Abstract
Background
Few prospective studies have assessed the role of bevacizumab and included a control arm with standard treatments for recurrent glioblastoma. We conducted a noncomparative phase II trial (AVAREG) to examine the efficacy of bevacizumab or fotemustine in this setting.
Methods
Eligible patients were randomized 2:1 to receive bevacizumab (10 mg/kg every 2 weeks) or fotemustine (75 mg/m2 on days 1, 8, and 15, then 100 mg/m2 every 3 weeks after a 35-day interval). The primary endpoint was 6-month overall survival (OS) rate (OS-6). No formal efficacy comparison was made between the treatment arms.
Results
Ninety-one patients were enrolled (bevacizumab n = 59; fotemustine n = 32). Median age was 57 years (range, 28–78 y), and patients had Eastern Cooperative Oncology Group performance status of 0 (n = 42), 1 (n = 35), or 2 (n = 14). OS-6 rate was 62.1% (95% confidence interval [CI], 48.4–74.5) with bevacizumab and 73.3% (95% CI, 54.1–87.7) with fotemustine. OS-6 rates were lower in bevacizumab-treated patients with MGMT promoter methylated tumors than in those with unmethylated tumors (50% and 85%, respectively), but higher in fotemustine-treated patients (87.5% and 50%, respectively). OS rates at 9 months were 37.9% (95% CI, 25.5–51.6) and 46.7% (95% CI, 28.3–65.7) with bevacizumab and fotemustine, respectively, and median OS was 7.3 months (95% CI, 5.8–9.2) and 8.7 months (95% CI, 6.3–15.4), respectively. Toxicity was as expected with the 2 agents.
Conclusion
Single-agent bevacizumab may have a role in patients with recurrent glioblastoma.
Keywords: AVAREG, bevacizumab, fotemustine, glioblastoma, overall survival
In Europe, the annual incidence rate of primary CNS tumors is ∼5/100 000.1 Using WHO classification, glioblastoma is the most common malignant CNS tumor.2 Effective glioblastoma treatment remains a challenge in oncology since time to progression after first-line therapy remains short. In recurrent glioblastoma, there are limited therapeutic options, and current disease control is disappointing; a meta-analysis of phase II trials reported that the proportion of patients without progressive disease (PD) at 6 months was only 15%.3
Nitrosoureas are commonly used treatments for recurrent glioblastoma.4–6 In Italy, the nitrosourea fotemustine has demonstrated a 6-month progression-free survival (PFS) rate (PFS-6) of 21% and a disease control rate of 42% in recurrent glioblastoma.7 Bevacizumab, a monoclonal antibody that inhibits vascular endothelial growth factor-A, was the first antiangiogenic therapy to be approved in oncology. Bevacizumab has important benefits for patients with glioblastoma (eg, the reduction of peritumoral edema that leads to reduced corticosteroid use) but is also associated with significant morbidity and side effects.8 In recurrent glioblastoma, bevacizumab has shown promising activity; a phase II study of bevacizumab, alone or in combination with irinotecan, reported objective response rates of 28.2% and 37.8%, and PFS-6 rates of 42.6% and 50.3%, respectively.9 These findings led to the provisional FDA approval of bevacizumab for recurrent glioblastoma in 2009. Recently, a randomized phase II study (BELOB) of single-agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine for patients with recurrent glioblastoma demonstrated that the combination met prespecified criteria for assessment in further phase III studies.10 Bevacizumab also had similar survival rates and PFS outcomes to single-agent lomustine monotherapy.10
Recently, the results of the randomized phase III trial, which compared the combination of bevacizumab and lomustine with lomustine alone (EORTC 26101), did not confirm the BELOB data, showing that the combination arm was not superior to lomustine alone.11
We report the results of a phase II, randomized trial of single-agent bevacizumab or fotemustine for recurrent glioblastoma. To avoid potential imaging biases due to vessel permeability alteration affecting PD assessment, the primary endpoint was 6-month overall survival (OS) rate (OS-6). In an attempt to improve disease assessment, we evaluated tumor response with both classical Macdonald criteria12 and the Response Assessment in Neuro-Oncology (RANO) criteria.13
Methods
Study Design
AVAREG (EudraCT: 2011-001363-46; NCT01474239) was a phase II, open-label, noncomparative, multicenter, randomized study. Patients with recurrent glioblastoma following first-line radiotherapy plus temozolomide (RT/TMZ) therapy were randomized in a 2:1 ratio to receive either bevacizumab or fotemustine. Patients were stratified by age (≤55 y vs >55 y)14 and previous surgery for recurrence (yes vs no). All patients provided written informed consent. Approval was obtained from institutional ethics committees of the participating centers.
Patients
Enrolled patients were (i) aged ≥18 years with histologically confirmed glioblastoma (WHO classification) and (ii) first recurrence of glioblastoma following standard front-line RT/TMZ.15 Additional inclusion criteria were: (iii) progression of documented disease as defined by RANO criteria at least 12 weeks after completion of RT/TMZ, unless the recurrence was outside the radiation field or was histologically documented, (iv) measurable disease by RANO criteria (bidimensional contrast-enhancing lesions with clearly defined margins by MRI, with 2 perpendicular diameters ≥10 mm visible on ≥2 axial slices); (v) WHO performance status (PS) 0–2; (vi) use of stable/decreasing corticosteroids within 7 days prior to randomization; (vii) and adequate hematologic, hepatic, and renal function. (viii) Full-dose anticoagulants were allowed if the patient’s international normalized ratio or activated partial thromboplastin time was within therapeutic limits (according to institutional guidelines) and he/she was on a stable dose of anticoagulants for at least 2 weeks before randomization (per the American Society of Clinical Oncology guidelines, low molecular weight heparins were preferred).
