Abstract
Background and Aims Climate warming has major impacts on seed germination of several alpine species, hence on their regeneration capacity. Most studies have investigated the effects of warming after seed dispersal, and little is known about the effects a warmer parental environment may have on germination and dormancy of the seed progeny. Nevertheless, temperatures during seed development and maturation could alter the state of dormancy, affecting the timing of emergence and seedling survival. Here, the interplay between pre- and post-dispersal temperatures driving seed dormancy release and germination requirements of alpine plants were investigated.
Methods Three plant species inhabiting alpine snowbeds were exposed to an artificial warming treatment (i.e. +1·5 K) and to natural conditions in the field. Seeds produced were exposed to six different periods of cold stratification (0, 2, 4, 8, 12 and 20 weeks at 0 °C), followed by four incubation temperatures (5, 10, 15 and 20 °C) for germination testing.
Key Results A warmer parental environment produced either no or a significant increase in germination, depending on the duration of cold stratification, incubation temperatures and their interaction. In contrast, the speed of germination was less sensitive to changes in the parental environment. Moreover, the effects of warming appeared to be linked to the level of (physiological) seed dormancy, with deeper dormant species showing major changes in response to incubation temperatures and less dormant species in response to cold stratification periods.
Conclusions Plants developed under warmer climates will produce seeds with changed germination responses to temperature and/or cold stratification, but the extent of these changes across species could be driven by seed dormancy traits. Transgenerational plastic adjustments of seed germination and dormancy shown here may result from increased seed viability, reduced primary and secondary dormancy state, or both, and may play a crucial role in future plant adaptation to climate change.
Keywords: Adaptation, Cerastium cerastoides, climate warming, Leucanthemopsis alpina, parental effects, plastic responses, seed dormancy, seed germination, seed phenology, Veronica alpina
INTRODUCTION
Climate has a dominant influence on several life-history traits of species. Among plant reproductive phases, seed germination and seedling establishment are probably the most sensitive to variation in climate conditions (Walck et al., 2011) and represent the major bottleneck to species recruitment (Lloret et al., 2004; Fay and Schultz, 2009; Dalgleish et al., 2010). The strong correlation between climate and plant regeneration from seeds has resulted in the evolution of specific germination requirements across many species (Baskin et al., 2000; Fenner and Thompson, 2005), which play a key role in plant distribution and vegetation dynamics (Silvertown and Charlesworth, 2001; Higgins et al., 2003; Fenner and Thompson, 2005; Neilson et al., 2005; McGill et al., 2006; Pearson, 2006; Thuiller et al., 2008).
In seasonal climates, which are characterized by cyclic variations in temperature and/or rainfall, seed germination is usually synchronized with the changes in environmental conditions, being delayed until the favourable period occurs (Fenner and Thompson, 2005; Baskin and Baskin, 2014). A key mechanism that has enabled the development of such behaviour is seed dormancy, an innate automatism that involves environmental stimuli to avoid seed germination at the time of the year when seedling emergence would not be successful (Mattana et al., 2012; Carta et al., 2014). Indeed, the favourable conditions inducing germination might not persist long enough for seedlings to survive and grow (Vleeshouwers et al., 1995; Geneve, 2003; Finch-Savage and Leubner-Metzger, 2006).
Seed dormancy occurs in all the major angiosperm clades, and the different dormancy types and classes reflect plant adaptation to different climatic and habitat conditions (Finch-Savage and Leubner-Metzger, 2006; Baskin and Baskin, 2014; Willis et al., 2014). A commonly accepted hierarchical system of classification distinguishes five classes of seed dormancy: physiological, morphological, morphophysiological, physical and a combinational dormancy (physical + physiological) (Baskin and Baskin, 2004). Within each class, it is possible to distinguish different levels, which provide additional information about dormancy. For example, three levels are distinguished within physiological dormancy: deep, intermediate or non-deep; the latter is the most common kind of seed dormancy. At maturity, seeds with physiological dormancy may be conditionally dormant, and germinate over only a very narrow range of temperatures (Baskin and Baskin, 2014). Indeed, although there are many environmental factors (dormancy-breaking factors), such as moisture, light or chemicals, that contribute to change the dormancy state, temperature is the major driving force for the release or the induction of physiological dormancy (Fenner and Thompson, 2005). Consequently, after perceiving an adequate/accurate thermal stimulus, seeds can forthwith overcome the conditional dormancy and germinate or broaden the range of germination conditions until eventually they become non-dormant (Fenner and Thompson, 2005; Baskin and Baskin, 2014).
The degree of primary dormancy may vary considerably across populations, and such variation can be associated with both genetic and environmental factors (Finch-Savage and Leubner-Metzger 2006). For example, recent studies highlighted that dormancy has a genetic basis, but may show significant adaptive changes in response to short-term climate variation during seed maturation (Fernàndez-Pascual et al., 2013). The seed maturation environment may even have a more major role in explaining the degree of dormancy across populations than genetic differentiation (Carta et al., 2016) and, in most cases, warmer pre-dispersal temperatures result in seeds with lower dormancy and higher germinability (Fenner, 1991; Gutterman, 2000). This pivotal role of temperature in driving seed dormancy responses highlights the need to understand how changing environmental conditions will affect seed germination patterns. A large body of evidence supports the existence of rapid climate change, the main effect of which is the rise in mean global temperatures (IPCC, 2013). This will be particularly evident in high-elevation biomes, making these ecosystems, inhabited by species highly specialized to low temperatures, among the environments most threatened by the predicted climate change (Diaz and Bradley, 1997; Kullman, 2004; Nogués-Bravo et al., 2007). As a consequence, in the last decades, an increasing number of studies have addressed the effects of climate warming on different life-history traits and functions of plants in arctic and alpine tundra ecosystems (Chapin et al., 1995; Hobbie and Chapin, 1998), including germination and dormancy responses (e.g. Milbau et al., 2009; Shevtsova et al., 2009; Mondoni et al., 2012; Hoyle et al., 2013; Briceño et al., 2015; Mondoni et al., 2015).
In general, seed germination of alpine/arctic species has been shown to vary considerably in response to climate warming, for example increasing, reducing or shifting in time (see references above), and such variation was probably due to the different species and approach used (Shevtsova et al., 2009). Nevertheless, all these studies have considered seeds developed and matured under the current climate (i.e. not under warming scenarios). However, warming is not a seasonal-selective event, but acts during the whole growing season; it is therefore necessary to consider the effect of the rise in temperature not only on dispersed seeds, but also during all the reproductive cycle phases. As mentioned above, temperatures during seed development and maturation could alter the state of dormancy in both fresh and stratified seeds. In particular, warmer temperatures during seed maturation, due to altitudinal or inter-annual variations, have resulted in reduced seed dormancy, increasing the germinability of both fresh (Fernández-Pascual et al., 2013; García-Fernández et al., 2015) and cold-stratified seeds (Fernández-Pascual and Jiménez-Alfaro, 2014), or decreasing the duration of cold stratification to satisfy pre-germination vernalization requirements (Cavieres and Arroyo, 2000). Thus, seed dormancy seems to be affected by temperature variations both in space, i.e. seeds of populations from lower and warmer elevations being less dormant than those from higher and colder elevations, and in time, i.e. maternal plastic responses to local seasonality, with the general rule ‘the warmer the climate, the shallower the dormancy’. However, exceptions to this general pattern have been found; for example, no differences were observed in the germination of fresh seeds of populations of Phacelia secunda from different elevations (Cavieres and Arroyo, 2000). Moreover, whether, and how, a warmer parental environment affects the thermal interval for seed germination before and after the cold stratification (i.e. just after seed dispersal and over winter) is still not understood. To this end, the study of climate warming effects on seed dormancy and germination should decouple the effects of pre- and post-dispersal temperatures and analyse their interplay; to the best of our knowledge, no study has yet focused on this purpose.
