014), loss of apical dominance and adventitious shoot formation in transgenic tobacco (Nicotiana tabacum) (Li et al., 1992), and more axillary stems in Arabidopsis (Kuroha et al., 2009); further research indicated that cell division genes, such as the gene encoding histone H4, were affected by CK (Kurakawa et al., 2007). Gibberellic acid is another important hormone that mainly regulates cell elongation, stimulating elongation of plant organs (Hedden, 1997). GAs are synthesized by gibberellin 20-oxidase (GA20-OX) and 3-oxidase and degraded by gibberellin 2-dioxygenase (Yamaguchi, 2008). For the GA signalling pathway, GID1 (GA insensitive dwarf1), which is the GA signal receptor and controls the binding of activity GA, interacts with the DELLA proteins, which negatively regulate stem growth and switch on the downstream gibberellin-response genes (Sun, 2010). In addition, the expansin and xyloglucan endotransglucosylase/hydrolase (XET) gene families are involved in cell expansion, cell separation and cell wall disassembly and are induced by GA (Jan et al., 2004; Claeys et al., 2014). Despite the widely recognized roles and mechanisms of CK and GA in regulating plant growth development, whether the development of rhizomes is directly regulated by hormones is not well documented, and the mechanisms underlying the regulation of rhizome formation and growth by CK or GA remain unclear.
Tall fescue (Festuca arundinacea) is a cool-season perennial grass species widely used as forage and turfgrass, whereas most tall fescue genotypes are non-rhizomatous or have short, determinate rhizomes (Burns and Chamblee, 1979; Jernstedt and Bouton, 1985; Saha et al., 2015,). Previous research has paid increasing attention to enhancing rhizome production in tall fescue and other perennial grass species (Cowan, 1956; Porter, 1958). Limited genetic variation exists in rhizome formation in tall fescue, and the number and length of rhizomes in tall fescue are affected by photoperiod and temperature (Charrier and Stewart, 2006; St John et al., 2009; Saxena et al., 2014). Furthermore, information is limited about the underlying mechanisms of rhizome initiation and development in tall fescue. We hypothesized that CK and GA may stimulate rhizome formation and growth of tall fescue by regulating proteins or genes involved in metabolic processes supporting cell division and elongation. Therefore, the objectives of this study were to investigate whether rhizome formation and growth could be promoted by CK and GA, and determine the major metabolic pathways underlying regulation of rhizome formation and growth of tall fescue by CK or GA through proteomic and gene expression analysis.
MATERIALS AND METHODS
Plant materials and growth conditions
A breeding selection from the Rutgers turfgrass breeding programme, ‘BR’, which forms short rhizomes, was examined in this study. Mature ‘BR’ plants were collected from the turfgrass research farm at Adelphia, NJ, and transplanted to plastic trays (54 × 27 × 6 cm) filled with fritted clay in a greenhouse. The growth conditions in the greenhouse were set up as a 14-h photoperiod, an average temperature of 20 °C and 780 μmol m−2 s−1 of photosynthetically active radiation (PAR) with natural sunlight and supplemented with sodium lamps on cloudy days. During the establishment phase, plants were irrigated three times per week and fertilized weekly with Hoagland’s nutrient solution (Hoagland and Arnon, 1950). Plants were kept at 6–7 cm canopy height by mowing weekly.
After 2 months of establishment, plants with the same number of tillers without rhizomes and roots were transferred to the hydroponic system with plastic boxes (56 × 54 × 15 cm) containing 20 L of half-strength Hoagland’s solution in growth chambers. Each box had 40 individual plants wrapped in sponge strips and held in position with foam board and aerated with a pump (115 V, 60 Hz; Tetra, Blacksburg, VA). Nutrient solution was changed every 4 d and pH was maintained at 6·5 every day. The growth conditions were controlled at a 14 h light/8 h darkness photoperiod and temperature of 20/18 °C (day/night) and PAR of 680 μmol m−2 s−1 at the canopy level.
Hormone treatments
After plants had acclimated to the hydroponic system for 5 d, hormone treatments were imposed. Thirty plants in each of four containers were maintained in either half-strength Hoagland’s solution or with addition of 6-benzylaminopurine (BAP, a synthetic cytokinin) (0, 0·1, 1, 3 and 5 µm) or GA3 (0, 0·1, 1 10 and 50 µm). Each hormone treatment was replicated in four containers, which were arranged in a randomized complete block design.
Prior to hormone treatment (0 d) and at 3, 6, 9 and 12 d of hormone treatment, the numbers of tillers, rhizomes and roots per plant were counted, and rhizome length of each plant was measured. Each trait was measured on 30 individual plants from each replicate of each treatment.
Endogenous cytokinin quantification
Isopentenyl adenosine (iPA) in rhizome nodes (crown tissues where rhizomes are initiated, excluding tillers and roots) and GA4 in the entire rhizomes were extracted at 12 d and purified according to Zhang et al. (2013). About 50 mg of frozen fresh tissue was ground with liquid nitrogen and mixed with 1·8 mL of Na-phosphate buffer (50 mm, pH 7·0) containing 0·02 % sodium diethyldithiocarbamate as an antioxidant, then purified twice with 1 mL of 1 % acetic acid and 1 mL of dichloromethane, and samples were dissolved in 210 µL of methanol and diluted to 700 µL in deionized H2O with 0·1 % formic acid.
Quantification was iPA was carried out by enzyme-linked immunosorbent assay (ELISA) as described by Zhang and Ervin (2004). Extracted samples were conjugated to bovine serum albumin (1:10 000 dilution), incubated overnight at 4 °C and washed three times with phosphate-buffered saline (50 mm, pH 7·2). Fifty microlitres of the iPA antibody (1:200 dilution) and 50 µL of the iPA extract were mixed and incubated at 37 °C for 1 h, 100 µL of alkaline phosphatase-labelled goat anti-mouse IgG (1:1000 dilution; Sigma, St Louis, MO) was added and incubation was continued for another 1 h at 37 °C. One hundred microlitres of substrate solution (3 mg mL−1 p-nitrophenyl phosphate in 10 % diethanolamine with 0·5 mm MgCl2, pH 9·8) was added and incubation was continued for 30 min at 37 °C. The colour reaction of each sample was determined by measuring absorbance at 405 nm and iPA concentration was calculated based on the standard curve.
