Abstract
Background
Although the vascular endothelial growth factor (VEGF)/VEGF receptor (VEGFR) system has become a prime target for antiangiogenic treatment, its biological role in glioblastoma beyond angiogenesis has remained controversial.
Methods
Using neutralizing antibodies to VEGF or placental growth factor (PlGF) or the tyrosine kinase inhibitor, cediranib, or lentiviral gene silencing, we delineated autocrine signaling in glioma cell lines. The in vivo effects of VEGFR1 and VEGFR2 depletion were evaluated in orthotopic glioma xenograft models.
Results
VEGFR1 and VEGFR2 modulated glioma cell clonogenicity, viability, and invasiveness in vitro in an autocrine, cell–line-specific manner. VEGFR1 silencing promoted mitogen-activated protein kinase/extracellular signal-regulated kinase (MAPK/ERK) signaling, whereas VEGFR2 silencing resulted in cell-type dependent activation of the protein kinase B (PKB)/AKT and MAPK/ERK pathways. These responses may represent specific escape mechanisms from VEGFR inhibition. The survival of orthotopic glioma-bearing mice was prolonged upon VEGFR1 silencing in the LNT-229, LN-308, and U87MG models and upon VEGFR2 silencing in LN-308 and U87MG. Disruption of VEGFR1 and VEGFR2 signaling was associated with decreased tumor size, increased tumor necrosis, or loss of matrix metalloproteinase 9 (MMP9) immunoreactivity. Neutralizing VEGF and PlGF by specific antibodies was superior to either antibody treatment alone in the VEGFR1-dependent LNT-229 model.
Conclusions
Differential dependence on autocrine signaling through VEGFR1 and VEGFR2 suggests a need for biomarker–stratified VEGF(R)-based therapeutic approaches to glioblastoma.
Keywords: angiogenesis, glioblastoma, PlGF, signaling, VEGF
Glioblastoma, the most common intrinsic brain tumor, is thought to originate from neuroglial progenitor cells. Glioblastoma cells are a rich source of angiogenic factors, notably vascular endothelial growth factor (VEGF),1,2 and placental growth factor (PlGF).3,4 Endothelial cells have been considered the major target of glioblastoma-derived proangiogenic messengers promoting tumor vascularization. In contrast, autocrine or paracrine effects, defined as cross-talk among tumor cell populations, have received little attention in glioblastoma.5–8
In mammals, VEGF signaling is a complex process involving various receptor molecules. The VEGFR1 (FLT1) tyrosine kinase receptor (TKR) mediates various biologic effects of VEGFA, B, and PlGF. VEGF or PlGF binding to VEGFR1 induces phosphorylation and activation of extracellular signal-regulated kinases 1 and 2 (ERK1/2) and p38 mitogen-activated protein kinase (MAPK); moreover, PlGF may stimulate the trans-phosphorylation of specific VEGFR2 tyrosine residues.9,10 Specifically, VEGF and PlGF expression by glioma cells may induce the accumulation of VEGFR1–positive bone marrow-derived myeloid cells in tumor tissue.7
Although VEGFR2 (KDR, FLK1) is considered the major mediator of VEGFA, C, and D bioactivity in both physiologic and pathologic angiogenesis, the mechanism of VEGF-induced phosphorylation of different tyrosine residues on VEGFR2 and the establishment of specific biological responses remain incompletely understood. In addition, VEGFR heterodimerization and interactions of VEGFR with coreceptors such as neuropilins (NRP), heparan sulfate proteoglycans or αvβ3 integrin further expand the complexity of signaling pathways activated by VEGF and PlGF homo- or heterodimers.11–14 Finally, VEGFC/D binding to VEGFR3 (FLT4) TKR is required for lymphangiogenesis and may play a role in developmental and tumor angiogenesis by modulating VEGFR2-mediated signals.15
Although VEGF receptors, NRP, integrins, and their ligands are expressed in several tumor cell types,6,8,16–18 it is unclear how distinct biological responses emanate from these receptors, specifically in glioblastoma. Autocrine VEGF effects mediated by VEGFR2 signaling have been proposed to promote glioblastoma cell invasion, viability, and tumor growth.6,19 In contrast, VEGF binding to VEGFR2 has also been reported to inhibit invasiveness by suppressing hepatocyte growth factor-dependent c-MET activity through recruitment of the phosphatase protein tyrosine phosphatase 1B (PTP1B) to the VEGFR2/MET heterocomplex.20
These overall conflicting data on autocrine VEGFR signaling led us to propose that responses to VEGFR pathway stimulation or inhibition in glioma are heterogeneous and may, among others, depend on the differential expression of VEGF family ligands and receptors. In fact, we report here that VEGFR1 or VEGFR2 signaling may exhibit distinct survival properties in human glioma models in vivo and that a thorough characterization of VEGFR signaling in tumor cells may facilitate patient enrichment for more successful clinical trials exploring VEGF(R) inhibition in the future.
