Abstract
Background and Aims Aerial surfaces of land plants are covered with a waxy cuticle to protect against water loss. The amount and composition of cuticular waxes on moss surfaces had rarely been investigated. Accordingly, the degree of similarity between moss and vascular plant waxes, and between maternal and offspring moss structure waxes is unknown. To resolve these issues, this study aimed at providing a comprehensive analysis of the waxes on the leafy gametophyte, gametophyte calyptra and sporophyte capsule of the moss Funaria hygrometrica.
Methods Waxes were extracted from the surfaces of leafy gametophytes, gametophyte calyptrae and sporophyte capsules, separated by gas chromatography, identified qualitatively with mass spectrometry, and quantified with flame ionization detection. Diagnostic mass spectral peaks were used to determine the isomer composition of wax esters.
Key Results The surfaces of the leafy gametophyte, calyptra and sporophyte capsule of F. hygrometrica were covered with 0·94, 2·0 and 0·44 μg cm–2 wax, respectively. While each wax mixture was composed of mainly fatty acid alkyl esters, the waxes from maternal and offspring structures had unique compositional markers. β-Hydroxy fatty acid alkyl esters were limited to the leafy gametophyte and calyptra, while alkanes, aldehydes and diol esters were restricted to the sporophyte capsule. Ubiquitous fatty acids, alcohols, fatty acid alkyl esters, aldehydes and alkanes were all found on at least one surface.
Conclusions This is the first study to determine wax coverage (μg cm–2) on a moss surface, enabling direct comparisons with vascular plants, which were shown to have an equal amount or more wax than F. hygrometrica. Wax ester biosynthesis is of particular importance in this species, and the ester-forming enzyme(s) in different parts of the moss may have different substrate preferences. Furthermore, the alkane-forming wax biosynthesis pathway, found widely in vascular plants, is active in the sporophyte capsule, but not in the leafy gametophyte or calyptra. Overall, wax composition and coverage on F. hygrometrica were similar to those reported for some vascular plant species, suggesting that the underlying biosynthetic processes in plants of both lineages were inherited from a common ancestor.
Keywords: Cuticular wax, Funaria hygrometrica, bryophyte, esters, leafy gametophyte, sporophyte, calyptra, very-long-chain, dehydration stress, maternal protection, gas chromatography
INTRODUCTION
Primary, above-ground plant surfaces are covered with a layered, waxy cuticle that decreases water loss across their vast surfaces (Riederer, 2006). The cuticle consists of two components, cutin and waxes, both biosynthesized by epidermal cells (Yeats and Rose, 2013). Cutin is a hydroxy acid- and dicarboxylic acid-rich polyester matrix deposited outside the epidermal secondary cell walls (Heredia, 2003). Within and on top of this scaffold is the cuticular wax, a mixture of very-long-chain (VLC) aliphatic compounds that usually includes alcohols, wax esters, aldehydes, alkanes and fatty acids (FAs; Jetter et al., 2006). It is the accumulated wax that forms the transpiration barrier and seals the plant surface (Schönherr, 1976; Haas and Schönherr, 1979; Isaacson et al., 2009), and thus was a key innovation to provide protection in non-aqueous environments during the colonization of dry land (Edwards, 1993). Determining the similarities between the waxes of distantly related plant lineages, such as mosses and vascular plants, is the first step toward envisioning the cuticle of their most recent common ancestor.
Chemical analyses of several moss species have revealed that at least some of their surfaces are coated with waxes that constitute approx. 0·1 % of the moss dry weight (Haas, 1982a; Xu et al., 2009; Buda et al., 2013), and micro-relief typical of crystalline wax projections has been detected on many species with scanning electron microscopy (SEM) (Proctor, 1979; Neinhuis and Jetter, 1995a; Koch et al., 2009). However, due to the small size and complex geometry of moss structures, the amount of wax they accumulate per surface area has not been determined as is commonly done for plants with large, relatively flat leaves. Accordingly, how much wax per unit area these early diverging plants can amass compared with well-studied vascular plants is unknown.
Moss surfaces can be coated with any or all of the ubiquitous wax compound classes found on vascular plants (Haas, 1982a; Xu et al., 2009; Buda et al., 2013), and in some cases with specialty wax compounds (Haas, 1982b; Neinhuis and Jetter, 1995b; Busta et al., 2016). For example, leafy gametophytes of Pogonatum urnigerum are covered with mainly aldehydes, while leafy gametophytes of Pogonatum aloides and Andreaea rupesteris bore principally wax esters and FAs (Haas, 1982a), those of Physcomitrella patens yielded mainly alcohols (Buda et al., 2013) and those of Syntrichia caninervis alkanes (Xu et al., 2009). In contrast, sporophytes of Polytrichales species were covered mainly with the specialty wax compound 10-nonacosanol (Neinhuis and Jetter, 1995b). Thus, diverse wax compositions exist among the moss cuticles that have been analysed; however, they are few in number, and waxes from both the offspring and maternal structures of a single species have not been analysed. Accordingly, it is unclear to what degree the waxes on different surfaces of a single moss species may vary in composition and overall amount.
The presence of all five ubiquitous compound classes in moss waxes suggests that moss wax biosynthesis may be similar to that of well-studied vascular plants. In these, wax biosynthesis begins with the elongation of long-chain fatty acyl-CoAs to produce a range of VLC acyl-CoAs (Samuels et al., 2008). The alkane-forming pathway then produces aldehydes with corresponding chain length ranges, and proceeds to generate alkanes through the removal of the terminal aldehyde head group carbon (Bernard et al., 2012). In parallel, VLC acyl-CoAs pass through the acyl reduction pathway to form alcohols as well as wax esters, which are wax dimers linking FAs and alcohols (Rowland et al., 2006; Li et al., 2008). Free FAs are also a ubiquitous wax compound class; however, their biosynthesis is not fully understood. To clarify the extent to which moss wax biosynthesis is similar to that of higher plants and to aid future biochemical investigations, detailed wax analyses of more moss species are required.
Mosses have two multicellular phases that are both exposed to the surrounding environment and are covered by a cuticle. The haploid leafy gametophyte is largely dominant and often lacks internal water conduction, instead relying on water uptake from the surrounding environment directly through the cuticle and cell walls, and is often desiccation-tolerant (Proctor et al., 2007). In contrast, the desiccation-resistant diploid sporophyte relies on the leafy gametophyte for water as it grows and elevates an undifferentiated group of cells above the protection of the boundary layer on a stalk. Meanwhile, a cap of maternal gametophyte tissue called the calyptra covers the immature sporophyte apex during early development, functioning to protect the dehydration-sensitive cells underneath prior to and during capsule formation, meiosis and spore production (Budke et al., 2013). At maturity, the calyptra falls off, and the spores are released and dispersed.
