Abstract
Background
The dichotomy between glioblastoma cell migration and proliferation is regulated by various parameters including oxygen tension. In glioblastoma stem-like cells, hypoxia induces downregulation of pentose phosphate pathway (PPP) enzymes and a flux shift towards glycolysis. We investigated whether the 2 parallel glucose metabolic pathways are intrinsically linked with cell function and whether these pathways are mechanistically involved in regulating functional programs.
Methods
Enzyme expression, migration, and proliferation under hypoxia were studied in multiple cell types. Rapidly and slowly dividing or migrating glioblastoma cells were separated, and enzyme profiles were compared. Glucose-6-phosphate dehydrogenase (G6PD) and Aldolase C (ALDOC), the most strongly inversely regulated PPP and glycolysis enzymes, were knocked down by short hairpin RNA.
Results
Hypoxia caused downregulation of PPP enzymes and upregulation of glycolysis enzymes in a broad spectrum of cancer and nonneoplastic cells and consistently stimulated migration while reducing proliferation. PPP enzyme expression was increased in rapidly dividing glioblastoma cells, whereas glycolysis enzymes were decreased. Conversely, glycolysis enzymes were elevated in migrating cells, whereas PPP enzymes were diminished. Knockdown of G6PD reduced glioblastoma cell proliferation, whereas ALDOC knockdown decreased migration. Enzyme inhibitors had similar effects. G6PD knockdown in a highly proliferative but noninvasive glioblastoma cell line resulted in prolonged survival of mice with intracerebral xenografts, whereas ALDOC knockdown shortened survival. In a highly invasive glioblastoma xenograft model, tumor burden was unchanged by either knockdown.
Conclusions
Cell function and metabolic state are coupled independently of hypoxia, and glucose metabolic pathways are causatively involved in regulating “go or grow” cellular programs.
Keywords: glioma, glycolysis, invasion, metabolism, pentose phosphate pathway
Glioblastomas typically consist of a tumor core containing highly proliferative cells and a diffusely invasive periphery with tumor cells that exhibit only low proliferation rates. Excessive proliferation and migration tend to be spatially and temporally disassociated behaviors, with cells either favoring proliferation at the expense of migration or migration at the expense of proliferation, a phenomenon conceptualized in the “go or grow” hypothesis.1
It has been shown that the balance between glioblastoma cell proliferation and invasion can be shifted by therapeutic intervention. Antiangiogenic treatment can inhibit glioblastoma growth in orthotopic xenograft models and reduce tumor cell proliferation; however, treated tumors typically display a striking compensatory increase in invasion.2,3 Inhibition of angiogenesis impairs intratumoral oxygen supply, and hypoxia is a potent driver of glioblastoma cell migration and invasion.4 Hypoxia can further enhance stem cell characteristics of glioblastoma cells, and cyclic fluctuations in oxygen tension, which occur during tumor progression, select for stem-like glioblastoma cells which possess outstanding metabolic adaptability.5,6 While investigating mechanisms of adaptation of glioblastoma stem-like (GS) cells to bidirectional fluctuations in oxygen tension, we recently discovered a reciprocal metabolic switch between the pentose phosphate pathway (PPP) and glycolysis.7 The PPP, which produces ribose-5-phosphate and NADPH for DNA/RNA and fatty acid synthesis, is an alternative anabolic pathway to the preparatory phase of glycolysis.8 Enzymes of the PPP are highly expressed under normoxic conditions, whereas acute hypoxia causes downregulation of PPP enzymes concomitant with upregulation of glycolysis enzymes. We confirmed this metabolic shift by flux analyses using [1,2-13C2]-d-glucose tracing, which showed that flux through the PPP is comparatively high under normoxia but shifts towards glycolysis upon hypoxia.7 Oxygenation of chronically hypoxic GS cells has opposite effects (ie, upregulation of the PPP and downregulation of glycolysis). Hypoxia further causes increased GS cell migration but with reduced proliferation, whereas oxygenation has opposite effects linking the metabolic switch to the go or grow potential of the cells. Likewise, in glioblastoma tissue sections, PPP enzymes are strongly expressed in highly proliferative regions but are downregulated in severely hypoxic pseudopalisades, while glycolysis enzymes exhibit an inverse pattern.7
In the present study, we investigated whether the hypoxia-induced reciprocal switch between PPP and glycolysis enzyme expression is restricted to stem-like glioblastoma cells or if it also occurs in other cell types. We further analyzed whether associations between the PPP and proliferation, as opposed to glycolysis and migration, necessarily depend on changes in oxygen tension or whether metabolic and functional programs are linked intrinsically with each other (ie, independent of hypoxia/oxygenation). Finally, we explored whether glycolysis and the PPP are directly and causatively involved in the dichotomous regulation of migration versus proliferation.
Materials and Methods
Cell Culture
Cell cultures were established from human tissue or blood samples with informed consent of the patients and approval by the local ethics committees in Hamburg, Munich, and Heidelberg. GS-11 and GS-12 cell lines were cultured as neurospheres, as described previously.9 Glioma cell lines G55 and U87, HuH7 (hepatocarcinoma), and MDA-MB231 (breast cancer) cells were cultured in Dulbecco's modified Eagle's medium (DMEM) with 10% fetal bovine serum (FBS; Life Technologies). Human astrocytes were maintained in astrocyte medium (Life Technologies) with 20% FBS. Human umbilical vein endothelial cells and human fibroblasts were isolated and cultured as described previously.10,11 Human mesenchymal stem cells (TB869 gbMSC) were isolated from a human glioblastoma and cultured in DMEM with 20% FBS. gbMSC cell surface marker profiles, as determined by fluorescence-activated cell sorting (FACS) analysis, were CD73+, CD90+, CD105+ (>95% of cells were positive for these markers) and CD14−, CD31−, CD34−, CD45− (<2% of cells were positive for these markers). gbMSCs grew adherent and could be differentiated into osteoblasts, adipocytes, and chondroblasts, thereby fulfilling all defining criteria for MSCs. Peripheral blood mononuclear cells were isolated from human blood by gradient centrifugation and cultured in RPMI1649 medium with 10% AB serum (MILAN Analytica).
Quantitative PCR Analysis
Real-time PCR analyses were performed as detailed in the Supplementary material and as described previously,7 using validated TaqMan Gene Expression Assays and a 7500 Fast Real-Time PCR System (Applied Biosystems).