Patients could have surgery for disease recurrence; residual/measurable disease was not required to determine recurrence, but surgery must have confirmed recurrence. An MRI scan within 48 hours of surgery and ≥28 days after surgery was required prior to administration of study drugs (baseline).
A local pathology report constituted adequate documentation for study inclusion. Tissue was collected following randomization and sent within 3 months for independent central review (ICR) to confirm diagnosis; samples were mandatory for study inclusion.
Patients were excluded if they had (i) prior antiangiogenic therapy for glioblastoma or MRI evidence of recent brain hemorrhage. Other exclusion criteria were (ii) history of clinically significant cardiovascular disease; (iii) history of pulmonary embolism/cerebral hemorrhage; (iv) uncontrolled hypertension or (v) an unhealed surgical wound.
Treatment
Patients received bevacizumab (10 mg/kg i.v.) every 2 weeks. Dose modification was not allowed; if necessary due to adverse events (AEs), treatment was discontinued or interrupted until the AE was resolved. Maximum allowable bevacizumab interruption was 42 days.
Fotemustine 75 mg/m2 i.v. was administered on days 1, 8, and 15 (induction phase); after a 35-day break, patients received fotemustine 100 mg/m2 i.v. every 3 weeks (maintenance phase). For patients randomized to the fotemustine arm, therapy was administered if platelets and granulocyte counts were >100 000/mm3 and >2000/mm3, respectively, according to the Italian fotemustine label. In case of hematologic toxicity, dose modification was permitted.
If platelet counts were >100 000/mm3 and granulocyte counts were >2000/mm3, 100% of the fotemustine dose was administered; if the platelet count was 100 000 ≥ n > 80 000/mm3 and/or the granulocyte count 2000 ≥ n > 1500/mm3, the fotemustine dose was administered at 75%. If granulocytes were 1500 ≥ n > 1000/mm3, the fotemustine dose was administered at 50%; if platelets were <80 000/mm3 and/or granulocytes were <1000/mm3, fotemustine was delayed.
Efficacy
Primary efficacy analyses were based on the intent-to-treat (ITT) population, which included all randomized patients with at least one administration of study drug. The primary endpoint was defined as the proportion of patients who were alive at 6 months (OS-6) after the start of treatment. This was calculated based on the total number of patients who did not die within 6 months after the start of treatment (out of the total of evaluable patients). Evaluable patients were defined as those observed for at least 6 months from the start of treatment or patients who died within 6 months of the start of treatment.
For the time-to-event analysis, OS was defined as the time in months from the start of treatment to the date of death due to any cause. The date of first drug infusion was used as the start date; if a patient was not known to have died, time was censored at the last date the patient was known to be alive. Secondary endpoints included OS; PFS-6; OS rate at 9 months (OS-9); OS rate at 12 months (OS-12); OS-6 by age (≤55 y or >55 y), and by previous surgery for recurrence (yes vs no). Since fotemustine was only a balancing arm, no formal efficacy comparison was made between the treatment arms.
Quality of Life
Quality of life (QoL) assessments, including the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire C30 (EORTC QLQ-C30),16 were carried out at the time of tumor assessments (at screening/baseline, 46 ± 3 days after the first administration of study drug, and after 56 ± 3 days until PD).
Safety
The safety population included all enrolled patients who received the study drug. AEs were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events v 4.0.
Assessments
Tumor response was evaluated according to RANO criteria. Although RANO criteria do not establish a PD cutoff point for the detection of nonenhancing lesions by T2/fluid-attenuated inversion recovery (FLAIR) sequences, based on previous clinical experience, a ≥25% increase in nonenhancing lesions in T2/FLAIR was assumed to be PD. The first tumor assessment was performed 46 ± 3 days following the first study drug administration. If there was uncertainty regarding PD, patients continued treatment, and a confirmatory MRI was performed after 4 weeks. Treatment was stopped if PD was confirmed, and the date at which PD was first noted was documented as the PD date. All MRI scans were collected for ICR. Disease assessment was also performed using Macdonald criteria to assess variations in different evaluation criteria. ICR for histologic diagnosis was performed according to the WHO 2007 classification criteria on hematoxylin and eosin-stained full sections.