Funaria hygrometrica is a well-studied model for moss biology (French and Paolillo, 1975; Nakosteen and Hughes, 1978; Shaw, 1991; Magdy et al., 2015); however, it was long unclear whether its various sporophyte and gametophyte structures are covered by cuticles. Transmission electron microscopy (TEM) investigation revealed the presence of a characteristic lipophilic coating on the leafy gametophyte and sporophyte capsule, and most interestingly an even thicker lipophilic layer on the gametophyte calyptra (Budke et al., 2011). Further analyses determined that during early sporophyte development the calyptra cuticle is fully formed and enhances capsule development and spore production by protecting the young sporophyte from dehydration, thus functioning similarly to cuticles of vascular plants (Budke et al., 2012, 2013). In contrast, the leafy gametophyte of F. hygrometrica is probably desiccation-tolerant and can absorb water via its cuticle, similar to the gametophytes of many other moss species. Thus, the gametophyte calyptra and leafy gametophyte of F. hygrometrica play distinct roles in the moss’s life cycle, and the ecophysiological functions of their cuticles may differ greatly. However, the exact water transport properties of these cuticles are unclear, and comprehensive analyses of the cuticular waxes covering the F. hygrometrica structures, including the sporophyte, are lacking. In a very recent study, we discovered the presence of novel β-hydroxy FA esters and diol esters in the gametophyte and sporophyte waxes of F. hygrometrica (Busta et al., 2016), but this needs to be complemented with comprehensive, quantitative information on all wax constituents.
To address the issues outlined above, presented here is a comprehensive analysis of the coverage and composition of the waxes on each main aerial surface of F. hygrometrica. In particular, we aimed to determine (1) the wax coverage on the leafy gametophyte, calyptra and sporophyte of F. hygrometrica; and (2) the compositional differences between the three moss structures. Overall, such chemical data may enable comparisons of the wax biosynthesis machinery between different moss structures and that of vascular plants.
MATERIALS AND METHODS
Moss growth conditions
Spores from four Funaria hygrometrica populations with developing sporophytes were collected in Connecticut (CONN-Budke #142, #144 and #145, and Goffinet #9027) and used to establish laboratory leafy gametophyte populations. These were grown for at least 4 months at room temperature with 16 h of light per day. Leafy gametophytes were treated as previously described to produce sporophytes (Budke et al., 2011).
Surface area measurement and wax extraction
Surface areas of F. hygrometrica leafy gametophytes, gametophyte calyptrae and sporophyte capsules were determined by averaging the surface areas of five individuals of each structure. Structures were dissected with a scalpel, flattened on a microscope slide with water, and photographed. Surface areas of the moss structures were determined from these images with Adobe Photoshop CS3 (Adobe Systems) by pixel counting and compared against a photograph of a ruler taken at the same magnification. Surface areas were also determined for the calyptrae by approximating the rostrum with a cylinder (surface area = height × width × 2π) and the inflated base with the frustum of a cone [surface area = π × (r1 + r2) × √h2 + (r1 – r2)2]. Using the pixel counting method, the average surface areas were 27 mm2 per leafy gametophyte, 4·3 mm2 per calyptra and 8·5 mm2 per sporophyte capsule.
To determine the wax coverage per unit mass, leafy gametophytes were allowed to dry at room temperature for at least 24 h and weighed multiple times until they reached a steady state. The average weight for 74 leafy gametophytes was 11·3 ± 2·9 mg (mean ± s.d., throughout).
Leafy gametophytes with a total surface area of 20 cm2 were isolated and dipped in two changes of chloroform for 30 s each to extract cuticular waxes. Tetracosane (10 μg) was added as an internal standard and the solvent was evaporated. Samples of calyptra and sporophyte capsule waxes were prepared using the same procedure. Cut ends of the leafy gametophyte and sporophyte stalk were held out of the chloroform to avoid internal lipid extraction.
Wax derivatization and gas chromatography (GC) conditions
Wax samples were dissolved in pyridine (10 μL, Sigma Aldrich), bis-N,O-trimethylsilyltrifluoroacetamide (BSTFA; 10 μL, Sigma Aldrich) was added, and the samples were incubated at 70 °C for 45 min. Excess derivatization reagents were then evaporated under a stream of N2 gas, and the dry residue was dissolved in CHCl3 (20 μL).
Derivatized samples were analysed quantitatively with a 6890N gas chromatograph (Agilent) equipped with an on-column injector and an HP-1 capillary column (Agilent, length 30 m, i.d. 320 μm, 1 μm film thickness). An aliquot of each sample was injected into the machine with H2 flowing through the column (2 mL min–1) and the oven set to 50 °C. After 2 min, the temperature was raised at 40 °C min–1 to 200 °C, held for 2 min, raised at 3 °C min–1 to 320 °C, and then held for 30 min. Analytes were detected with a flame ionization detector (FID; Agilent) at 250 °C that burned H2 (30 mL min–1) in air (200 mL min–1). The flame was shaped with N2 (20 mL min–1).
The samples were analysed qualitatively with a separate 6890N gas chromatograph (Agilent) equipped with the same injector, column and oven program, but a column flow of He (1·4 mL min–1) and a 5793N Mass Selective Detector (EI, 70 eV, m/z 50–800, 1 scan s–1).
Wax quantification and ester analysis
Peaks in the GC-FID chromatograms were integrated and their sum area was used to determine the total wax load by comparing them against that of the internal standard. The identity of each GC-FID peak was determined using the information from corresponding peaks in the GC-mass spectrometry (GC-MS) chromatograms. Finally, the amount of each wax component was determined by comparing its GC-FID peak area against the area of the internal standard.
Statistical analysis
Data were analysed using R 3.2.0 (R Core Team, 2015). An analysis of variance (ANOVA) was used to assess whether there were differences in coverage between moss structures. Following a significant ANOVA, Tukey post-hoc tests (P < 0·01) were conducted to determine significant differences between pairs of moss structures.
RESULTS
This study provides a comprehensive analysis of the wax mixtures covering the leafy gametophyte, gametophyte calyptra and sporophyte capsule surfaces of Funaria hygrometrica. Triplicates of each moss structure were sampled by surface extraction and investigated first with GC-MS to identify individual homologues of various compound classes, and then by GC-FID to quantify them. However, positional isomers could not be separated under the GC conditions employed here, so their distributions were assessed using further GC-MS analyses. Based on the relative abundances of characteristic MS fragments within each GC peak, isomer distributions were calculated for all wax ester classes.
Total wax amounts, compound class and chain length distributions
The F. hygrometrica leafy gametophyte, calyptra and sporophyte capsule surfaces were covered by 0·94 ± 0·13, 2·0 ± 0·2 and 0·44 ± 0·10 μg cm–2 of total wax, respectively (Fig. 1). Within each of the three wax mixtures, compounds with an additive coverage of 0·83 μg cm–2 (88 %), 1·8 μg cm–2 (92 %) and 0·32 μg cm–2 (75 %) were identified, respectively. The dry weight of the leafy gametophyte material was also determined, revealing that the (GC-determined) wax amount represented 0·07 % of the material’s dry weight.
Fig. 1.

Wax coverage on three major structures of Funaria hygrometrica. The total amount of wax from each structure is expressed in terms of wax mass (μg) per surface area extracted (cm2). Bar heights and error bars represent the mean and s.d. of three independent samples, respectively. Letters indicate significant differences based on Tukey post-hoc tests (P < 0·01) and ANOVA (F2,6 = 111·3, P < 0·001).