We analysed GA4 according to Zhang et al. (2013), using an Agilent liquid chromatography–tandem mass spectrometry (LC–MS/MS) system with an ESI sample introduction interface (Agilent, Santa Clara, CA), consisting of 1290 UPLC (ultra-performance liquid chromatography) and 6490 QQQ (triple quadrupole). The HPLC separation was performed on Agilent Zorbax Extend-C18 analytical (4·6 × 50 mm, 5 µm) and guard (4·6 × 12 mm, 5 µm) columns. The analytes were eluted with water (mobile phase A) and methanol (B) in 0·1 % formic acid in a gradient of 0–4·5 min B increasing from 30 % to 80 %, 4·5–5 min B increasing to 100 %, 5–7 min B at 100 %, and B decreasing to 30 % at 7·5 min. The injection volume was 10 µL and flow rate was 0·5 mL min−1. The column temperature was 40 °C. The internal standard was C13-labelled indole-3-acetic acid. The source parameters were as follows: nebulizer pressure 310 kPa; dry gas temperature 250 °C; sheath gas temperature 200 °C and gas flow 8 mL min−1. We determined GA4 based on retention times, ion products and GA4 standards.
Protein extraction and two-dimensional electrophoretic separation
Proteins were extracted from rhizome nodes or entire rhizomes (the same as those used for the hormone samples) using the acetone/trichloroacetic acid (TCA) protein extraction method (Xu et al., 2010) with modifications. Tissues were washed three times with deionized water and ∼1·5 g of frozen fresh tissue was ground using liquid nitrogen. Six millilitres of ice-cold precipitation solution (10 % TCA, 0·07 % 2-mercaptoethanol in acetone) was added and the sample was homogenized and left overnight. Precipitated proteins were pelleted by centrifugation at 10414 g for 15 min at 4 °C and washed three times with rinse solution (0·07 % 2-mercaptoethanol in acetone) to remove pigments and lipids. The pellets were vacuum-dried and suspended in resolubilization solution (8 m urea, 1 % CHAPS (3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate), 1 % IPG (immobilized pH gradient) buffer [GE Healthcare], 2 m thiourea, 1 % dithiothreitol [DTT]) and sonicated for 1 h at 4 °C. The supernatants were obtained after centrifugation for 15 min at 4 °C. Protein concentration was quantified according to Bradford (1976).
Proteins were separated using two-dimensional electrophoresis according to Burgess and Huang (2014) with minor modifications. Briefly, Immobiline DryStrips (pH3-10; GE Healthcare, Piscataway, NJ) were rehydrated with 250 µg of protein samples in rehydration solution (8 m urea, 1 % CHAPS (3-[(3-Cholamidopropyl)dimethylammonio]-1-propanesulfonate), 1 % IPG (immobilized pH gradient) buffer [GE Healthcare], 2 M thiourea, 1 % DTT, 0·002 % bromophenol blue). The voltage settings for first-dimensional isoelectric focusing were 30 V for 12 h, 100 V for 3 h, 200 V for 3 h, 500 V for 1 h, 1000 V for 1 h, 5000 V for 1 h and 8000 V to a total of 80 kVh. The samples were then incubated in equilibration buffer (50 mm Tris–HCl (pH 8·8), 6 m urea, 2 % sodium dodecyl sulphate [SDS], 0·002 % bromophenol blue, 30 % glycerol, 1 % DTT) for 15 min twice, and again incubated in the same buffer with 2·5 % iodoacetamide replacing DTT for 20 min. For the second-dimensional electrophoresis, a Hoefer SE 600 Ruby electrophoresis unit (GE Healthcare, Piscataway, NJ) was used with 12·5 % SDS–polyacrylamide gel. Voltage settings were 5 mA per gel for 45 min and 18 mA per gel for 7 h. Gels were stained with Coomassie brilliant blue (CBB) G-250 (17 % w/v ammonium sulphate, 34 % v/v methanol, 3 % v/v O-phosphoric acid, 0·07 % w/v CCB) and scanned with a Typhoon FLA 9500 (GE Healthcare Piscataway, NJ). Gel for each treatment was replicated four times and the images were analysed using SameSpots software (Nonlinear USA, Durham, NC). Protein spots were normalized to the total volume of spots on the gel and automatically matched. Gels with hormone treatment were compared with normal control gels and differentially expressed protein (DEP) spots were selected for further analysis (P ≤ 0·05).
Protein identification and functional analysis
Protein spots were manually excised and washed with 30 % acetonitrile in 50 mm ammonium bicarbonate before DTT reduction and iodoacetamide alkylation according to Xu and Huang (2008). Trypsin was used for digestion at 37°C overnight. The resulting peptides were extracted with 30 µL of 1 % trifluoroacetic acid followed by C18 Ziptip desalting, then mixed with 7 mg ml−1 α-cyano-4-hydroxycinnamic acid and subjected to matrix-assisted laser desorption/ionization time-of-flight MS (MALDI-TOF-MS) analysis (Applied Biosystems, Framingham, MA). Proteins were identified using the Peptide Mass Fingerprinting module of the Mascot search engine (V2.2; Matrix Science Boston, MA, USA) against the green plant NCBI database. The following parameters were set: trypsin, methionine oxidation, carboxyamidomethylation of cysteine, two missed cleavages, precursor mass tolerance 50 ppm and fragment mass tolerance 0·6 Da. Confidence interval values >95 % for at least two peptides were considered as successful identification.
For the classification and functional analysis of identified proteins, protein homologues were identified against the Arabidopsis database (https://www.arabidopsis.org) and gene ontology (GO) was categorized against the Agrigo database (http://bioinfo.cau.edu.cn/agriGO/analysis.php) for biological process and cellular component analysis; the threshold was set at –log10(P value)>4. Functional classification and regulation network analysis were performed using MapMan software (http://mapman.gabipd.org/web/guest;jsessionid=D943DDE0DBAF371F1B1D5C4F1A2E1597.ajp13_mapman_gabipd_org) and the KEGG pathway database (http://www.genome.jp/kegg/pathway.html).
Gene expression analysis by quantitative real-time PCR (qRT-PCR)
Total RNA was extracted from the same treatments as those used for protein analysis using Trizol reagent (Gibco BRL, Life Technologies, Grand Island, NY) according to the manufacturer’s instructions, as previously described (Ma et al., 2016). A Turbo DNA-Free Kit (Ambion, Austin, TX) was used to remove DNA contamination. A High-Capacity cDNA Reverse Transcription Kit (Life Technologies, Grand Island, NY) was used for RNA reverse transcription according to the manufacturer’s manual. Power SYBR Green PCR Master Mix (Life Technologies, Grand Island, NY) was used for cDNA amplification on the StepOnePlus Real-Time PCR System (Applied Biosystems, Foster City, CA). Corresponding homologous genes of tall fescue were obtained from the biofuel feedstock genomics resource (http://bfgr.plantbiology.msu.edu/integrated_searches.shtml). Details of primer sequences and gene bank IDs are provided in Supplementary Data Table S1.