Immunoblot Analysis
Western blot analysis was performed as detailed in the Supplementary material and as described previously.7 Western blot scans of x-ray films were quantified by densitometry using the ImageJ program.
Cell Proliferation Assays
Proliferation of GS cells was quantified by manual cell counts, and proliferation of G55 cells was measured using a colorimetric assay12 as detailed in the Supplementary material.
Migration Assay
Glioma cell migration was analyzed using modified Boyden chamber assays as described previously.9 Briefly, the lower wells of a 96-well modified Boyden chamber (Neuro Probe) were filled with serum-free medium containing 0.1% bovine serum albumin. Wells were covered with an 8-µm pore size filter coated with 5 µg/mL laminin (Invitrogen) for GS-11 cells or with 0.875% collagen (StemCell Technologies) for other cell types. Sextuplicates of 1.5 × 104 cells in 50 mL assay medium were seeded into the upper wells. For inhibitor experiments, 6-aminonicotinamide (6-AN) or 2-deoxyglucose (2-DG) were added to the upper and lower wells. After 5 hours or 24 hours (GS-11 cells) of incubation, migrated cells on the underside of the membrane were stained and counted.
Short Hairpin RNA Transduction
Lentiviral transduction of short hairpin RNA (shRNA) targeting aldolase C (ALDOC), glucose-6-phosphate dehydrogenase (G6PD) or nonsilencing control shRNA was performed as outlined in the Supplementary material. shRNA-expressing cells were selected using puromycin.
In vivo Tumor Models
Animal experiments were approved by the local authority in Hamburg. Tumor xenografts were generated as described previously.3 Briefly, dissociated G55 cells (4 × 104) or GS-11 cells (1.5 × 105) in 4 µL medium were injected stereotactically into the striatum of 6–8 week old anesthetized NMRI/Foxn1nu mice or SHrNTM Hairless NOD.SCID mice, respectively (Harlan Laboratories). The endpoint of the G55 xenograft model was the occurrence of symptoms such as weight loss ≥10% or neurological symptoms. The endpoint of the GS-11 model was 7.5 months after tumor injection. Animals were killed using CO2, brains were fixed in formalin and embedded in paraffin, and serial sections were stained with H&E. Quantification of invasive growth in the GS-11 model was performed as described previously9,13,14 and as detailed in the Supplementary material.
Immunostaining of paraffin sections for Ki-67 was performed as described previously using a mouse monoclonal antibody (Dako,) and the Histofine detection system (Medac).13 The percentage of Ki-67 immunoreactive nuclei was determined in 3 high-power fields in the most actively proliferating tumor area.
PKH67 Staining and Cell Separation
2 × 107 G55 or GS-11 cells were labeled using the PKH67 Fluorescent Cell Linker Kit (Sigma-Aldrich) and incubated in standard media. Optimal time points for separation of fast dividing (PKHlow) and slowly dividing (PKHhigh) populations were determined in pilot experiments to maximize differences in fluorescence intensity as well as in population size for each cell type. Cell sorting was performed using a FACS Aria III flow cytometer (BD Biosciences).
Separation of Migrated and Nonmigrated Cells
Transwell Permeable Supports (Corning Incorporated) were coated with laminin (5 µg/mL) or collagen (0.875%) for GS-11 or G55 cells, respectively, and were installed in 6-well plates. 2 × 106 cells were seeded into each transwell. After 6 hours (G55) or 28 hours (GS-11) of incubation, nonmigrated cells from the upper side of the transwell membrane, as well as migrated cells from the underside, were scraped off and collected separately. Cells from 6 transwell assays were pooled for RNA and protein extraction.
Statistical Analyses
Differences in gene expression and in functional assays were analyzed using the unpaired t test and the SigmaStat 2.0 program. Survival analyses were performed using the MedCalc program (Kaplan-Meier analysis, log-rank test).
Results
The switch from PPP to glycolysis and from proliferation to migration is a common response mechanism to hypoxia in tumor cells and normal cells
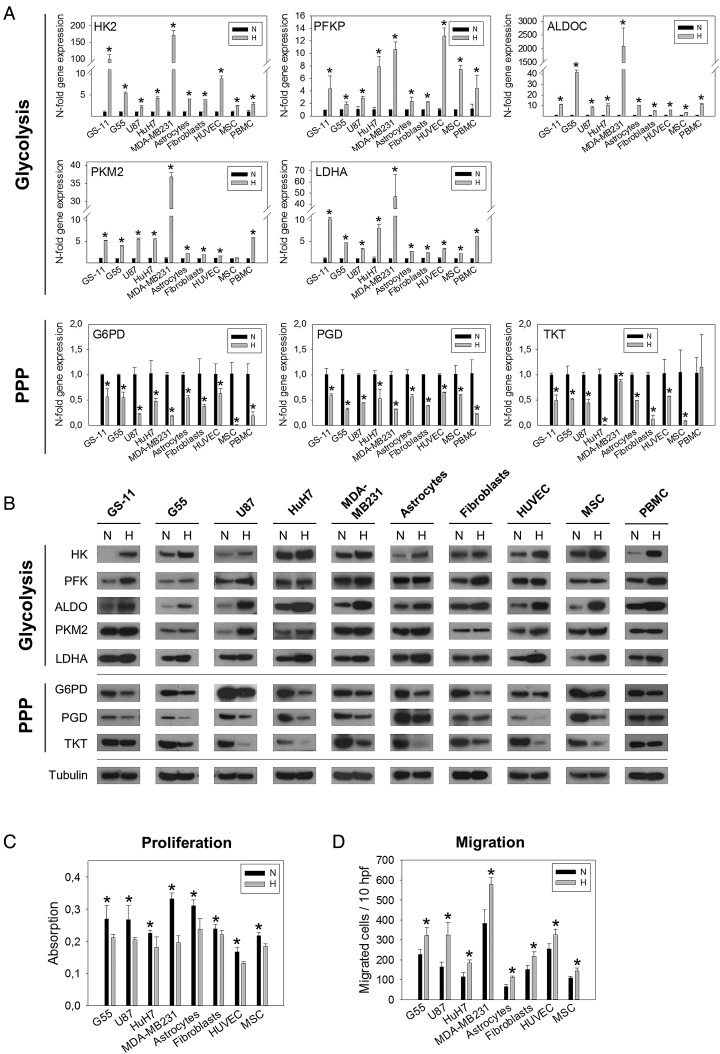