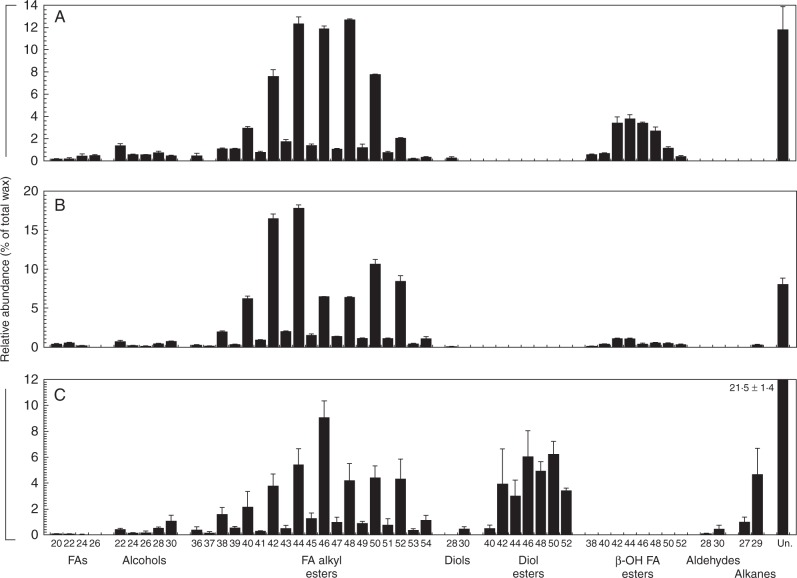
On the leafy gametophyte, FA alkyl esters (67 % of the total wax) and β-hydroxy FA alkyl esters (17 %) were the most abundant compound classes, accompanied by FAs (1 %), alcohols (4 %) and diols (0·3 %) (Supplementary Data Table S1). On the calyptra, the FA alkyl esters were the major compound class (85 %), with minor admixtures of FAs (1 %), alcohols (2 %), diols (0·07 %), β-hydroxy FA alkyl esters (4 %) and alkanes (0·3 %). The sporophyte capsule wax harboured mainly FA alkyl esters (40 %), together with FA esters of primary,secondary alkanediols (28 %), alkanes (6 %), FAs (0·2 %), alcohols (3 %), diols (0·6 %) and aldehydes (0·6 %).
Many of the compounds identified on the leafy gametophyte were present as homologous series. The detected FAs had even carbon numbers ranging from C20 to C26, with relative abundances increasing towards the longer chain lengths (Fig. 2A). The homologous series of alcohols had even carbon numbers that ranged from C22 to C30, with a bimodal distribution peaking at C22 and C28. FA alkyl esters had even and odd carbon numbers between C36 and C54, and a relatively broad maximum around C44, C46 and C48. Similarly, the β-hydroxy FA alkyl esters had even carbon numbers ranging from C38 to C52, and with homologues C42–C48 dominating. Only one diol, octacosane-1,3-diol, was detected in the leafy gametophyte wax.
Fig. 2.
Relative abundance of wax compounds on three major structures of Funaria hygrometrica. The amount of each wax compound is expressed as a percentage of the total amount of wax extracted from the leafy gametophyte (A), the gametophyte calyptra (B) and the sporophyte capsule (C). Labels on the x-axis indicate the compound class and carbon number of each compound. Bar heights and error bars represent the mean and s.d. of three independent samples, respectively. Abbreviations: FAs, fatty acids; FA alkyl esters, fatty acid alkyl esters; 28 Diol, octacosane-1,3-diol; 30 Diol, triacontane-1,7-diol; β-OH FA esters, β-hydroxy fatty acid esters; Un., the sum of unidentified GC peaks.
In the calyptra wax, largely the same individual compounds were detected as in the leafy gametophyte wax, leading to fairly similar overall chain length ranges within the compound classes from both moss structures (Fig. 2B). In addition, the calyptra wax contained β-hydroxy FA alkyl esters with the C42 and C44 homologues dominating, and a single diol, octacosane-1,3-diol. However, in contrast to the qualitative similarities, several of the chain length distributions within the calyptra wax differed quantitatively from those in the leafy gametophyte wax. Shorter FA homologues had higher relative abundances in the calyptra wax. Both the alcohols and the alkyl esters had bimodal distributions, peaking at C22 and C30, and at C42/44 and C50/52, respectively.
The sporophyte capsule yielded FAs with even carbon numbers between C20 and C24, all with relatively similar abundances (Fig. 2C). The alcohols with even carbon numbers C22–C30 were detected in a bimodal distribution, thus similar to those in the calyptra wax. Sporophyte capsule wax had FA alkyl esters with both even and odd carbon numbers that ranged from C36 to C52 and were dominated by the C46 homologue. In addition, FA esters of primary,secondary alkanediols (diol esters) with even carbon numbers C42–C52 were present in similar abundances, accompanied by small amounts of the C40 homologue. Finally, C28 and (predominantly) C30 aldehydes were found, as well as C27 and (predominantly) C29 alkanes and triacontane-1,7-diol.
Ester isomer distributions
Unlike other wax constituents, esters are dimers, formed by linking FA and alcohol monomers. Esters of a single overall chain length can result from several different combinations of FA and alcohol homologues (e.g. C46 esters can be made up of C20 acid + C26 alcohol, C22 acid + C24 alcohol, or other combinations), giving rise to isomerism within each ester homologue in a series. Therefore, the next stage of the analysis was directed toward quantifying both the acid and alcohol chain length profiles within individual ester homologues of substantial abundance, and the distribution of all esterified acids and alcohols across all ester homologues.
In the wax of the leafy gametophyte, the prevalent C46, C48 and C50 alkyl ester homologues all contained mainly C24 FA linked to C22, C24 and C26 alcohol, respectively (Fig. 3A, B; Supplementary Data Table S2). The accompanying shorter ester homologues consisted of somewhat shorter FAs and alcohols, namely C20 acid and C20 alcohol, C20 acid and C22 alcohol, as well as C22 acid and C22 alcohol. Similarly, a longer (C52) ester homologue was formed by combination of somewhat longer building blocks, C26 acid and C26 alcohol. Finally, the esters with extreme chain lengths (C38 and C54) were composed of multiple, more evenly distributed acids and alcohols, including relatively short acids and alcohols (C18–C22) in the C38 esters and extremely long acids and alcohols (up to C32) in the C54 esters. Overall, the different FA alkyl ester homologues in the leafy gametophyte wax had fairly similar acid and alcohol chain lengths, thus positioning the ester functional group near the centre of the overall backbone structure.
Fig. 3.