Statistical analysis
All data were subjected to analysis of variance according to the general linear model of SAS 9.0 (SAS Institute, Cary, NC). Treatment means were separated using Fisher’s protected least significant difference (LSD) test at P ≤0·05.
RESULTS
Rhizomatous phenotypes
Rhizomatous phenotypes of 3-month old ‘BR’ plants are illustrated in Fig. 1A. Each plant had an average of two rhizomes and the average rhizome length was 3·97 ± 0·17 cm, including two internodes. Three days after transplanting to the hydroponic system, a new rhizome bud was initiated from the crown node (Fig. 1B), and after 6 d each plant had an average of 0·78 ± 0·20 rhizome buds initiated, with average length 1·96 ± hx00A0;0·28 cm (Fig. 1D). Twelve days after transplantation the number of rhizomes had increased to 1·46 ± 0·24 and the length of rhizomes had increased to 4·33 ± 0·44 cm (Fig. 1C, E).
Fig. 1.
Phenotypes of ‘BR’ tall fescue. (A) Rhizomatous phenotypes that had been grown in fritted clay for 3 months in the greenhouse. (B) A rhizome bud was observed 3 d after transplanting to the hydroponic system. (C) Rhizomatous phenotypes in hydroponics after 12 d in the growth chamber. (D) Average numbers of rhizomes per plant in hydroponics. Values are mean ± s.e. for 30 plants. (E) Average length of rhizomes in hydroponics. Values are mean ± s.e. for 30 rhizomes. Columns marked with different letters indicate significant differences among treatments based on the LSD value (P≤0·05); FW, fresh weight.
Effects of BAP on rhizome formation
To investigate the effects of CK on the initiation of rhizomes, crowns were treated with BAP in the hydroponic solution. The number of rhizomes per plant increased during the 12-d treatment period in BAP-untreated control plants and plants treated with either 0·1 or 1 µm BAP (Fig. 2A). The number of rhizomes per plant increased significantly from 0·78 and 1·45 in the BAP-untreated plants to 1·75 and 2·1 at 6 and 12 d of treatment with 0·1 µm BAP, respectively; corresponding figures were 1·83 and 2·43 with 1 µm BAP treatment (Fig. 2A). Plants treated with 3 and 5 µm BAP displayed phytotoxicity symptoms (leaf deformation) and no increased rhizome numbers were observed (data not shown). In addition, during treatment with BAP we investigated the number of tillers and new root formation, but there were no significant differences between the BAP treatment and the control (Supplementary Data Fig. S1A, B).The 1 µm BAP treatment resulted in a 2·5-fold increase in endogenous iPA content compared with the untreated control (Fig. 2B).
Fig. 2.
Effects of BAP on rhizomes of ‘BR’ tall fescue. (A) Average number of rhizomes per individual plant treated with different concentrations of BAP in hydroponics. (B) Endogenous iPA content in rhizome nodes of ‘BR’ tall fescue treated with 1 µm BAP. Columns marked with different lowercase letters indicate significant differences among treatments at a given day of sampling based on the LSD value (P≤0·05). Columns marked with different uppercase letters indicate significant differences among sampling time points for a given treatment based on the LSD value (P≤0·05); FW, fresh weight.
Proteins in rhizome nodes induced by BAP
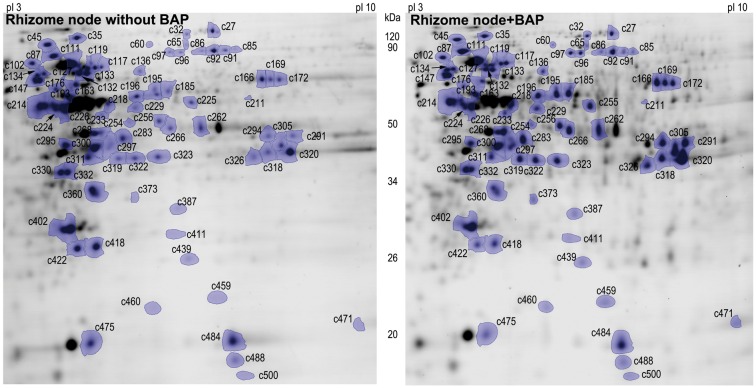
More than 502 protein spots were detected on each gel of the rhizome nodes (Fig. 3, Supplementary Data Table S2). Through mass spectrometry and protein database analysis, a total of 76 DEPs were successfully identified with BAP treatment compared with the untreated control (Table S2), including 60 upregulated and 16 downregulated proteins. GO category enrichment indicated that BAP caused rhizome nodes to express proteins involved in various biological processes, which were mainly metabolic processes, i.e. cellular metabolism, primary metabolism, carbohydrate metabolism, monosaccharide metabolism and glucose metabolism, responses to stress, oxidoreductase activity, protein folding and glycolysis. These DEPs were located in intracellular spaces, cytoplasm, organelles, membrane-bounded organelles, mitochondria and envelopes according to the cellular component (Fig. 4A). Functional classification showed that they were mainly involved in redox regulation (15·79 %), glycolysis (11·84 %), amino acid metabolism (11·84 %), stress response (10·53 %), cell development (7·89 %), the tricarboxylic acid cycle (6·58 %), photosynthesis (5·26 %), protein synthesis (3·95 %) and transport protein (3·95 %) (Fig. 4B, Table S2).
Fig. 3.
Two-dimensional SDS–PAGE gels of rhizome nodes with BAP treatment in ‘BR’ tall fescue. Differentially expressed protein spots are marked. There were at least four replicated gels for each treatment (P ≤ 0·05).
Fig. 4.
Cluster analysis and functional distribution of differentially expressed proteins of rhizome nodes with BAP treatment in ‘BR’ tall fescue. (A) Biological process and cellular components were analysed against the Arabidopsis database (http://bioinfo.cau.edu.cn/agriGO/analysis.php) (threshold −log10(P value) > 4). (B) Functional distribution was grouped using MapMan software (http://mapman.gabipd.org/web/guest/home).