To investigate whether the hypoxia-induced switch from the PPP to glycolysis is confined to GS cells or is a general mechanism, we performed enzyme expression analyses on a spectrum of different human cell types. Glioblastoma cell lines grown under adherent conditions (U87, G55), cell lines derived from other tumor entities (HuH7, MDA-MB-231), and non-transformed cells such as normal human astrocytes, fibroblasts, umbilical vein endothelial cells, glioblastoma-associated mesenchymal stem cells (MSCs), and peripheral blood mononuclear cells were included. All cell types were exposed to 1% hypoxia, and transcript levels were compared with normoxic controls (21% O2) after 24, 48, 72, and 96 hours. The enzymes analyzed represent the key components of the different glucose pathways, which showed strongest hypoxic induction in GS cells7 and included hexokinase 2 (HK2), 6-phosphofructokinase platelet type (PFKP), ALDOC, (all preparatory phase of glycolysis), pyruvate kinase M2 (PKM2), (pay-off phase of glycolysis), lactate dehydrogenase A (LDHA), (lactate production), glucose-6-phosphate dehydrogenase (G6PD), 6-phosphogluconate dehydrogenase (PGD), (both oxidative part of the PPP), and transketolase (TKT), (nonoxidative part of the PPP). qPCR revealed that the expression of glycolysis enzymes was consistently upregulated by hypoxia, whereas expression of PPP enzymes was downregulated in the vast majority of cell types (Fig. 1A). We had focused the analysis on enzyme isoforms described as most relevant in brain and/or cancer; despite a certain degree of tissue specificity, these isoforms are expressed virtually ubiquitously, which we confirmed by directly comparing mRNA levels between different cell types under normoxia (Supplementary material, Fig. S1). To assess whether changes in gene expression corresponded to protein levels, immunoblot analyses were performed after 48 hours, and the results consistently confirmed that hypoxia induced downregulation of PPP enzyme expression, concomitant with upregulation of glycolysis enzymes (Fig. 1B, Supplementary material, Fig. S2).
Fig. 1.
Effect of hypoxia on enzyme expression and cell function. (A) Quantification of glycolysis and pentose phosphate pathway (PPP) enzyme transcripts by qPCR in different cell types exposed to hypoxia (H), (1% O2). Relative quantities were calculated and normalized to normoxic (N) controls. Asterisks indicate significant maximal upregulation or downregulation of transcripts, which typically occurred at 48 hours (P < .05). (B) Immunoblot analysis of glycolysis and PPP enzymes after 48 hours of hypoxia versus normoxia. Densitometric analysis is presented in Supplementary material, Fig. S1. (C) Cell proliferation was quantified after 3 days of growth using a colorimetric assay. Values are means ± SD of quadruplicate determinations. (D) Cell migration was analyzed in modified Boyden chamber assays. After 5 hours of incubation, migrated cells were counted in 10 high power fields (hpf). Values are means ± SD of sextuplicate determinations. Asterisks in (C) and (D) indicate significance (P < .05).
We next analyzed whether the hypoxia-induced shift from proliferation to migration is limited to GS cells or if it is a common phenomenon. After 3 days of hypoxic incubation, proliferation of all cell types was significantly decreased compared with normoxic controls (Fig. 1C). Conversely, cell migration was found to be invariably enhanced by incubation under hypoxia for 5 hours in modified Boyden chamber assays (Fig. 1D).
Associations of the PPP with proliferation and of glycolysis with migration are independent of changes in oxygen tension
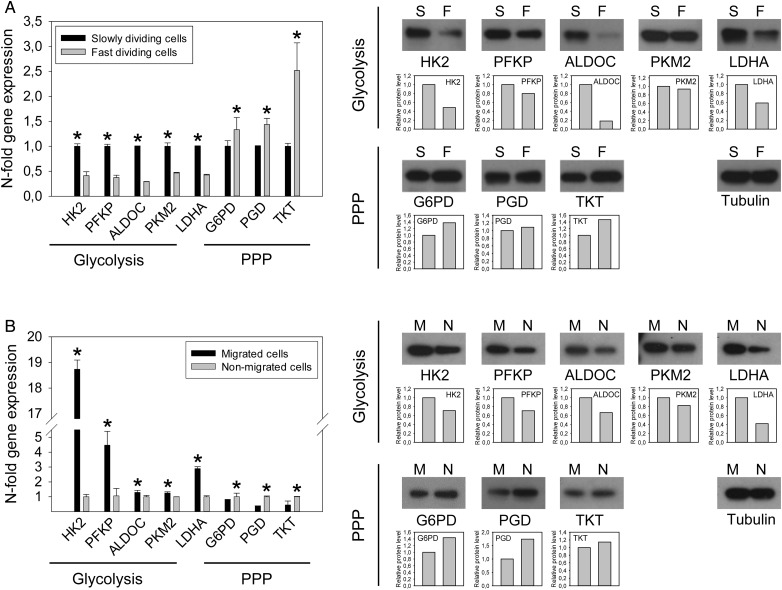
To investigate whether metabolic pathways and functional behaviors are coupled independently of fluctuations in oxygen tension, we isolated subpopulations of rapidly versus slowly proliferating cells from the generally fast-growing G55 cell line and the relatively slow-growing GS-11 line. Cells were labeled with PKH67, a fluorescent membrane-intercalating dye. With each cell division, fluorescence intensity of the daughter cells is approximately halved, so that fast-dividing clones exhibit lower fluorescence intensity than slowly dividing cells. Time points for FACS-separation of fast dividing (PKHlow) and slowly dividing (PKHhigh) subpopulations were optimized for each line to maximize differences in fluorescence intensity as well as population size for further analyses and were 2 days for G55 cells and 14 days for GS-11 cells. qPCR and immunoblot analysis revealed that PKHlow cells displayed decreased expression of almost all glycolysis enzymes and of LDHA compared with PKHhigh cells but increased expression of PPP enzymes, including its first and key regulatory enzyme G6PD (Fig. 2A, Supplementary material, Fig. S3A).
Fig. 2.