Understanding the mechanisms affecting the development of seed dormancy plays a crucial role to forecast the future dynamics of alpine plant populations and communities in a warmer climate, since changes in thermal conditions are expected to affect both seed dormancy and germination requirements, which may preclude, delay or enhance regeneration from seeds (Walck et al., 2011). This is especially true in topographically fragmented and azonal habitats, such as alpine snowbed communities, which develop in sites characterized by a very short snow-free period. Fast alterations in plant community composition and structure have recently been observed in these habitats (e.g. Elumeeva et al., 2013; Carbognani et al., 2014a; Sandvik and Odland, 2014) that are especially sensitive to changes in environmental conditions (e.g. Carbognani et al., 2012; Petraglia et al., 2013, 2014a, b), and are retained among the most threatened vegetation types of the alpine life zone under the current climate change (Björk and Molau, 2007). Moreover, as growth and reproduction of snowbed plants are strongly controlled by micro-climatic conditions (e.g. Galen and Stanton, 1995; Hülber et al., 2010; Petraglia et al., 2014a; Carbognani et al., 2016), the predicted occurrence of longer and warmer snow-free periods will significantly advance reproductive phenophases (Petraglia et al., 2014a; Carbognani et al., 2016).
On these bases, the hypothesis that, in the next decades, seeds of snowbed plants will be dispersed during periods characterized by considerably warmer temperatures cannot be ruled out. Nevertheless, still little is known about the effects (direct and indirect) of climate warming on seed germination in snowbed environments, and even less about the effects on seed dormancy. Here, we present the first attempt to evaluate the influence of a warmer parental plant environment on seed dormancy and seed germination requirements in an alpine snowbed habitat. To this aim, three alpine species inhabiting snowbeds were exposed to an experimental warming and to natural conditions in the field. The germinability of seeds produced was tested in the laboratory after six cold stratification periods combined with four incubation temperatures. In particular, we asked the following questions. (1) Does a warmer pre-dispersal environment (i.e. during seed development and maturation) influence the temperature requirements and the speed of germination of both fresh and cold-stratified seeds? (2) Are such responses further characterized by reduced duration of cold stratification requirements to overcome seed dormancy?
MATERIALS AND METHODS
Study area
The study was performed in a snowbed area of about 2 ha located in the high Gavia Valley (Stelvio National Park, 46°20'N 10°30'E, 2700 m a.s.l.), in the Italian Rhaetian Alps.
The study site, occurring on siliceous bedrock, is located within the alpine vegetation belt, where Carex curvula grassland is the climax vegetation. A more detailed description of the vegetation types was provided by Carbognani et al. (2012).
Pre-dispersal treatment
To simulate a moderate climate warming, we used the open top chambers (OTCs; Marion et al., 1997) proposed by the International Tundra Experiment (ITEX), that are known to increase the mean daily air temperature by about 1·5 K; such an increase is consistent with the most conservative warming scenario of mountain areas for 2055 (Nogués-Bravo et al., 2007). The chambers were constructed by modifying the ITEX protocol as follows: tetragonal chambers 28 cm in height, made of 3 mm thick polymethylmethacrylate sheets (ACRIDITE® ACRISUN, Plastidite, Trieste, Italy), with basal width of 90–127 cm and summit width of 50–71 cm (Carbognani et al., 2014b).
The warming treatment started in 2008. From the end of June to mid July 2008, a total of 20 plots were randomly located within the study at snowmelt dates. The OTCs were placed in ten randomly selected plots; the other ten plots were left as natural control. Every year, the OTCs were placed in the field immediately after the snowmelt and removed at the end of the growing season. Hereafter, the warming treatment will be referred to as ‘W’ and the control (i.e. plants exposed to natural climate) as ‘C’.
Microclimatic features
In each plot, the snowmelt dates were monitored by direct observations; soil (–5 cm depth) temperatures of the snow-free period from 2008 to 2014 were recorded hourly using 12 probes in total, six for W plots and six for C plots (Onset, Cape Cod, MA, USA and Tecnosoft, Milan, Italy).
Seed collection
Between 11 September and 4 October 2013, seeds of Cerastium cerastoides (L.) Britton (Caryophyllaceae), Leucanthemopsis alpina (L.) Heywood (Asteraceae) and Veronica alpina L. (Scrophulariaceae) were collected at the time of natural dispersal from both W and C plots. The choice of the species was made on the basis of their frequency and abundance in the study site. Seeds collected from different plots of the same treatment were pooled together. After collection, seeds were processed (cleaned) and stored at room temperature until the beginning of the experiment, which occurred within 1 week after the last collection. The high initial viability (>80 %) of seed samples was checked through germination tests under conditions previously found to be optimal for germination (20 °C constant + addition of 250 mg L–1 GA3, at a 12 h daily photoperiod).
Cold stratification and incubation temperatures
Seeds were exposed to six different periods of cold stratification (0, 2, 4, 8, 12 and 20 weeks) at 0 °C in complete darkness using a cooled incubator (LMS Ltd, Sevenoaks, UK). After each interval, seeds were incubated for germination at constant temperatures at 5, 10, 15 and 20 °C. Constant temperatures were chosen to assess the effect of determinate temperature levels on seed germination (which would not have been possible under alternating regimes). In this regard, preliminary germination tests confirmed that our species did not require the trigger effect of diurnal temperature variation (e.g. Mooney and Billings, 1961; but see Liu et al., 2013), i.e. germinated well (>90 %) under constant temperatures (data not shown). For each species, pre-dispersal treatment, stratification periods and incubation temperatures, three samples of 20 seeds (C. alpina and L. alpina) or two samples of 25 seeds (C. cerastoides) were sown on agar held in 50 mm diameter Petri dishes and tested for germination. All germination tests were carried out in temperature- and light-controlled incubators (LMS 250A; LMS Ltd) using a 12 h daily photoperiod (photosynthetically active radiation 40–50 μmol m–2 s–1). Plates were checked weekly for germination and seeds were scored as germinated once radicle protrusion and elongation was >2 mm. At the completion of each germination test (4 weeks after sowing), non-germinated seeds were cut-tested to confirm whether there were empty seeds. Non-germinated empty seeds were excluded from the following analyses.
Data analysis
Germination of fresh seeds.
Influences of species, pre-dispersal treatments (i.e. warmer seed developmental environment vs. control seed developmental environment) and incubation temperatures on germination of fresh seed (i.e. without cold stratification) were evaluated by means of a Generalized Linear Model (GLM) with quasibinomial error structure (due to overdispersion) and logit link function. In this GLM, final germination (i.e. number of emerged seedlings out of the number of seeds sown) was the response variable, whereas species (as a three-level categorical variable), treatment (as a two-level categorical variable, C and W), temperature (as a continuous variable, 5, 10, 15 and 20 °C), and their two-way interactions were the fixed effects. In addition, species-specific GLMs were performed with final germination as response variable, and pre-dispersal treatment, sowing temperature and their interaction as explanatory variables.
Moreover, to study the effects of warmer incubation temperatures on the speed of seedling emergence, the mean time to germination (MTG) was calculated using the formula:
where ni is the number of seeds that emerged within consecutive intervals of time, ti the time between the beginning of the test and the end of a particular interval of measurement, and N the total number of seeds that emerged. The MTG was calculated using the day of sowing as initial time. The influences of species, pre-dispersal treatments, incubation temperatures and their two-way interactions on the MTG were analysed by means of GLM with gamma error structure and inverse link function. Within each combination of species, pre-dispersal treatment, stratification periods and incubation temperatures, seed lots that failed to germinate more than 1 % were excluded from the analysis, although they are still present in the figures for comparative purposes.