Statistical Analysis
The OS-6 rate for bevacizumab7 was compared with the expected proportion used for sample size calculation with application of the exact binomial test. Statistical significance was assessed with a 1-sided α error of 10%. The 1-tailed statistical hypotheses were p0 ≤ 0.60 (null hypothesis) vs pA >0.77 (alternative hypothesis), where p is the estimated probability of survival at 6 months. The proportion was provided with the exact 95% CI computed using the exact binomial method (Clopper-Pearson). Efficacy analyses were performed at a 1-sided α = 0.10 level of significance. With a power of 0.90 and β = 0.10, 50 patients were required for the bevacizumab arm; ≥34 observed patients were required to prove that the outcome was positive (OS-6 ≥ 77%). Due to the randomization ratio of 2:1, 25 patients were needed for the balancing fotemustine arm. Taking into account an expected dropout rate of ∼17%, the number of required patients was increased to 60 (bevacizumab) and 30 (fotemustine). Kaplan–Meier methodology was used to determine OS-6 and 95% CI. Cox proportional hazard model was evaluated for exploratory OS analyses: covariates included age, previous surgery for recurrence, sex, and Eastern Cooperative Oncology Group (ECOG) PS.
The Cox proportional hazard model corrected by site and treatment arm was used to assess the association of demographic characteristics and clinical features (ie, age class, PS, previous surgery for recurrence) with OS and PFS. The results, expressed as hazard ratios (HRs) with 95% CI, were reported for each factor. The WHO PS was considered as the PS parameter to be included in this model. All values of the Karnofsky performance scale were properly converted.
MGMT Methylation Assessment and IDH1/2 Mutations
As retrospective analyses, we determined O6-methylguanine-DNA methyltransferase (MGMT) methylation status, using a methylation-specific PCR method,17 and isocitrate dehydrogenase 1/2 (IDH1/2) mutation status in patient tumor samples.18
Results
Baseline Characteristics
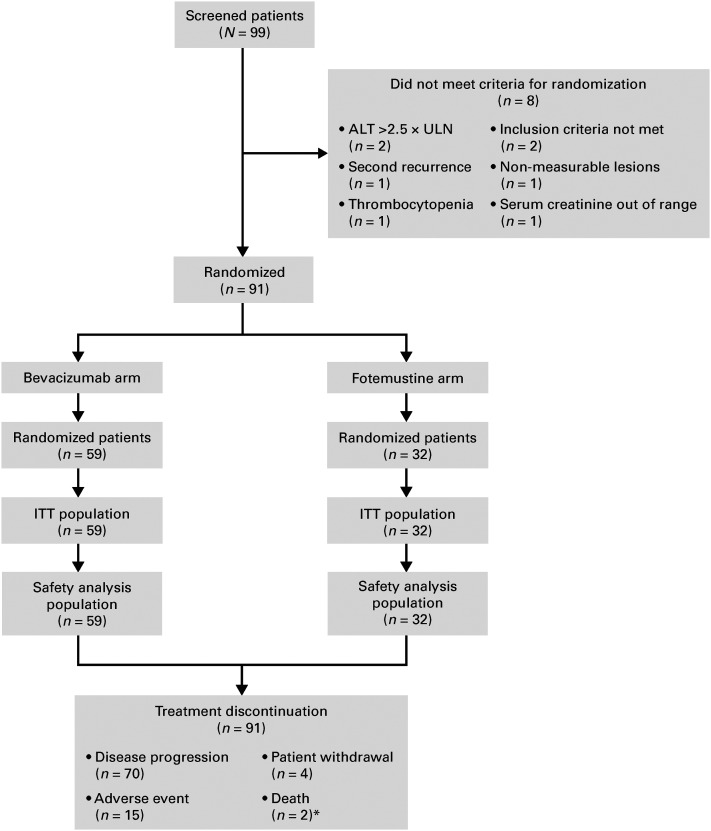
Between November 2011 and September 2012, 91 patients with recurrent glioblastoma were enrolled at 10 sites in Italy. Patients were randomly assigned to receive bevacizumab (n = 59) or fotemustine (n = 32) (Fig. 1). Clinical characteristics are reported in Table 1. Twenty-one patients underwent surgical resection before study inclusion, 13 (22%) in the bevacizumab arm and 8 (25%) in the fotemustine arm. No significant heterogeneity was found between treatment arms for age (P = .79), ECOG PS (P = .24), corticosteroid use (P = .39), or T1-weighted tumor size (P = .4). Time from diagnosis to MRI at screening was 331 days in the bevacizumab arm and 462 days in the fotemustine arm (P = .07).
Fig. 1.
CONSORT diagram.: Abbreviations: ALT, alanine aminotransferase; ITT, intent-to-treat; ULN, upper limit of normal. *, One patient had a serious adverse event classified as pneumonitis, to which the investigator attributed death as it was the cause of treatment discontinuation.
Table 1.
Patient characteristics and demographics
| Characteristic | Bevacizumab (n = 59) | Fotemustine (n = 32) |
|---|---|---|
| Median age, years, (range) | 59 (37–74) | 56 (28–78) |
| Sex, n (%) | ||
| Male | 39 (66%) | 23 (72%) |
| Female | 20 (34%) | 9 (28%) |
| ECOG PS, n (%) | ||
| 0 | 29 (49%) | 13 (41%) |
| 1 | 19 (32%) | 16 (50%) |
| 2 | 11 (19%) | 3 (9%) |
| Median days from diagnosis to MRI at screening (range) | 331 (163–2271) | 462 (162–1383) |
| Corticosteroids at baseline, n (%) | 42 (71%) | 20 (62%) |
| Total of product of diameters at baseline (range), mm2 | ||
| T1 | 1024 (147–7746) | 757 (110–3309) |
| T2/FLAIR | 4193 (266–18 100) | 4131 (77–25 480) |
| Re-surgery before study entry, n (%) | 13 (22%) | 8 (25%) |
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group performance status; FLAIR, fluid-attenuated inversion recovery.