Expression of glycolysis and pentose phosphate pathway (PPP) enzymes in G55 cell subpopulations. (A) Fast and slowly dividing cell subpopulations were isolated by fluorescence-activated cell sorting (FACS) from PKH67-labelled cells (slowly: 8.9%, fast: 9.4% of all cells). qPCR and immunoblot analysis revealed downregulation of glycolysis enzymes in fast dividing (F) versus slowly dividing (S) cells, paralleled by upregulation of PPP enzymes. (B) Migrated (M) and nonmigrated (N) cells were separated using transwell assays. Migrated cells displayed increased expression of glycolysis enzymes and downregulation of PPP enzymes. Asterisks mark significant differences (P < .05).
Rapidly migrating and nonmigrating cells were separated using transwell assays. After 6 hours (G55) or 28 hours (GS-11) of incubation, migrated cells from the underside and nonmigrated cells from the upper side of the membrane were collected separately. Expression of almost all glycolysis enzyme transcripts was significantly increased in migratory cells compared with nonmigrated cells, whereas PPP enzyme expression was regulated inversely (Fig. 2B, Supplementary material, Fig. S3B).
Inhibition of G6PD Decreases Cell Proliferation but Increases Migration
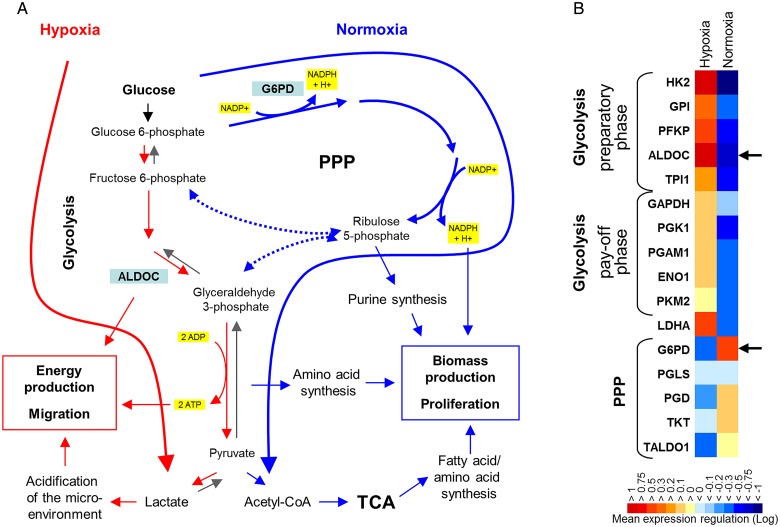
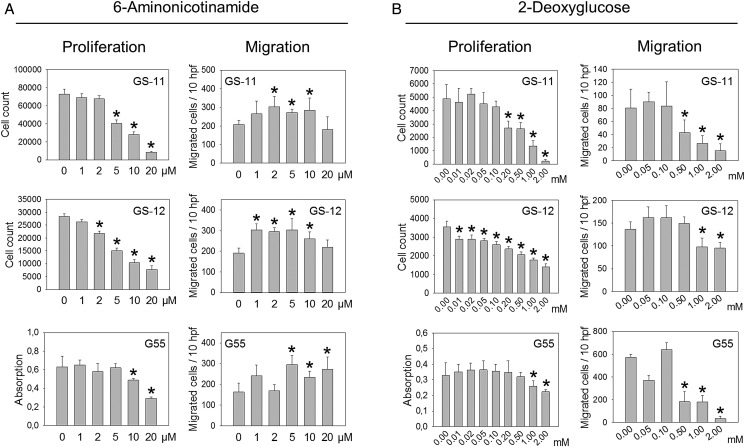
Since associations between metabolic and functional state are obviously independent of changes in oxygen tension, we asked whether different glucose pathways are mechanistically involved in the dichotomous regulation of cell motility and growth. We addressed this question by using enzyme inhibitors and enzyme knockdown of G6PD and ALDOC. G6PD is the first and key rate-controlling enzyme of the PPP, and G6PD and ALDOC are the strongest inversely hypoxia-regulated enzymes of the PPP and glycolysis in GS lines7 (Fig. 3A and B). Incubation with 6-AN, an antimetabolite and competitive inhibitor of G6PD, reduced the proliferation of GS-11, GS-12, and of G55 cells in a dose-dependent fashion with >50% inhibition at 10–20 µM in all lines (Fig. 4A). Conversely, migration of all cell lines was enhanced by 6-AN, with maximum stimulation of 80.8% in G55 (Fig. 4A).
Fig. 3.
Enzyme regulation by hypoxia and normoxia. (A) Under normoxic conditions, pentose phosphate pathway (PPP) enzyme expression and flux are upregulated to facilitate biomass production and cell cycling (blue arrows). Acute hypoxia causes downregulation of PPP enzymes and shifts glucose metabolism towards direct glycolysis (red arrow), facilitating rapid energy production and cell migration. (B) Gene expression profiling showed that enzymes of the preparatory phase of glycolysis (HK2, GPI, PFKP, ALDOC) are more strongly upregulated by hypoxia than enzymes of the pay-off phase (original microarray data are published in Kathagen et al.7). G6PD and ALDOC emerged as the most strongly inversely hypoxia-regulated enzymes of both pathways. Colors represent mean n-fold enzyme transcript regulation in 4 GS cell lines exposed to acute hypoxia (48 h) versus normoxia. Abbreviations: HK2, hexokinase 2; GPI, glucose-6-phosphate isomerase; PFKP, 6-phosphofructokinase platelet type; ALDOC, aldolase C; TPI1, triosephosphate isomerase 1; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; PGK1, phosphoglycerate kinase 1; PGAM1, phosphoglycerate mutase 1; ENO1, enolase 1; PKM2, pyruvate kinase M2; LDHA, lactate dehydrogenase A chain; G6PD, glucose-6-phosphate dehydrogenase; PGLS, 6-phosphogluconolactonase; PGD, 6-phosphogluconate dehydrogenase; TKT, transketolase; TALDO1, transaldolase 1.
Fig. 4.
Functional effects of 6-AN and 2-DG. Proliferation and migration were analyzed in the presence or absence of 6-AN (A) or 2-DG (B). GS cell proliferation was analyzed by cell counting after 7 days of incubation, and G55 proliferation was determined after 4 days using a colorimetric assay. Migration was analyzed in modified Boyden chamber assays with 24 hours (GS cells) or 5 hours (G55) of incubation. All experiments were performed at 21% O2. Values are means ± SD of sextuplicate determinations. Asterisks indicate significant inhibition or stimulation (P < .05).