Germination after cold stratification.
Differences in final seed germination between species, incubation temperatures and stratification periods were assessed using GLM with quasibinomial error structure and logit link function. In this model, seed germination (i.e. number of emerged seedlings out of the number of seeds sown) was the response variable, whereas species (as a three-level categorical variable), treatment (as a two-level categorical variable, C and W), temperature (as a continuous variable, 5, 10, 15 and 20 °C), stratification (as a continuous variable, 0, 2, 4, 8, 12 and 20 weeks at 0 °C), and their two-way interactions were the explanatory variables. In addition, in order to gain a better understanding of the influences of variation in incubation temperatures and stratification periods on seed germination, further GLMs were performed at the species level, and model selection was carried out comparing residual deviance and the Akaike information criterion (AIC) of different models. In particular, the relationships between temperature and stratification with germination were evaluated testing the explanatory power of GLMs fitted with different combinations of explanatory variables (incubation temperatures and stratification periods) as linear, log-transformed or polynomial terms. Then, for each species, the minimal adequate model was obtained excluding non-significant terms following Crawley (2013).
In these analyses, the experimental units were the Petri dishes (each including 20–25 seeds) to which post-dispersal treatments (incubation temperatures and stratification periods) were independently applied. Nevertheless, to check for potential influence due to non-independence of seeds within a Petri dish (e.g. substrate, neighbour and incubator effects), all models were re-run including Petri dish as a random factor, with the same structures and selection procedures previously described (with the exception of error structure, since in the case of overdispersion, quasibinomial distribution was not required due to the presence of the observation-level random effect). However, in all the cases, the two different approaches produced the same outcomes and, consequently, only the results of the GLMs were reported.
All analyses were performed using R software (version 3.1.2, R Core Team, 2014).
RESULTS
Microclimatic features
During the period 2008–2014, snowmelt at the experimental site occurred on average during the 27th week of the year (WOY), between the 25th WOY (in 2011) and the 29th WOY (in 2008). The mean weekly soil temperature during this week has been detected to be between 7·4 °C (in 2014) and 11·4 °C (in 2010) in C and between 8·4 °C (in 2014) and 12·0 °C (in 2010) in W (Table 1A). During the snow-free period, which lasted 12–13 weeks, the OTCs (W) produced a mean soil warming of 1·5 K. The increase in temperature caused advancement (of about 10 d) of the phenological phases and, coherently, of seed dispersal timing in W. Indeed, seed dispersal occurred between WOY 35 and 36 in W and between WOY 36 and 37 in C, with a corresponding difference of soil temperature of about 3 K (Table 1A). The snowfall usually occurs at WOY 40–41, in the first half of October (Table 1A).
Table 1.
(A) Weekly soil temperatures (mean ±; s.e. in °C) during the snow-free period of the years 2008–2014 in control (C) and warmed (W) plots. (B) Monthly mean soil temperatures of the year 2013
| (A) | Soil temperature (°C) |
||
|---|---|---|---|
| Week of the year | Equivalent time of a normal year | C | W |
| 27 | 2–8 July | 9·7 ± 0·1 | 11·0 ± 0·1 |
| 28 | 9–15 July | 10·2 ± 0·3 | 11·0 ± 0·2 |
| 29 | 16–22 July | 8·8 ± 0·2 | 10·6 ± 0·2 |
| 30 | 23–29 July | 8·8 ± 0·1 | 10·3 ± 0·1 |
| 31 | 30 July–5 August | 9·8 ± 0·2 | 11·3 ± 0·2 |
| 32 | 6–12 August | 9·4 ± 0·1 | 10·7 ± 0·1 |
| 33 | 13–19 August | 8·1 ± 0·3 | 9·5 ± 0·3 |
| 34 | 20–26 August | 8·3 ± 0·3 | 9·8 ± 0·3 |
| 35 | 27 August–2 September | 8·1 ± 0·1 | 9·5 ± 0·1 |
| 36 | 3–9 September | 7·6 ± 0·1 | 9·2 ± 0·2 |
| 37 | 10–16 September | 4·9 ±0·5 | 6·1 ± 0·4 |
| 38 | 17–23 September | 4·7 ± 0·2 | 6·3 ± 0·2 |
| 39 | 24–30 September | 5·9 ± 0·2 | 7·2 ± 0·3 |
| 40–41 | 1–14 October | Snowfall | |
| Different styling indicates the significant events of snowmelt (underlined), seed dispersal (bold) and snowfall (italic). | |||
| (B) |
2013 soil temperature (°C) |
||
|---|---|---|---|
| Month | Weeks of the year (2013) | C | W |
| July | 29–31 | 10·8 ± 0·8 | 12·7 ± 0·7 |
| August | 31–35 | 7·7 ± 1·1 | 9·3 ± 1·1 |
| September | 35–40 | 4·9 ± 1·2 | 6·5 ± 1·1 |
Seeds of the studied species were collected in 2013, between the 35th and 36th WOY in W and between the 36th and the 37th WOY in C; the mean monthly temperature of this growing season, consistent with the multi-year monthly mean, is shown in Table 1B.
Germination of fresh seeds
Germination phenology.
On fresh seeds (i.e. without cold stratification), significant differences in the final germination were observed among species, whose seeds showed a higher germination proportion under warmer incubation temperatures but were not influenced by the pre-dispersal treatment (Table 2; Supplementary Data Table S1). Furthermore, the effects of both pre-dispersal treatment and incubation temperatures were not consistent among the species studied (i.e. significant interaction terms).
Table 2.
Analysis of deviance table of GLMs for the influence of species, pre-dispersal treatment, incubation temperature and their interactions on final germination and mean time to germinate of fresh seeds (i.e. without stratification)
| Factor |
d.f. | Dev. | Res. d.f. | Dev. res | F-value | P-value |
|---|---|---|---|---|---|---|
| Final germination | ||||||
| Species (Sp) | 2 | 276·9 | 61 | 711·9 | 33·30 | <0·001 |
| Pre-dispersal treatment (Tr) | 1 | 1·2 | 60 | 710·7 | 0·30 | 0·589 |
| Incubation temperature (T) | 1 | 465·4 | 59 | 245·3 | 111·93 | <0·001 |
| Sp × Tr | 2 | 26·9 | 57 | 218·4 | 3·24 | 0·047 |
| Sp × T | 2 | 42·4 | 55 | 176·0 | 5·10 | 0·009 |
| Tr × T | 1 | 6·1 | 54 | 168·9 | 1·46 | 0·232 |
| Mean time to germinate | ||||||
| Species (Sp) | 2 | 0·4 | 45 | 8·8 | 7·47 | 0·002 |
| Pre-dispersal treatment (Tr) | 1 | 0·0 | 44 | 8·8 | 0·15 | 0·702 |
| Incubation temperature (T) | 1 | 7·6 | 43 | 1·2 | 293·02 | <0·001 |
| Sp × Tr | 2 | 0·2 | 41 | 1·1 | 3·11 | 0·056 |
| Sp × T | 2 | 0·1 | 39 | 1·0 | 1·44 | 0·250 |
| Tr × T | 1 | 0·0 | 38 | 1·0 | 0·02 | 0·895 |
The models were performed with quasibinomial error and logit link function for the final germination, and gamma error and inverse link function for the mean time to germinate. Seed lots that failed to germinate >1 % were excluded from the analysis of mean time to germinate.
Degrees of freedom (d.f.), deviance (Dev.), residual degrees of freedom (Res. d.f.), residual deviance (Dev. res), F- and P-values are shown.
Significant values are highlighted in bold.
