Abstract
Background and Aims Plant species differ greatly in the three-dimensional arrangements of their flowers (inflorescence architecture). However, the nature of selection responsible for this diversity is poorly understood. Studies that examine among-species variation suggest that inflorescence architecture affects pollinator behaviour, and so should influence plant mating. However, few studies consider the consequences of within-population architectural variation for pollinator behaviour.
Methods We manipulated inflorescence architecture of Delphinium glaucum to contrast bumblebee responses to normal and one-sided (secund) inflorescences.
Key Results The ‘dimensionality’ of manipulated inflorescences did not affect the number of flowers that bees visited; however, bees moved upward proportionally more on secund inflorescences.
Conclusions This study shows that realistic within-population variation in inflorescence architecture can manipulate pollinator behaviour. These results bear important consequences for plant mating success and the coordinated evolution of inflorescence architecture and floral specialization within inflorescences. These results also question why secund inflorescences are rare, for which we propose four testable explanations.
Keywords: Floral display, evolution, pollination, behaviour, Delphinium glaucum
INTRODUCTION
The three-dimensional arrangement of flowers (‘inflorescence architecture’) varies greatly among angiosperms (Weberling, 1989), presumably because it promotes reproductive performance in different mating environments (Harder and Prusinkiewicz, 2013). Inflorescence traits can influence mating quantity and quality through their effects on pollination and resource allocation (Wyatt, 1982). For animal-pollinated species, the number of flowers displayed simultaneously increases pollinator attraction and the number (but not the proportion) of flowers visited per pollinator (reviewed by Ohashi and Yahara, 2001), with consequences for among-flower self-pollination and pollen export (Harder and Barrett, 1995; Harder et al., 2000; Jersáková and Johnson, 2007; Karron and Mitchell, 2012). In contrast, few studies have addressed the roles of the three-dimensional arrangement of flowers in manipulating pollinator behaviour, pollination and plant mating.
Several studies used either large-scale manipulation (Fishbein and Venable, 1996: compound versus simple umbels) or artificial inflorescences (Jordan and Harder, 2006; Hirabayashi et al., 2006; Ishii et al., 2008: racemes, panicles and umbels) to contrast pollinator responses to different classes of inflorescences. Such categorical architectural differences influence pollinator attraction (Fishbein and Venable, 1996; Ishii et al., 2008) and, especially, the consistency of the foraging path within inflorescences (Jordan and Harder, 2006; Ishii et al., 2008), but they weakly affect the number of flowers visited (Hirabayashi et al., 2006; Jordan and Harder, 2006). By simulating pollen dispersal based on the bee behaviour observed during their experiments, Jordan and Harder (2006) further demonstrated that differences in the consistency of foraging paths among inflorescence types could affect pollen export and selfing rate.
The preceding studies provide limited information on the nature of selection on inflorescence architecture, as they considered more extreme architectural differences than feasibly occur in natural populations. The only relevant study in this context considered the effects of natural variation in the horizontal angle between adjacent flowers (helical angle) in spiralled inflorescences of Spiranthes sinensis on pollinator behaviour and pollen removal (Iwata et al., 2012). Helical angle negatively affected the number of flowers that bees probed and pollen removal. However, because this study did not manipulate inflorescences, the effects of inflorescence architecture were not isolated from those of unmeasured, correlated factors (e.g. resource status).
The simple design of racemes facilitates the study of inflorescence architecture, because manipulations can readily mimic natural variation. Racemes vary in floral density both within and among species, and less commonly in whether flowers face all directions or are presented on only one side (secund racemes: e.g. Digitalis, Lathyrus, Convallaria). However, the functional significance of floral density and sidedness has received little attention in natural populations (but see Hainsworth et al., 1983). In particular, sidedness has received little attention, likely because ‘normal’ inflorescences can be very rare (and difficult to study) in natural populations that display both normal and secund inflorescences (e.g. Digitalis purpurea; Fig. 1).
Fig. 1.

Digitalis purpurea inflorescences naturally displaying flowers either (A) 180° (on left) or (B) 360° around the flowering stalk. The latter occurs rarely in this species.