2-DG is a glucose analog that inhibits HK, the rate-limiting enzymatic step of glycolysis that is also essential for fueling the PPP with glucose-6-phosphate (Fig. 4B). 2-DG reduced the proliferation of all 3 cell lines, and GS-lines were more sensitive already at lower concentration (0.01–0.2 mM) than G55 cells (Fig. 4B). Furthermore, migration of all cell lines was also inhibited by 2-DG, and the effects became significant at 0.5 or 1.0 µM (Fig. 4B).
Downregulation of G6PD and ALDOC Has Opposite Functional Effects in Vitro
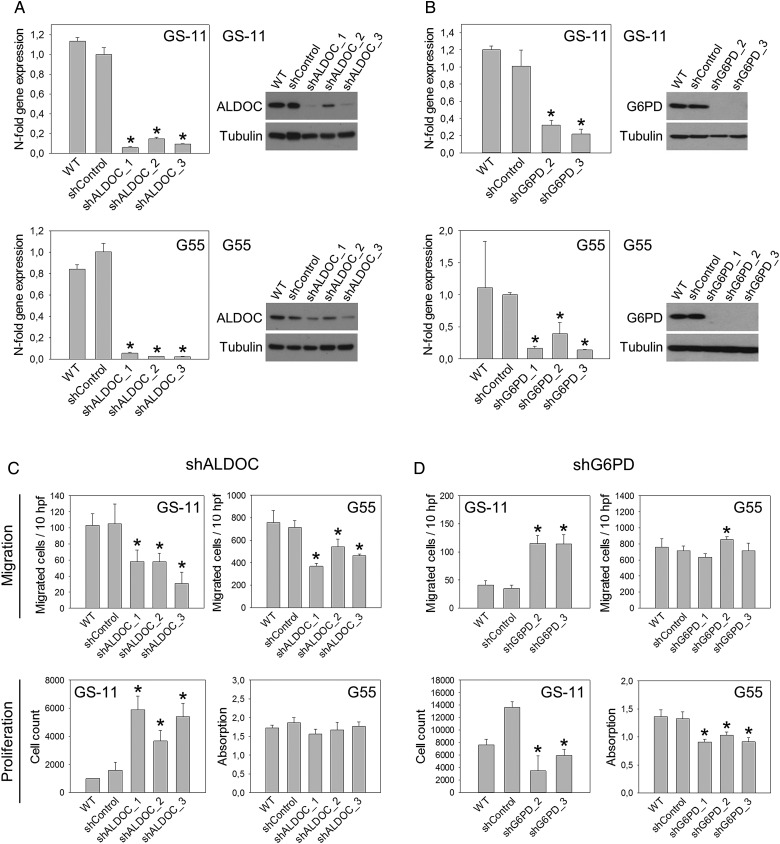
ALDOC is the most strongly hypoxia-induced enzyme in GS cells and other cell types (Fig. 1A and B). Since no specific ALDOC inhibitor is available,15 we knocked down its expression using shRNA. Efficient downregulation of ALDOC mRNA and protein was achieved by transduction with 3 different shRNAs in GS-11 and G55 cells (Fig. 5A). Downregulation of G6PD was successful with 2 shRNAs in GS-11 and 3 in G55 cells (Fig. 5B).
Fig. 5.
Aldolase C (ALDOC) and glucose-6-phosphate dehydrogenase (G6PD) knockdown in vitro. (A) Quantification of ALDOC expression by qPCR and immunoblot analysis in GS-11 and G55 cells transduced with 3 different ALDOC shRNAs or nonsilencing shRNA (shControl) and in untransduced wild-type (WT) cells. Gene expression values are means ± SD of triplicate determinations, asterisks indicate significant downregulation compared with shControls (P < .05). (B) Quantification of G6PD in cells transduced with shRNAs targeting G6PD or nonsilencing shRNA. α-tubulin served as loading control. Effects of ALDOC knockdown (C) and G6PD knockdown (D) on cell migration and proliferation were analyzed as described in Fig. 4. Values in (C)and (D) are means ± SD of sextuplicate determinations. Asterisks indicate significant differences versus shControls (P < .05).
ALDOC knockdown resulted in significantly reduced migration of all GS-11 and G55 sublines (Fig. 5C). Migration of GS-11 cells was decreased maximally by 70.5% (shALDOC_3), and G55 cell migration was reduced by up to 48.3% (shALDOC_1) compared with nonsilencing controls (shControl), (P < .05). Proliferation of GS-11 shALDOC cells was increased up to 3.7-fold (shALDOC_1) compared with shControls, but G55 proliferation was not affected by ALDOC knockdown.
Knockdown of G6PD resulted in decreased cell proliferation. Growth of GS-11 cells was reduced by maximally 74.7% (shG6PD_2) compared with shControls, and G55 proliferation was decreased by up to 31.5% (shG6PD_1), (P < .05). Furthermore, G6PD knockdown in GS-11 cells led to a 3.3-fold increase in migration (P < .05).
Knockdown of ALDOC and G6PD Has Partially Opposite Functional Effects in Vivo
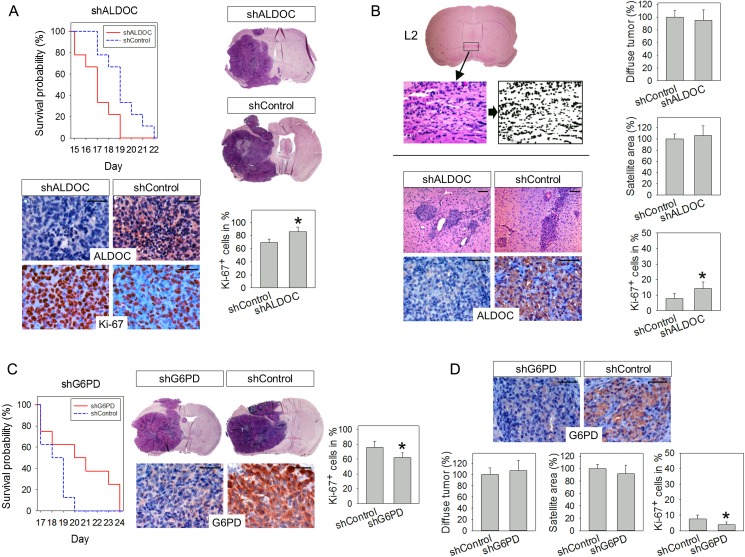
Based on the in vitro observations, we next analyzed the impact of both pathways on tumor initiation and progression in vivo by implanting one of the knockdown and shControl cell lines into the brains of immunocompromised mice. G55 cells typically form highly proliferative, but largely noninvasive tumors, which are usually fatal within 3 weeks.3 In contrast, GS-11 tumors are highly invasive but grow slowly, so that development of tumor-related symptoms usually takes at least 7 months.7 Mice with orthotopic G55-shALDOC_1 xenografts had a significantly shorter survival (median, 17 d) than animals with shControl xenografts (median, 19 d), (P < .05), (Fig. 6A). Histologically, tumor morphologies were similar, with little invasion and no obvious difference in necrosis formation. Immunohistochemistry for Ki-67 revealed that the tumor cell proliferation was 16.4% higher in shALDOC_1 tumors compared with shControls (P < .05).