Site-determined histologic diagnosis of glioblastoma was subsequently confirmed by ICR in 83% (bevacizumab arm) and 72% (fotemustine arm) of patients. Histologic samples were insufficient for ICR in 7% (bevacizumab arm) and 12% (fotemustine arm) of cases.
In the bevacizumab arm, 6 patients (10%) at ICR were diagnosed as anaplastic gliomas (n = 4), anaplastic oligodendroglioma (n = 1), and probably GBM but tissue was insufficient o confirm a specific diagnosis (n = 1). In the fotemustine arm, 5 patients (16%) at ICR were diagnosed as anaplastic astrocytomas (n = 2), anaplastic gliomas (n = 2), and malignancy glioma with necrosis but with scarce tissue to define a certain diagnosis (n = 1).
All 91 patients in the ITT population were analyzed for efficacy. At the time of this analysis, all patients had discontinued treatment; the reasons are reported in Fig. 1.
Steroid Use
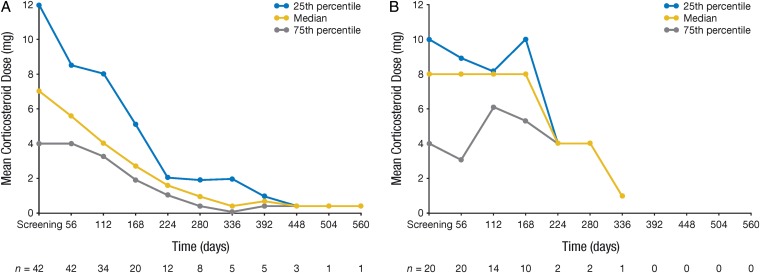
Steroid analysis took into account only the 62 patients (68.13%) in the ITT population who reported information regarding dexamethasone assumption at screening (42 in the bevacizumab group and 20 in the fotemustine group). In the bevacizumab arm after 8 weeks of treatment, most patients (59.62%) reported no change in dexamethasone dose, while 23.08% reported a dosage decrease. Only 17.31% of patients had an increase in dexamethasone therapy >2 mg.
The dexamethasone mean dose was calculated as total dose of drug taken by the patient during each 56-day period divided by the number of completed days in each period. Patients taking dexamethasone at baseline received decreasing doses over time in the bevacizumab arm (Fig. 2A); this trend was less evident for patients randomized to the fotemustine arm (Fig. 2B).
Fig. 2.
Change in corticosteroid dose over time for patients treated with (A) bevacizumab (n = 42) and (B) fotemustine (n = 20).
Overall Survival (Intention toTreat Population)
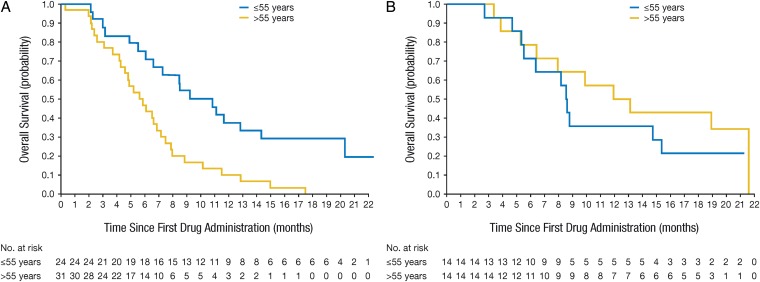
The estimated OS-6 rates were 62.1% (95% CI, 48.4–74.5) in the bevacizumab arm and 73.3% (95% CI, 54.1–87.7) in the fotemustine arm. The OS-9 rates were 37.9% (95% CI, 25.5–51.6) and 46.7% (95% CI, 28.3–65.7), and the OS-12 rates were 25.9% (95% CI, 15.3–39.0) and 40.0% (95% CI, 22.7–59.4) with bevacizumab and fotemustine, respectively. Median OS was 7.3 months (95% CI, 5.8–9.2) with bevacizumab and 8.7 months (95% CI, 6.3–15.4) with fotemustine. When analyzed by age, OS-6 and OS-9 rates were numerically higher in younger patients compared with older patients in the bevacizumab arm (Fig. 3A): OS-6 and OS-9 were 77.8% and 59.3% in patients aged ≤55 years, respectively, and 48.4% and 19.3% in patients aged >55 years, respectively. In patients treated with fotemustine, OS-6 and OS-9 were 66.7% and 33% in patients aged ≤55 years, respectively, and 80% and 60% in patients aged >55 years, respectively (Fig. 3B).
Fig. 3.
Overall survival for patients randomized to receive (A) bevacizumab (n = 59) and (B) fotemustine (n = 32).