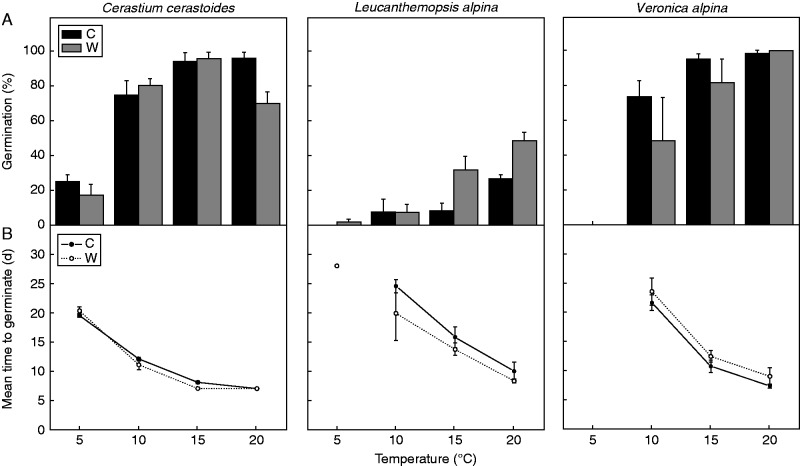
The increase in incubation temperatures resulted in a significant increase in germination percentage in all the species and treatments tested (Table 3; Supplementary Data Table S2). At the lowest incubation temperature (i.e. 5 °C), germination percentage was very low or, in some cases, null in all the species and treatments (Fig. 1A). In particular, at 5 °C, seeds of C. cerastoides germinated between 20 and 25 %, the germination of W seeds of L. alpina remained <1 %, while no emergence was found in control seeds of L. alpina and in V. alpina. At 10 °C, about 60–80 % of seeds of C. cerastoides and V. alpina germinated, and under higher incubation temperatures (i.e. 15 and 20 °C) the final germination percentage of these species remained, on average, at high levels. Conversely, germination of L. alpina remained low (<7 %) until the temperature of 15 °C for W seeds and 20 °C for C seeds, after which it showed a significant, but moderate (up to 40–50 % in W and to 30 % in C), increase. The pre-dispersal warming had no effects on the germination of C. cerastoides and V. alpina (Table 3), but it increased seed germination of L. alpina (Table 3; Fig. 1A). Despite this, the final germination of this latter species remained lower (<50 %) in comparison with those of the other two species.
Table 3.
Analysis of deviance tables of species-specific GLMs (with quasibinomial error and logit link function) for the effects of pre-dispersal treatment and incubation temperature on fresh seed final germination
| Factor | d.f. | Dev. | Res. d.f. | Dev. res | F-value | P-value |
|---|---|---|---|---|---|---|
| Final germination | ||||||
| Cerastium cerastoides | ||||||
| Pre-dispersal treatment (Tr) | 1 | 3·6 | 14 | 158·2 | 0·71 | 0·417 |
| Incubation temperature (T) | 1 | 93·4 | 13 | 64·8 | 18·52 | 0·001 |
| Tr × T | 1 | 6·5 | 12 | 58·3 | 1·30 | 0·277 |
| Leucanthemopsis alpina | ||||||
| Pre-dispersal treatment (Tr) | 1 | 7·6 | 16 | 47·9 | 5·99 | 0·028 |
| Incubation temperature (T) | 1 | 29·2 | 15 | 18·7 | 22·94 | <0·001 |
| Tr × T | 1 | 0·1 | 14 | 18·6 | 0·07 | 0·797 |
| Veronica alpina | ||||||
| Pre-dispersal treatment (Tr) | 1 | 3·8 | 16 | 91·9 | 1·23 | 0·286 |
| Incubation temperature (T) | 1 | 51·6 | 15 | 40·3 | 16·85 | 0·001 |
| Tr × T | 1 | 0·1 | 14 | 40·2 | 0·031 | 0·863 |
Degrees of freedom (d.f.), deviance (Dev.), residual degrees of freedom (Res. d.f.), residual deviance (Dev. res), F- and P-values are shown.
Significant values are highlighted in bold.
Fig. 1.
(A) Final germination percentage (mean ± s.e.) of control (C) and warmed (W) seeds and (B) mean time to germinate (in days, mean ± s.e.) of control and warmed seeds of C. cerastoides, L. alpina and V. alpina under the four incubation temperatures. Seed lots that failed to germinate more than 1 % were excluded from the analyses of the mean time to germination, although they are still present in the figures for comparative purposes.
Mean time to germinate.
Significant differences in the MTG of fresh seeds were found between both species and incubation temperatures tested (with faster germination under warmer temperatures), but no effect of the pre-dispersal warming was found (Table 2). We found that the increase in incubation temperatures produced a significant reduction of the MTG in all the species, homogeneously between both the pre-dispersal treatments and the species (of about 1 d per 1 K of temperature increase); however, some species-specific patterns were found (Fig. 1B). In particular, at the coldest temperature (i.e. 5 °C), C. cerastoides was the only species able to germinate (values >5 %) and its seeds required almost 3 weeks (20 d) to germinate. Similarly, when the other species started to germinate, at 10 °C, the MTG was 20–25 d. With the increase in incubation temperatures, the MTG was progressively reduced in all the species, with the lowest value at 20 °C.
Germination after cold stratification
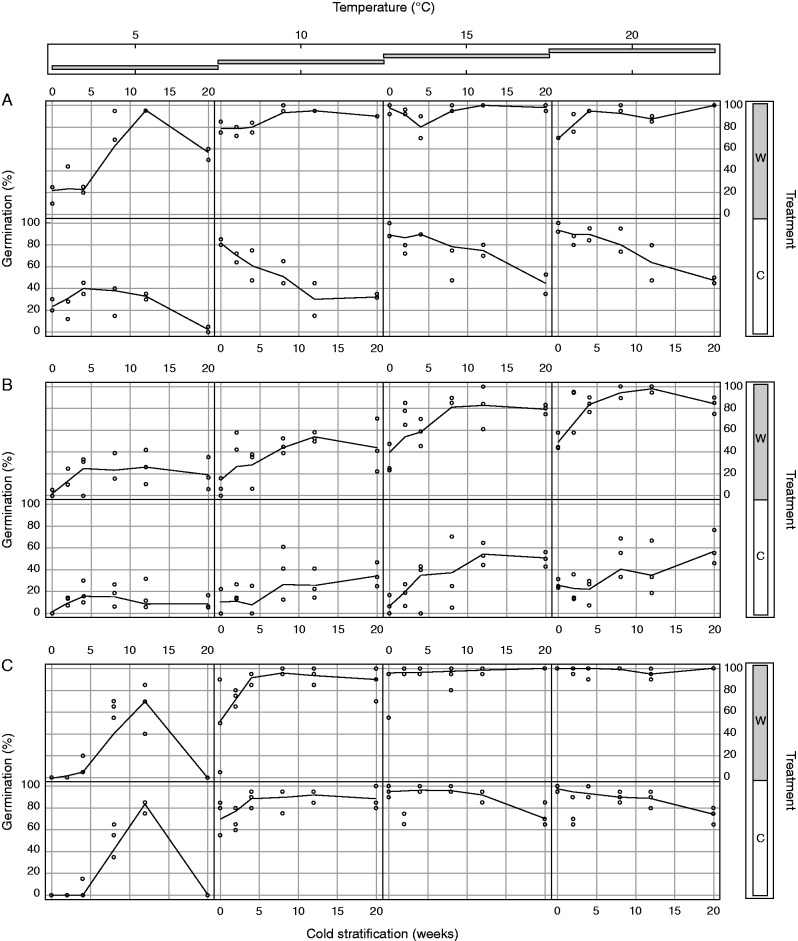
Our analyses showed significant differences in germination percentage within all the factors (species, pre-dispersal treatment, incubation temperature and cold stratification period) tested (Table 4; Supplementary Data Table S3). In particular, germination was on average higher in C. cerastoides and V. alpina compared with L. alpina, in W compared with C seeds, and under warmer incubation temperature and longer cold stratification periods (Fig. 2). Furthermore, significant interactions revealed non-consistent responses of species to all the other factors, and non-additive effects of pre-dispersal treatment with different incubation temperatures and stratification periods. Indeed, species-specific GLMs (Table 5) showed that W seeds, compared with C seeds, had higher germination with increasing length of stratification period (in C. cerastoides, Table 5), with increasing temperature of incubation (in L. alpina, Table 5) or with increasing both the length of stratification period and the incubation temperature (in V. alpina, Table 5).