We report the first manipulative field study of the effects of realistic within-population variation in inflorescence architecture on pollinator behaviour [see Friedman and Harder (2004) for a comparable study of wind-pollinated species]. We examine bumblebee behaviour on three-dimensional and one-sided (secund) inflorescences of Delphinium glaucum (Ranunculaceae), a species that produces a typical raceme with spiral phyllotaxis (secund displays have not been observed). Therefore, our experiment helps illuminate selection during the early stages of the evolution of secund architecture in a population with ‘normal’ orientation. Pollinator behaviour, in particular movement paths and the number of flowers visited, strongly influences siring success and self-pollination in flowering plants (e.g. Barrett et al., 1994; Harder and Barrett, 1995; Harder et al., 2000; Karron et al., 2004). Hence, we assume that analyses of pollinator behaviour inform selection on inflorescence architecture. In general, our manipulations should illustrate aspects of selection on inflorescence traits that have not been addressed by previous manipulative studies because they mimic architectural variation observable in nature (e.g. Fig. 1).
MATERIALS AND METHODS
We studied Delphinium glaucum on Sibbald Flats, Alberta, Canada (51°02′ N; 114°49′ W). Its protandrous flowers open acropetally; hence, older, female-phase flowers occur below younger, male-phase flowers (Ishii and Harder, 2006). All experimental inflorescences (save two) originally displayed both female- and male-phase flowers (Ishii and Harder, 2012). To standardize flower age among treatments, we removed all female-phase flowers and left the oldest male-phase flower at the basal position. All male-phase flowers offer both nectar and pollen.
We randomly assigned inflorescences to one of three treatments, each displaying eight male-phase flowers (Fig. 2). The unmanipulated (U) treatment (n = 26 inflorescences) displayed flowers with natural orientation and density. For the low-density (LD) treatment (n = 20) we removed every second flower (based on pedicel origin on the main stem). For the secund (S) treatment (n = 28) we removed flowers from one side, leaving eight flowers on the remaining side. Therefore, U inflorescences presented flowers over roughly half the length (mean ± s.e. = 3·51 ± 0·21 cm) of the other treatments (LD, 6·46 ± 0·33 cm; S, 5·78 ± 0·20 cm). Hence, treatments differed in density, sidedness (or ‘dimensionality’) or both. This experimental design allows tests of two orthogonal, a priori comparisons: LD + U versus S assesses the general effect of inflorescence sidedness, whereas LD versus U examines the effect of density in the absence of sidedness differences. We also considered the a posteriori comparison of S versus LD, for which we used the Dunn–Šidák correction for multiple comparisons.
Fig. 2.
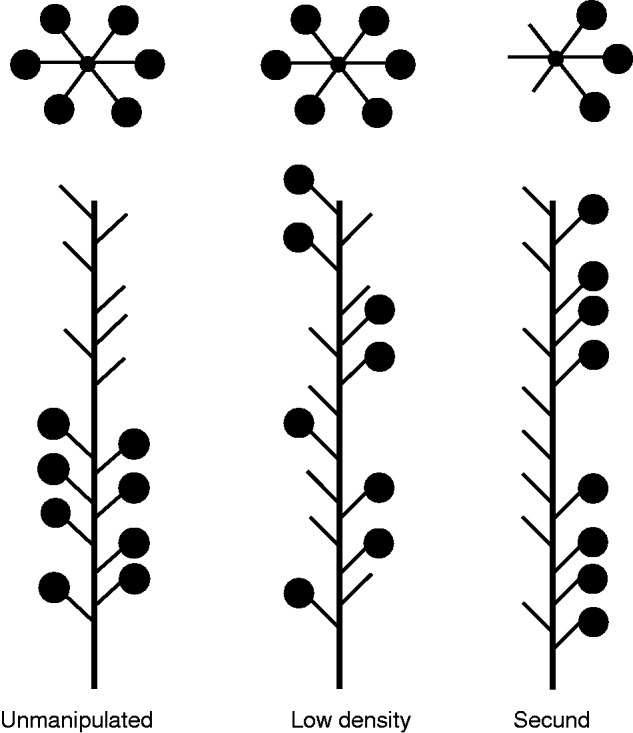
Top and side views of manipulated inflorescences presented to bumblebees foraging on Delphinium glaucum. Before presentation to bees, portions of the inflorescences above remaining flowers (denoted by filled circles) were removed; the excess stem remains in the schematic of the unmanipulated treatment to clarify patterns of flower removal.