In order to understand how CK could stimulate rhizome formation, it was of particular interest to identify proteins upregulated in rhizome nodes by BAP treatment. Among proteins involved in redox regulation, seven were upregulated, including peroxidase (c373, c387), catalase (c166, c169, c172), ascorbate peroxidase (c402) and disulphide isomerase (c147). Among proteins involved in glycolysis six were upregulated, including phosphoglycerate kinase (PGK) (c283), GAPDH (c305, c318, c320), fructose-bisphosphate aldolase (FBP) (c294) and phosphopyruvate hydratase (c218). Among proteins involved in amino acid metabolism, nine were upregulated, including methionine synthases (c85, c86, c91, c92, c96 and c97), adenosylhomocysteinase (c185), adenosylmethionine synthase (c256) and alanine aminotransferase (c225). In addition, four proteins involved in the tricarboxylic acid cycle were upregulated, including pyruvate dehydrogenase (PDH) (c295), malate dehydrogenase (c323), succinate dehydrogenase (SDH) (c136), aconitate hydratase (c32) and isocitrate dehydrogenase (CDH) (c266). Furthermore, five cell division and growth-related proteins were also upregulated with BAP treatment in the rhizome nodes, including α-tubulin (c224, c226), β-tubulin (c214), cell division cycle protein (c35) and actin (c268) (Fig. 3, Supplementary Table S2).
Expression analysis of cytokinin-related genes in rhizome nodes
While proteomics is a powerful approach for identifying abundant proteins, proteins of low abundance involved in CK metabolism or signalling were not found in the proteomic profiles of rhizome nodes. In order to further determine whether CK regulation of rhizome formation involved different components of CK metabolism and the signalling pathway, transcript levels of several cytokinin-related genes were examined using qRT–PCR analysis. The CK synthesis gene IPT and the metabolic gene CKX were upregulated with BAP treatment in rhizome nodes. The transcript level of the CK signalling pathway receptor, histidine kinase HK1 and the response regulator orthologous genes RR1 and RR6 were also increased in BAP-treated rhizome nodes compared with the untreated control, but the expression level of HK2 was not different with or without BAP treatment (Fig. 5A). Expression levels of cell-division-related gene were also examined, and the results indicated that cyclin D2 (CYCD), histone H4 (His4), proliferating cell nuclear antigen (PCNA) and cyclin-dependent kinase B (CDKB) were also increased by BAP treatment compared with the control (Fig. 5B).
Fig. 5.
Expression levels of genes in rhizome nodes of ‘BR’ tall fescue with BAP treatment. (A) Expression levels of genes responsible for CK metabolism and the signalling pathway in rhizome nodes with BAP treatment. (B) Expression levels of genes involved in cell division in crown nodes with BAP treatment. FaIPT, tRNA dimethylallyltransferase 2-like; FaCKX, cytokinin oxidase; FaHK1, histidine kinase 1; FaHK2, histidine kinase 2; FaRR1, response regulator 1; FaRR6, response regulator 6; FaCYCD, cyclin-D2-2; FaHis4, histone h4; FaPCNA, proliferating cell nuclear antigen; FaCDKB, cyclin-dependent kinase B1-1.
Effects of GA3 on rhizome elongation
To further investigate whether GA3 may enhance rhizome growth, changes in the length of rhizomes of plants treated with GA3 were examined. Plants treated with GA3 had longer rhizomes than untreated control plants at 12 d of treatment (Fig. 6A). Compared with the untreated control, the average length of rhizomes with 10 µm GA3 treatment was increased by 24·86 % at 12 d; however, GA3 treatment at other concentrations had no significant effects on rhizome length (Fig. 6B). Additionally, the content of endogenous GA4 was increased by 16 % compared with the untreated control (Fig. 6C).
Fig. 6.

Effects of GA3 on rhizomes of ‘BR’ tall fescue. (A) Rhizomatous phenotypes with GA3 treatment in hydroponics after 12 d in the growth chamber. White arrows indicate rhizomes. (B) Average length of rhizomes with GA3 treatment in hydroponics. Values are mean ± s.e. of 30 rhizomes. Vertical bars are LSD values (P ≤ 0·05) indicating significant differences among treatments at a given day. (C) Endogenous GA4 content in rhizomes treated with 10 µm GA3. Columns marked with different letters indicate significant differences among treatments based on the LSD value (P ≤ 0·05); FW, fresh weight.
Identification and functional classification of proteins with GA3 treatment in rhizomes
In order to identify proteins and associated metabolic processes that could be altered by GA3 and associated with GA stimulation of rhizome elongation, protein profiles were analysed and compared in the rhizomes of plants treated with GA3 and untreated control plants. Two-dimensional electrophoresis separated more than 530 proteins spots in each rhizome (Fig. 7). A total of 37 DEPs were successfully identified in rhizomes treated with GA3 compared with those of the untreated control: 30 were upregulated and 7 were downregulated by GA3 treatment (Fig. 7, Supplementary Data Table S3). GO annotation indicated that the biological processes of the rhizome altered by GA3 treatment mainly included cellular processes, metabolic processes, primary metabolic processes, response to stress and metabolism of nitrogen compounds, cellular ketones, oxoacids and carboxylic acid (Fig. 8A). Based on the cellular component analysis, those DEPs were mainly located in intracellular spaces, organelles, membrane bounded organelles, cytoplasm, plastids, chloroplasts, mitochondria, membranes and envelopes (Fig. 8A). The GA3-responsive DEPs were mainly classified into the following functional categories: glycolysis (18·92 %), amino acid metabolism (18·92 %), stress response (13·5 %), photosynthesis (10·81 %), redox regulation (5·41 %), and transport protein (5·41 %) according to MapMan functional analysis (Fig. 8B).
Fig. 7.
Two-dimensional SDS–PAGE gels of tall fescue rhizomes with GA3 treatment. Differentially expressed proteins are marked. There were least four repeat gels for each treatment (P≤0·05).
Fig. 8.
Cluster analysis and functional distribution of differentially expressed proteins of rhizomes with GA3 treatment in ‘BR’ tall fescue. (A) Biological processes and cellular components were analysed against the Arabidopsis database (http://bioinfo.cau.edu.cn/agriGO/analysis.php) (threshold −log10(P value) > 4). (B) Functional distribution was grouped using MapMan software (http://mapman.gabipd.org/web/guest/home).