Materials and Methods
In Vitro Studies
Detailed information on reagents, cell lines, cell culture, viability, clonogenicity, and spherogenicity assays is summarized in Supplementary material, Note 1. Details on real-time quantitative reverse transcription-PCR (qRT-PCR) and primers are provided with Supplementary material, Table S1, and details on immunoblotting, flow cytometry, and ELISA are provided in Supplementary material, Note 2.
The nonsilencing control (#RHS4348) and the silencing microRNA-adapted shRNA (shRNAmir) pGIPZ lentiviral vectors (#RHS4531-V3LHS_403557; #RHS4531-V3LHS_302174) containing a single VEGFA (TCTGTATCGATCGTTCTGT) or VEGFR1-targeting hairpin sequence (TGAACCTGAACTAGATCCT) provided in bacterial stocks of E. coli were purchased from Thermo Scientific Open Biosystems. Lentiviral infectious particles were produced in HEK 293T cells using pGIPZ shRNAmir lentiviral vector, pCMV-dR8.91 second-generation packaging, and pMD2.G envelope plasmids. To generate stable VEGFR2 gene-silenced cells, glioma cells were transduced with VEGFR2-silencing shRNA lentiviral particles (# sc-29318-V, Santa Cruz Biotechnology) containing 3 target-specific constructs: ACTGTGGTGATTCCATGTCTTCAAGAGAGACATGGAATCACCACAGTTTTTT; ACTTGTAAACCGAGACCTATTCAAGAGATAGGTCTCGGTTTACAAGTTTTTT; and CACCTGTTTGCAAGAACTTTTCAAGAGAAAGTTCTTGCAAACAGGTGTTTTT. BLAST analysis showed that the VEGFR1 targeting sequence (TGAACCTGAACTAGATCCT) may target the HRNR (Hornerin) gene (expect value (E) of 11); therefore, we performed a quantitative PCR analysis to exclude this possibility in VEGFR1-silenced cells (data not shown).
Nonsilencing shRNA virus was used as a negative control (#sc-108080). In all cases, stable transduced clones were selected with 4 µg/mL puromycin and used for analysis and assays after 1–2 passages post selection. A pool of 3 target-specific PlGF siRNA and control siRNA was purchased from Santa Cruz and transfected with TransIT-X2 Dynamic Delivery system (Mirus Bio LLC).
The invasive potential of glioma cells was measured by spheroid invasion assay. Glioma spheroids were generated from the respective cell lines by seeding 1–5 × 103 cells in 100 µL of media onto 96-well plate base-coated with 1% nobel agar/PBS medium substrate. After 2–3 days in culture, spheroids with a mean diameter of 200 µm were transferred to collagen I matrix-coated wells and covered by complete Dulbecco's modified Eagle's medium. Every 24 hours for 3 days, the pixel area covered by cells sprouting from these spheroids was determined after subtraction of the initial spheroid pixel area at time zero. Image J software (NIH) was used to determine the invasion area.