Individual inflorescences, supported in a vial attached to the end of a hand-held stick (Thomson, 1981), were presented to bumblebees (Bombus flavifrons) already foraging for nectar on Delphinium glaucum. We videotaped a bee’s behaviour on the focal inflorescence and viewed the recording in slow motion to record the number of flowers visited and the direction (up versus down) travelled between two flowers. The relative positions of each flower’s perianth determined a bee’s travel direction, which proved more appropriate to the study of pollinator (and therefore pollen) movement than using each flower’s point of attachment to the inflorescence stem. A bee ‘visited’ a flower if it stopped beating its wings while on the flower.
Statistical methods
The effect of inflorescence type on the number of flowers probed per inflorescence visit was assessed with a generalized linear model (McCullagh and Nelder, 1989) that considered a Poisson distribution and a ln-link function (genmod procedure of SAS/STAT® version 13.2; SAS Institute Inc., 2014). Statistical inference involved a likelihood ratio (G) test.
Variation in the proportion of upward flights while bees visited inflorescences was under-dispersed compared with a binomial distribution and was modelled with a multiplicative binomial distribution (Altham, 1978) using the nlmixed procedure of SAS/STAT® version 13.2 (SAS Institute Inc., 2014). Parameter q of this distribution, which characterizes the dispersion compared with a binomial distribution (q = 1), was estimated as 1·47 (s.e. = 0·217; comparison with q = 1, t74 = 2·16, P < 0·05), indicating significant under-dispersion (ΔAIC for binomial distribution = 7·6).
RESULTS
Architecture affected bee movement within inflorescences, but not visit intensity. Bees visited an average of 4·9 flowers per inflorescence visit (lower 95 % confidence limit = 4·49, upper confidence limit = 5·4), with no difference among inflorescence types (G2 = 0·39, P > 0·75; nU = 26, nLD = 20, nS = 28 inflorescences). In contrast, the proportion of upward flights between flowers differed significantly among types (Fig. 3). Specifically, bees moved upward significantly more often on secund inflorescences than on inflorescences with a third dimension (LD and U: G1 = 5·06, P < 0·025), but they moved upward with equal frequency on the two three-dimensional types (U versus LD; G1= 0·003, P > 0·95). Thus, dimensionality, but not flower density, affected bee movement on the experimental inflorescences. Comparison of the two manipulated types also detected no significant difference in bee movement (S versus LD: G1 = 3·32, P>0·15, Dunn–Šidák multiple comparison).
Fig. 3.
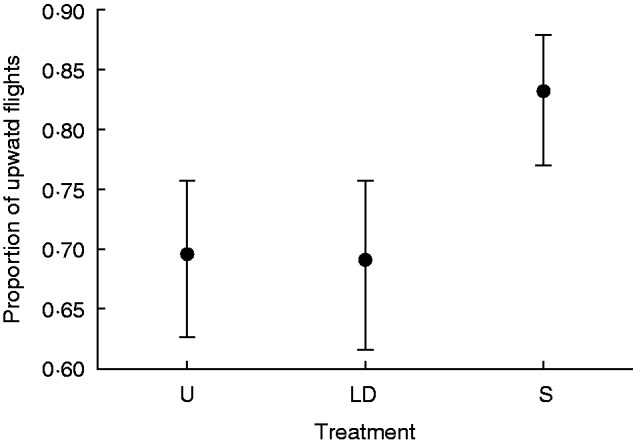
Proportion of movements (± s.e.) between flowers that were upward on three treatment inflorescences. U, unmanipulated; LD, low density; S, secund.
DISCUSSION
Inflorescence characteristics influence plant mating by manipulating abiotic and biotic pollen vectors (Harder and Prusinkiewicz, 2013). Bee responses in our experiment provide field confirmation of accumulated experimental evidence from studies with artificial inflorescences (Hirabayashi et al., 2006; Jordan and Harder, 2006; Ishii et al., 2008) that the three-dimensional arrangement of flowers primarily affects the patterns of pollinator movement among flowers. Importantly, our results clarify that even architectural differences within the scope of a species’ natural variation (Fig. 1) can influence pollinator movement, with likely effects on pollination and plant mating.