Fig. 6.
In vivo effects of Aldolase C (ALDOC) and glucose-6-phosphate dehydrogenase (G6PD) knockdown. (A) Mice engrafted with G55-shALDOC_1 cells had a shorter survival than mice with shControl tumors (P = .015). Tumor morphologies in both groups were similar (H&E staining). Immunohistochemically, reduced expression of ALDOC was detected in shALDOC_1 tumors compared with shControls. Tumor cell proliferation (Ki-67) was increased in shALDOC_1 tumors. (B) Diffuse tumor burden in mice engrafted with GS-11-shALDOC_1 cells was assessed by analyzing 20 landmark areas (at 4 coronal levels [L1-L4] in 5 defined regions each). Exemplary analysis of diffuse invasion is shown for L2 (anterior commissure level). Digital images of all 20 regions were acquired from H&E-stained sections, transformed into black and white, and the percentage of black pixels was quantified. Values acquired for identical regions in normal murine brain were subtracted, resulting in the net area occupied by tumor cell nuclei. Values for all regions were pooled and expressed as diffuse tumor burden (means ± SD in %). Micronodular tumor burden was assessed by measuring the cumulative micronodular tumor area at 4 coronal levels. Immunohistochemically, reduced ALDOC expression was present in knockdown tumors, while tumor cell proliferation was increased in GS-11-shALDOC_1 tumors. (C) Survival of mice with G55-shG6PD_2 tumors was prolonged relative to controls (P = .046). G6PD expression and tumor cell proliferation were reduced in knockdown tumors. (D) G6PD expression was reduced in GS-11-shG6PD_2 tumors. Diffuse and micronodular tumor burden was analyzed as described for (B). G6PD expression and proliferation of knockdown tumors was decreased compared with controls. Asterisks in (A)-(D) indicate significance (P < .05); size bars are 50 µm.
The GS-11 in vivo experiment was terminated 7.5 months after tumor cell injection to facilitate evaluation of tumor invasion at a single identical time point, at which no animal had yet died of tumor-related symptoms. Histologically, GS-11 tumors exhibited both perivascular invasion as well as diffuse invasion (Fig. 6B). On cross sections, perivascular invasion typically appeared as micronodular or finger-like structures. To quantify the extent of this type of invasive growth, the cumulative micronodular area on 4 different defined coronal sections was measured. In addition, diffusely infiltrative tumor growth was quantified by measuring the net area occupied by tumor cell nuclei in 20 representative regions using a previously developed method.9,13,14 Neither the analysis of micronodular tumor growth nor quantification of diffusely invasive growth revealed a significant difference between shALDOC_1 and shControl tumors (Fig. 6B). However, the proliferation rate of shALDOC_1 tumors was increased by 6.5% compared with controls (P < .05), (Fig. 6B).
The effect of G6PD knockdown on the in vivo growth of G55 tumors was contrary to that of ALDOC knockdown; ie, the survival of animals engrafted with G55- shG6PD_2 cells was prolonged (median, 20.5 d) compared with shControls (median, 18.5 d), (P < .05). Tumor cell proliferation was decreased by 18.1% in shG6PD_2 tumors compared with shControls (Fig. 6C). In the GS-11 model, knockdown of G6PD had no significant effect on micronodular or diffusely invasive growth; however, proliferation rates were decreased by 3.6% compared with controls (P < .05), (Fig. 6D).
Discussion
The major novel findings of this study are as follows: (i) hypoxia-induced downregulation of PPP enzymes concomitant with upregulation of glycolysis enzymes is a universal phenomenon that is consistently associated with increased migration and decreased proliferation in different cell types; (ii) associations between the PPP and proliferation versus glycolysis and migration exist independently of changes in oxygen tension; and (iii) the PPP and glycolysis are directly mechanistically involved in the preferential activation of the go versus grow cellular programs.
We previously discovered a metabolic switch in glioblastoma stem-like cells from the PPP to glycolysis and vice versa in response to hypoxia and oxygenation.7 Our current findings extend these observations by showing that the switch is not limited to GS cells but is a common phenomenon that also occurs in other types of cancer cells as well as in non-neoplastic cells that do not exhibit the Warburg phenotype. Upregulation and downregulation of glycolysis and PPP enzymes in response to hypoxia were not generally stronger in tumor cells, indicating that normal cells share a comparable capacity of adaptation to fluctuating microenvironmental challenges. We further observed a uniform regulation of go versus grow cellular programs. The finding that hypoxia increased the migration of epithelial cancer cell types supports the notion that hypoxia acts as a major driver of cancer progression by provoking acquisition of invasive and metastatic properties.16 The observation that migration of non-neoplastic cells prevalent in adult brain (including astrocytes, MSCs, fibroblasts, and mononuclear cells) was also accelerated by hypoxia indicates that enhanced movement of stromal cells governed by hypoxic tumor gradients may foster mutual stimulatory interactions between tumor and stromal cells in glioblastomas.
Notably, coupling of functional and metabolic regulation is also evident in glioblastoma tissue in situ. Immunohistochemical analyses showed that the expression of glycolysis enzymes is strongly increased in pseudopalisading cells, whereas PPP enzyme expression is reduced.7 Pseudopalisading cells most likely represent a wave of tumor cells that actively migrate away from a severely hypoxic necrotic area arising after a microvascular insult.17 In contrast, expression of PPP enzymes is elevated in highly proliferative tumor regions where expression of glycolysis enzymes is comparatively low. This distribution pattern indicates that the PPP, which supplies ribose-5-phosphate and NADPH for biosynthetic processes, is elevated in rapidly proliferating cells but suppressed under acute severe hypoxic stress, favoring glycolysis to protect cells against hypoxic damage.