Isomer distribution within the FA alkyl esters in Funaria hygrometrica waxes. Relative amounts of fatty acids (A, C, E) and alcohols (B, D, F) are expressed as a percentage of the total amount of fatty acids or alcohols in each FA alkyl ester homologue, respectively. The amount and chain length of the fatty acids bound in a single FA alkyl ester can be read in the height and colour or the bars above the carbon number corresponding to that FA alkyl ester. FA alkyl esters with varying carbon numbers are plotted for the leafy gametophyte (A, B), the gametophyte calyptra (C, D) and the sporophyte capsule (E, F). Bar heights and error bars represent the mean and s.d. of three independent samples, respectively. Bar colours denote fatty acid and alcohol chain lengths as indicated in the key. In a few cases, alkyl esters of extreme chain length (C36 or C54) were above the limit of homologue quantification, but were too low in abundance to analyse for their isomer composition.
The FA alkyl esters in the calyptra wax may be grouped into two series of homologues distinguished by their isomer patterns. The first group consisted of the C38–C44 ester homologues, all characterized by having the same predominant alcohol (C22) linked to varying acids C16–C22 (Fig. 3C, D; Supplementary Data Table S3). In the other group, the C50–C54 ester homologues each contained predominantly C30 alcohol, bound to C20–C24 acids. The two groups of esters, distinguishable by their isomer compositions, were also reflected in the bimodal distribution of ester homologues (compare Fig. 2B). The isomer compositions of the remaining ester homologues (C46 and C48) were intermediate between the two dominating ester series.
The FA alkyl esters of the sporophyte capsule could also be divided into two groups of homologues. One group of esters (C50 and C52) was formed mainly by C30 alcohol bound to different acids (C20 and C22 acids, respectively) (Fig. 3E, F; Supplementary Data Table S4). This group of esters thus had isomer compositions similar to those in the calyptra wax. Conversely, another group of esters (C38 and C40) was formed mainly by one acid (C16), linked to different alcohols (C22 and C24 alcohols, respectively). Neighbouring ester homologues (C36 and C42) also contained substantial amounts of C16 acid, together with acids of other chain lengths. These two ester groups intersected at the most abundant ester homologue (C46), which consisted mainly of C16 acid and C30 alcohol.
Finally, the overall chain length profiles of alkyl ester-bound FAs and alcohols in each moss structure were determined by summing the amounts of constituent acids or alcohols with the same carbon number across all ester homologues. This revealed that the leafy gametophyte had alkyl ester-bound FAs with mainly even carbon numbers spanning C16–C28, within which C20–C24 were the most abundant (Supplementary Data Tables S2–S4; Fig. 4A). These acids were esterified predominantly with C22–C26 alcohols (Fig. 4B). On the calyptra, mostly even carbon number acid homologues ranging from C16 to C24 were observed, with C20 and C22 predominating (Fig. 4C). In this moss structure, the corresponding ester alcohols had a distinct bimodal distribution, peaking at C22 and C30 (Fig. 4D). The sporophyte capsule esters contained FAs ranging from C14 to C30, in a bimodal distribution peaking at C16 and C22, and accompanied by alcohols with a bimodal distribution peaking at C22 and C30 (Fig. 4E and F).
Fig. 4.
Relative abundance of FA alkyl ester-bound fatty acids and alcohols in Funaria hygrometrica waxes. Relative amounts of fatty acids (A, C, E) and alcohols (B, D, F) are expressed as a percentage of the total amount of fatty acids or alcohols from all FA alkyl ester homologues in the wax mixture from a single structure, respectively. FA alkyl esters with varying carbon numbers are plotted for the leafy gametophyte (A and B), the gametophyte calyptra (C and D) and the sporophyte capsule (E and F). Bar heights and error bars represent the mean and s.d. of three replicate samples, respectively.
Hydroxy ester isomer distributions
β-Hydroxy FA esters may also occur as isomer mixtures, arising from the combination of β-hydroxy FAs and alcohols of varying chain lengths. Again using diagnostic MS fragments, the isomer composition of each β-hydroxy FA ester homologue from the wax of each moss structure was determined. The prominent C42, C44 and C46 β-hydroxy FA esters from the leafy gametophyte contained C20, C22 and C24 β-hydroxy FAs, respectively, mainly in combination with C22 alcohol (Fig. 5A, B; Supplementary Data Table S5) Longer β-hydroxy FA esters contained largely C24 β-hydroxy FA and alcohols ranging from C22 to C28. Thus, the isomer composition of the β-hydroxy FA esters resembled those of corresponding alkyl ester homologues in the leafy gametophyte wax.
Fig. 5.
Isomer distribution within the β-hydroxy fatty acid esters in Funaria hygrometrica waxes. Relative amounts of β-hydroxy fatty acids (A, C) and alcohols (B, D) are expressed as a percentage of the total amount of β-hydroxy FAs or alcohols in each β-hydroxy FA ester homologue, respectively. The amount and chain length of the fatty acids bound in a single β-hydroxy FA ester can be read in the height and colour of the bars above the carbon number corresponding to that β-hydroxy FA ester. Esters with varying carbon numbers are plotted for the leafy gametophyte (A and B) and the gametophyte calyptra (C and D). Bar heights and error bars represent the mean and s.d. of three independent samples, respectively. Bar colours denote specific chain lengths as indicated in the key. β-OH fatty acid (ester), β-hydroxy fatty acid (ester).
On the calyptra, the prominent C42 and C44 β-hydroxy FA esters were formed by C20 and C22 β-hydroxy FAs together with C22 alcohol (Fig. 5C, D; Supplementary Data Table S6). Longer β-hydroxy FA esters contained mostly C20 β-hydroxy FA, esterified with particularly long alcohols ranging from C26 to C32.
Next, the total profiles of esterified β-hydroxy FAs and β-hydroxy FA-bound alcohols were determined using the same process described for the alkyl esters. Esterified β-hydroxy FAs on the leafy gametophyte and the calyptra had mainly even carbon numbers from C16 to C30. On the leafy gametophyte, C20, C22 and C24 β-hydroxy FAs were most abundant, while on the calyptra the C20 homologue was most common (Tables S5 and S6; Fig. 6A, C). The accompanying esterified alcohols had even carbon numbers from C14 to C36, with a normal distribution peaking at C22 on the leafy gametophyte and a bimodal distribution peaking at both C22 and C30 on the calyptra (Fig. 6B, D).
Fig. 6.
Relative abundance of ester-bound β-hydroxy fatty acids and alcohols in Funaria hygrometrica waxes. Relative amounts of β-hydroxy FAs (A, C) and alcohols (B, D) are expressed as a percentage of the total amount of FAs or alcohols from all the β-hydroxy FA ester homologues in the wax mixture from a single structure, respectively. β-Hydroxy FA esters with varying carbon numbers are plotted for the leafy gametophyte (A and B) and the gametophyte calyptra (C and D). Bar heights and error bars indicate the mean and s.d. of three independent samples, respectively.
Fatty acid esters of primary,secondary alkanediols (diol esters) exhibit the potential for isomerism not only via combination of various FA and alkanediol chain lengths, but also through variability in the position of the secondary hydroxyl group on the diol moiety. Inspection of the MS fragmentation patterns of the sporophyte capsule diol esters revealed that they contained such positional isomers. In particular, diagnostic α-fragments indicated that the secondary hydroxyl function was located mainly on C-7 of the diol moiety, but with admixtures of corresponding C-5 and C-9 isomers in each ester homologue (Fig. 7A). Across all homologues, the average ratio of esterified 1,5-, 1,7- and 1,9-diols was approx. 1:4:1 (Fig. 7B). It should be noted that the diol esters did not exhibit additional isomerism due to combinations of FA and alkanediol chain lengths, as did the other ester classes described above. Instead, all the diol esters contained only C30 diols, esterified with varying FA homologues.