Bees foraged ‘naively’ on the secund treatment because Delphinium glaucum does not produce secund inflorescences naturally. Therefore, our results inform selection on rare mutations that induce the development of a secund inflorescence. Given that previous experiments involving ‘experienced’ bees on artificial inflorescences yielded similar results to our experiment (e.g. Hirabayashi et al., 2006; Jordan and Harder, 2006; Ishii et al., 2008), we expect that the nature of selection on inflorescence architecture will change little as rare phenotypes increase in frequency. Thus, if the secund phenotype is favoured when rare, it can spread to fixation. Whether inflorescence architecture experiences frequency-dependent selection awaits investigation.
Our study involved variations on the structure of vertical racemes, which elicit upward movement by bumblebees (Darwin, 1862; Waddington and Heinrich, 1979; Corbet et al., 1981). Reduction of inflorescence dimensionality to a secund form enhanced this tendency, increasing the percentage of upward movements from 68 to 83 (Fig. 3). In general agreement with this result, bumblebees similarly moved upward during 76 % of within-inflorescence flights on D. purpurea (Best and Bierzychudek, 1982), a species that typically produces secund inflorescences (Fig. 1). Secund flower presentation likely reinforces a bees’ proclivity for upward movement by simplifying the number of economical foraging paths and reducing the chance that rewarding flowers will be overlooked (Best and Bierzychudek, 1982).
Previous experiments indicate that pollinator movement consistency creates dissimilar conditions for pollen import and export among floral positions (e.g. Harder and Barrett, 1995; Harder et al., 2000), thereby affecting opportunities for floral specialization within inflorescences. If all flowers in a display present and receive pollen simultaneously and pollinators follow consistent paths, flowers in earlier-visited positions will import more outcross pollen, but export less pollen, than flowers visited later because of among-flower self-pollination and the associated pollen discounting (Harder et al., 2000; Jersáková and Johnson, 2007). Because of such contrasting female and male performance, foraging consistency should select for differential sex allocation (Brunet and Charlesworth, 1995) or other means of sexual segregation (e.g. dichogamy; Lloyd and Webb, 1986; Jordan and Harder, 2006). Our results indicate that relatively small differences in inflorescence architecture could affect such selection for sexual segregation within floral displays.
Although increased foraging consistency can select for sexual segregation among flower positions, it need not always increase reproductive success. Foraging consistency can benefit reproduction when pollinators visit female(-phase) flowers before male(-phase) flowers by effectively limiting among-flower self-pollination and pollen discounting (Harder et al., 2000; Jersáková and Johnson, 2007). However, foraging consistency by individual pollinators provides little benefit when flowers in all positions function simultaneously through female and male roles (Jordan and Harder, 2006). Hence, sex segregation should magnify mating pattern differences between architectures (Jordan and Harder, 2006). However, the consequences of consistent foraging for mating success may change when multiple, successive pollinators collectively determine mating patterns (see below).
If secund racemes generally promote consistent pollinator movement, why are they relatively rare among bee-pollinated angiosperms? Digitalis purpurea provides a familiar example (Fig. 1), but even within Digitalis only about half of the species exhibit this trait (Braüchler et al., 2004). Secund inflorescences may be rare for four non-exclusive reasons. (1) Secund flower presentation may often provide limited marginal mating benefits in combination with other inflorescence traits. Vertical inflorescences generally induce upward movement by bumblebees (Waddington and Heinrich, 1979), and commonly present male(-phase) flowers above female(-phase) flowers, effectively limiting the costs of geitonogamy (Harder et al., 2000; Jersáková and Johnson, 2007). Thus, although secund inflorescences promote consistent pollinator movement, their additional mating benefits may be limited. (2) Secund inflorescences may impose costs not associated with the presentation of flowers in all directions. All else being equal, a secund inflorescence is approximately twice as long as a typical raceme with similar flower number and density, which could introduce production and maintenance costs and impose biomechanical stress. Alternatively, secund inflorescences may be less attractive to pollinators. (3) Pollination dynamics associated with multiple pollinator visits may reduce the mating benefits of secund inflorescences. On secund inflorescences, consistent movement by individual pollinators should generate a strong correlation in reward standing crop among flowers. Consequently, pollinators could quickly learn that an empty flower signals low future rewards on other flowers on the same inflorescence, prompting them to depart after visiting few flowers (Hodges, 1985). By contrast, on typical racemes, which offer more foraging paths, empty flowers should provide less information about future rewards and be less likely to induce a pollinator’s departure (Kadmon and Schmida, 1992; Richards et al., 2009). Thus, the number of flowers visited per pollinator may vary more for secund inflorescences than on typical racemes. As pollination involves diminishing returns on the number of flowers visited per pollinator (Harder and Barrett, 1995), extensive variation in this behaviour could decrease mean pollen export and receipt of secund inflorescences (Richards et al., 2009). (4) Finally, developmental constraints on the divergence angle between successive primordia on a stem (phyllotaxis) may preclude production of primordia along only one side (Smith et al., 2006). Indeed, secund Digitalis (and Convallaria and Orthilia) initiate flower buds in the typical spiral pattern: their secund inflorescences arise secondarily by coordinated bending of floral pedicels that reorients flowers to face the same direction.
