Abstract
Background
Higher-grade meningiomas (HGMs; World Health Organization grades II and III) pose a clinical problem due to high recurrence rates and the absence of effective therapy. Preclinical development of novel therapeutics requires a disease model that recapitulates the genotype and phenotype of patient HGM. Oncolytic herpes simplex virus (oHSV) has shown efficacy and safety in cancers in preclinical and clinical studies, but its utility for HGM has not been well characterized.
Methods
Tumorsphere cultures and serial orthotopic xenografting in immunodeficient mice were used to establish a patient-derived HGM model. The model was pathologically and molecularly characterized by immunohistochemistry, western blot, and genomic DNA sequencing and compared with the patient tumor. Anti-HGM effects of oHSV G47Δ were assessed using cell viability and virus replication assays in vitro and animal survival analysis following intralesional injections of G47Δ.
Results
We established a serially transplantable orthotopic malignant meningioma model, MN3, which was lethal within 3 months after tumorsphere implantation. MN3 xenografts exhibited the pathological hallmarks of malignant meningioma such as high Ki67 and vimentin expression. Both the patient tumor and xenografts were negative for neurofibromin 2 (merlin) and had the identical NF2 mutation. Oncolytic HSV G47Δ efficiently spread and killed MN3 cells, as well as other patient-derived HGM lines in vitro. Treatment with G47Δ significantly extended the survival of mice bearing subdural MN3 tumors.
Conclusions
We established a new patient-derived meningioma model that will enable the study of targeted therapeutic approaches for HGM. Based on these studies, it is reasonable to consider a clinical trial of G47Δ for HGM.
Keywords: higher-grade meningioma, HGM, NF2, orthotopic tumor model, oHSV, patient-derived xenografts
Meningiomas are the most common primary tumor arising within the central nervous system.1 Nearly 80% of meningiomas are benign and classified as World Health Organization (WHO) grade I, and most of these can be cured by surgery alone. However, 20% of the tumors fall in the category of higher-grade meningiomas (HGMs), which are WHO grade II (atypical, which include the rare chordoid and clear cell histological variants) and WHO grade III (malignant or anaplastic, which include the papillary and rhabdoid histological variants).2–4 Tumor relapse poses a significant clinical challenge, as 40% of grade II and up to 80% of grade III relapse after surgery and radiation within 5 years.2,3 At recurrence, HGM is frequently refractory to additional surgery and radiotherapy. Outcomes of HGM are currently unfavorable, with 5-year survival rates of 72% and 32% for grades II and III, respectively.2,3 Clinical investigations testing systemic chemotherapies and molecular targeted therapies in HGM have not improved clinical outcomes.5,6 The development of effective therapies for HGM is needed.
A critical obstacle hindering progress in meningioma research and preclinical development of novel HGM therapies is the lack of reliable and clinically relevant preclinical models.7,8 Previously reported meningioma models relied on primary, early passage cell lines that typically undergo cell senescence after a few passages.9 Only a few human meningioma-derived cell lines have been widely used to generate orthotopic growth of tumors in mice. The Ben-Men-1 meningioma cell line is immortalized with human telomerase reverse transcriptase (hTERT) transduction and creates small nonfatal xenografts10; however, such transgene-driven tumorigenesis may not be representative of most recurrent tumors. IOMM-Lee is a human malignant meningioma cell line and exhibits extremely aggressive behavior in vivo, with median mice survival after intracranial engrafting of only 12–19 days.7,11,12 IOMM-Lee has a complex karyotype likely due to long-term culture,8 potentially limiting its generalizability for studying disease-specific biology and novel treatments. Recent genomic analysis studies revealed that 40%–50% of meningiomas harbor mutations in NF2 and that activating alterations in AKT and SMO were exclusively present in NF2 wild-type meningiomas.13,14 Identification of these clinically actionable genetic drivers highlights the need of preclinical models that preserve patient-specific genotypes.
Oncolytic viruses (OVs) are emerging cancer therapeutics that have potential utility for a wide variety of cancer types. OV is replication competent, either genetically engineered or naturally occurring, and selectively replicates in tumor cells, causing cell death.15 Oncolytic herpes simplex virus type 1 (oHSV) represents one of the most extensively studied OVs. For clinical translation, oHSV has undergone genetic engineering to reduce neurovirulence and confer specificity toward tumor cells.16 G47Δ is a newer-generation oHSV that lacks the neurovirulence gene γ34.5 and has a lacZ gene insertion inactivating the UL39 gene, required for viral growth in nondividing cells.17 An additional deletion within α47 in G47Δ allows increased major histocompatibility complex class I expression and early expression of Us11, enhancing the growth of γ34.5 mutants.17 The efficacy and safety of G47Δ have been demonstrated in a number of cancer types in animal models, including those based on glioblastoma stem cells.18–22 G47Δ is currently being evaluated in an early-phase clinical trial for recurrent glioblastoma23 (http://apps.who.int/trialsearch/Trial2.aspx?TrialID=JPRN-UMIN000002661), and promising anecdotal results have been reported (9th International Oncolytic Virus Therapeutics Conference, Boston, June 13–16, 2015). The therapeutic efficacy of G47Δ in orthotopic meningioma has not been explored, although our group showed anti-meningioma effects of another oHSV, G207, in an orthotopic meningioma model generated with tissue fragment implantation.24 Because meningiomas are known to have intercellular gap junctions,25 and because HSV can transfer via cell-to-cell contact,26 we hypothesized that oHSV would be an effective therapeutic for these otherwise treatment-refractory tumors.
In this work we report the establishment and characterization of a new patient-derived malignant meningioma xenograft model, MN3. Orthotopic engraftment of MN3 cells cultured as tumorspheres generates tumors in mice that mimic the phenotype of the human disease and are lethal and serially transplantable. This unique HGM model is an ideal tool for a better understanding of meningioma biology, as well as for testing new therapeutic approaches in a clinically representative setting. Using the MN3 model, we investigated the therapeutic activity of oHSV G47Δ to develop an effective treatment for meningioma. We demonstrate significant efficacy of G47Δ in the MN3 HGM model both in vitro and in vivo.