Fig. 7.

Isomer distribution within diol esters in Funaria hygrometrica sporophyte wax. For diol esters with varying carbon numbers, relative amounts of diol isomers are expressed as a percentage of the total amount of diols in each diol ester homologue (A), or of the total amounts of diols in all diol ester homologues (B). Bar heights and error bars represent the mean and s.d. of three independent samples, respectively. Bar colours denote specific chain lengths as indicated in the key.
DISCUSSION
The present study aimed to provide a comprehensive analysis of cuticular waxes on the aerial surfaces of the moss Funaria hygrometrica. We found that (1) the leafy gametophyte, gametophyte calyptra and sporophyte capsule were all covered by cuticular waxes, with amounts in the low end of the range reported for vascular plants (approx. 1 μg cm–2); (2) the waxes on all three moss structures contained large portions of alkyl esters with minor admixtures of other ubiquitous compound classes; (3) each of the moss structures was distinguished by its own characteristic compound class(es) and/or chain length profile(s); and (4) ester isomer patterns differed between ester classes and moss structures. In the following we will discuss each of these four major findings.
Cuticular wax coverage on Funaria hygrometrica
To put wax amounts into context with cuticle structure and function, and to enable comparison with vascular plant species, they are preferably measured as coverages (i.e. in units of μg per cm2 surface area). However, the complex geometry of mosses makes measuring their surface area particularly challenging and has impeded determination of their wax coverages thus far. In this study, the surface area of each moss structure to be extracted was first measured by counting pixels in a photograph of dissected and flattened representative structures. In parallel, the geometrically simplest structures, the calyptrae, were approximated with geometric shapes to calculate their surface areas. The results from the geometric method were within 5 % of the values from the pixel counting approach, validating the latter.
Based on our approximations for the surface areas of the moss structures, the leafy gametophyte, calyptra and sporophyte capsule of F. hygrometrica had wax coverages of 0·5–2·0 μg cm–2. These findings enable direct comparisons with species of other lineages for the first time. Leaves of seedless vascular plants such as the fern Osmunda regalis have a wax coverage of approx. 20 μg cm–2 (Jetter and Riederer, 2000), while those of other ferns range from 5 to 15 μg cm–2 (J. Li and R. Jetter, unpubl. res.). Wax coverage on seed plant surfaces also varies widely, for example reaching 20–30 μg cm–2 for needles of gymnosperms such as Taxus baccata (Wen et al., 2006) and typically ranging from 1 to 30 μg cm–2 on angiosperm leaves (Rashotte et al., 2001; Szafranek and Synak, 2006; Yeats et al., 2012; Ni et al., 2013). Thus, the wax amounts on F. hygrometrica surfaces fall within the low end of the range of wax coverages on vascular plants.
Prior to this study, total wax amounts on mosses had only been reported for leafy gametophytes, and as wax amounts per plant dry weight rather than per surface area. To enable comparisons with these previous reports, the dry weight of F. hygrometrica leafy gametophyte material was determined in parallel with wax extraction. Using the GC results, the wax represented approx. 0·07 % of the leafy gametophyte dry weight. This finding is similar to other moss leafy gametophytes, where wax amounts ranged from 0·02 % dry weight for Syntrichia caninervis (Xu et al., 2009) and Physcomitrella patens (Buda et al., 2013) to 0·05, 0·08 and 0·12 % for Andreaea rupestris, Pogonatum aloides and Pogonatum urnigerum (Haas, 1982a), and 5 % for Saelania glaucescens (Haas, 1982b).
Finally, the wax coverages of the three F. hygrometrica structures can also be compared with previous reports on the relative thicknesses of their respective cuticles. TEM investigations had shown the calyptra to have the thickest cuticle of the three moss structures, more than twice that of the other two structures, when considering all lipophilic layers of the cuticle (Budke et al., 2011). Our chemical results parallel the anatomical observations, revealing wax coverages roughly proportional to apparent cuticle thicknesses. However, it must be noted that moss cuticles, like those of vascular plants, consist of both wax and cutin (Buda et al., 2013). Hence, TEM data may reflect cutin amounts more than those of waxes, and can therefore be expected to correlate only loosely with wax amounts.
Cuticular wax constituents common to all surfaces of Funaria hygrometrica
The wax mixtures from the leafy gametophyte, calyptra and sporophyte capsule all contained FAs, alcohols and FA alkyl esters, and the sporophyte capsule and calyptra also bore aldehydes and alkanes. Chain lengths ranged from C20 to C30 for monomeric compounds, and from C36 to C54 for ester-linked, dimeric compounds. The same compound classes and chain lengths have been reported for the cuticular wax mixtures of other mosses (Haas, 1982a; Xu et al., 2009; Buda et al., 2013) as well as many vascular plant species, indicating that mosses can regularly have a full complement of the ubiquitous wax compounds. It is interesting to note that, therefore, entire wax-forming pathways similar or identical to those in vascular plants are generally operational in mosses, and a full set of wax biosynthesis gene orthologues is probably present. It seems plausible that a common ancestor of mosses and vascular plants had the ubiquitous wax compound biosynthesis machinery in place, and that further differences in relative amounts of wax products between various plant lineages would have resulted from differential regulation of the common wax biosynthesis genes.
In particular, the wax mixtures on the leafy gametophyte, calyptra and sporophyte capsule of F. hygrometrica contained high percentages of alkyl esters. Interestingly, comparable alkyl ester amounts had been reported before for only relatively few plant species, including the leafy gametophytes of the mosses A. rupesteris and P. aloides (Haas, 1982a), the leaves of vascular plants such as Camelina sativa (Razeq et al., 2014), Copernicia cerifera (Kolattukudy, 1976) and Quercus ilex (Martins et al., 1999), and the seed oil of Simmondsia chinensis (Benzioni and Forti, 1989). However, it must be noted that some GC configurations and settings may bias against alkyl esters due to their high molecular weights. For this reason, they may have been underestimated or entirely overlooked in some previous plant wax analyses.
Alcohols and alkyl esters, the products of the acyl reduction biosynthetic pathway, accounted for >90 % of the wax mass on each of the three F. hygrometrica structures. Similarly high amounts of acyl reduction products have been reported for diverse plant species, albeit mostly in the form of free (rather than esterified) alcohols, e.g. the mosses P. patens (Buda et al., 2013), A. rupesteris and P. aloides (Haas, 1982a), the ferns O. regalis (Jetter and Riederer, 2000) and Pteridium aquilinum (Baker and Gaskin, 1987), the cycad genus Encephalartos (Osborne and Stevens, 1996) and the grass genera Triticum and Agropyron (Tulloch and Hoffman, 1973; Tulloch, 1983).