The GA3-upregulated proteins in rhizomes could play important roles in GA stimulation of rhizome elongation. Seven proteins involved in amino acid metabolism were upregulated, including methionine synthases (r113, r114, r117, r118, r120) and s-adenosylmethionine synthases (r285 and r289). Three GAPDH (r344, r367 and r375), three FBP (r338, r339 and r340) proteins and alcohol dehydrogenase (r290) involved in glycolysis were also upregulated with GA3 treatment. In addition, the abundance of UDP-glucose 6-dehydrogenase (r224), involved in cell wall development, glutamine synthetase (r307), involved in nitrogen metabolism and glycine-rich RNA binding protein (r586), involved in RNA regulation and transcription, were increased with GA3 treatment in rhizome tissues (Fig. 7, Supplementary Data Table S3).
Expression analysis of gibberellin-related and cell growth-related genes in rhizomes
Gibberellin biosynthesis and signalling pathway gene expression levels were also examined in order to determine the possible involvement of different components of GA metabolism and signalling pathways regulating rhizome elongation. The transcript levels of ent-kaurene oxidase (KO), GA20-OX1 and DELLA were increased in GA3-treated rhizomes compared with the control, but the expression levels of GA20-OX2 and SPINDLY were not significantly different from the control (Fig. 9). Several cell-elongation-related genes that were available in the tall fescue EST database were also analysed, including expansin (EXP) and endotransglucosylase/hydrolases (XTH) family genes. The transcript levels of EXPA5, EXPANA11, EXPB2, XET1 and XET2 were increased in rhizomes treated with GA3 compared with the untreated control, but EXPB11 with GA3 treatment was not different from the control (Fig. 9).
Fig. 9.
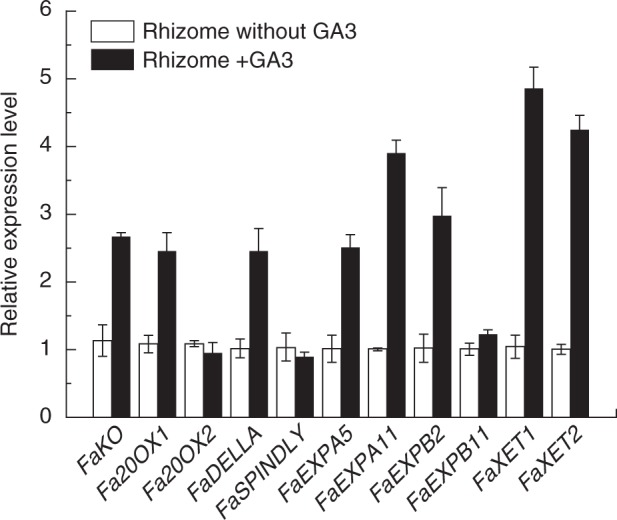
Expression level of genes in rhizomes of ‘BR’ tall fescue with GA3 treatment. FaKO, ent-kaurene; FaGA20OX1, gibberellin 20-oxidase1; FaGA20OX2, gibberellin 20-oxidase2; FaEXPA5, EXPANSIN A5; FaEXPA11, EXPANSIN A11; FaEXPB2, EXPANSIN B2; FaEXPB11, EXPANSIN B11; FaXET1, xyloglucan endotransglycosylase1; FaXET2, xyloglucan endotransglycosylase 2.
DISCUSSION
While numerous studies reported effects of CK and GA on various plant growth and development processes, to our knowledge this is the first report that demonstrates the stimulative effects of CK on rhizome formation and of GA on rhizome elongation in a perennial grass species. This finding is of great significance for the development of rhizomatous grass species, which are known to be more resilient and to have a greater potential for recuperation from stress damage, as described in the Introduction. The proteomic and transcript analyses in this study showed that the underlying mechanisms of regulation of rhizome formation by CK and of rhizome elongation by GA are complex, involving multiple metabolic processes and multiple components of CK and GA metabolism and signalling pathways, as discussed in detail below.
Proteomic analysis identified 76 proteins either up- or downregulated by CK treatment, which were classified into multiple functional categories, including redox regulation (15·79 %), glycolysis (11·84 %), amino acid metabolism (11·84 %), stress response (10·53 %), cell development (7·89 %), the tricarboxylic acid cycle (6·58 %), photosynthesis (5·26 %), protein synthesis (3·95 %) and transport protein (3·95 %). The respiratory pathways, including glycolysis, the tricarboxylic acid cycle and the mitochondrial electron transport chain, are essential for plant growth and development, being involved in energy provision, amino acid biosynthesis and a wide range of other physiological functions (Plaxton, 1996; Fernie et al., 2004). In our study, 36 out of 76 (47·36 %) proteins were involved in the respiratory pathways; most of them were upregulated with BAP treatment compared with the untreated control (Fig. 10). For instance, FBP (c294), GAPDH (c318, c305, c320), PGK (c283 and c297) and bifunctional enolase 2 (c218) – involved in the glycolysis pathway in the cytosol –showed greater accumulation, and have also been shown to be upregulated in wild rice rhizomes (Hu et al., 2011; He et al., 2014). Also showing higher abundance in rhizome nodes with BAP treatment were PDH (c295), aconitate hydratase (c32), CDH (c266), SDH (c136), malate dehydrogenase (c323) and ATPase complex subunits (c132, c195, c193, c196), involved in the tricarboxylic acid cycle and the electron transport chain in mitochondria. Along with the process of energy metabolism, the biosynthesis of amino acids, including methionine, alanine, serine and glycine, was also enhanced with BAP treatment. It has been reported that metabolites involved in amino acid and carbohydrate metabolites and metabolites in the tricarboxylic acid cycle were increased in ipt-transgenic creeping bentgrass with associated higher endogenous CKs (Merewitz et al., 2011, 2012). Furthermore, higher accumulation of amino acid and intermediate products of the tricarboxylic acid cycle associated with increased contents of endogenous iPA, trans-zeatin and trans-zeatin riboside were found in the Arabidopsis m132 mutant (Yu et al., 2012). Energy metabolism-related genes and proteins such as sucrose synthase, GAPDH and alcohol dehydrogenase were identified in rhizomes of perennial rice (He et al., 2014), wild sorghum (Jang et al., 2006), bamboo (Wang et al., 2009) and horsetail (Balbuena et al., 2012). Our results suggest that integrated networks involved in respiratory energy and amino acid metabolism could be activated by CK, which provides energy products to support cell division and the subsequent initiation of rhizomes in tall fescue.
Fig. 10.