Table 4.
Analysis of deviance table of GLM for the influence of species (Sp), pre-dispersal treatment (Tr), incubation temperature (T), cold stratification period (Sf) and their interactions on seed germination proportion
| Factor | d.f. | Dev. | Res. d.f. | Dev. res | F-value | P-value |
|---|---|---|---|---|---|---|
| Sp | 2 | 621·1 | 381 | 3822·3 | 69·17 | <0·001 |
| Tr | 1 | 223·4 | 380 | 3598·9 | 49·75 | <0·001 |
| T | 1 | 1522·1 | 379 | 2076·8 | 339·00 | <0·001 |
| Sf | 1 | 50·8 | 378 | 2026·0 | 11·32 | 0·001 |
| Sp × Tr | 2 | 90·6 | 376 | 1935·4 | 10·09 | <0·001 |
| Sp × T | 2 | 98·6 | 374 | 1836·8 | 10·98 | <0·001 |
| Sp × Sf | 2 | 81·2 | 372 | 1755·7 | 9·04 | <0·001 |
| Tr × T | 1 | 70·3 | 371 | 1685·4 | 15·66 | <0·001 |
| Tr × Sf | 1 | 54·2 | 370 | 1631·1 | 12·08 | <0·001 |
| T × Sf | 1 | 1·8 | 369 | 1629·3 | 0·40 | 0·526 |
The model was performed with quasibinomial error and logit link function.
Degrees of freedom (d.f.), deviance (Dev.), residual degrees of freedom (Res. d.f.), residual deviance (Dev. res), F- and P-values are shown.
Significant values are highlighted in bold.
Fig. 2.
Seed germination (in percentage) of (A) C. cerastoides, (B) L. alpina and (C) V. alpina. For each pre-dispersal treatment (C, control; W, warmed), the influences on seed germination of the length of cold stratification periods and the four incubation temperatures are shown.
Table 5.
Species-specific GLM results for the responses of seed germination proportion to the different incubation temperature (T), stratification period (Sf), pre-dispersal treatment (Tr) and their interactions
| Estimate | s.e. | t-value | P-value | |
|---|---|---|---|---|
| C. cerastoides | ||||
| (Intercept) | –5·6850 | 0·7610 | –7·47 | <0·001 |
| log(T) | 2·8030 | 0·3237 | 8·66 | <0·001 |
| Sf | 0·0928 | 0·0274 | 3·39 | 0·001 |
| Sf2 | –0·0007 | 0·0002 | –3·81 | <0·001 |
| Tr | –0·1221 | 0·2595 | –0·47 | 0·639 |
| log(T) × Sf | –0·0409 | 0·0117 | –3·51 | 0·001 |
| log(T) × Sf2 | 0·0003 | 0·0001 | 3·43 | 0·001 |
| Sf × Tr | 0·0280 | 0·0044 | 6·43 | <0·001 |
| L. alpina | ||||
| (Intercept) | –4·8571 | 0·5674 | –8·56 | <0·001 |
| log(T) | 1·1410 | 0·2115 | 5·39 | <0·001 |
| Tr | –2·0266 | 0·7429 | –2·73 | 0·007 |
| log(Sf) | 0·2996 | 0·0344 | 8·72 | <0·001 |
| log(T) × Tr | 1·3783 | 0·2936 | 4·69 | <0·001 |
| V. alpina | ||||
| (Intercept) | –6·0075 | 1·4577 | –4·12 | <0·001 |
| log(T) | 2·9854 | 0·5783 | 5·16 | <0·001 |
| Sf | 0·0734 | 0·0189 | 3·88 | <0·001 |
| Tr | –4·0819 | 1·2556 | –3·25 | 0·002 |
| log(T) × Sf | –0·0283 | 0·0072 | –3·94 | <0·001 |
| log(T) × Tr | 1·6460 | 0·5109 | 3·22 | 0·002 |
| Sf × Tr | 0·0132 | 0·0060 | 2·20 | 0·030 |
Estimated coefficient, standard error, t-value and P-value are shown.
Significant values are highlighted in bold.
The germination of all the three species showed a saturated pattern in response to increasing incubation temperatures (Table 5), with greater effects of increasing temperature on germination under colder than warmer conditions (i.e. log-transformed temperatures had the best fit to data). However, in L. alpina, the effect of the incubation temperatures was consistent under different stratification periods (Table 5; Fig. 2B). In contrast, in the other two species, seeds incubated at the lowest temperature (i.e. 5 °C) showed different patterns of germination in response to variation in the length of stratification period compared with those of seeds incubated under warmer temperature levels (Table 5; Fig. 2A, C).
Finally, seed germination of the species studied differed markedly in their response to the duration of the cold stratification. In particular, seed germination of C. cerastoides (which had ‘stratification’ as the polynomial term in the best model) showed an initial increase followed by a decrease of germination percentage with the increase of the cold stratification period (Table 5; Fig. 2A). However, in this species, the increase of germination after the shortest cold stratification periods was extremely low in seeds produced at the control (C) plots and therefore the general tendency was to lose germinability with increasing duration of stratification in this pre-dispersal treatment (Fig. 2A). On the other hand, L. alpina seed germination seemed to have a saturated response to an increase in cold stratification duration, with more pronounced increases of germination during shorter durations of cold stratification (i.e. the log-transformed stratification period had the best fit to data) (Table 5; Fig. 2B). In the case of V. alpina, unlike the previous species, the best relationship between cold stratification period and seed germination was linear, with (after the effects of the others factors) a constant increase of germination with increasing duration of cold stratification (Table 5; Fig. 2C).
DISCUSSION
Germination of fresh seeds
The present study described how pre- and post-seed dispersal climate warming influenced seed dormancy and germination requirements of three alpine species inhabiting snowbeds. We have shown that, after dispersal, germination was similar between seeds produced in the control (C) and under warming (W) at lower incubation temperatures (i.e. 5 and 10 °C) in all species, but that seeds of L. alpina produced in W showed a slight increase in germination under warmer incubation temperatures (i.e. 15 and 20 °C) (Fig. 1A). These results alone indicate that germination/dormancy changes of fresh seeds driven by a warmer parental growth environment are limited and may be expected only if seeds experience warm (e.g. >15 °C) conditions after dispersal. Interestingly, temperatures recorded at the study area at the time of seed dispersal are usually lower (see Table 1) than those able to stimulate an increase of seed germination in W seeds of L. alpina (15 and 20 °C), indicating that no changes of seedling emergence should be expected in a future warmer climate on fresh seeds of this species. However, the possibility that a warmer parental growth environment (than that used here, about +1·5 K) could have higher effects on germination of fresh seeds cannot be ruled out.