Progression-free Survival and Response Rate
PFS-6 according to RANO criteria was 26.3% (95% CI, 15.5–39.7) and 10.7% (95% CI, 2.3–28.3) for the bevacizumab and fotemustine arms, respectively; median PFS was 3.38 (95% CI, 3.15–4.37) and 3.45 months (95% CI, 1.87–3.84), respectively. PFS-6 was 40.7% in patients aged ≤55 years and 13.3% in patients aged >55 years treated with bevacizumab. For patients treated with fotemustine, PFS-6 was 7.7% in patients aged ≤55 years and 13.3% for patients aged >55 years. Response rates according to RANO criteria were 29% and 9% for patients treated with bevacizumab and fotemustine, respectively. Nonenhancing PD was recorded in 4 patients, all of whom had been randomized to the bevacizumab arm (6.8%); survival of these 4 patients was 6, 13, 20.1+, and 21.2+ months at the time of analysis. Concordance between RANO and Macdonald criteria was found in 96.3% of cases. Central assessment showed a PFS-6 of 19.6% (95% CI, 9.2–30.1) and 10.7% (95% CI, 0.0–22.2) in the bevacizumab and fotemustine arms, respectively.
Exploratory Overall Survival Subgroup Analyses
In the bevacizumab arm, patients aged ≥55 years had a higher risk of death (HR, 2.02; 95% CI, 0.99–4.11; P = .05) compared with those aged ≤55 years. Females had a significantly lower risk than males (HR, 0.22; 95% CI, 0.10–0.49; P = .0002), while patients with an ECOG PS of zero had a significantly lower risk versus ECOG PS 1 (HR, 3.70; 95% CI, 1.55–8.83; P = .0031) or 2 (HR, 7.71; 95% CI, 2.39–24.93; P = .0006). In the fotemustine arm, only ECOG PS had a significant effect on OS; patients with an ECOG PS of zero had a significantly lower risk compared with patients with an ECOG PS of 2 (HR, 3.10; 95% CI, 2.02–24.58; P = .011).
Postprogression Therapy
Thirty-two (54%) and 19 (58%) patients, in the bevacizumab and fotemustine arms, respectively, received further systemic treatments following PD. Post-PD, bevacizumab was continued in one patient (4%; bevacizumab arm) and administered to 4 patients (21%; fotemustine arm). Other postprogression treatments are listed in Table 4. Median OS for patients who received post-PD therapy was 11.2 months (bevacizumab arm) and 13.1 months (fotemustine arm). For patients who did not receive post-PD therapy, median OS was 4.6 months (bevacizumab arm) and 5.5 months (fotemustine arm).
Table 4.
Postprogression treatment
| Bevacizumab (n = 59) | Fotemustine (n = 32) | Total (n= 91) | |
|---|---|---|---|
| 1st postprogression treatment, n (%) | 32 (54.2%) | 19 (59.4%) | 51 (56.0%) |
| Nitrosoureas | 27 (45.8%) | 5 (15.6%) | 32 (35.2%) |
| Bevacizumab | 1 (1.7%) | 4 (12.5%) | 5 (5.5%) |
| Temozolomide | 1 (1.7%) | 3 (9.4%) | 4 (4.4%) |
| Platinum derivatives | 0 | 7 (21.9%) | 7 (7.7%) |
| Other | 3 (5.1%) | 0 | 3 (3.3%) |
| 2nd postprogression treatment, n (%) | 5 (8.5%) | 6 (18.8%) | 11 (12.1%) |
| Nitrosoureas | 1 (1.7%) | 1 (3.1%) | 2 (2.2%) |
| Bevacizumab | 0 | 0 | 0 |
| Temozolomide | 2 (3.4%) | 3 (9.4%) | 5 (5.5%) |
| Platinum derivatives | 1 (1.7%) | 0 | 1 (1.1%) |
| Other | 1 (1.7%) | 2 (6.3%) | 3 (3.3%) |
Quality of Life (EORTC QLQ-C30)
The mean QoL score at baseline was 58.05 ± 26.40 in the bevacizumab arm and 66.13 ± 24.90 in the fotemustine arm, comprising physical functioning (71.95 ± 25.65 bevacizumab, 78.92 ± 25.07 fotemustine), emotional functioning (73.56 ± 23.47 bevacizumab, 74.19 ± 23.41 fotemustine), and social functioning (72.99 ± 28.07 bevacizumab, 81.18 ± 24.24 fotemustine). At day 46 ± 3, physical functioning showed an average increase of 10.37 ± 19.07 in the bevacizumab arm and 7.08 ± 12.76 in the fotemustine arm. However, the most important deteriorations in scores were observed in the fotemustine arm for fatigue (−13.19 ± 25.73), nausea (−8.33 ± 14.91), insomnia (−8.33 ± 33.33), and appetite loss (−10.42 ± 23.47). Emotional functioning showed a decrease (−8.02 ± 23.62) in patients treated with bevacizumab. At the third assessment, the EORTC QLQ-C30 questionnaire was completed by only 15 (13.56%) bevacizumab-treated patients and 8 (25.00%) fotemustine-treated patients. In the following weeks, even lower responder rates were reported.
Safety
Median duration of treatment exposure, defined as the time between the first and last drug administration was 97 days (range, 1–570) and 80 days (range, 1–526) in the bevacizumab and fotemustine arms, respectively. Toxicity profiles were in line with known bevacizumab19 and fotemustine7 toxicities. Adverse events were recorded for 49 patients (83.1%) who received bevacizumab and 27 patients (84.4%) who received fotemustine (Table 2). Serious AEs (SAEs) resulted in 11 bevacizumab-treated patients (18.6%) and 3 fotemustine-treated patients (9.4%) permanently discontinuing the study. Two patients (3.4%) in the bevacizumab arm and 2 patients (6.3%) in the fotemustine arm had an adjustment or interruption in treatment due to SAEs.