Materials and Methods
Isolation and Culture of MN3 and Other Meningioma Cells
Fresh tumor specimen was obtained at a surgery of a recurrent malignant meningioma (MN3) in a sporadic patient (without neurofibromatosis). The tissue was minced into small pieces and digested in 0.1% trypsin (Invitrogen) and DNase (Sigma) for 40 min at 37°C. After washes in Hank's Balanced Salt Solution (Gibco), cells were cultured in Neurobasal medium (Invitrogen) supplemented with 1× B27 (Gibco), 0.5× N2 (Gibco), heparin (2 µg/mL; Sigma), l-glutamine (3 mM; Cellgro), 0.5× penicillin/streptomycin/amphotericin B complex (Cellgro), epidermal growth factor (20 ng/mL; R and D Systems), fibroblast growth factor 2 (20 ng/mL; Peprotech), and platelet-derived growth factor BB (15 ng/mL; Peprotech). The cultures were fed every 3 days with one-third volume of fresh medium. Culture passage was performed by dissociation of cell spheres by TrypLE Express (Invitrogen). The patient passed away 12 months after the surgery due to the progression of disseminated meningioma. The same isolation and culture methods were applied to subsequently obtained patient meningioma specimens: MN5 (WHO grade II), MN6 (grade I), and MN7 (grade III). In contrast, MN465 (grade I, merlin positive), MN475 (grade II, merlin positive), MN298 (grade III, merlin positive),27 and MN1 (grade III) were primary cultured human meningioma lines grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 15% fetal calf serum. These procedures were performed in accordance with institutional review board–approved protocols.
Orthotopic Xenografting
Seven- to eight-week-old female severe combined immunodeficient (SCID) mice were purchased from the National Cancer Institute. Serum-cultured MN1 (passage 1) or MN298 (passage 7) cells (300 000 cells) or tumorsphere MN3 (50 000 cells) or MN5 or MN6 (150 000 cells) were suspended in 3–5 µL DMEM and stereotactically implanted into the frontal subdural region (2.5 mm lateral from bregma and 1.0 mm deep from the skull) of SCID mice under anesthesia with intraperitoneal pentobarbital. Mice were monitored regularly and euthanized when they developed significant neurologic signs or poor general conditions (>15% body weight loss). Tumors were excised sterilely and subjected to digestion and culture as described above and to molecular analysis. Cells were serially implanted into new animals after brief (24–48 h) culture.
Immunohistochemistry
Formalin-fixed tumor samples were embedded in paraffin, and 7-µm-thick sections were obtained. Hematoxylin and eosin (H&E) staining was performed using standard procedures. Immunohistochemistry was performed using the Vectastain Elite Kit (Vector Laboratories) as described previously.22,28 Primary antibodies used included: anti-Ki67 (MIB-1; Dako), Vimentin (Dako), epithelial membrane antigen (Dako), and nestin (Santa Cruz Biotechnology). Signals were visualized with diaminobenzidine (Dako). Hematoxylin was used to counterstain nuclei. Antigen retrieval with microwave treatment in 10 mmol/L sodium citrate buffer (pH 6) was used for all staining. All specimens were examined under a microscope (Nikon) equipped with a digital camera (RT color SPOT) connected to SPOT imaging software (Diagnostic Instruments).
Immunocytochemistry
Dissociated cells were cytospun onto slides and fixed with 4% paraformaldehyde and 70% ethanol. After blocking with 10% goat serum (Vector), cells were incubated with anti-vimentin antibody, followed by incubation with secondary antibody, Alexa Fluor 546 goat anti-mouse immunoglobulin G (Invitrogen).
Western Blot
Tumor tissues and cells were lysed in radioimmunoprecipitation assay buffer (Boston Bioproducts) with protease and phosphatase inhibitors (Roche Diagnostics). Protein concentrations were determined by modified Bradford assay (Bio-Rad). Protein (20 µg) was separated on a sodium dodecyl sulfate polyacrylamide gel; transferred to polyvinylidene difluoride membranes (Bio-Rad); blocked with 5% nonfat dry milk in 20 mM Tris-buffered saline (pH 7.5), 150 mM NaCl, 0.1% Tween 20; and incubated with primary antibodies at 4°C overnight. Primary antibodies were against NF2 (merlin; provided by V.R.)27 and vinculin (Thermo). Membranes were washed and incubated with horseradish peroxidase–conjugated secondary antibodies (1:5000, anti-mouse or anti-rabbit; Promega). Signals were visualized with an electrochemiluminescence kit (Amersham Bioscience or Bio-Rad) on Kodak films.
Whole Exome Sequencing
As previously described,13 150 bp insert libraries were prepared by Covaris sonication, followed by solid phase reversible immobilization size-selection (Agencourt AMPure XP beads) and ligation to molecular barcoded adaptors for multiplexed analysis. Exome hybrid capture was performed using the Agilent Sure Select All Exon v2.0 hybrid capture kit as described previously.29
Genomic Polymerase Chain Reaction–Based Sequencing
Genomic PCR-based sequencing was used to probe the alterations identified in NF2, DTNB, MSLNL, and CARM1 by whole exome sequencing, as well as to detect mutations in the promoter of TERT. DNA was extracted from the blood and original tumor of the patient and an MN3 xenograft (fourth generation). PCR products were amplified from genomic DNA templates with Platinum Taq polymerase per manufacturer's protocol using intron-based primers spanning the coding sequences. PCR products were then Sanger sequenced (Beckman Coulter Genomics).
Oncolytic Herpes Simplex Viruses
G207 lacks γ34.5 and has a lacZ insertion inactivating ICP6.30 G47Δ lacks γ34.5 and α47 and has a lacZ insertion inactivating ICP6.17 MG18L contains deletion of the Us3 gene and a lacZ insertion inactivating ICP6.31 G47Δ-mCherry and G47Δ-Us11-fluc are recombinant oHSVs derived from a bacterial artificial chromosome plasmid carrying the G47Δ genome. G47Δ-mCherry expresses mCherry driven by immediate early 4/5 HSV promoter.32 G47Δ-Us11-fluc expresses firefly luciferase driven by the late Us11 HSV promoter.33 Viruses were grown, purified, and titered on Vero (African green monkey kidney) cells (ATCC).
Virus Yield Assay
Meningioma cells were plated at 8000 cells/well in 48-well plates. The next day, cells were infected with G207 or G47Δ at multiplicity of infection (MOI) = 1.25 in triplicate, and 24 h and 48 h later cells and media were harvested for plaque assay on Vero cells.
In vitro Studies with Oncolytic Herpes Simplex Viruses
To measure cytotoxicity of oHSV, cells were plated into 96-well plates at 4000–5000 cells per well. The next day the cells were treated with oHSV at indicated doses. Ninety-six hours after infection, we performed cell viability assays by MTS (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium). To measure late virus gene expression, cells seeded in 96-well plates were infected with the luciferase reporter oHSV G47Δ-Us11-fluc. Luciferase activity was assayed by adding D-Luciferin (Gold Bio) to cells to 2 mM, followed by detection of bioluminescence with a microplate reader (Synergy-HT Bio-Tek).