It is of note that both acyl reduction and alkane pathway-dominated waxes have been found in mosses. For example, P. urnigerum leafy gametophyte wax is rich in aldehydes, while A. rupesteris and P. aloides leafy gametophytes bear mostly alkyl esters (Haas, 1982a), and Pogonatum belangeri and P. urnigerum sporophytes are mostly covered with secondary alcohols (Neinhuis and Jetter, 1995b). Overall, this shows that the balance of substrate flux between wax biosynthesis pathways is set differently by each moss species, and even varies between closely related species, as in Pogonatum.
Distinguishing features in the wax mixtures of the three F. hygrometrica structures
The parallel investigation of three F. hygrometrica structures enables comparisons between their characteristic wax compositions. For example, the sporophyte capsule wax contained large quantities of alkanediol esters, accompanied by a small percentage of corresponding free diols. In sharp contrast, the same or similar diols were not detected in the wax mixtures on the gametophyte structures, and they may thus serve as specific markers for the sporophyte capsule. It remains to be seen whether comparable alkanediol esters occur in other plant species, now that their GC-MS characteristics are available for use in future wax analyses (Busta et al., 2016).
The sporophyte capsule wax also contained substantial amounts of alkanes and traces of aldehydes, while only minute amounts of alkanes were on the calyptra and neither was on the leafy gametophyte. Overall, the sporophyte capsule had a unique wax composition with only partial resemblance to the wax mixtures on the gametophyte moss structures. Conversely, the wax of the F. hygrometrica gametophyte structures comprised characteristic β-hydroxy FA esters that were absent from sporophyte capsule wax (or at best present in trace amounts below the detection limit). The β-hydroxy FA esters may therefore be regarded as leafy gametophyte markers in this moss species.
The F. hygrometrica calyptra and leafy gametophyte waxes were further distinguished from each other by the presence and absence, respectively, of C29 alkane. The calyptra physically touches the sporophyte capsule during development, potentially leading to contamination of the calyptra wax preparation with minor amounts of sporophyte capsule wax material. However, the absence of diol esters (as sporophyte capsule wax markers) in the calyptra samples rules out cross-contamination. Thus, the accumulation of alkanes distinguished the calyptra from the leafy gametophyte. This suggests that the alkane-forming biosynthesis pathway is indeed active in only one of the gametophyte structures, the calyptra, and otherwise in the sporophyte capsule. Overall, the different F. hygrometrica structures all have distinct compound class compositions. This suggests that the balance of substrate flux between wax biosynthesis pathways is set differently in each moss structure. This may reflect the differences in their ability to absorb water through their cuticle and to prevent water loss.
Lastly, the overall chain length distributions in the wax of each moss structure can be assessed by examining the homologue profiles across all compound classes, including esterified compounds. The leafy gametophyte wax contained predominantly C24 and C26 compounds, while the calyptra and sporophyte capsule waxes also bore substantial amounts of C30 compounds. The major chain lengths involved in both cases are reminiscent of those predominating in certain angiosperm waxes, suggesting that the biosynthetic machineries determining chain length profiles may be similar in mosses and vascular plants. In Arabidopsis thaliana, the ketoacyl-CoA synthetase enzyme encoded by CER6 and the BAHD-family protein encoded by CER2 are known to, together, be important for chain length elongation from C24 and C26 towards C30 (Millar and Kunst, 1997; Haslam et al., 2012). Elongation beyond C30 is facilitated by the CER2-like enzyme CER26. Several candidate CER6 orthologues had been annotated in the P. patens genome (Lang et al., 2005), but their putative roles in FA elongation have not been tested to date. In contrast, no CER2-like genes (including CER26) were annotated in the P. patens genome, and sequence similarity-based searches revealed putative BAHD proteins distantly related to CER2 but no direct orthologue. It may thus be speculated that F. hygrometrica has an orthologue of CER6 and perhaps CER2, and that one (or both) of these genes is (are) differentially regulated between the leafy gametophyte (very little expression), the calyptra (low expression) and the sporophyte capsule (high expression). Based on the absence of monomeric wax compounds with >30 carbons, it seems likely that this moss species does not express a CER26 orthologue. Nevertheless, the wax biosynthesis machinery in both mosses and vascular plants has probably been inherited from a common ancestor. This parallels other observations of compounds produced in both mosses and vascular plants by related genes, for example callose, where homologous biosynthesis genes had been identified in A. thaliana and P. patens (Huang et al., 2009; Schuette et al., 2009).
Isomer patterns in different wax ester classes on the three Funaria hygrometrica structures
Wax esters are formed from the combination of two (very-) long-chain compounds, a fatty acyl component (bearing a carboxyl group) and an alkyl component (bearing a hydroxyl group). The possible incorporation of acyl and alkyl moieties with diverse chain lengths (and maybe additional hydroxyl functions) into the broad array of F. hygrometrica esters warranted detailed analysis. Using the isomer data presented here, the chain length profiles within the diverse ester classes were explored to gain insights into their biosynthesis.
The chain lengths of wax ester-bound alcohols on the three F. hygrometrica structures varied from C14 to C36 (Figs 4 and 6). The overall range of free alcohols in the total wax mixture from all F. hygrometrica structures matched those of the respective esterified alcohols (Fig. 2), indicating that free alcohols may serve as substrates for alkyl ester formation. Previous analyses of wax ester composition in angiosperms have led to similar observations and conclusions (Lai et al., 2007; Razeq et al., 2014), and feeding experiments further confirmed that the pool of wax alcohols serves as substrate for wax ester biosynthesis in Arabidopsis (Li et al., 2008).
FA alkyl esters consist of FAs and alcohols. The chain length range of ester-bound FAs was C16 to C30 (Fig. 4), within which falls the chain length range of free FAs in the total wax mixture (Fig. 2). Thus, a wide range of acyl precursor chain lengths appears to serve as substrates for ester formation in F. hygrometrica. The overall chain length ranges of both esterified acyls and alkyls indicate that ester moieties are directly recruited from the respective wax precursor pools. This finding is in sharp contrast to A. thaliana, where the chain length profile of ester-bound FAs peaks sharply at C16 (Lai et al., 2007), probably reflecting predominance of this chain length in the substrate pool available to the ester synthase involved.
While the qualitative information on ester isomer ranges reveals possible substrate pools, the quantitative acyl and alkyl chain length profiles may further inform about substrate specificities of the ester synthase(s) involved. The F. hygrometrica leafy gametophyte wax contained FA alkyl esters and β-hydroxy FA esters, both made up almost exclusively of C20–C24 acyl (Figs 4A and 6A) and C22–C26 alkyl moieties (Figs 4B and 6B). However, the free FA and alcohol pools accompanying these esters in the leafy gametophyte wax contained substantial amounts of C26 FA, and C28 and C30 alcohols (Fig. 2). A direct comparison of these profiles (Supplementary Data Fig. S1) indicates that, in leafy gametophyte FA alkyl ester formation, the C26 FA is selected against, while in both FA and β-hydroxy FA alkyl ester formation C28 and C30 alcohols are selected against (Supplementary Data Fig. S1). The very similar substrate biases in the biosynthesis of both ester classes suggest that they are formed by the same enzyme, or by two enzymes with very similar characteristics.