Apart from hypoxia, a variety of other parameters can regulate the dichotomous balance between proliferative versus migratory functional programs including extracellular matrix components, growth factors, microRNAs, and transcription factors.1,18–21 Therefore, we investigated whether metabolic enzyme expression and functional cellular behavior are also coupled without hypoxic challenging. Expression of PPP enzymes was elevated in rapidly dividing cells, whereas glycolysis enzymes were diminished. Conversely, glycolysis enzymes were elevated in rapidly migrating cells while PPP enzymes were diminished, indicating that coupling between functional and metabolic states is independent of changes in oxygen tension and that the metabolic state is intrinsically linked to the dichotomous activation of go or grow cellular programs. While the importance of the PPP for rapid cell proliferation is rather obvious since the PPP generates building bricks and reductive power to fuel anabolic processes, the association between glycolysis and cell migration is less straightforward to explain. It has been shown that glycolytic enzymes are enriched in pseudopodia and that glycolytic energy rather than oxidative phosphorylation is the primary energy source for cancer cell motility and cytoskeletal rearrangement.22–24 Furthermore, lactate can stimulate tumor cell migration and invasion.25 Interestingly, several glycolytic enzymes exhibit additional noncanonical functions, some of which are related to migration. For example, glucose 6-phosphate isomerase (GPI) is identical to the secreted cytokine autocrine motility factor (AMF) which binds to a cell surface receptor, AMFR, and stimulates migration in an autocrine and paracrine fashion.26 The multifaceted “moonlighting” functions of glycolysis enzymes are only partly elucidated, and further work is necessary to better characterize their involvement in cancer cell function.
To determine whether glycolysis and the PPP are causatively involved in the dichotomous regulation of migration versus proliferation, we used enzyme inhibitors and shRNA. 6-AN reduced glioblastoma cell proliferation while stimulating migration, whereas 2-DG reduced both proliferation and migration, indicating that interference at the enzymatic branching point that supports both pathways affects both associated functional states. To substantiate these observations, expression of ALDOC and G6PD were knocked down. G6PD knockdown resulted in reduced glioblastoma cell proliferation, whereas knockdown of ALDOC decreased cell migration. In GS-11 cells, decreased migration achieved with ALDOC knockdown was accompanied by increased proliferation, and reduced proliferation of G6PD knockdown cells was accompanied by increased migration. These findings support the results of the enzyme inhibitor experiments and provide evidence that glycolysis and the PPP are mechanistically relevant for the dynamic regulation of migration versus proliferation.
In vivo, knockdown of ALDOC and G6PD had opposite effects on the growth of G55-derived tumors. Whereas mice engrafted with G6PD knockdown cells survived longer, animals with ALDOC knockdown tumors died sooner than controls. G55 cells exhibit a very high proliferation rate in vitro and in vivo and form rapidly expanding, noninvasive nodular tumors in the mouse brain. Hence, G55 tumors probably rely greatly on the PPP for sustained rapid growth, while the preparatory phase of glycolysis may be less important. This is supported by the finding that the Ki-67 rate was reduced in shG6PD tumors but increased in shALDOC tumors. In vitro, enhanced proliferation after ALDOC knockdown occurred only in GS11 cells but not in G55 cells, indicating that the shift towards proliferation depends on cell type as well as microenvironmental conditions. In the GS-11 model, no significant differences in total tumor burden were detected between ALDOC or G6PD knockdown tumors and controls, although proliferation was slightly increased in shALDOC tumors and decreased in shG6PD tumors. Naturally, the invasive growth of GS-11 cells in vivo requires both cell migration and proliferation, and it is impossible to differentiate with the currently available histological techniques to what degree distant tissue infiltration is due to actual tumor cell migration/invasion or rather to continuous infiltrative growth of the proliferating tumor. Considering the opposite in vitro effects of ALDOC and G6PD knockdown on migration and proliferation, the impairment of either function alone is obviously not sufficient to affect the invasive tumor growth of GS-11 cells in vivo.
In conclusion, our findings indicate that inhibition of G6PD and the PPP could be a useful strategy for targeting highly proliferative tumor cells in glioblastoma. In contrast, ALDOC, as the most strongly hypoxia-induced glycolytic enzyme, may rather be important for securing cell survival under severe hypoxic stress and for cell migration and thus may be a useful target for inhibiting invasion. Ideally, both pathways should be blocked simultaneously to target both the highly proliferative as well as the invasive tumor component; this might be achieved by inhibiting HK2, for example by using inorganic phosphate or azole antifungal agents as reported recently by others.26,27 HK2 catalyzes an irreversible reaction essential for both glycolysis as well as the PPP. It is strongly upregulated in tumor cells and has additional antiapoptotic moonlighting functions adding to its attractiveness as a therapeutic metabolic target. Future studies will show whether inhibition of HK2 can effectively inhibit glioblastoma growth in vivo.27,28
Supplementary Material
Supplementary material is available online at Neuro-Oncology (http://neuro-oncology.oxfordjournals.org/).
Funding
This work was supported by the Anni Hofmann Stiftung (KL, CHM, RG), the Deutsche Forschungsgemeinschaft (LA1300/4-1), the Forschungsförderungsfonds des Universitätsklinikums Hamburg-Eppendorf (AK), and the Rudolf Bartling Stiftung (KL).
Supplementary Material
Acknowledgments
We thank Svenja Zapf, Katharina Kolbe, and Regina Peters for expert technical assistance and the FACS core facility of the UKE for help with cell sorting.
Conflict of interest statement. None declared.