In vivo Studies with Oncolytic Herpes Simplex Virus G47Δ
To determine the therapeutic efficacy of G47Δ for orthotopic MN3 meningioma, 12 SCID mice were stereotactically implanted with MN3 cells (75 000 cells in 3 µL) as described above, and then randomized into 2 groups. One group was treated with 2 G47Δ injections (2 × 106 plaque-forming units [pfu] in 3 µL) intratumorally at days 14 and 25 post tumor cell implantation, and the second group received intratumoral phosphate buffered saline (3 µL) injections. Mice were monitored daily and euthanized when they developed significant neurologic deficits or lost more than 15% of their starting body weight.
To assess the efficiency of G47Δ infection in vivo, orthotopic MN3 xenografts were injected with a single inoculation of G47Δ (1 × 106 pfu in 3 µL). Twenty-four hours later, mice were euthanized with cardiac perfusion of 4% paraformaldehyde. X-gal staining was performed on cryosections (7 µm thick) of the brains22 to detect G47Δ-infected cells. The institutional animal care and use committee approved all the animal procedures.
Statistical Analysis
Student's t-test (2-tailed) was used to analyze differences between the 2 groups. Kaplan–Meier analysis and log-rank tests were used to analyze overall survival of mice receiving different treatments.
Results
Establishing a New Patient-Derived Malignant Meningioma Model
Our initial attempt to establish tumorigenic HGM lines involved engraftment of malignant meningioma cells primarily cultured in 15% fetal calf serum. Implantation of 300 000 MN298 cells led to no tumor formation after 8-month follow-up (data not shown). Implantation of 300 000 MN1 cells generated a small multilayered nodular lesion at the subdural space, which was composed of vimentin-positive meningothelial tumor cells after 3 months (Supplementary Fig. S1). However, the lesion did not progress to a sizable mass or cause symptoms, and we were unable to establish serial transplantation to new mice.
We reasoned that tumorsphere culture in serum-free medium supplemented with mitogenic growth factors would promote enrichment of meningioma cells with stemlike features and tumorigenic potential, as was the case with glioblastoma and other brain tumors.22,34 A surgical specimen of a recurrent malignant meningioma (MN3) was digested and subjected to sphere culture conditions. Cell spheres or aggregates resembling glioblastoma tumorspheres formed within 2 days (Fig. 1A). Emergence of similar cell spheres/aggregates was also observed in 3 other patient meningioma specimens, 1 benign (WHO grade I, case MN6), 1 atypical (grade II, MN5), and 1 malignant (grade III, MN7) (Supplementary Fig. S2).
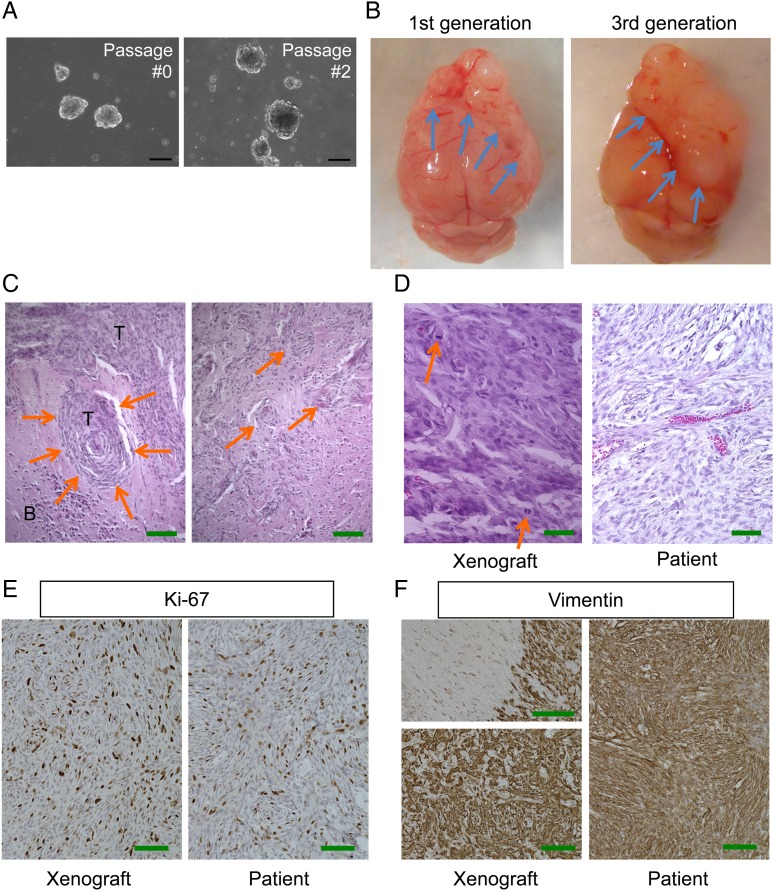
Fig. 1.
Pathological characterization of patient-derived malignant meningioma model MN3. (A) Microscopic pictures of MN3 tumorspheres cultured at passages 0 and 2. Scale bars, 100 µm. (B) Macroscopic appearance of lethal subdurally transplanted MN3 tumors located at the frontal region of mouse brains. Left, first generation. Right, third generation. Arrows point to brain-tumor boundaries. (C) H&E staining of MN3 xenografts showing whorl formation by tumor cells (left, arrows) and irregular tongue-like protrusions indicative of invasion to the brain (right, arrows). T, tumor; B, brain. Scale bars, 200 µm. (D) Higher magnification pictures of H&E-stained MN3 xenografts (left) and patient tumor sections (right), showing histological similarities between the two. Arrows show mitoses. (E and F) Immunohistochemistry for Ki67 (MIB-1) and vimentin, showing high proliferative activity and mesenchymal protein expression, respectively, in the matched patient (right) and xenograft (left). Left panels in (F) show a tumor–brain interface with negative staining in the brain parenchyma on the left (upper panel) and homogeneous staining at the tumor core (lower panel). Scale bars in (D)–(F), 100 µm.
Implantation of briefly (48 h) cultured MN3 cells (50 000 cells) into the frontal subdural region of the brain of SCID mice resulted in body weight loss and decreased activity of the mice after 10–12 weeks, which necessitated euthanasia. Autopsy revealed a large (6 mm), elastic hard mass covering and compressing both frontal lobes (Fig. 1B). Staining by H&E revealed fibroblastic meningioma with densely cellular foci and focal “whorl formations” by spindle-shaped tumor cells (Fig. 1C). At higher magnification, both the patient tumor and xenografts contained cells with nuclear atypia and high nuclear/cytoplasmic ratio (Fig. 1D). In xenografts, the interface with adjacent brain showed irregular tongue-like tumor protrusions (Fig. 1C), consistent with brain invasion, and scattered mitotic figures (Fig. 1D), features of HGMs. Leptomeningeal dissemination and metastasis to distant organs were not observed. Xenografts, after excision and 24–48 h sphere culture, were serially transplantable to new SCID mice (currently up to the sixth generation). We did not observe noticeable alterations in tumor phenotypes such as tumor growth patterns and aggressiveness in subsequent generations (Fig. 1B), highlighting the stable and stem cell nature of the model. Unlike MN3, implantation of MN5 (WHO grade II) and MN6 (grade I) did not initiate tumor formation in SCID mice after the follow-up of 8 months.