On the calyptra, FA esters and β-hydroxy FA alkyl esters were again both present. Here, ester-bound acyl moieties were mainly C18, C20, C22 and C24 in both ester classes (Fig. 4C and Fig. 6C), while the ester alkyls had consistent, bimodal distributions peaking at C22 and C30. The accompanying FAs and alcohols (Fig. 2) had homologue profiles very similar to those of ester acyls and alcohols (Fig. S1), suggesting that the ester-forming enzyme(s) in this moss structure does (do) not exhibit strong substrate chain length specificity.
Finally, the F. hygrometrica sporophyte capsule wax contained both FA alkyl esters and FA diol esters. The acyl moieties in the FA alkyl esters were mainly C16, C20, C22 and C24 (Fig. 4E), and the FA diol esters predominantly C16, C18 and C20 acyls (calculated from the total carbon number of the diol ester by subtracting the carbon number of the sole diol homologue, C30). Conversely, mainly C30 and some C22 alkyl units were incorporated into the esters of the sporophyte capsule (Fig. 4F). The ester isomer profiles thus match those of free FAs and alcohols as well as diols in the same wax mixture, except for the additional esterification of C16 acyl components. Overall, it seems that the ester-forming enzyme(s) in this moss structure have access to substantial amounts of C16 fatty acyl-CoA, and that they may exhibit a preference for this substrate, unlike those in the other two structures.
In conclusion, the detailed analyses of wax esters on three unique F. hygrometrica structures suggest that incorporation of FAs and alcohols into wax esters proceeds with characteristic bias against or preference for different substrate chain lengths in each moss structure. These disparate patterns indicate that different enzymes may be active in each. Nevertheless, wax coverage on all three moss structures was similar to those found on vascular plants. Additional compositional comparisons suggest that the wax biosynthesis machinery in members of both lineages may be inherited from a common ancestor, but fine-tuned in each species and even between organs/structures in a single species, presumably to optimize function. Further studies may illuminate whether and how the different wax coverages and compositions on the three moss structures affect their surface properties and ecophysiological functions. High wax coverage on the calyptra may provide more durable protection for the developing sporophyte capsule, which is unable to survive even mildly desiccating conditions during early development without the protective calyptra cuticle (Budke et al., 2013).
SUPPLEMENTARY DATA
Supplementary data are available online at www.aob.oxfordjournals.org and consist of the following. Table S1: wax coverage on three F. hygrometrica organs. Table S2: amount of esterified compounds in each alkyl ester homologue on the leafy gametophyte of F. hygrometrica. Table S3: amount of esterified compounds in each alkyl ester homologue on the gametophyte calyptra of F. hygrometrica. Table S4: amount of esterified compounds in each alkyl ester homologue on the sporophyte capsule of F. hygrometrica. Table S5: amount of esterified compounds in each β-hydroxy ester homologue on the leafy gametophyte of F. hygrometrica. Table S6: amount of esterified compounds in each β-hydroxy ester homologue on the gametophyte calyptra of F. hygrometrica. Figure S1: comparison of ester-bound and free alkyl and acyl chains in F. hygrometrica waxes.
ACKNOWLEDGEMENTS
This work has been made possible by generous support from the Natural Sciences and Engineering Research Council (Canada), the Canada Research Chairs Program, the Canada Foundation for Innovation, the US National Science Foundation (DEB-1146295) and a Katherine Esau Postdoctoral Fellowship to J.M.B.
LITERATURE CITED
- Baker EA, Gaskin RE. 1987. Composition of leaf epicuticular waxes of Pteridium sub-species. Phytochemistry 26: 2847–2848. [Google Scholar]
- Benzioni A, Forti M. 1989. Jojoba In: G Robbelen, RK Downey, A Ashri, eds. Oil crops of the world. New York: McGraw-Hill, 448–461. [Google Scholar]
- Bernard A, Domergue F, Pascal S, et al. 2012. Reconstitution of plant alkane biosynthesis in yeast demonstrates that Arabidopsis ECERIFERUM1 and ECERIFERUM3 are core components of a very-long-chain alkane synthesis complex. The Plant Cell 24: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buda GJ, Barnes WJ, Fich EA, et al. 2013. An ATP binding cassette transporter is required for cuticular wax deposition and desiccation tolerance in the moss Physcomitrella patens. The Plant Cell 25: 4000–4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budke JM, Goffinet B, Jones CS. 2011. A hundred-year-old question: is the moss calyptra covered by a cuticle? A case study of Funaria hygrometrica. Annals of Botany 107: 1279–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budke JM, Goffinet B, Jones CS. 2012. The cuticle on the gametophyte calyptra matures before the sporophyte cuticle in the moss Funaria hygrometrica (Funariaceae). American Journal of Botany 99: 14–22. [DOI] [PubMed] [Google Scholar]
- Budke JM, Goffinet B, Jones CS. 2013. Dehydration protection provided by a maternal cuticle improves offspring fitness in the moss Funaria hygrometrica. Annals of Botany 11: 781–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busta L, Budke JM, Jetter R. 2016. Identification of β-hydroxy fatty acid esters and primary, secondary-alkanediol esters in cuticular waxes of the moss Funaria hygrometrica. Phytochemistry 121: 38–49. [DOI] [PubMed] [Google Scholar]
- Edwards D. 1993. Cells and tissues in the vegetative sporophytes of early land plants. New Phytologist 125: 225–247. [DOI] [PubMed] [Google Scholar]
- French AJC, Paolillo DJ. 1975. On the role of the calyptra in permitting expansion of capsules in the moss Funaria. The Bryologist 78: 438–446. [Google Scholar]
- Haas K. 1982a. Surface wax of Andreaea and Pogonatum species. Phytochemistry 21: 657–659. [Google Scholar]
- Haas K. 1982b. The surface lipids of Saelania moss gametophytes: a comparison with cuticular wax of higher plants In: DF Cutler, KL Alvin, CE Price, eds. The plant cuticle. London: Academic Press, 225–230. [Google Scholar]
- Haas K, Schönherr J. 1979. Composition of soluble cuticular lipids and water permeability of cuticular membranes from Citrus leaves. Planta 146: 399–403. [DOI] [PubMed] [Google Scholar]
- Haslam T, Mañas Fernàndez A, Zhao L, et al. 2012. Arabidopsis ECERIFERUM2 is a component of the fatty acid elongation machinery required for fatty acid extension to exceptional lengths. Plant Physiology 160: 1164–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heredia A. 2003. Biophysical and biochemical characteristics of cutin, a plant barrier biopolymer. Biochimica et Biophysica Acta 1620: 1–7. [DOI] [PubMed] [Google Scholar]