Fresh seeds of all species did not germinate, or germinated to very low percentages at 5 °C, a temperature similar to the temperatures they currently experience after dispersal (Table 1), but germination increased significantly after cold stratification (Fig. 2), indicating that seedling emergence of the species studied is probably programmed to occur after the winter season has passed. Seed germination of alpine plants is indeed known to occur in spring, after they have experienced autumn and winter seasons (Körner, 2003). However, high germination percentages of C. cerastoides and V. alpina seeds (approx. 80 %) were found at 10 °C, i.e. a temperature similar to those occurring under warming during the seed dispersal time (9·5 °C, Table 1). Thus, germination before the winter season should increase in these species in a warmer climate, which may have important implications for seedling survival and establishment (see Mondoni et al., 2012, 2015). Interestingly, our data also highlighted that such an increase in germination percentage occurred similarly for seeds produced under both W and C, regardless of the climatic scenarios experienced before seed dispersal. Further, we have shown that an increase of seedling emergence at the end of the snow-free season is possible only for seeds exposed to approx. 10 °C for at least approx. 10 and 20 d for C. cerastoides and V. alpina, respectively (see MTG, Fig. 1B). However, a recent study showed that a short-term heat event may also affect the timing of germination and lead seeds to germinate before the winter season (Orsenigo et al., 2015), suggesting that the early germination could be possible even when the exposure of fresh seeds to warm temperatures is shorter than those shown here.
Considering the warming rate predicted for the next decades in the European mid-latitude mountains (ranging from about +0·3 to 0·5 K per decade; Nogués-Bravo et al., 2007) and the consequent antedating of phenological phases in alpine snowbed plants (Carbognani et al., 2016), a significant increase of germination at the end of the snow-free season should be expected. This is especially true for C. cerastoides, i.e. among the snowbed species at the study site, the one with the earliest seed dispersal (M. Carbognani et al., unpubl. res.).
Germination after cold stratification
Seeds of our species showed a certain degree of non-deep physiological dormancy (sensu Baskin and Baskin, 2014), which varied in its depth depending on the species. Indeed, seed germination increased significantly with an increase in the period of cold stratification in all species, with a wide variation across them (Table 4). In particular, fresh seeds of C. cerastoides germinated at all temperatures, between approx. 20 % (at 5 °C) and 80–100 % (at > 10 °C) (Fig. 1A), and its W-treated seeds showed a significant increase to approx. 90 % at 5 °C after 3 months of cold stratification (Fig. 2A). Indeed, the germination of seeds developed under the current climate declined with an increase in the stratification period, indicating either a loss of viability or the development of a secondary dormancy (see below). Seeds of V. alpina showed similar responses but did not germinate at 5 °C in the absence of cold stratification. Conversely, seed germination was low in fresh seeds of L. alpina at all temperatures (about 30–40 %), increasing mostly after a long period of cold stratification (i.e. Fig. 2B; Table 5). On the basis of this evidence, although these species should be considered as non-deep physiologically dormant, the different temperature windows for germination and durations of cold stratification required to break dormancy indicate that L. alpina is the strongest (physiologically) dormant species, followed by V. alpina and C. cerastoides. Moreover, the increase in stratification period generally resulted in a significant increase in germination percentage (Table 4, Fig. 2), but the species studied responded differently to the interplay of cold stratification and warming treatment. In seeds of C. cerastoides, the increase in stratification duration had opposite responses in seeds produced under W and C, increasing and reducing the germination percentage, respectively, and similar responses were observed in V. alpina (see Sf × Tr, Table 5). Conversely, parental warming did not affect the germination response to cold stratification in L. alpina (Table 5). The significant temperature × treatment interaction found in the cold-stratified seeds of L. alpina (Table 5) indicates that incubation temperatures had different responses in seeds produced under W and C in this species. These are interesting and novel observations indicating that changes in germination requirements induced by the pre-dispersal warming environment depended on the seed dormancy state, with more deeply dormant species showing major changes in response to incubation temperature on both fresh and cold-stratified seeds (i.e. L. alpina) and less dormant species in response to the stratification (i.e. C. cerastoides and V. alpina) (Tables 3 and 5). Moreover, changes of pre-dispersal temperatures affected germination response to both stratification and incubation temperatures (at least on cold-stratified seeds) in V. alpina, which showed an intermediate dormancy behaviour between C. cerastoides and L. alpina (see above).
As a result, higher seed maturation temperatures consistently increased the germination in all species, although to a different extent across them (Fig. 2). The higher seed maturation temperatures had no effect on the MTG, but it widened the suitable temperature range for germination and reduced the cold stratification time to break dormancy in L. alpina. Indeed, at 15 °C, germination was extended to approx. 40 % when fresh seeds of this species matured under W, while it was <10 % on seeds produced in the C. Moreover, seed germination was significantly higher in W-treated seeds of L. alpina, compared with C seeds, after only 2 weeks of cold stratification (Fig. 2B), indicating that under warming some species will produce seeds with a shorter chilling requirement to overcome dormancy. This behaviour occurred at all incubation temperatures, except 5 °C, probably because the low temperature constrains the germination (Fig. 2B).
The higher germination responses observed for both fresh and cold-stratified seeds produced under W, compared with those developed under C, could be due to changes in the dormancy patterns and/or the seed viability of our species. Warmer maturation conditions are known to lead to better quality of seeds (see reviews by Roach and Wulff, 1987; Wulff, 1995; Gutterman, 2000), and it has been suggested that plants grown in a warmer environment produce longer lived seeds (Bernareggi et al., 2015). In this regard, the different behaviour of W and C seeds of C. cerastoides might suggest that the current alpine climate is not optimal for seed development and maturation, thereby reducing the survival capacities during the long-lasting winter conditions. Similar conclusions may explain the lower germination of C vs. W seeds of L. alpina. However, after 20 weeks of cold stratification seed germination of V. alpina dropped to 0 % at 5 °C, but remained almost complete at the other incubation temperatures, indicating that seeds were still viable. This behaviour reflects the condition of fresh seeds (Fig. 1A, C), suggesting that the prolonged permanence at 0 °C could have induced a secondary dormancy state in seeds of this species. Indeed, it is possible that non-dormant seeds that do not meet adequate conditions for germination enter secondary dormancy (Brandel, 2005; Leymarie et al., 2008; Baskin and Baskin, 2014) and, therefore, similar mechanisms may also explain the decreased germination with increasing duration of the cold stratification period in C. cerastoides and the lower germination of C vs. W seeds in L. alpina.
Summarizing, this study suggests that climate warming will increase the likelihood of seed germination both just after dispersal and during the winter season (i.e. after cold stratification) due to the effects on seed phenology (e.g. advanced seed dispersal) and on environmental conditions (e.g. early snowmelt, warmer temperatures during the snow-free period). However, the consequences that these changes will have on plant reproduction success still remain partially investigated. For example, while germination immediately after seed dispersal has always been considered to be disadvantageous because of constraints on seedlings to cope with the harsh winter conditions, a recent study in a glacier foreland showed that a high percentage (approx. 60 and 75 %) of autumn-emerged seedlings can survive through the winter (Mondoni et al., 2015). However, survival over the winter involves elevated energy consumption by seedlings (Maruta, 1994), which may reduce the growing capacities in spring. Moreover, the higher germination of W-treated vs. C seeds after long periods of stratification suggests an increase in reproductive success. A persistent soil seed bank in several alpine species is thought to be an ecological adaptation to the low chance of establishment in these environments (Schwienbacher et al., 2010). Hence, higher germination may not necessarily be beneficial for alpine plants, as summer drought and late-winter/early spring soil freeze–thaw events are expected to increase due to climate warming (Barriopedro et al., 2011), as already shown in some mountain regions (Rikiishi et al., 2004; Scherrer et al., 2004; Mote et al., 2005), reducing the likelihood of seedlings to survive. In this context, here we have shown that the degree of (physiological) dormancy will play a primary role in modulating the effects of (pre- and post-dispersal) warming on seed germination, which may have important effects on plant regeneration from seeds and, consequently, on vegetation dynamics.