Animal Studies
The effects of antiangiogenic treatments or VEGFR depletion on tumor growth and the survival of glioma-bearing mice were examined in immunodeficient Crl:CD1-Foxn1nu nude mice (Charles River). Mice were xenografted with 75 000 LNT-229 or 100 000 LN-308 or U87MG cells. Cells were stereotactically implanted into the right striatum of 6- to 12-week-old mice. Neurological symptoms were assessed daily according to the Cantonal Veterinary Office Zurich guidelines (grade 0: no visible impairment; grade 1: reduced activity, slight balance and coordination impairments; grade 2: reduced activity, 15% weight loss compared with peak weight, slight paralysis of left legs, moderate signs of pain). Seven animals were used to assess survival, defined as the timepoint of the onset of symptoms (grade 2). Data are presented as the number of surviving mice over the time. For histology, 3 prerandomized animals per group were euthanized when the first animal became symptomatic.
Animal experiments were conducted under valid licence and permission of the Cantonal Veterinary Office Zurich and Federal Food Safety and Veterinary Office. Mice were anesthetized by intraperitoneal injection of fentanyl (Sintetica SA, )/midazolam (Roche Pharma)/medetomidine (Orion Pharma mixture and combined with analgesia using carprofen (Pfizer AG). Details on histology and immunohistochemistry are provided in Supplementary material, Note 3.
Statistical Analyses
Detailed information on statistical analysis is summarized in Supplementary material, Note 4.
Results
Glioma Cell Lines Show Different Levels of Constitutive and Inducible VEGFR1 and VEGFR2 Phosphorylation
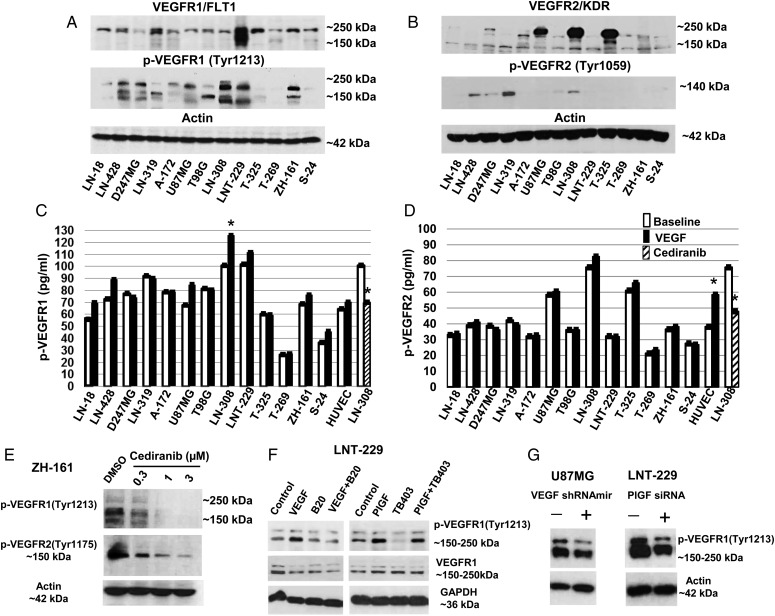
To select appropriate models, we first screened our glioma cell line panel for the expression of VEGF and PlGF and their receptors at the mRNA and protein levels (Supplementary material, Notes 5 and 6; Supplementary material, Fig. S1–S6). Compared with long-term cell (LTC) lines, glioma-initiating cell lines (GIC) expressed lower levels of PlGF mRNA but were more responsive to hypoxia with regard to VEGF release (Supplementary material, Fig. S1D, F and G). Most cell lines exhibited VEGFR1 protein on the cell surface (Supplementary material, Fig. S2C), whereas VEGFR2 was not detected on the surface by flow cytometry (Supplementary material, Fig. S2D). However, immunoblot (Fig. 1A and B) and intracellular flow cytometry (Supplementary material, Fig. S2E and F) revealed significant intracellular levels of both receptors. Immunoblot showed the highest VEGFR2 protein levels in U87MG, LN-308, and T-325 (Fig. 1B). Total protein levels did not correlate to phosphorylation of the major tyrosine site, VEGFR1Tyr1213 or VEGFR2Tyr1059 (Fig. 1A and B) or the total levels of p-VEGFR1 or p-VEGFR2 measured by capture ELISA (Fig. 1C and D), suggesting multiple effects of interacting mechanisms triggered by various ligands.
Fig. 1.