Table 2.
Selected adverse events in the safety population (grades 3/4)
| Adverse Event | Grade | Bevacizumab (n = 59) |
Fotemustine (n = 32) |
||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Leukopenia | 3 | 1 | 1.7 | 1 | 3.1 |
| 4 | 0 | 0 | 0 | 0 | |
| Neutropenia | 3 | 0 | 0 | 3 | 9.4 |
| 4 | 1 | 1.7 | 1 | 3.1 | |
| Pancytopenia | 3 | 0 | 0 | 1 | 3.1 |
| 4 | 0 | 0 | 1 | 3.1 | |
| Thrombocytopenia | 3 | 0 | 0 | 5 | 15.6 |
| 4 | 0 | 0 | 2 | 6.3 | |
| Intestinal perforation | 3 | 0 | 0 | 0 | 0 |
| 4 | 2 | 3.4 | 0 | 0 | |
| Anal abscess | 3 | 0 | 0 | 1 | 3.1 |
| 4 | 0 | 0 | 0 | 0 | |
| Cerebral hemorrhage | 3 | 0 | 0 | 0 | 0 |
| 4 | 1 | 1.7 | 0 | 0 | |
| Cerebral ischemia | 3 | 1 | 1.7 | 0 | 0 |
| 4 | 0 | 0 | 0 | 0 | |
| Pulmonary embolism | 3 | 1 | 1.7 | 0 | 0 |
| 4 | 0 | 0 | 0 | 0 | |
| Acute myocardial infarction | 3 | 1 | 1.7 | 0 | 0 |
| 4 | 0 | 0 | 0 | 0 | |
| Hypertension | 3 | 1 | 1.7 | 0 | 0 |
| 4 | 0 | 0 | 0 | 0 | |
MGMT Promoter Methylation and IDH1/2 Mutations
Tumor samples were available for MGMT methylation analysis in 73 patients (80.2%); the MGMT promoter was methylated in 45 of these tumors (62% overall; 58% and 68% of patients treated with bevacizumab and fotemustine, respectively). In patients treated with bevacizumab, OS-6 rates were lower in patients with MGMT promoter methylated tumors than in those with MGMT unmethylated tumors (50% and 85%, respectively), while in patients treated with fotemustine, OS-6 rates were higher in patients with MGMT promoter methylated tumors than in those with MGMT unmethylated tumors (87.5% and 50%, respectively). Other relevant outcomes are listed in Table 3.
Table 3.
MGMT methylation status in relation to other clinical outcomes
| Clinical Outcome | Bevacizumab |
Fotemustine |
||
|---|---|---|---|---|
| MGMT Methylated (n = 28) | MGMT Unmethylated (n = 20) | MGMT Methylated (n = 17) | MGMT Unmethylated (n = 8) | |
| OS-9, % (SE) | 29% (0.09) | 45% (0.11) | 63% (0.12) | 25% (0.15) |
| OS-12, % (SE) | 21% (0.00) | 25% (0.10) | 56% (0.12) | 13% (0.12) |
| Median OS, months (95% CI) | 5.8 (4.3–7.4) | 8.7 (6.6–11.6) | 16.0 (8.2–21.6) | 5.9 (2.6–11.9) |
Abbreviations: CI, confidence interval; OS, overall survival; OS-9, OS rate at 9 months; OS-12, OS rate at 12 months.
The Cox proportional hazards model was performed to assess the prognostic value of the methylation status of the MGMT promoter on survival. In the bevacizumab arm, nonmethylated patients had a significantly lower risk in comparison with MGMT methylated patients (HR, 2.28; P = .0464). In the fotemustine arm, the estimates suggested that MGMT methylated patients had a significantly lower risk in comparison with nonmethylated ones (HR, 0.19; P = .0481).
Tumor material was available for IDH1/2 analysis in 52 patients (57%), including patients with other diagnoses at ICR; only 2 patients were found to harbor an IDH1 mutation (one in each treatment arm).
Discussion
Since 2005, phase II clinical trials of bevacizumab in recurrent glioblastoma have shown encouraging efficacy results, with reported response rates of up to 60%, PFS-6 rates of up to 50%,, and post-PD survival ranging between 7 and 10 months.9,15 Indirect comparisons with historical data have suggested a potential role for bevacizumab following glioblastoma disease recurrence, and the FDA granted provisional approval of bevacizumab for the treatment of recurrent glioblastoma in 2009. In Europe, bevacizumab was not licensed for recurrent glioblastoma due to insufficient comparative data with standard chemotherapy and concerns regarding the potential bias of the antiangiogenic agent for PD assessment. To avoid risk of pseudoresponse,20,21 clinical trials started to use PFS as a secondary endpoint of bevacizumab efficacy, with OS rates after PD being the primary endpoint.
The phase II BELOB and AVAREG studies, designed with a nitrosourea control arm, were launched in Europe to assess the role of bevacizumab in recurrent glioblastoma. The BELOB study enrolled 153 patients who received bevacizumab monotherapy, lomustine monotherapy, or the combination of bevacizumab plus lomustine.10 OS-9 rates of 43%, 38%, and 63% were reported with lomustine alone, bevacizumab alone, or bevacizumab plus lomustine, respectively. Median OS and OS-12 were almost superimposable between single-agent lomustine and single-agent bevacizumab: median OS was 8 months for each group, and OS-12 was 30% and 26%, respectively.