SUPPLEMENTARY DATA
Supplementary data are available online at www.aob.oxfordjournals.org and consist of the following. Table S1: results of minimal adequate GLMs concerning the effects of species, pre-dispersal treatment, incubation temperature and their interactions on final germination and mean time to germination of fresh seeds (i.e. without stratification). Table S2: species-specific minimal adequate GLM (with quasibinomial error and logit link function) results for the influence of pre-dispersal treatment and incubation temperature on fresh seed final germination. Table S3: GLM results for the effects of species, pre-dispersal treatment, incubation temperature, cold stratification period and their interactions on seed germination proportion.
ACKNOWLEDGEMENTS
We thank the Stelvio National Park for fieldwork authorization and facilities.
LITERATURE CITED
- Barriopedro D, Fischer EM, Luterbacher J, Trigo RM, García-Herrera R. 2011. The hot summer of 2010: redrawing the temperature record map of Europe. Science 332: 220–224. [DOI] [PubMed] [Google Scholar]
- Baskin CC, Baskin JM. 2014. Seeds: ecology, biogeography, and evolution of dormancy and germination, 2nd edn London: Academic Press. [Google Scholar]
- Baskin JM, Baskin CC. 2004. A classification system for seed dormancy. Seed Science Research 14: 1–16. [Google Scholar]
- Baskin JM, Baskin CC, Li X. 2000. Taxonomy, ecology, and evolution of physical dormancy in seeds. Plant Species Biology 15: 139–152. [Google Scholar]
- Bernareggi G, Carbognani M, Petraglia A, Mondoni A. 2015. Climate warming could increase seed longevity of alpine snowbed plants. Alpine Botany 125: 69–78. [Google Scholar]
- Björk RG, Molau U. 2007. Ecology of alpine snowbeds and the impact of global change. Arctic, Antarctic and Alpine Research 39: 34–43. [Google Scholar]
- Brandel M. 2005. The effect of stratification temperatures on the level of dormancy in primary and secondary dormant seeds of two Carex species. Plant Ecology 178: 163–169. [Google Scholar]
- Briceño VF, Hoyle GL, Nicotra AB. 2015. Seeds at risk: how will a changing alpine climate affect regeneration from seeds in alpine areas? Alpine Botany 125: 59–68. [Google Scholar]
- Carbognani M, Bernareggi G, Perucco F, Tomaselli M, Petraglia A. 2016. Micro-climatic controls and warming effects on flowering time in alpine snowbeds. Oecologica 1–13. doi:10.1007/s00442-016-3669-3. [DOI] [PubMed] [Google Scholar]
- Carbognani M, Petraglia A, Tomaselli M. 2012. Influence of snowmelt time on species richness, density and production in a late snowbed community. Acta Oecologica 43: 113–120. [Google Scholar]
- Carbognani M, Tomaselli M, Petraglia A. 2014a. Current vegetation changes in an alpine late snowbed community in the south-eastern Alps (N-Italy). Alpine Botany 124: 105–113. [Google Scholar]
- Carbognani M, Petraglia A, Tomaselli M. 2014b. Warming effects and plant trait control on the early-decomposition in alpine snowbeds. Plant and Soil 376: 277–290. [Google Scholar]
- Carta A, Probert RJ, Moretti M, Peruzzi L, Bedini G. 2014. Seed dormancy and germination in three Crocus ser. Verni species (Iridaceae): implications for evolution of dormancy within the genus. Plant Biology 16: 1065–1074. [DOI] [PubMed] [Google Scholar]
- Carta A, Probert RJ, Puglia G, Peruzzi L, Bedini G. 2016. Local climate explains degree of seed dormancy in Hypericum elodes L. (Hypericaceae). Plant Biology 18: 76–82. [DOI] [PubMed] [Google Scholar]
- Cavieres LA, Arroyo MTK. 2000. Seed germination response to cold stratification period and thermal regime in Phacelia secunda (Hydrophyllaceae): altitudinal variation in the mediterranean Andes of central Chile. Plant Ecology 149: 1–8. [Google Scholar]
- Chapin FS III, Shaver GR, Giblin AE, Nadelhoffer KJ, Laundre JA. 1995. Responses of arctic tundra to experimental and observed changes in climate. Ecology 76: 694–711. [Google Scholar]
- Crawley MJ. 2013. The R book. Chichester, UK: John Wiley & Sons. [Google Scholar]
- Dalgleish HJ, Koons DN, Adler PB. 2010. Can life-history traits predict the response of forb populations to changes in climate variability? Journal of Ecology 98: 209–217. [Google Scholar]
- Diaz HF, Bradley RS. 1997. Temperature variations during the last century at high elevation sites. Climatic Change 36: 253–279. [Google Scholar]
- Elumeeva T, Onipchenko VG, Egorov AV, et al. 2013. Long-term vegetation dynamic in the Northwestern Caucasus: which communities are more affected by upward shifts of plant species? Alpine Botany 123: 77–85. [Google Scholar]
- Fay PA, Schultz MJ. 2009. Germination, survival, and growth of grassland forb seedlings: effects of soil moisture variability. Acta Oecologica 35: 679–684. [Google Scholar]
- Fenner M. 1991. The effects of the parent plant environment on seed germinability. Seed Science Research 1: 75–84. [Google Scholar]
- Fenner M, Thompson K. 2005. The ecology of seeds. Cambridge: Cambridge University Press. [Google Scholar]
- Fernández-Pascual E, Jiménez-Alfaro B. 2014. Phenotypic plasticity in seed germination relates differentially to overwintering and flowering temperatures. Seed Science Research 24: 273–280. [Google Scholar]
- Fernández-Pascual E, Jiménez-Alfaro B, Caujapé-Castells J, Jaén-Molina R, Díaz TE. 2013. A local dormancy cline is related to the seed maturation environment, population genetic composition and climate. Annals of Botany 112: 937–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch-Savage WE, Leubner-Metzger G. 2006. Seed dormancy and the control of germination. New Phytologist 171: 501–523. [DOI] [PubMed] [Google Scholar]
- Galen C, Stanton ML. 1995. Responses of snowbed plant-species to changes in growing-season length. Ecology 76: 1546–1557. [Google Scholar]
- García-Fernández A, Escudero A, Lara-Romero C, Iriondo JM. 2015. Effects of the duration of cold stratification on early life stages of the Mediterranean alpine plant Silene ciliata. Plant Biology 17: 344–350. [DOI] [PubMed] [Google Scholar]
- Geneve RL. 2003. Impact of temperature on seed dormancy. HortScience 38: 336–341. [Google Scholar]
- Gutterman Y. 2000. Maternal effects on seeds during development In: Fenner M, ed. Seeds: the ecology of regeneration in plant communities, 2nd edn Wallingford, UK: CABI Publishing, 59–84. [Google Scholar]
- Higgins SI, Clark JS, Nathan R, et al. 2003. Forecasting plant migration rates: managing uncertainty for risk assessment. Journal of Ecology 91: 341–347. [Google Scholar]
- Hobbie SE, Chapin FS III. 1998. The response of tundra plant biomass, aboveground production, nitrogen, and CO2 flux to experimental warming. Ecology 79: 1526–1544. [Google Scholar]
- Hoyle GL, Venn SE, Steadman KJ, et al. 2013. Soil warming increases plant species richness but decreases germination from the alpine soil seed bank. Global Change Biology 19: 1549–1561. [DOI] [PubMed] [Google Scholar]
- Hülber K, Winkler M, Grabherr G. 2010. Intraseasonal climate and habitat-specific variability controls the flowering phenology of high alpine plant species. Functional Ecology 24: 245–252. [Google Scholar]