Autocrine and induced vascular endothelial growth factor receptor (VEGFR) activation in glioma cells. (A, B) The levels of total VEGFR1 and VEGFR2 as well as of phosphorylated VEGFR1Tyr1213 or VEGFR2Tyr1059 were assessed by immunoblot, using actin as a loading control. (C, D) Constitutive and VEGF-evoked total phosphorylation levels of VEGFR1 or VEGFR2 were determined by ELISA (*P < .05). (E) VEGFR1 and VEGFR2 phosphorylation in response to increasing concentrations of cediranib (2 h) in ZH-161 cells were detected by immunoblot. (F) Effects of VEGF or PlGF stimulation or neutralizing anti-VEGF (B20) or anti-placental growth factor (PlGF) (TB403) antibodies on VEGFR1Tyr1213 in LNT-229 cells were assessed by immunoblot; cells were incubated for 15 minutes with VEGF (500 ng/mL) or PlGF(1 + 2) (200 ng/mL) alone or in combination with neutralizing VEGF or PlGF antibodies (100 µg/mL). (G) Effects of lentivirus-mediated VEGFA shRNAmir (left) or PlGF siRNA (right) on constitutive p-VEGFR1Tyr1213 in U87MG or LNT-229 cells, respectively, were evaluated by immunoblot.
Next, we studied the phosphorylation status of VEGFR1 and VEGFR2 (total p-VEGFR) at baseline and after stimulation with recombinant VEGF. p-VEGFR1 was induced by VEGF only in LN-308 and p-VEGFR2 only in human umbilical vein endothelial cells (HUVEC) (Fig. 1C and D). The specificity of the ELISA was supported by stimulation of HUVEC with VEGF and the reduction by cediranib in LN-308. Incomplete correlation between Figs. 1A–D is likely a result of comparing single versus all phosphorylated residues of VEGFR.
Constitutive VEGFR1 and VEGFR2 phosphorylation was sensitive to the pan-VEGFR inhibitor, cediranib, in ZH-161 cells with IC50 values of ∼0.3 µM (Fig. 1E), indicating autocrine activation. Sensitivity to cediranib thus confirmed the specificity of the 2 main bands detected by immunoblot. p-VEGFR2 was less sensitive to cediranib than p-VEGFR1.
To separate constitutive versus inducible VEGFR1 phosphorylation at Tyr1213, LNT-229 cells were unstimulated or exposed to ligand for 15 minutes after 12 hours of serum starvation and washing. The cells were preincubated for 2 hours in serum-free condition with neutralizing antibodies to VEGF (B20) or PlGF (TB403) before stimulation with VEGF or PlGF. Exogenous VEGF and PlGF stimulated VEGFR1Tyr1213 phosphorylation in LNT-229. B20 or TB403 interfered only with exogenous VEGF- or PlGF-induced p-VEGFR1Tyr1213 (Fig. 1F). In contrast, constitutive p-VEGFR1Tyr1213 was reduced in response to PlGF or VEGFA gene silencing in U87MG or LNT-229 cells, respectively (Fig. 1G; Supplementary material, Fig. S7A and B), confirming their autocrine activity.
Not surprisingly, we determined that higher VEGFR1 or VEGFR2 mRNA expression levels were associated with higher tumor grade and worse prognosis (Supplementary material, Fig. S8).