Differentially expressed proteins in rhizome nodes involved in the respiratory metabolism pathway according to the KEGG pathway in ‘BR’ tall fescue. Red colour indicates proteins that were upregulated with BAP treatment compared with the control, and black colour indicates proteins that were downregulated with BAP treatment compared with the control.
Cytokinins may regulate rhizome formation by the direct effects of increasing endogenous cytokinin content and regulatory effects through CK responses or signalling. In this study, the endogenous iPA content increased along with increased transcript levels of the CK synthesis gene IPT, the CK signalling pathway receptor, histidine kinase HK1 and the response regulator orthologous genes RR1 and RR6. These results suggest that exogenous application of CK regulates expression levels of related genes, such as JcRRA9 and JcRRA17 in Jatropha (Pan et al., 2014) and SIHK4, SIRRAs and SICKX in tomato (Lycopersicon esculentum) roots and leaves (Gupta et al., 2013; Shi et al., 2013), or are affected in the CK-deficient ipt mutant compared with the wild type in Arabidopsis (Nishiyama et al., 2012). In addition, cell-division-related gene expression levels of cyclin D2 (CYCD), histone H4 (His4), proliferating cell nuclear antigen (PCNA) and cyclin-dependent kinase B (CDKB) were also upregulated by BAP treatment. CYCD, His4 and PCNA which are involved in RNA and DNA replication at G1 and S phase, and CDKB, which is involved in the G2-M phase of cell cycle, were found to be involved in bud dormancy development in pea (Pisum sativum) and sorghum (Stafstrom and Sussex, 1992; Kebrom et al., 2010a, b), which suggested that cell-division-related genes involved in the CK signalling pathway were upregulated and responsible for shoot apical meristem and axillary meristem development (Müller and Leyser, 2011; Schaller et al., 2014). Our results suggest that CK stimulation of rhizome formation in tall fescue involves both direct and regulatory effects, which could lead to the upregulation of expression levels of downstream genes controlling cell division and cycles, and thereby subsequently increase the numbers of rhizomes in tall fescue.
It is widely known that GA plays key roles in controlling cell elongation. In this study, rhizome elongation was indeed promoted by GA3 treatment. Proteomic analysis of rhizomes for proteins regulated by GA that could contribute to enhanced rhizome elongation found that 21 out of 37 (56·75 %) GA-responsive proteins in the rhizome of tall fescue were involved in energy metabolism and amino acid biosynthesis. Most of them were also confirmed to be abundant in rhizomes of other plant species without GA treatment, including wild rice (Hu et al., 2011; He et al., 2014) and sacred lotus (Nelumbo nucifera) (Kim et al., 2013), or showed greater accumulation in the stolons of strawberry (Fragaria ananassa) (Fang et al., 2011). For instance, GAPDH (r344, r367 and r375), FBP (r338, r339 and r340) and phosphoglycerate mutase (r185), which are involved in glycolysis, were increased with GA3 treatment in rhizomes compared with the untreated control, and methionine synthase (r113, r114, r117, r118 and r120) and adenosylmethionine synthase, which are involved in methionine metabolism, were increased with GA3 treatment in rhizomes. In addition, proteins responsive to protein synthesis were identified in rhizomes of tall fescue with GA3 treatment in this study, including protein disulphide isomerase-like (r192), heat shock protein (HSP) 90 protein (r77), mitochondrial HSP (r146 and r153) and HSP 70 (r128). These data suggest that GA stimulation of rhizome elongation could result mainly from the activation of respiratory and amino acid metabolism, although multiple metabolic processes were altered due to GA3 treatment in the rhizome of tall fescue. Several GA-associated genes were identified in the rhizomes of sorghum (Jang et al., 2006), wild rice (He et al., 2014), bamboo (Wang et al., 2009) and lotus (Cheng et al., 2013) without GA treatment. In our study, the endogenous GA4 content increased along with increased transcript levels of ent-kaurene (KO) and GA 20 oxidase (GA20-OX) in rhizomes treated with GA3, and the expression level of DELLA was also increased with GA3 treatment, which might be related to the rapid degradation of DELLA protein during rhizome elongation. Our results indicate that GA synthesis and signalling pathway genes were increased with the application of GA3, which might enhance the expression of downstream genes, such as cell wall growth genes. Indeed, the transcript levels of expansins and XET genes were higher in rhizomes treated with GA3 compared with the untreated control. A previous study showed that internode elongation was possibly promoted by upregulation of XET and expansins associated with higher levels of endogenous GA4 in wheat (Triticum aestivum) (Zhang et al., 2007), and higher XET activity was detected with exogenous application of GA3 in elongation leaves of barley (Hordeum vulgare) (Smith et al., 1996). The combination of results from proteomic profiling and transcription analysis suggested that application of GA3 enhanced the expression of genes involved in energy and protein synthesis and upregulated cell wall loosening genes expression and subsequently promoted rhizome elongation.
Conclusions
Based on phenotypic responses to hormones and proteomic profiling, as well as gene expression related to the regulation of rhizome formation and elongation by CK or GA, we have proposed a potential regulatory mechanism of rhizome initiation and elongation in tall fescue (Fig. 11). The increased number of rhizomes with CK treatment and the increased length of rhizomes resulting from GA3 treatment could be associated with the activation of respiratory metabolism, amino acid metabolism, redox regulation, the stress response, major carbohydrate metabolism and upregulation of hormone-responsive gene expression, thereby, through their integrated actions, providing energy products to support cell division and subsequent rhizome development in tall fescue. The biological functions and associated molecular factors of CK- or GA-regulated proteins imparting enhanced rhizome initiation and elongation deserve further investigation, which will provide new insights into the development of rhizomatous perennial grass species
Fig. 11.
A model for hormone-regulated rhizome development in tall fescue ‘BR’.
SUPPLEMENTARY DATA
Supplementary data are available online at www.aob.oxfordjournals.org and consist of the following. Figure S1: effects of 6-benzylaminopurine (BAP) on tillers and new roots of ‘BR’ tall fescue. (A) Average numbers of tillers per plant in hydroponics conditions. (B) Average numbers of new roots per plant in hydroponic conditions. Values are mean ± s.e. of 30 rhizomes. Columns marked with different letters indicate significant differences among treatments based on the LSD value (P≤0·05·). Table S1: sequences of tall fescue primers used for qRT–PCR analysis. Table S2: differentially expressed proteins in rhizome nodes treated with BAP. Table S3: differentially expressed proteins in rhizomes treated with GA3.