This study demonstrates that realistic within-population variation in the three-dimensional arrangement of flowers within inflorescences can alter pollinator behaviour, and therefore should respond to natural selection by altering plant mating. Our Discussion highlights two complementary predictions: (1) architectures that induce consistent foraging should experience stronger selection for sexual segregation, yet (2) inflorescences in which the sex functions are already beneficially segregated experience little selection for architectural traits that further increase foraging consistency, such as secund inflorescences. These predictions suggest limits on the extent to which inflorescence architecture benefits mating, and are readily testable. Manipulative field experiments that allow multiple pollinators to participate in plant mating are most likely to inform mechanisms of selection, whereas comparative analyses of within-clade variation in inflorescence architecture can address the long-term consequences of our hypothesized interactions between movement consistency and sexual segregation.
ACKNOWLEDGEMENTS
We thank T. Whidden, T. Moushian and C. M. Payne for assistance. The work was supported by a Discovery Grant from the Natural Sciences and Engineering Research Council of Canada (L.D.H.) and a grant from the Biotechnology and Biological Sciences Research Council to R. A. Ennos to support C.Y.J.
Competing interests. The authors declare that they have no competing interests.
Authors’ contributions. C.Y.J. and L.D.H. conceived and designed the study; all authors analysed the data and wrote the manuscript; C.Y.J. collected the data. All authors gave final approval for publication.
LITERATURE CITED
- Altham PME. 1978. Two generalizations of the binomial distribution. Applied Statistics 27: 162–167. [Google Scholar]
- Barrett SCH, Harder LD, Cole WW. 1994. Effects of flower number and position on self-fertilization in experimental populations of Eichhornia paniculata (Pontederiaceae). Functional Ecology 8: 526–535. [Google Scholar]
- Best LS, Bierzychudek P. 1982. Pollinator foraging on foxglove (Digitalis purpurea): a test of a new model. Evolution 36: 70–79. [DOI] [PubMed] [Google Scholar]
- Braüchler C, Meimberg H, Heubl G. 2004. Molecular phylogeny of the genera Digitalis L. and Isoplexis (Lindley) Loudon (Veronicaceae) based on ITS- and trnL-F sequences. Plant Systematics and Evolution 248: 111–128. [Google Scholar]
- Brunet J, Charlesworth D. 1995. Floral sex allocation in sequentially blooming plants. Evolution 49: 70–79. [DOI] [PubMed] [Google Scholar]
- Corbet SA, Cuthill I, Fallows M, Harrison T, Hartley G. 1981. Why do nectar-foraging bees and wasps work upwards on inflorescences? Oecologia 51: 79–83. [DOI] [PubMed] [Google Scholar]
- Darwin CR. 1862. On the various contrivances by which British and foreign orchids are fertilised by insects. London: John Murray. [PMC free article] [PubMed] [Google Scholar]
- Fishbein M, Venable DL. 1996. Evolution of inflorescence design: theory and data. Evolution 50: 2165–2177. [DOI] [PubMed] [Google Scholar]
- Friedman J, Harder LD. 2004. Inflorescence architecture and wind pollination in six grass species. Functional Ecology 18: 851–860. [Google Scholar]
- Hainsworth FR, Mercier T, Wolf LL. 1983. Floral arrangements and hummingbird feeding. Oecologia 58: 225–229. [DOI] [PubMed] [Google Scholar]
- Harder LD, Barrett SCH. 1995. Mating cost of large floral displays in hermaphrodite plants. Nature 373: 512–515. [Google Scholar]
- Harder LD, Prusinkiewicz P. 2013. The interplay between inflorescence development and function as a crucible of architectural diversity. Annals of Botany 112: 1477–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder LD, Barrett SCH, Cole WW. 2000. The mating consequences of sexual segregation within inflorescences of flowering plants. Proceedings of the Royal Society of London, Series B 267: 315–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirabayashi Y, Ishii HS, Kudo G. 2006. Significance of nectar distribution for bumblebee behaviour within inflorescences, with reference to inflorescence architecture and display size. Ecoscience 13: 351–359. [Google Scholar]