Orthotopic MN3 Meningioma Xenografts Recapitulate the Phenotypic Features of the Patient Tumor
We further characterized the phenotypes of the MN3 model, comparing it with the original patient tumor. Immunohistochemistry for the cell proliferation marker Ki67 showed high expression in both patient and xenograft tumor tissue (Fig. 1E), with the average staining indices of 27% and 44% in the patient and xenografts, respectively. In both the patient and xenografts, virtually all tumor cells were strongly immunopositive for vimentin, a mesenchymal cell marker (Fig. 1F), along with positive vimentin staining of cultured MN3 cells by immunofluorescence (Supplementary Fig. S3A). Immunohistochemistry showed lack of expression of epithelial membrane antigen in both patient tumor and xenografts, suggesting a similar histological phenotype of the tumors (Supplementary Fig. S3B). Intriguingly, there was a strong diffuse staining of nestin, a neural stem/progenitor cell marker, in the xenograft tissue, whereas it was mostly confined to vascular endothelial cells in the primary tumor tissue (Supplementary Fig. S3C).
The MN3 Xenografts Retain the NF2 Mutation the Patient Tumor Harbored
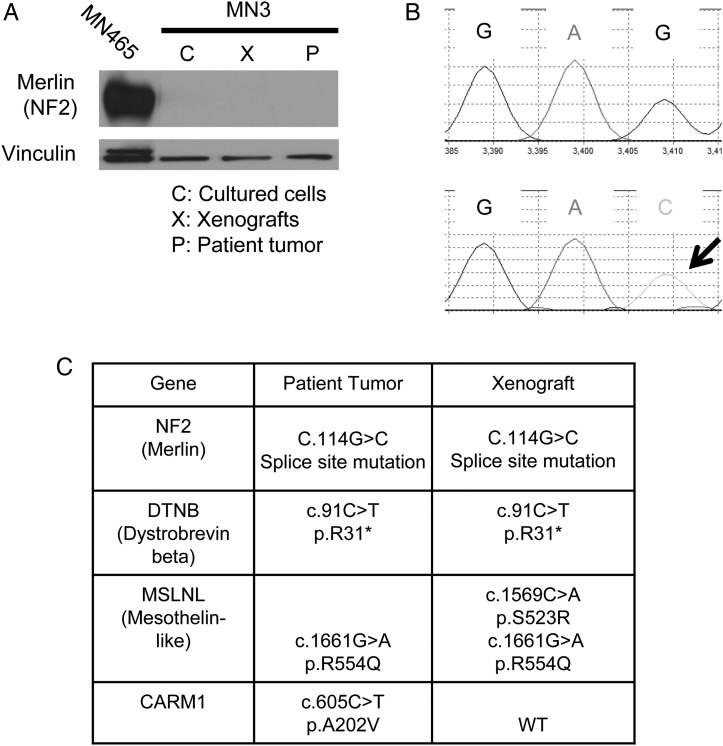
The tumor suppressor gene NF2 is mutated in about 50% of meningiomas, including HGM, which plays a role in meningioma pathogenesis.2,3,13,14 Western blot analysis of the MN3 patient and xenograft tissue as well as cultured MN3 cells revealed that all of these lacked expression of NF2 (merlin) (Fig. 2A). Whole exome sequencing of the patient tumor DNA identified a homozygous c.114G >C point mutation at the splice site between exons 1 and 2 of NF2, a somatic mutation predicted to disrupt mRNA processing (Supplementary Table S1), and we confirmed the identical mutation in the MN3 xenografts by genomic PCR-based sequencing of NF2 (Fig. 2B). Thus, the MN3 model maintained the disrupted NF2 status of the patient meningioma. In addition to NF2, whole exome sequencing of the patient tumor revealed 3 genetic mutations that have been previously reported as potentially cancer associated at COSMIC (Catalogue of Somatic Mutations in Cancer, http://cancer.sanger.ac.uk/cosmic): a nonsense mutation in DTNB (dystrobrevin beta; found in ovarian cancer) and missense mutations in MSLNL (mesothelin-like; p.R554W found in gastric cancer) and CARM1 (found in glioma, lung cancer, and melanoma) (Fig. 2C, Supplementary Table S1). Focused sequencing of xenograft DNA identified the same mutations in DTNB and MSLNL, along with an additional mutation in MSLNL, but not in CARM1. TERT promoter mutations have been identified in a subset of HGM35 and meningiomas that undergo malignant progression.36 Sequencing of the MN3 patient tumor and xenograft revealed that neither has any TERT promoter mutations (data not shown).
Fig. 2.
Molecular characterization of the MN3 meningioma model. (A) Western blot for the NF2 product merlin in the patient tumor, xenografts, and cultured cells. MN465 cells were used as a positive control for merlin. Vinculin was used as a loading control. Irrelevant lanes between the lanes of MN465 and MN3 were removed during image processing of the blot. (B) Genomic PCR-based sequencing showing G to C alteration at C.114 in NF2 in the xenograft DNA. (C) Summary of mutation status of NF2, DTNB, MSLNL, and CARM1 in the patient tumor (whole exome sequencing) and xenografts (fourth generation, focused sequencing). WT, wild type.
Taken together, we have developed a new, patient-derived orthotopic malignant meningioma model, MN3, which is serially transplantable and lethal and mimics the original patient tumor both phenotypically and genetically.