ACKNOWLEDGEMENTS
This research was supported by the China Postdoctoral Science Foundation (Grant No. 2014M561672), the National Natural Science Foundation of China (Grant No. 31572153), and Rutgers Center of Turfgrass Science.
LITERATURE CITED
- Ashikari M, Sakakibara H, Lin S, et al. 2005. Cytokinin oxidase regulates rice grain production. Science 309: 741–745. [DOI] [PubMed] [Google Scholar]
- Balbuena TS, He R, Salvato F, Gang DR, Thelen JJ. 2012. Large-scale proteome comparative analysis of developing rhizomes of the ancient vascular plant Equisetum hyemale. Frontiers in Plant Science 3: 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Battista J, Bouton J. 1990. Greenhouse evaluation of tall fescue genotypes for rhizome production. Crop Science 30: 536–541. [Google Scholar]
- Bradford MM. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry 72: 248–254. [DOI] [PubMed] [Google Scholar]
- Burgess P, Huang B. 2014. Root protein metabolism in association with improved root growth and drought tolerance by elevated carbon dioxide in creeping bentgrass. Field Crops Research 165: 80–91. [Google Scholar]
- Burns JC, Chamblee DS. 1979. Adaptation In: Buckner R, Bush L, eds. Tall fescue. Agronomy Monograph 20: 9–30. [Google Scholar]
- Charrier S, Stewart A. 2006. Breeding of rhizomatous turf tall fescue In: CF Mercer, ed. Breeding for success: diversity in action. In: Proceedings of the 13th Australasian Plant Breeding Conference, Christchurch, New Zealand, 18–21 April 2006, 383–387. [Google Scholar]
- Cheng L, Li S, Yin J, Li L, Chen X. 2013. Genome-wide analysis of differentially expressed genes relevant to rhizome formation in lotus root (Nelumbo nucifera Gaertn). PLoS One 8: e67116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Hwang I. 2007. Cytokinin: perception, signal transduction, and role in plant growth and development. Journal of Plant Biology 50: 98–108. [Google Scholar]
- Claeys H, De Bodt S, Inzé D. 2014. Gibberellins and DELLAs: central nodes in growth regulatory networks. Trends in Plant Science 19: 231–239. [DOI] [PubMed] [Google Scholar]
- Cowan JR. 1956. Tall fescue. Advances in Agronomy 8: 283–318. [Google Scholar]
- Cox T, Bender M, Picone C, et al. 2002. Breeding perennial grain crops. Critical Reviews in Plant Sciences 21: 59–91. [Google Scholar]
- Fang XP, Ma HS, Lu DZ, Yu H, Lai W, Ruan S. 2011. Comparative proteomics analysis of proteins expressed in the I-1 and I-2 internodes of strawberry stolons. Proteome Science 9: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernie AR, Carrari F, Sweetlove LJ. 2004. Respiratory metabolism: glycolysis, the TCA cycle and mitochondrial electron transport. Current Opinion in Plant Biology 7: 254–261. [DOI] [PubMed] [Google Scholar]
- Gao S, Fang J, Xu F, et al. 2014. CYTOKININ OXIDASE/DEHYDROGENASE4 integrates cytokinin and auxin signaling to control rice crown root formation. Plant Physiology 165: 1035–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Shi X, Lindquist IE, Devitt N, Mudge J, Rashotte AM. 2013. Transcriptome profiling of cytokinin and auxin regulation in tomato root. Journal of Experimental Botany 64: 695–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha S, Vankova R, Yamaguchi-Shinozaki K, Shinozaki K, Tran L-SP. 2012. Cytokinins: metabolism and function in plant adaptation to environmental stresses. Trends in Plant Science 17: 172–179. [DOI] [PubMed] [Google Scholar]
- He R, Salvato F, Park J-J, et al. 2014. A systems-wide comparison of red rice (Oryza longistaminata) tissues identifies rhizome specific genes and proteins that are targets for cultivated rice improvement. BMC Plant Biology 14: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedden P. 1997. Gibberellin biosynthesis. eLS, doi:10.1002/9780470015902.a0023720. [Google Scholar]
- Hoagland DR, Arnon DI. 1950. The water-culture method for growing plants without soil. Circular. California Agricultural Experiment Station 347: 1–32. [Google Scholar]
- Hu C, Jin A, Zhang Z. 1995. Change of endohormone in mixed bud on Lei bamboo rhizome during differentiation. Journal of Zhejiang Forestry College 13: 1–4. [Google Scholar]
- Hu F, Wang D, Zhao X, et al. 2011. Identification of rhizome-specific genes by genome-wide differential expression analysis in Oryza longistaminata. BMC Plant Biology 11: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan A, Yang G, Nakamura H, et al. 2004. Characterization of a xyloglucan endotransglucosylase gene that is up-regulated by gibberellin in rice. Plant Physiology 136: 3670–3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang CS, Kamps TL, Skinner DN, Schulze SR, Vencill WK, Paterson AH. 2006. Functional classification, genomic organization, putatively cis-acting regulatory elements, and relationship to quantitative trait loci, of sorghum genes with rhizome-enriched expression. Plant Physiology 142: 1148–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernstedt J, Bouton J. 1985. Anatomy, morphology, and growth of tall fescue rhizomes. Crop Science 25: 539–542. [Google Scholar]
- Kebrom TH, Brutnell TP, Finlayson SA. 2010a. Suppression of sorghum axillary bud outgrowth by shade, phyB and defoliation signalling pathways. Plant, Cell & Environment 33: 48–58. [DOI] [PubMed] [Google Scholar]
- Kebrom TH, Brutnell TP, Hays DB, Finlayson SA. 2010b. Vegetative axillary bud dormancy induced by shade and defoliation signals in the grasses. Plant Signaling & Behavior 5: 317–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M-J, Nelson W, Soderlund CA, Gang DR. 2013. Next-generation sequencing-based transcriptional profiling of sacred lotus “China antique”. Tropical Plant Biology 6: 161–179. [Google Scholar]
- Kurakawa T, Ueda N, Maekawa M, et al. 2007. Direct control of shoot meristem activity by a cytokinin-activating enzyme. Nature 445: 652–655. [DOI] [PubMed] [Google Scholar]
- Kuroha T, Tokunaga H, Kojima M, et al. 2009. Functional analyses of LONELY GUY cytokinin-activating enzymes reveal the importance of the direct activation pathway in Arabidopsis. The Plant Cell 21: 3152–3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Beuselinck P. 1996. Rhizomatous Lotus corniculatus L.: II. Morphology and anatomy of rhizomes. Crop Science 36: 407–411. [Google Scholar]