- IPCC (International Panel on Climate Change). 2013. Summary for policymakers In: Stocker TF, Qin D, Plattner G-K, et al. , eds. Climate change 2013: the physical science basis. Contribution of working group I to the fifth assessment report of the Intergovernmental Panel on Climate Change. Cambridge: Cambridge University Press, 3–29. [Google Scholar]
- Körner C. 2003. Alpine plant life: functional plant ecology of high mountain ecosystems, 2nd edn Berlin: Springer-Verlag. [Google Scholar]
- Kullman L. 2004. Long-term geobotanical observations of climate change impacts in the Scandes of West-Central Sweden. Nordic Journal of Botany 24: 445–467. [Google Scholar]
- Leymarie J, Robayo-Romero ME, Gendreau E, Benech-Arnold RL, Corbineau F. 2008. Involvement of ABA in induction of secondary dormancy in barley (Hordeum vulgare L.) seeds. Plant and Cell Physiology 49: 1830–1838. [DOI] [PubMed] [Google Scholar]
- Liu K, Baskin JM, Baskin CC, Bu H, Du G, Ma M. 2013. Effect of diurnal fluctuating versus constant temperatures on germination of 445 species from the eastern Tibet Plateau. PLoS One 8: e69364. doi:10.1371/journal.pone.0069364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloret F, Peñuelas J, Estiarte M. 2004. Experimental evidence of reduced diversity of seedlings due to climate modification in a Mediterranean-type community. Global Change Biology 10: 248–258. [Google Scholar]
- Marion GM, Henry GHR, Freckman DW, et al. 1997. Open-top design for manipulating field temperature in high-latitude ecosystems. Global Change Biology 3: 20–32. [Google Scholar]
- Maruta E. 1994. Seedling establishment of Polygonum cuspidatum and Polygonum weyrichii var. alpinum at high-altitudes of Mt Fuji. Ecological Research 9: 205–213. [Google Scholar]
- Mattana E, Pritchard HW, Porceddu M, Stuppy WH, Bacchetta G. 2012. Interchangeable effects of gibberellic acid and temperature on embryo growth, seed germination and epicotyl emergence in Ribes multiflorum ssp. sandalioticum (Grossulariaceae). Plant Biology 14: 77–87. [DOI] [PubMed] [Google Scholar]
- McGill BJ, Enquist BJ, Weiher E, Westoby M. 2006. Rebuilding community ecology from functional traits. Trends in Ecology and Evolution 21: 178–185. [DOI] [PubMed] [Google Scholar]
- Milbau A, Graae BJ, Shevtsova A, Nijs I. 2009. Effects of a warmer climate on seed germination in the subarctic. Annals of Botany 104: 287–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondoni A, Rossi G, Orsenigo S, Probert RJ. 2012. Climate warming could shift the timing of seed germination in alpine plants. Annals of Botany 110: 155–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondoni A, Pedrini S, Bernareggi G, et al. 2015. Climate warming could increase recruitment success in glacier foreland plants. Annals of Botany 116: 907–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney HA, Billings WD. 1961. Comparative physiological ecology of arctic and alpine populations of Oxyria digyna. Ecological Monographs 31: 1–29. [Google Scholar]
- Mote PW, Hamlet AF, Clark MP, Lettenmaier DP. 2005. Declining mountain snowpack in western North America. Bulletin of the American Meteorological Society 86: 39–49. [Google Scholar]
- Neilson RP, Pitelka LF, Solomon AM, et al. 2005. Forecasting regional to global plant migration in response to climate change. Bioscience 55: 749–759. [Google Scholar]
- Nogués-Bravo D, Araújo MB, Errea MP, Martínez-Rica JP. 2007. Exposure of global mountain systems to climate warming during the 21st Century. Global Environmental Change 17: 420–428. [Google Scholar]
- Orsenigo S, Abeli T, Rossi G, et al. 2015. Effects of autumn and spring heat waves on seed germination of high mountain plants. PLoS One 10: e0133626. doi:10.1371/journal.pone.0133626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson RG. 2006. Climate change and the migration capacity of species. Trends in Ecology and Evolution 21: 111–113. [DOI] [PubMed] [Google Scholar]
- Petraglia A, Carbognani M, Tomaselli M. 2013. Effects of nutrient amendments on modular growth, flowering effort and reproduction of snowbed plants. Plant Ecology and Diversity 6: 475–486. [Google Scholar]
- Petraglia A, Tomaselli M, Petit Bon M, Delnevo N, Chiari G, Carbognani M. 2014a. Responses of flowering phenology of snowbed plants to an experimentally imposed extreme advanced snowmelt . Plant Ecology 215: 759–768. [Google Scholar]
- Petraglia A, Tomaselli M, Mondoni A, Brancaleoni L, Carbognani M. 2014b. Effects of nitrogen and phosphorus supply on growth and flowering phenology of the snowbed forb Gnaphalium supinum L. Flora 209: 271–278. [Google Scholar]
- R Core Team. 2014. R: a language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria. [Google Scholar]
- Rikiishi K, Hashiya E, Imai M. 2004. Linear trends of the length of snow-cover season in the Northern Hemisphere as observed by the satellites in the period 1972–2000. Annals of Glaciology 38: 229–237. [Google Scholar]
- Roach DA, Wulff RD. 1987. Maternal effects in plants. Annual Review of Ecology and Systematics 18: 209–235. [Google Scholar]
- Sandvik SM, Odland A. 2014. Changes in alpine snowbed–wetland vegetation over three decades in northern Norway. Nordic Journal of Botany 32: 377–384. [Google Scholar]
- Scherrer SC, Appenzeller C, Laternser M. 2004. Trends in Swiss Alpine snow days: the role of local- and large-scale climate variability. Geophysical Research Letters 31: L13215. doi:10.1029/2004GL020255. [Google Scholar]
- Schwienbacher E, Marcante S, Erschbamer B. 2010. Alpine species seed longevity in the soil in relation to seed size and shape – a 5-year burial experiment in the Central Alps. Flora 205: 19–25. [Google Scholar]
- Shevtsova A, Graae BJ, Jochum T, et al. 2009. Critical periods for impact of climate warming on early seedling establishment in subarctic tundra. Global Change Biology 15: 2662–2680. [Google Scholar]
- Silvertown JW, Charlesworth D. 2001. Introduction to plant population biology, 4th edn London: Blackwell. [Google Scholar]
- Thuiller W, Albert C, Araújo MB, et al. 2008. Predicting global change impacts on plant species’ distributions: future challenges. Perspectives in Plant Ecology, Evolution and Systematics 9: 137–152. [Google Scholar]
- Vleeshouwers LM, Bouwmeester HJ, Karssen CM. 1995. Redefining seed dormancy: an attempt to integrate physiology and ecology. Journal of Ecology 83: 1031–1037. [Google Scholar]
- Walck JL, Hidayati SN, Dixon KW, Thompson K, Poschlod P. 2011. Climate change and plant regeneration from seed. Global Change Biology 17: 2145–2161. [Google Scholar]
- Willis C, Baskin CC, Baskin JM, et al. 2014. The evolution of seed dormancy: environmental cues, evolutionary hubs, and diversification of the seed plants. New Phytologist 203: 300–309. [DOI] [PubMed] [Google Scholar]
- Wulff RD. 1995. Environmental maternal effects on seed quality and germination In: Kigel J, Galili G, eds. Seed development and germination. New York: Marcel Dekker, 491–506. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.