Oncolytic Herpes Simplex Virus G47Δ Infects, Spreads, and Kills MN3 Cells In vitro
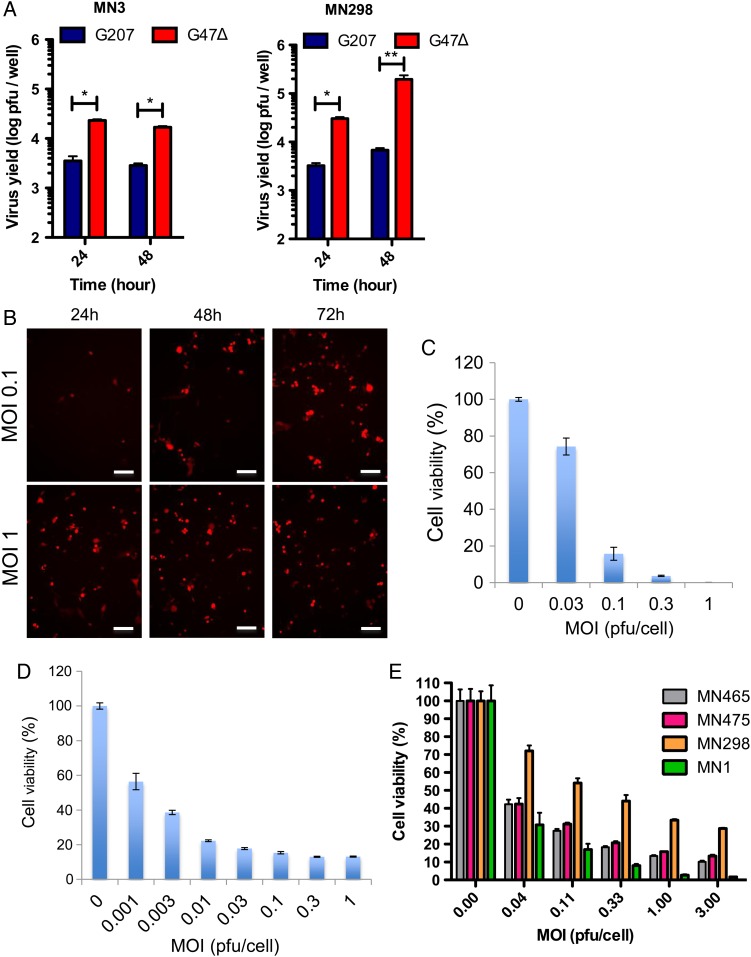
We previously reported therapeutic effects of multimutated oHSV G207 in a preclinical model of meningioma.24 G207, however, has limitations in its replication potential and potency against cancer stem cells.22 G47Δ shows a comparable safety profile to G207 upon brain inoculation in HSV-permissive hosts,17 demonstrated preclinical potency against a number of cancer models including cancer stem cells,18,19,21,22,37 and has been under clinical evaluation for malignant gliomas and prostate cancer.16,23 Therefore, we investigated the therapeutic activity of G207 and G47Δ in our meningioma models. Single-step virus growth assay in MN3 cells revealed that G47Δ was able to replicate, while G207 yield did not surpass the input (Fig. 3A). In monolayer cultured MN298 cells, G47Δ exhibited much more robust amplification compared with G207 (Fig. 3A). MN3 infection with G47Δ-Us11-fluc, in which the Us11 late gene promoter drives firefly luciferase expression, showed that luciferase activity was dose dependent at 24 h post-infection (p.i.) and increased from 24 to 48 h p.i. at a low MOI of 0.1, indicative of virus replication (Supplementary Fig. S4A). Infection of MN3 cells with G47Δ-mCherry, which expresses the mCherry reporter protein under the immediate early 4/5 promoter, resulted in dose-dependent infection at 24 h p.i. and virus spread from 24 to 72 hours p.i. at MOI = 0.1 (Fig. 3B, expression of mCherry in red). Treatment of MN3 cells with G47Δ showed efficient and dose-dependent cell killing, with half-maximal inhibitory concentration at MOI = 0.06 (Fig. 3C). MG18L, which has a different genetic background than G47Δ,31 had comparable potency in vitro (Supplementary Fig. S4B). Thus, MN3 cells are permissive to oHSV replication and killing.
Fig. 3.
In vitro efficacy of oHSV G47Δ for MN3 and other patient-derived meningioma cells. (A) Virus yield assay in MN3 cells (left, in stem cell medium) and MN298 cells (right, 10% fetal calf serum medium). Cells were infected with G207 or G47Δ at MOI = 1.25, and virus yield was measured at 24 and 48 h post-infection. Input = 10 000 pfu. *P < .0001, **P < .005. (B) Fluorescent microscopic pictures showing spread of G47Δ-mCherry (red) in MN3 cells. MN3 cells were infected at MOI 0.1 and 1, and microscopic pictures were captured at 24, 48, and 72 h p.i. Scale bars, 100 µm. (C and D) MTS cell viability assay showing the cytotoxic activity of G47Δ against MN3 cells (C) and MN7 (D). (E) MTS cell viability assay showing the cytotoxic activity of G47Δ-mCherry against different serum-cultured meningioma primary cultures in vitro. In (C–E), MTS assay was done at day 4 post oHSV infection.
We further tested the efficacy of G47Δ in other patient-derived meningioma cell lines. G47Δ efficiently spread and killed MN7 malignant meningioma tumorsphere cells that we isolated from a different patient (Supplementary Fig. S4C, Fig. 3D). In addition, several serum-cultured primary human meningioma cells showed sensitivity to G47Δ-mediated killing (Fig. 3E). These results thus show that oHSV G47Δ is very cytotoxic against meningiomas with different malignancy grades and molecular backgrounds at relatively low MOI.