- Huang L, Chen XY, Rim Y, et al. 2009. Arabidopsis glucan synthase-like 10 functions in male gametogenesis. Journal of Plant Physiology 166: 344–352. [DOI] [PubMed] [Google Scholar]
- Isaacson T, Kosma DK, Matas AJ, et al. 2009. Cutin deficiency in the tomato fruit cuticle consistently affects resistance to microbial infection and biomechanical properties, but not transpirational water loss. The Plant Journal 60: 363–377. [DOI] [PubMed] [Google Scholar]
- Jetter R, Riederer M. 2000. Composition of cuticular waxes on Osmunda regalis fronds. Journal of Chemical Ecology 26: 399–412. [Google Scholar]
- Jetter R, Kunst L, Samuels AL. 2006. Composition of plant cuticular waxes In: M Riederer, C Muller, eds. Biology of the plant cuticle. Oxford: Blackwell, 145–181. [Google Scholar]
- Koch K, Frahm J-P, Pollawatn R. 2009. The cuticle of the Buxbaumia viridis sporophyte. Flora 204: 34–39. [Google Scholar]
- Kolattukudy PE. 1976. Chemistry and biochemistry of natural waxes. Amsterdam: Elsevier. [Google Scholar]
- Lai C, Kunst L, Jetter R. 2007. Composition of alkyl esters in the cuticular wax on inflorescence stems of Arabidopsis thaliana cer mutants. The Plant Journal 50: 189–196. [DOI] [PubMed] [Google Scholar]
- Lang D, Eisinger J, Reski R, et al. 2005. Representation and high-quality annotation of the Physcomitrella patens transcriptome demonstrates a high proportion of proteins involved in metabolism in mosses. Plant Biology 7(3): 238–250. [DOI] [PubMed] [Google Scholar]
- Li F, Wu X, Lam P, et al. 2008. Identification of the wax ester synthase/acyl-coenzyme A: diacylglycerol acyltransferase WSD1 required for stem wax ester biosynthesis in Arabidopsis. Plant Physiology 148: 97–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magdy M, Werner O, McDaniel SF, Goffinet B, Ros RM. 2015. Genomic scanning using AFLP to detect loci under selection in the moss Funaria hygrometrica along a climate gradient in the Sierra Nevada Mountains, Spain. Plant Biology 18: 280–288. [DOI] [PubMed] [Google Scholar]
- Martins CMC, Mesquita SMM, Vaz WLC. 1999. Cuticular waxes of the Holm (Quercus ilex L. subsp. ballota (Desf.) Samp.) and cork (Q. suber L.) oaks. Phytochemical Analysis 10: 1–5. [Google Scholar]
- Millar A, Kunst L. 1997. Very-long-chain fatty acid biosynthesis is controlled through the expression and specificity of the condensing enzyme. The Plant Journal 12: 121–131. [DOI] [PubMed] [Google Scholar]
- Nakosteen PC, Hughes KW. 1978. Sexual life cycle of three species of Funariaceae in culture. The Bryologist 81: 307–314. [Google Scholar]
- Neinhuis C, Jetter R. 1995a. Ultrastructure and chemistry of epicuticular wax crystals in Polytrichales sporophytes. Journal of Bryology 18: 339–406. [Google Scholar]
- Neinhuis C, Jetter R. 1995b. Epicuticular wax of Polytrichales sporophytes. Journal of Bryology 18: 399–406. [Google Scholar]
- Ni Y, Guo Y-J, Wang J, et al. 2013. Responses of physiological indexes and leaf epicuticular waxes of Brassica napus to Sclerotinia sclerotiorum infection. Plant Pathology 63: 174–184. [Google Scholar]
- Osborne R, Stevens JF. 1996. Epicuticular waxes and glaucousness of Encephalartos leaves. Phytochemistry 42: 1335–1339. [Google Scholar]
- Proctor MCF. 1979. Surface wax on the leaves of some mosses. Journal of Bryology 10: 531–538. [Google Scholar]
- Proctor MCF, Oliver MJ, Wood AJ, Stark LR, Cleavitt NL, Mishler BD. 2007. Desiccation-tolerance in bryophytes: a review. The Bryologist 110: 595–621. [Google Scholar]
- Rashotte AM, Jenks MA, Feldmann KA. 2001. Cuticular waxes on eceriferum mutants of Arabidopsis thaliana. Phytochemistry 57: 115–123. [DOI] [PubMed] [Google Scholar]
- Razeq FM, Kosma DK, Rowland O, Molina I. 2014. Extracellular lipids of Camelina sativa: characterization of chloroform-extractable waxes from aerial and subterranean surfaces. Phytochemistry 106: 188–196. [DOI] [PubMed] [Google Scholar]
- R Core Team. 2015. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria.
- Riederer M. 2006. Introduction: biology of the plant cuticle In: M Riederer, C Muller, eds. Biology of the plant cuticle. Oxford: Blackwell Publishing Ltd, 1–10. [Google Scholar]
- Rowland O, Zheng H, Hepworth SR, Lam P, Jetter R, Kunst L. 2006. CER4 encodes an alcohol-forming fatty acyl-coenzyme A reductase involved in cuticular wax production in Arabidopsis. Plant Physiology 142: 866–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels L, Kunst L, Jetter R. 2008. Sealing plant surfaces: cuticular wax formation by epidermal cells. Annual Review of Plant Biology 59: 683–707. [DOI] [PubMed] [Google Scholar]
- Schönherr J. 1976. Water permeability of isolated cuticular membranes: the effect of cuticular waxes on diffusion of water. Planta 131: 159–164. [DOI] [PubMed] [Google Scholar]
- Schuette S, Wood AJ, Geisler M, Geisler-Lee J, Ligrone R, Renzaglia KS. 2009. Novel localization of callose in the spores of Physcomitrella patens and phylogenomics of the callose synthase gene family. Annals of Botany 103: 749–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw AJ. 1991. The genetic structure of sporophytic and gametophytic populations of the moss, Funaria hygrometrica Hedw. Evolution 45: 1260–1274. [DOI] [PubMed] [Google Scholar]
- Szafranek BM, Synak EE. 2006. Cuticular waxes from potato (Solanum tuberosum) leaves. Phytochemistry 67: 80–90. [DOI] [PubMed] [Google Scholar]
- Tulloch AP. 1983. Epicuticular waxes from Agropyron dasystachyum, Agropyron riparium and Agropyron elongatum. Phytochemistry 22: 1605–1613. [Google Scholar]
- Tulloch AP, Hoffman LL. 1973. Leaf wax of Triticum aestivum. Phytochemistry 12: 2217–2223. [Google Scholar]
- Wen M, Buschhaus C, Jetter R. 2006. Nanotubules on plant surfaces: chemical composition of epicuticular wax crystals on needles of Taxus baccata L. Phytochemistry 67: 1808–1817. [DOI] [PubMed] [Google Scholar]
- Xu S-J, Jiang P-A, Wang Z-W, Wang Y. 2009. Crystal structures and chemical composition of leaf surface wax depositions on the desert moss Syntrichia caninervis. Biochemical Systematics and Ecology 37: 723–730. [Google Scholar]
- Yeats TH, Buda GJ, Wang Z, et al. 2012. The fruit cuticles of wild tomato species exhibit architectural and chemical diversity, providing a new model for studying the evolution of cuticle function. The Plant Journal 69: 655–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeats TH, Rose JKC. 2013. The formation and function of plant cuticles. Plant Physiology 163: 5–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.