- Li Y, Hagen G, Guilfoyle TJ. 1992. Altered morphology in transgenic tobacco plants that overproduce cytokinins in specific tissues and organs. Developmental Biology 153: 386–395. [DOI] [PubMed] [Google Scholar]
- Ma X, Zhang J, Huang B. 2016. Cytokinin-mitigation of salt-induced leaf senescence in perennial ryegrass involving the activation of antioxidant systems and ionic balance. Environmental and Experimental Botany 125: 1–11. [Google Scholar]
- Merewitz EB, Gianfagna T, Huang B. 2011. Photosynthesis, water use, and root viability under water stress as affected by expression of SAG12-ipt controlling cytokinin synthesis in Agrostis stolonifera. Journal of Experimental Botany 62: 383–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merewitz EB, Du H, Yu W, Liu Y, Gianfagna T, Huang B. 2012. Elevated cytokinin content in ipt transgenic creeping bentgrass promotes drought tolerance through regulating metabolite accumulation. Journal of Experimental Botany 63: 1315–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller D, Leyser O. 2011. Auxin, cytokinin and the control of shoot branching. Annals of Botany 107: 1203–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama R, Le DT, Watanabe Y, et al. 2012. Transcriptome analyses of a salt-tolerant cytokinin-deficient mutant reveal differential regulation of salt stress response by cytokinin deficiency. PLoS One 7: e32124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan B-Z, Chen M-S, Ni J, Xu Z-F. 2014. Transcriptome of the inflorescence meristems of the biofuel plant Jatropha curcas treated with cytokinin. BMC Genomics 15: 974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaxton WC. 1996. The organization and regulation of plant glycolysis. Annual Review of Plant Biology 47: 185–214. [DOI] [PubMed] [Google Scholar]
- Porter HL. 1958. Rhizomes in tall fescue. Agronomy Journal 50: 493–494. [Google Scholar]
- Sacks E, Dhanapala M, Tao D, Cruz MS, Sallan R. 2006. Breeding for perennial growth and fertility in an Oryza sativa/O. longistaminata population. Field Crops Research 95: 39–48. [Google Scholar]
- Saha MC, Talukder S, Azhaguvel P, Mukhergee S, Chekhovskiy K. 2015. Deciphering drought tolerance in tall fescue [Lolium arundinaceum (Schreb.) Darbysh.] In: Budak H, Spangenberg G, eds. Molecular breeding of forage and turf. Cham, Switzerland: Springer, 1–7. [Google Scholar]
- Saxena P, Huang B, Bonos SA, Meyer WA. 2014. Photoperiod and temperature effects on rhizome production and tillering rate in tall fescue [(Schreb.) Darby.]. Crop Science 54: 1205–1210. [Google Scholar]
- Schaller GE, Street IH, Kieber JJ. 2014. Cytokinin and the cell cycle. Current Opinion in Plant Biology 21: 7–15. [DOI] [PubMed] [Google Scholar]
- Shi X, Gupta S, Lindquist IE, Cameron CT, Mudge J, Rashotte AM. 2013. Transcriptome analysis of cytokinin response in tomato leaves. PLoS One 8: e55090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RC, Matthews PR, Schünmnn PH, Chandler PM. 1996. The regulation of leaf elongation and xyloglucan endotransglycosylase by gibberellin in ‘Himalaya’ barley (Hordeum vulgare L.). Journal of Experimental Botany 47: 1395–1404. [Google Scholar]
- St John R, Fry J, Bremer DJ, Keeley S. 2009. Establishment rate and lateral spread of Festuca arundinacea cultivars. International Turfgrass Society Research Journal 11: 481–487. [Google Scholar]
- Stafstrom JP, Sussex IM. 1992. Expression of a ribosomal protein gene in axillary buds of pea seedlings. Plant Physiology 100: 1494–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun T. 2010. Gibberellin-GID1-DELLA: a pivotal regulatory module for plant growth and development. Plant Physiology 154: 567–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao D, Hu F, Yang Y, et al. 2001. Rhizomatous individual was obtained from interspecific BC2F1 progenies between Oryza sativa and Oryza longistaminata. Rice Genetics Newsletter 18: 11–13. [Google Scholar]
- Wang K, Peng H, Lin E, et al. 2009. Identification of genes related to the development of bamboo rhizome bud. Journal of Experimental Botany 61: 551–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Huang B. 2008. Root proteomic responses to heat stress in two Agrostis grass species contrasting in heat tolerance. Journal of Experimental Botany 59: 4183–4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Sibicky T, Huang B. 2010. Protein profile analysis of salt-responsive proteins in leaves and roots in two cultivars of creeping bentgrass differing in salinity tolerance. Plant Cell Reports 29: 595–615. [DOI] [PubMed] [Google Scholar]
- Yamaguchi S. 2008. Gibberellin metabolism and its regulation. Annual Review of Plant Biology 59: 225–251. [DOI] [PubMed] [Google Scholar]
- Yu H, Du X, Zhang F, et al. 2012. A mutation in the E2 subunit of the mitochondrial pyruvate dehydrogenase complex in Arabidopsis reduces plant organ size and enhances the accumulation of amino acids and intermediate products of the TCA cycle. Planta 236: 387–399. [DOI] [PubMed] [Google Scholar]
- Zhang T, Zhao X, Wang W, et al. 2014. Deep transcriptome sequencing of rhizome and aerial-shoot in Sorghum propinquum. Plant Molecular Biology 84: 315–327. [DOI] [PubMed] [Google Scholar]
- Zhang X, Ervin E. 2004. Cytokinin-containing seaweed and humic acid extracts associated with creeping bentgrass leaf cytokinins and drought resistance. Crop Science 44: 1737–1745. [Google Scholar]
- Zhang X, Ervin EH, Evanylo GK, Li J, Harich K. 2013. Corn and soybean hormone and antioxidant metabolism responses to biosolids under two cropping systems. Crop Science 53: 2079–2089. [Google Scholar]
- Zhang Y, Ni Z, Yao Y, Nie X, Sun Q. 2007. Gibberellins and heterosis of plant height in wheat (Triticum aestivum L.). BMC Genetics 8: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Lambrides CJ, Fukai S. 2014. Drought resistance and soil water extraction of a perennial C4 grass: contributions of root and rhizome traits. Functional Plant Biology 41: 505–519. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.