G47Δ Significantly Prolonged Survival of Mice Bearing Orthotopic MN3 Meningiomas
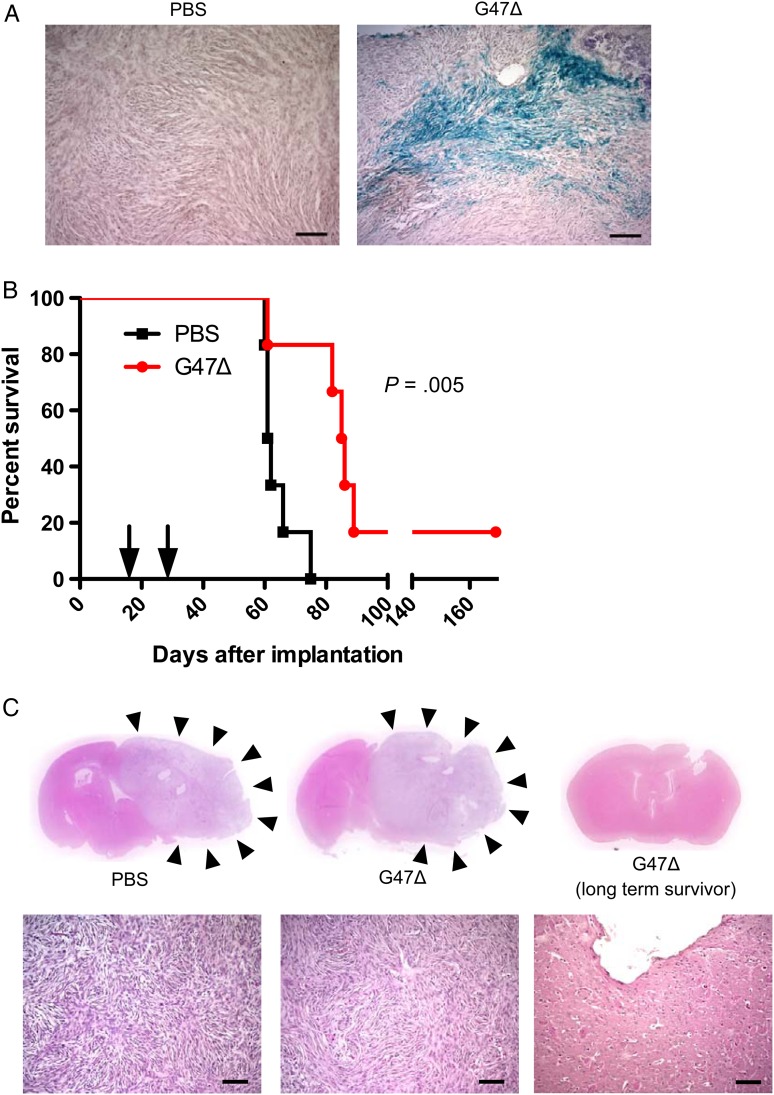
We next explored the therapeutic potential of G47Δ in the MN3-derived orthotopic meningioma model. A single injection of G47Δ into an established large tumor mediated efficient infection of tumor cells within the tumor tissue (Fig. 4A). Finally, we sought to determine whether G47Δ exerts therapeutic efficacy in the MN3 model in vivo. Two intratumoral injections of G47Δ significantly prolonged survival of mice bearing subdural MN3 meningioma, compared with the control group (P = .005, log-rank test; Fig. 4B). All animals that were euthanized due to neurologic or systemic symptoms had large intracranial tumors (Fig. 4C). One animal in the G47Δ group lived long-term without exhibiting symptoms and was euthanized on day 169. In this animal, we found a localized defect of the brain tissue near the implantation site and no evidence of tumor (Fig. 4C). There were no signs of adverse effects such as encephalitis associated with G47Δ, confirming the safety of the treatment.
Fig. 4.
In vivo efficacy of oHSV G47Δ in orthotopic MN3 xenografts. (A) X-gal staining of MN3 xenografts 24 h after injection of phosphate buffered saline (PBS, left) or G47Δ (1 × 106 pfu/3 µL, right). Scale bars, 100 µm. (B) Kaplan–Meier analysis showing significant extension of mouse survival after 2 intratumoral injections of G47Δ (2 × 106 pfu/3 µL each) vs PBS (n = 6/group) in the orthotopic MN3 xenograft model. Arrows show the times when G47Δ or PBS was injected (days 14 and 25 after tumor cell implantation). One animal in the G47Δ group survived long without exhibiting symptoms and was euthanized on day 169. (C) H&E staining of brain sections (upper panels) and microscopic images (lower panels) prepared from representative animals in the survival study shown as (B). Left, PBS treated, and middle, G47Δ treated, show growth of large and lethal tumors (pointed to by arrowheads), while right, G47Δ treated, reveals a small tissue defect near the implantation site with no sign of tumor.
Discussion
Establishment of a stable and reproducible preclinical model of HGM is key to facilitating the development and testing of novel therapeutic approaches. Here, we describe a new patient-derived orthotopic model of malignant meningioma, MN3. Previously, generation of orthotopic subdural tumors from cultured cells isolated from patient meningiomas with different malignancy grades has been reported.7 However, establishing cell lines or xenografts as a reproducible resource that is available to the research community has been difficult. Widely used traditional models include Ben-Men-110 and IOMM-Lee,7,11,12 but exogenous TERT transduction and nonlethal in vivo growth of the former and the complex karyotype and extreme in vivo aggressiveness of the latter reduce the clinical relevance of these meningioma models.
The MN3 model has a number of characteristics that make it unique. (i) Subdural engraftment of a relatively small number of genetically unaltered cells (50 000 cells) creates intracranial tumors exhibiting the histopathological features of “malignant meningioma” and expressing a standard meningioma marker, vimentin. (ii) Xenografts are serially transplantable and reproducibly lethal within 2–3 months. (iii) The model recapitulates the molecular driver mutations, which were found in the meningioma from which the model was derived. (iv) Tumorsphere cultures enable a variety of in vitro cell-based assays (eg, screens for effective therapeutics). Therefore, MN3 is ideally suited for studies that seek to address disease-specific molecular biological questions as well as for development of novel targeted agents.
In an attempt to maintain stem-like tumorigenic properties and patient-specific genetic abnormalities, we applied the culture method that we optimized to establish patient-derived glioblastoma stem-like cells.22,28 Culture in serum, particularly for the long term, is known to alter the status of cancer-driving genetic mutations and karyotypes of the original tumor.38 This was the case in a primary culture of meningioma recently reported that exhibited accelerated cell proliferation along with marked changes in cytogenetics and telomerase activity after extended passage in 10% fetal bovine serum.39 We avoided long-term culture even without serum, and employed serial passaging via orthotopic xenografts in combination with short-term tumorsphere culture. Although meningioma stem cells have been isolated from patients,40,41 it is still not clear whether cellular hierarchy exists in meningiomas and whether certain markers accurately define meningioma stem cells. Nestin is expressed in developing meninges during a limited period,42 and we unexpectedly found upregulated expression of nestin in MN3 xenografts compared with the patient tumor. Although this may imply a stem-like phenotype of the model, further study is necessary to determine whether our MN3 cells meet the criteria of cancer stem cells and are suitable for studies on cancer stem cell biology.
The majority of sporadic meningiomas exhibit inactivation of the NF2 gene, supporting the vital role of its tumor suppressor product, merlin, in meningioma pathogenesis.43 Studies employing next-generation sequencing revealed that meningiomas comprise genomically diverse groups of tumors and are primarily categorized into 2 subtypes based on NF2 status.13,14 The MN3 model has disrupted NF2 with no detectable merlin expression and the same splice site point mutation as the original tumor. This validates the maintenance of molecular drivers in the tumor from which the model was derived and supports the utility of the model to seek molecularly targeted strategies. Merlin is a multifunctional protein and regulates a number of cancer-promoting signaling pathways, including those of phosphatidylinositol-3 kinase–Akt–mammalian target of rapamycin,44,45 Ras–Raf–extracellular signal-regulated kinase,46 and focal adhesion kinase.47 The MN3 model therefore should serve as a prototypic model for systematic investigations to identify therapeutic targets of NF2-disrupted meningioma. Additional models with different genetic backgrounds would improve our ability to identify targeted therapies, and we are continuing our effort to build a set of patient-derived meningioma models.