References
- 1. Giese A, Bjerkvig R, Berens ME, Westphal M. Cost of migration: invasion of malignant gliomas and implications for treatment. J Clin Oncol. 2003;21 (8):1624–1636. [DOI] [PubMed] [Google Scholar]
- 2. Keunen O, Johansson M, Oudin A et al. Anti-VEGF treatment reduces blood supply and increases tumor cell invasion in glioblastoma. Proc Natl Acad Sci USA. 2011;108 (9):3749–3754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kunkel P, Ulbricht U, Bohlen P et al. Inhibition of glioma angiogenesis and growth in vivo by systemic treatment with a monoclonal antibody against vascular endothelial growth factor receptor-2. Cancer Res. 2001;61 (18):6624–6628. [PubMed] [Google Scholar]
- 4. Fack F, Espedal H, Keunen O et al. Bevacizumab treatment induces metabolic adaptation toward anaerobic metabolism in glioblastomas. Acta Neuropathol. 2015;129 (1):115–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Griguer CE, Oliva CR, Gobin E et al. CD133 is a marker of bioenergetic stress in human glioma. PLoS One. 2008;3 (11):e3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Heddleston JM, Li Z, McLendon RE, Hjelmeland AB, Rich JN. The hypoxic microenvironment maintains glioblastoma stem cells and promotes reprogramming towards a cancer stem cell phenotype. Cell Cycle. 2009;8 (20):3274–3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kathagen A, Schulte A, Balcke G et al. Hypoxia and oxygenation induce a metabolic switch between pentose phosphate pathway and glycolysis in glioma stem-like cells. Acta Neuropathol. 2013;126 (5):763–780. [DOI] [PubMed] [Google Scholar]
- 8. Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324 (5930):1029–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schulte A, Gunther HS, Phillips HS et al. A distinct subset of glioma cell lines with stem cell-like properties reflects the transcriptional phenotype of glioblastomas and overexpresses CXCR4 as therapeutic target. Glia. 2011;59 (4):590–602. [DOI] [PubMed] [Google Scholar]
- 10. Kasten U, Plottner N, Johansen J, Overgaard J, Dikomey E. Ku70/80 gene expression and DNA-dependent protein kinase (DNA-PK) activity do not correlate with double-strand break (dsb) repair capacity and cellular radiosensitivity in normal human fibroblasts. Br J Cancer. 1999;79 (7–8):1037–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lamszus K, Schmidt NO, Ergun S, Westphal M. Isolation and culture of human neuromicrovascular endothelial cells for the study of angiogenesis in vitro. J Neurosci Res. 1999;55 (3):370–381. [DOI] [PubMed] [Google Scholar]
- 12. Martens T, Schmidt NO, Eckerich C et al. A novel one-armed anti-c-Met antibody inhibits glioblastoma growth in vivo. Clin Cancer Res. 2006;12 (20 Pt 1):6144–6152. [DOI] [PubMed] [Google Scholar]
- 13. Martens T, Laabs Y, Gunther HS et al. Inhibition of glioblastoma growth in a highly invasive nude mouse model can be achieved by targeting epidermal growth factor receptor but not vascular endothelial growth factor receptor-2. Clin Cancer Res. 2008;14 (17):5447–5458. [DOI] [PubMed] [Google Scholar]
- 14. Zamykal M, Martens T, Matschke J et al. Inhibition of intracerebral glioblastoma growth by targeting the insulin-like growth factor 1 receptor involves different context-dependent mechanisms. Neuro Oncol. 2015;17 (8):1076–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Scatena R, Bottoni P, Pontoglio A, Mastrototaro L, Giardina B. Glycolytic enzyme inhibitors in cancer treatment. Expert Opin Investig Drugs. 2008;17 (10):1533–1545. [DOI] [PubMed] [Google Scholar]
- 16. Semenza GL. The hypoxic tumor microenvironment: A driving force for breast cancer progression. Biochim Biophys Acta. 2016;1863 (3):382–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brat DJ, Castellano-Sanchez AA, Hunter SB et al. Pseudopalisades in glioblastoma are hypoxic, express extracellular matrix proteases, and are formed by an actively migrating cell population. Cancer Res. 2004;64 (3):920–927. [DOI] [PubMed] [Google Scholar]
- 18. Ghosh P, Beas AO, Bornheimer SJ et al. A G{alpha}i-GIV molecular complex binds epidermal growth factor receptor and determines whether cells migrate or proliferate. Mol Biol Cell. 2010;21 (13):2338–2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Godlewski J, Bronisz A, Nowicki MO, Chiocca EA, Lawler S. microRNA-451: A conditional switch controlling glioma cell proliferation and migration. Cell Cycle. 2010;9 (14):2742–2748. [PubMed] [Google Scholar]
- 20. Horing E, Harter PN, Seznec J et al. The “go or grow” potential of gliomas is linked to the neuropeptide processing enzyme carboxypeptidase E and mediated by metabolic stress. Acta Neuropathol. 2012;124 (1):83–97. [DOI] [PubMed] [Google Scholar]
- 21. Wang SD, Rath P, Lal B et al. EphB2 receptor controls proliferation/migration dichotomy of glioblastoma by interacting with focal adhesion kinase. Oncogene. 2012;31 (50):5132–5143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Beckner ME, Chen X, An J, Day BW, Pollack IF. Proteomic characterization of harvested pseudopodia with differential gel electrophoresis and specific antibodies. Lab Invest. 2005;85 (3):316–327. [DOI] [PubMed] [Google Scholar]
- 23. Beckner ME, Stracke ML, Liotta LA, Schiffmann E. Glycolysis as primary energy source in tumor cell chemotaxis. J Natl Cancer Inst. 1990;82 (23):1836–1840. [DOI] [PubMed] [Google Scholar]
- 24. Shiraishi T, Verdone JE, Huang J et al. Glycolysis is the primary bioenergetic pathway for cell motility and cytoskeletal remodeling in human prostate and breast cancer cells. Oncotarget. 2015;6 (1):130–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Goetze K, Walenta S, Ksiazkiewicz M, Kunz-Schughart LA, Mueller-Klieser W. Lactate enhances motility of tumor cells and inhibits monocyte migration and cytokine release. Int J Oncol. 2011;39 (2):453–463. [DOI] [PubMed] [Google Scholar]
- 26. Fairbank M, St-Pierre P, Nabi IR. The complex biology of autocrine motility factor/phosphoglucose isomerase (AMF/PGI) and its receptor, the gp78/AMFR E3 ubiquitin ligase. Mol Biosyst. 2009;5 (8):793–801. [DOI] [PubMed] [Google Scholar]
- 27. Agnihotri S, Vartanian A, Burrell K et al. Identification of the azole class of antifungals as potent inhibitors of hexokinase II mediated tumour metaboism in glioblastoma. Neuro Oncol. 2014; 16 (suppl 5):v213. [Google Scholar]
- 28. Patra KC, Wang Q, Bhaskar PT et al. Hexokinase 2 is required for tumor initiation and maintenance and its systemic deletion is therapeutic in mouse models of cancer. Cancer Cell. 2013;24 (2):213–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.