- Hodges CM. 1985. Bumble bee foraging: energetic consequences of using a threshold departure rule. Ecology 66: 188–197. [Google Scholar]
- Ishii HS, Harder LD. 2006. The size of individual Delphinium flowers and the opportunity for geitonogamous pollination. Functional Ecology 20: 1115–1123. [Google Scholar]
- Ishii HS, Harder LD. 2012. Phenological associations of within- and among-plant variation in gender with floral morphology and integration in protandrous Delphinium glaucum. Journal of Ecology 100: 1029–1038. [Google Scholar]
- Ishii HS, Hirabayashi Y, Kudo G. 2008. Combined effects of inflorescence architecture, display size, plant density and empty flowers on bumble bee behaviour: experimental study with artificial inflorescences. Oecologia 156: 341–350. [DOI] [PubMed] [Google Scholar]
- Iwata T, Nagasaki O, Ishii HS, Ushimaru A. 2012. Inflorescence architecture affects pollinator behaviour and mating success in Spiranthes sinensis (Orchidaceae). New Phytologist 193: 196–203. [DOI] [PubMed] [Google Scholar]
- Jersáková J, Johnson SD. 2007. Protandry promotes male pollination success in a moth-pollinated orchid. Functional Ecology 21: 496–504. [Google Scholar]
- Jordan CY, Harder LD. 2006. Manipulation of bee behavior by inflorescence architecture and its consequences for plant mating. American Naturalist 167: 496–509. [DOI] [PubMed] [Google Scholar]
- Karron JD, Mitchell RJ, Holmquist KG, Bell JM, Funk B. 2004. The influence of floral display size on selfing rates in Mimulus ringens. Heredity 92(3): 242–248. [DOI] [PubMed] [Google Scholar]
- Kadmon R, Schmida A. 1992. Departure rules used by bees foraging for nectar: a field test. Evolutionary Ecology 6: 142–151. [Google Scholar]
- Karron JD, Mitchell RJ. 2012. Effects of floral display size on male and female reproductive success in Mimulus ringens. Annals of Botany 109: 563–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd DG, Webb CJ. 1986. The avoidance of interference between the presentation of pollen and stigmas in angiosperms. I. Dichogamy. New Zealand Journal of Botany 24: 135–162. [Google Scholar]
- McCullagh P, Nelder JA. 1989. Generalized linear models. London: Chapman and Hall. [Google Scholar]
- Ohashi K, Yahara T. 2001. Behavioural responses of pollinators to variation in floral display size and their influences on the evolution of floral traits In: Chittka L, Thomson JD, eds. Cognitive ecology of pollination. Cambridge, UK: Cambridge University Press, 274–296. [Google Scholar]
- Richards SA, Williams NM, Harder LD. 2009. Variation in pollination: causes and consequences for plant reproduction. American Naturalist 174: 382–398. [DOI] [PubMed] [Google Scholar]
- SAS Institute Inc. 2014. SAS/STAT® 13.2 user’s guide. Cary, NC: SAS Institute. [Google Scholar]
- Smith RS, Guyomarc'h S, Mandel T, Reinhardt D, Kuhlemeier C, Prusinkiewicz P. 2006. A plausible model of phyllotaxis. Proceedings of the National Academy of Sciences of the USA 103: 1301–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson JD. 1981. Field measures of flower constancy in bumblebees. American Midland Naturalist 105: 377–380. [Google Scholar]
- Waddington KD, Heinrich B. 1979. The foraging movements of bumblebees on vertical ‘inflorescences’: an experimental analysis. Journal of Comparative Physiology 134: 113–117. [Google Scholar]
- Weberling F. 1989. Morphology of flowers and inflorescences. Cambridge: Cambridge University Press. [Google Scholar]
- Wyatt R. 1982. Inflorescence architecture: how flower number, arrangement, and phenology affect pollination and fruit-set. American Journal of Botany 69: 585–594. [Google Scholar]