Antitumor potency of G47Δ in a variety of tumor models, including benign NF2-mutated schwannomas21,48 and cancer stem cell models,22,37 increases its translational potential. We demonstrate promising therapeutic effects of oHSV G47Δ in the MN3 HGM model both in vitro and in vivo. MN3 cells are permissive to G47Δ infection, as shown by significant virus replication and spread. In the past, a few publications explored OV therapy in preclinical meningioma models testing oncolytic adenovirus49 and oHSV,9,24,50 with the only OV study in an animal model published in 1995.24 In this work, Yazaki et al showed that the serum-cultured human malignant meningioma cell lines F5, GPSM4, and GPSM5 were permissive to G207 (Δγ34.5, ICP6–), a parental oHSV from which G47Δ was derived. In a subdural tumor model established in athymic mice with an F5 tumor fragment, intratumoral injections of 5 × 106 pfu G207 increased survival of mice compared with mock treatment. Unfortunately, reliable orthotopic tumor formation with F5 requires engraftment of subcutaneous tumor tissue fragments, while in our MN3 model 50 000–75 000 cells are sufficient to induce 100% tumor take. In the current study, G47Δ demonstrated much greater virus replication than G207 in cultured meningioma cells. We further show in vitro G47Δ efficacy against several patient-derived meningioma lines of different grade, supporting its broad utility. These studies support consideration of a clinical trial of G47Δ for refractory HGM.
Supplementary Material
Funding
This work was supported by grants from the National Institutes of Health (R01NS032677 to R.L.M. and R01CA160762 to S.D.R.), Burroughs Wellcome Career Award 1007616.02 (to D.P.C.), and NF Inc, MA (to V.R.).
Supplementary Material
Acknowledgments
Conflict of interest statement. None declared.
References
- 1. Ostrom QT, Gittleman H, Liao P et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2007–2011. Neuro Oncol. 2014;16(suppl 4):iv1–i63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Choy W, Kim W, Nagasawa D et al. The molecular genetics and tumor pathogenesis of meningiomas and the future directions of meningioma treatments. Neurosurg Focus. 2011;30 (5):E6. [DOI] [PubMed] [Google Scholar]
- 3. Riemenschneider MJ, Perry A, Reifenberger G. Histological classification and molecular genetics of meningiomas. Lancet Neurol. 2006;5 (12):1045–1054. [DOI] [PubMed] [Google Scholar]
- 4. Mawrin C, Perry A. Pathological classification and molecular genetics of meningiomas. J Neurooncol. 2010;99 (3):379–391. [DOI] [PubMed] [Google Scholar]
- 5. Preusser M, Berghoff AS, Hottinger AF. High-grade meningiomas: new avenues for drug treatment? Curr Opin Neurol. 2013;26 (6):708–715. [DOI] [PubMed] [Google Scholar]
- 6. Wen PY, Quant E, Drappatz J et al. Medical therapies for meningiomas. J Neurooncol. 2010;99 (3):365–378. [DOI] [PubMed] [Google Scholar]
- 7. McCutcheon IE, Friend KE, Gerdes TM et al. Intracranial injection of human meningioma cells in athymic mice: an orthotopic model for meningioma growth. J Neurosurg. 2000;92 (2):306–314. [DOI] [PubMed] [Google Scholar]
- 8. Ragel BT, Couldwell WT, Gillespie DL et al. A comparison of the cell lines used in meningioma research. Surg Neurol. 2008;70 (3):295–307; discussion 307. [DOI] [PubMed] [Google Scholar]
- 9. Markert JM, Coen DM, Malick A et al. Expanded spectrum of viral therapy in the treatment of nervous system tumors. J Neurosurg. 1992;77 (4):590–594. [DOI] [PubMed] [Google Scholar]
- 10. Puttmann S, Senner V, Braune S et al. Establishment of a benign meningioma cell line by hTERT-mediated immortalization. Lab Invest. 2005;85 (9):1163–1171. [DOI] [PubMed] [Google Scholar]
- 11. Lee WH. Characterization of a newly established malignant meningioma cell line of the human brain: IOMM-Lee. Neurosurgery. 1990;27 (3):389–395; discussion 396. [PubMed] [Google Scholar]
- 12. Wilisch-Neumann A, Kliese N, Pachow D et al. The integrin inhibitor cilengitide affects meningioma cell motility and invasion. Clin Cancer Res. 2013;19 (19):5402–5412. [DOI] [PubMed] [Google Scholar]
- 13. Brastianos PK, Horowitz PM, Santagata S et al. Genomic sequencing of meningiomas identifies oncogenic SMO and AKT1 mutations. Nat Genet. 2013;45 (3):285–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Clark VE, Erson-Omay EZ, Serin A et al. Genomic analysis of non-NF2 meningiomas reveals mutations in TRAF7, KLF4, AKT1, and SMO. Science. 2013;339 (6123):1077–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Russell SJ, Peng KW, Bell JC. Oncolytic virotherapy. Nat Biotechnol. 2012;30 (7):658–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ning J, Wakimoto H. Oncolytic herpes simplex virus–based strategies: toward a breakthrough in glioblastoma therapy. Front Microbiol. 2014;5:303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Todo T, Martuza RL, Rabkin SD et al. Oncolytic herpes simplex virus vector with enhanced MHC class I presentation and tumor cell killing. Proc Natl Acad Sci U S A. 2001;98 (11):6396–6401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Antoszczyk S, Spyra M, Mautner VF et al. Treatment of orthotopic malignant peripheral nerve sheath tumors with oncolytic herpes simplex virus. Neuro Oncol. 2014;16 (8):1057–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fukuhara H, Martuza RL, Rabkin SD et al. Oncolytic herpes simplex virus vector g47delta in combination with androgen ablation for the treatment of human prostate adenocarcinoma. Clin Cancer Res. 2005;11 (21):7886–7890. [DOI] [PubMed] [Google Scholar]
- 20. Liu R, Martuza RL, Rabkin SD. Intracarotid delivery of oncolytic HSV vector G47delta to metastatic breast cancer in the brain. Gene Ther. 2005;12 (8):647–654. [DOI] [PubMed] [Google Scholar]
- 21. Prabhakar S, Messerli SM, Stemmer-Rachamimov AO et al. Treatment of implantable NF2 schwannoma tumor models with oncolytic herpes simplex virus G47delta. Cancer Gene Ther. 2007;14 (5):460–467. [DOI] [PubMed] [Google Scholar]