In AVAREG, better PFS-6 and response rates were found in the bevacizumab arm as the antiangiogenic effect of bevacizumab directly affects the primary imaging-based outcome measure (contrast MRI, Macdonald, and RANO criteria). OS-6, the primary endpoint, was 62% and 73% for bevacizumab and fotemustine, respectively. OS-9 (38% and 47%), median OS (7.2 and 8.7 months), and OS-12 (26% and 40%) were comparable between the treatment arms, despite a numerical imbalance of baseline characteristics (more patients with PS 2, larger tumors, and corticosteroid use at baseline in the bevacizumab arm vs the fotemustine arm), suggesting that patients in the bevacizumab arm had slightly worse disease. However, AVAREG results were consistent with the BELOB trial; the almost superimposable survival results found between nitrosoureas and single-agent bevacizumab should be considered an opportunity, with 2 agents providing therapeutic alternatives for patients and oncologists, especially in peculiar conditions (ie, comorbidities or hematologic toxicities with alkylating agents).
Recently, results from the EORTC 26101 (NCT01290939) phase III trial, which compared the combination of bevacizumab and lomustine with lomustine alone, have been presented and showed no survival advantage for the combination arm, with median survival in the range of 9 months in both arms. This study confirmed that the combination of bevacizumab and chemotherapy does not have a role in recurrent GBM, but we do not yet have robust data on the sequence of these drugs.
Currently, no validated biomarkers for recurrent glioblastoma exist, and molecular selection of patients cannot yet be implemented. However, age, toxicity, and comorbidities should be considered during treatment selection for recurrent glioblastoma. Age, a recognized prognostic factor for glioblastoma,22,23 may be helpful in selecting effective treatments. In AVAREG, age was a stratification factor; bevacizumab-treated patients aged ≤55 years had higher OS-6 rates (77.8%) compared with the >55 years group (48.4%); HR for survival in the bevacizumab arm for patients aged >55 years vs ≤55 years was 2.0 (95% CI, 1.0–4.1; P = .05);. This potential predictive role was not seen in patients treated with fotemustine.
MGMT methylation status has a role in predicting survival in newly diagnosed glioblastoma17 as well as in recurrent disease10,24 for patients treated with alkylating agents. A similar effect was also seen in patients treated with bevacizumab in the BELOB trial. Despite the low sample size, we found that MGMT methylation was predictive of efficacy of fotemustine in the recurrence setting, with patients with methylated tumors living longer (HR, 0.19; P = .0481) than those with nonmethylated tumors. On the contrary, bevacizumab provided an increased survival for patients with MGMT unmethylated tumors compared with methylated tumors (HR, 2.28; P = .0464). The reasons for these differences across the AVAREG and BELOB trials are challenging. Furthermore, the numbers of patients treated with bevacizumab and with MGMT methylation evaluated (48 in our trial and 42 in the BELOB trial) are too limited to draw any firm conclusions.
Bevacizumab was associated with a reduction in corticosteroid use over time, as previously reported.25 This, combined with the identified relationship with age, suggests that younger patients with large tumors and edema could benefit from bevacizumab administration and decreased corticosteroid intake. However, AVAREG was not powered for this exploratory analysis, and definitive conclusions cannot be drawn.
Bevacizumab was well-tolerated, with the reported AEs in line with its known toxicity profile. Grades 3–4 hematologic toxicities, nausea, and vomiting were more frequent with fotemustine, while hypertension was more common with bevacizumab. These differing toxicity profiles could be used to optimize treatment. In the case of hematologic toxicities arising during first-line RT/TMZ, bevacizumab may be preferred upon disease recurrence, but for patients with severe cardiovascular disorders or other conditions (eg, diverticulitis), nitrosoureas may be the best option. In the EORTC 26101, dose reductions or delays were allowed in case of treatment-related toxicities in the combination arm. In addition, if one of the agents were to be stopped for any reason other than PD, patients were allowed to continue on single-agent therapy.
In conclusion, we observed that survival rates with bevacizumab in recurrent glioblastoma appeared to be similar to those obtained with fotemustine after front-line RT/TMZ therapy, but toxicity profiles were different. As in other cancer types, further efforts should be made to identify clinical and biologic predictors to improve outcomes and provide clinically meaningful therapy options for glioblastoma patients.
Funding
The AVAREG (NCT01474239) study was sponsored by F. Hoffmann-La Roche Ltd.
Acknowledgments
We would like to thank all patients who participated in the study and clinical personnel involved in data collection including Dr Stefania Bartolini, Dr Marco Bartolotti, Dr Rosalba Poggi, and Dr Alexandro Paccapelo, together with Dr Simona Doria from Roche S.p.A. Support for third-party writing assistance for this manuscript was provided by F. Hoffmann-La Roche Ltd.
Conflict of interest statement. A.A. Brandes: travel grants from Merck Serono Ltd and Pfizer Ltd; E. Franceschi: travel grant from F. Hoffmann-La Roche Ltd; E. Proietti: employee of Roche S.p.A. Italy as Medical Manager. All other authors have no conflicts of interest to declare.