- 22. Wakimoto H, Kesari S, Farrell CJ et al. Human glioblastoma-derived cancer stem cells: establishment of invasive glioma models and treatment with oncolytic herpes simplex virus vectors. Cancer Res. 2009;69 (8):3472–3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Todo T. Active immunotherapy: oncolytic virus therapy using HSV-1. Adv Exp Med Biol. 2012;746:178–186. [DOI] [PubMed] [Google Scholar]
- 24. Yazaki T, Manz HJ, Rabkin SD et al. Treatment of human malignant meningiomas by G207, a replication-competent multimutated herpes simplex virus 1. Cancer Res. 1995;55 (21):4752–4756. [PubMed] [Google Scholar]
- 25. Arishima H, Sato K, Kubota T. Immunohistochemical and ultrastructural study of gap junction proteins connexin26 and 43 in human arachnoid villi and meningeal tumors. J Neuropathol Exp Neurol. 2002;61 (12):1048–1055. [DOI] [PubMed] [Google Scholar]
- 26. Mateo M, Generous A, Sinn PL et al. Connections matter—how viruses use cell-cell adhesion components. J Cell Sci. 2015;128 (3):431–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. James MF, Lelke JM, Maccollin M et al. Modeling NF2 with human arachnoidal and meningioma cell culture systems: NF2 silencing reflects the benign character of tumor growth. Neurobiol Dis. 2008;29 (2):278–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wakimoto H, Mohapatra G, Kanai R et al. Maintenance of primary tumor phenotype and genotype in glioblastoma stem cells. Neuro Oncol. 2012;14 (2):132–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fisher S, Barry A, Abreu J et al. A scalable, fully automated process for construction of sequence-ready human exome targeted capture libraries. Genome Biol. 2011;12 (1):R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mineta T, Rabkin SD, Yazaki T et al. Attenuated multi-mutated herpes simplex virus-1 for the treatment of malignant gliomas. Nat Med. 1995;1 (9):938–943. [DOI] [PubMed] [Google Scholar]
- 31. Kanai R, Wakimoto H, Martuza RL et al. A novel oncolytic herpes simplex virus that synergizes with phosphoinositide 3-kinase/Akt pathway inhibitors to target glioblastoma stem cells. Clin Cancer Res. 2011;17 (11):3686–3696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cheema TA, Wakimoto H, Fecci PE et al. Multifaceted oncolytic virus therapy for glioblastoma in an immunocompetent cancer stem cell model. Proc Natl Acad Sci U S A. 2013;110 (29):12006–12011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sgubin D, Wakimoto H, Kanai R et al. Oncolytic herpes simplex virus counteracts the hypoxia-induced modulation of glioblastoma stem-like cells. Stem Cells Transl Med. 2012;1 (4):322–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Singh SK, Clarke ID, Terasaki M et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63 (18):5821–5828. [PubMed] [Google Scholar]
- 35. Koelsche C, Sahm F, Capper D et al. Distribution of TERT promoter mutations in pediatric and adult tumors of the nervous system. Acta Neuropathol. 2013;126 (6):907–915. [DOI] [PubMed] [Google Scholar]
- 36. Goutagny S, Nault JC, Mallet M et al. High incidence of activating TERT promoter mutations in meningiomas undergoing malignant progression. Brain Pathol. 2014;24 (2):184–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Li J, Zeng W, Huang Y et al. Treatment of breast cancer stem cells with oncolytic herpes simplex virus. Cancer Gene Ther. 2012;19 (10):707–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lee J, Kotliarova S, Kotliarov Y et al. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell. 2006;9 (5):391–403. [DOI] [PubMed] [Google Scholar]
- 39. Michelhaugh SK, Guastella AR, Varadarajan K et al. Development of patient-derived xenograft models from a spontaneously immortal low-grade meningioma cell line, KCI-MENG1. J Transl Med. 2015;13:227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hueng DY, Sytwu HK, Huang SM et al. Isolation and characterization of tumor stem-like cells from human meningiomas. J Neurooncol. 2011;104 (1):45–53. [DOI] [PubMed] [Google Scholar]
- 41. Rath P, Miller DC, Litofsky NS et al. Isolation and characterization of a population of stem-like progenitor cells from an atypical meningioma. Exp Mol Pathol. 2011;90 (2):179–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Petricevic J, Forempoher G, Ostojic L et al. Expression of nestin, mesothelin and epithelial membrane antigen (EMA) in developing and adult human meninges and meningiomas. Acta Histochem. 2011;113 (7):703–711. [DOI] [PubMed] [Google Scholar]
- 43. Lomas J, Bello MJ, Arjona D et al. Genetic and epigenetic alteration of the NF2 gene in sporadic meningiomas. Genes Chromosomes Cancer. 2005;42 (3):314–319. [DOI] [PubMed] [Google Scholar]
- 44. James MF, Han S, Polizzano C et al. NF2/merlin is a novel negative regulator of mTOR complex 1, and activation of mTORC1 is associated with meningioma and schwannoma growth. Mol Cell Biol. 2009;29 (15):4250–4261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. James MF, Stivison E, Beauchamp R et al. Regulation of mTOR complex 2 signaling in neurofibromatosis 2–deficient target cell types. Mol Cancer Res. 2012;10 (5):649–659. [DOI] [PubMed] [Google Scholar]
- 46. Lim JY, Kim H, Kim YH et al. Merlin suppresses the SRE-dependent transcription by inhibiting the activation of Ras-ERK pathway. Biochem Biophys Res Commun. 2003;302 (2):238–245. [DOI] [PubMed] [Google Scholar]
- 47. Shapiro IM, Kolev VN, Vidal CM et al. Merlin deficiency predicts FAK inhibitor sensitivity: a synthetic lethal relationship. Sci Transl Med. 2014;6 (237):237ra268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Messerli SM, Prabhakar S, Tang Y et al. Treatment of schwannomas with an oncolytic recombinant herpes simplex virus in murine models of neurofibromatosis type 2. Hum Gene Ther. 2006;17 (1):20–30. [DOI] [PubMed] [Google Scholar]
- 49. Grill J, Lamfers ML, van Beusechem VW et al. Oncolytic virotherapy of meningiomas in vitro with replication-competent adenovirus. Neurosurgery. 2005;56 (1):146–153; discussion 153–154. [DOI] [PubMed] [Google Scholar]
- 50. Liu TC, Zhang T, Fukuhara H et al. Dominant-negative fibroblast growth factor receptor expression enhances antitumoral potency of oncolytic herpes simplex virus in neural tumors. Clin Cancer Res. 2006;12 (22):6791–6799. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.