References
- 1. Crocetti E, Trama A, Stiller C et al. Epidemiology of glial and non-glial brain tumours in Europe. Eur J Cancer. 2012;48 (10):1532–1542. [DOI] [PubMed] [Google Scholar]
- 2. Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359 (5):492–507. [DOI] [PubMed] [Google Scholar]
- 3. Wong ET, Hess KR, Gleason MJ et al. Outcomes and prognostic factors in recurrent glioma patients enrolled onto phase II clinical trials. J Clin Oncol. 1999;17 (8):2572–2578. [DOI] [PubMed] [Google Scholar]
- 4. Brandes AA, Tosoni A, Amistà P et al. How effective is BCNU in recurrent glioblastoma in the modern era? A phase II trial. Neurology. 2004;63 (7):1281–1284. [DOI] [PubMed] [Google Scholar]
- 5. Batchelor TT, Mulholland P, Neyns B et al. Phase III randomized trial comparing the efficacy of cediranib as monotherapy, and in combination with lomustine, versus lomustine alone in patients with recurrent glioblastoma. J Clin Oncol. 2013;31 (26):3212–3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wick W, Puduvalli VK, Chamberlain MC et al. Phase III study of enzastaurin compared with lomustine in the treatment of recurrent intracranial glioblastoma. J Clin Oncol. 2010;28 (7):1168–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brandes AA, Tosoni A, Franceschi E et al. Fotemustine as second-line treatment for recurrent or progressive glioblastoma after concomitant and/or adjuvant temozolomide: a phase II trial of Gruppo Italiano Cooperativo di Neuro-Oncologia (GICNO). Cancer Chemother Pharmacol. 2009;64 (4):769–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chamberlain MC. Bevacizumab for the treatment of recurrent glioblastoma. Clin Med Insights Oncol. 2011;5:117–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Friedman HS, Prados MD, Wen PY et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27 (28):4733–4740. [DOI] [PubMed] [Google Scholar]
- 10. Taal W, Oosterkamp HM, Walenkamp AM et al. Single-agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): a randomised controlled phase II trial. Lancet Oncol. 2014;15 (9):943–953. [DOI] [PubMed] [Google Scholar]
- 11. Wick W, Brandes AA, Gorlia T et al. Phase III trial exploring the combination of bevacizumab and lomustine in patients with first recurrence of a glioblastoma: the EORTC 26101 trial. Neuro Oncol. 2015; 17 (suppl 5):v1. [Google Scholar]
- 12. Macdonald DR, Cascino TL, Schold SC Jr, Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8 (7):1277–1280. [DOI] [PubMed] [Google Scholar]
- 13. Wen PY, Macdonald DR, Reardon DA et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28 (11):1963–1972. [DOI] [PubMed] [Google Scholar]
- 14. Kreisl TN, Kim L, Moore K et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2009;27 (5):740–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stupp R, Mason WP, van den Bent MJ et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352 (10):987–996. [DOI] [PubMed] [Google Scholar]
- 16. EORTC Quality of Life Group. Available at: http://groups.eortc.be/qol/. Accessed September 25, 2015.
- 17. Hegi ME, Diserens AC, Gorlia T et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352 (10):997–1003. [DOI] [PubMed] [Google Scholar]
- 18. Meyer J, Pusch S, Balss J et al. PCR- and restriction endonuclease-based detection of IDH1 mutations. Brain Pathol. 2010;20 (2):298–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Brandes AA, Bartolotti M, Tosoni A, Poggi R, Franceschi E. Practical management of bevacizumab-related toxicities in glioblastoma. Oncologist. 2015;20 (2):166–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brandes AA, Franceschi E, Gorlia T et al. Appropriate end-points for right results in the age of antiangiogenic agents: future options for phase II trials in patients with recurrent glioblastoma. Eur J Cancer. 2012;48 (6):896–903. [DOI] [PubMed] [Google Scholar]
- 21. Reardon DA, Galanis E, DeGroot JF et al. Clinical trial end points for high-grade glioma: the evolving landscape. Neuro Oncol. 2011;13 (3):353–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gorlia T, Stupp R, Brandes AA et al. New prognostic factors and calculators for outcome prediction in patients with recurrent glioblastoma: a pooled analysis of EORTC Brain Tumour Group phase I and II clinical trials. Eur J Cancer. 2012;48 (8):1176–1184. [DOI] [PubMed] [Google Scholar]
- 23. Carson KA, Grossman SA, Fisher JD, Shaw EG. Prognostic factors for survival in adult patients with recurrent glioma enrolled onto the new approaches to brain tumor therapy CNS consortium phase I and II clinical trials. J Clin Oncol. 2007;25 (18):2601–2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Weller M, Tabatabai G, Kästner B et al. MGMT promoter methylation is a strong prognostic biomarker for benefit from dose-intensified temozolomide rechallenge in progressive glioblastoma: the DIRECTOR trial. Clin Cancer Res. 2015;21 (9):2057–2064. [DOI] [PubMed] [Google Scholar]
- 25. Chinot OL, Wick W, Mason W et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014;370 (8):709–722. [DOI] [PubMed] [Google Scholar]