VEGFR1 and VEGFR2 Gene Silencing Affect Major Signaling Pathways in Glioma Cells
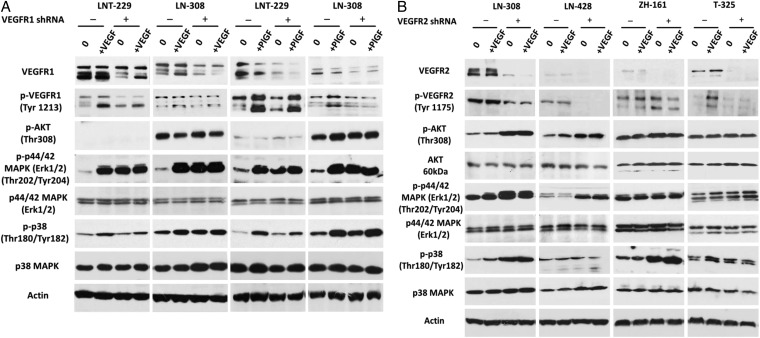
Untreated or ligand-stimulated control or VEGFR-depleted cells were analyzed by immunoblot to gain insight into the molecular changes triggered by VEGFR gene silencing. To test VEGF signaling, we used recombinant VEGF112 (Ala207 - Arg318).21 Both VEGF and PlGF induced phosphorylation of p44/42 (ERK1/2) and p38 MAPK in LNT-229 and LN-308 (Fig. 2A). In contrast, p-AKT levels were largely unaffected. VEGFR1 gene silencing alone resulted in an increase of p44/42 (ERK1/2) phosphorylation compared with the control cells in both models, and this stimulation was not superinduced by exogenous VEGF or PlGF. Phospho-p38 MAPK increased in VEGFR1-depleted LN-308. VEGFR1 gene silencing attenuated the VEGF or PlGF-induced stimulation of p38 MAPK to a different extent, raising the possibility of VEGFR1-dependent signaling of VEGF and PlGF to p38 MAPK. VEGFR2 gene silencing increased p-VEGFR2Tyr1175 in ZH-161, p-AKTThr308, and p-p44/42 (ERK1/2)Thr202/Tyr204 in LN-308 and LN-428 and phospho-p38 MAPK in LN-308 and ZH-161. Yet, when VEGFR2 expression was silenced, p-p44/42 (ERK1/2) and p-p38 MAPK were no longer induced by VEGF in LN-308 (Fig. 2B).
Fig. 2.
Altered downstream signaling in vascular endothelial growth factor receptor (VEGFR)1- and VEGFR2-depleted glioma cells. (A) Stably VEGFR1 gene-silenced LNT-229 or LN-308 cells or (B) VEGFR2 gene-silenced LN-308, LN-428, ZH-161 or T-325 cells, or corresponding controls were assayed for changes in downstream signaling by immunoblot. After 12 hours of serum starvation, subconfluent cells were untreated or stimulated with VEGF (500 ng/mL) or placental growth factor (PlGF) (1 + 2) (100 + 100 ng/mL) as indicated for 15 minutes.
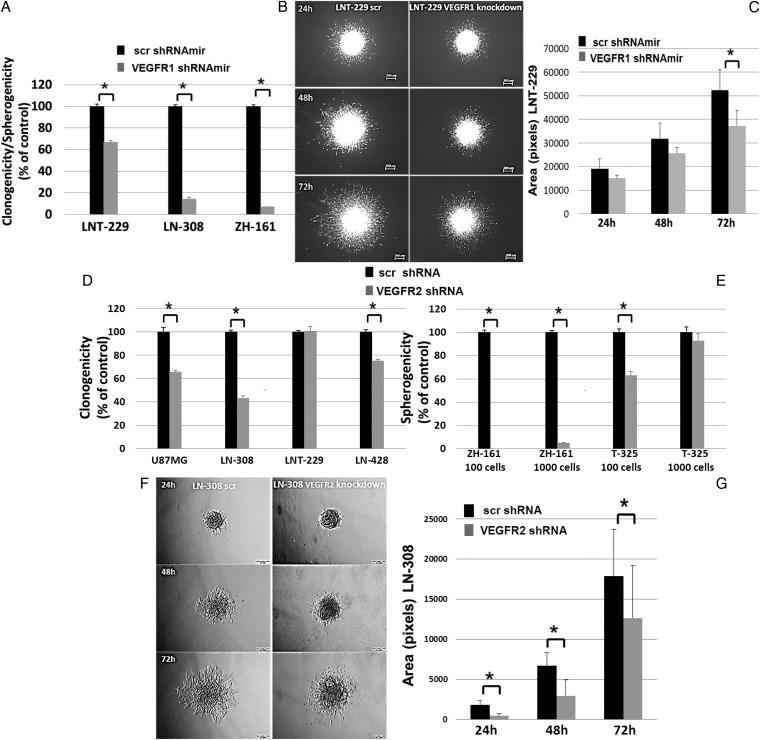
VEGFR1 and VEGFR2 Signaling in Glioma Cells Modulates Clonogenic Survival and Invasion in a Cell–line-dependent Manner
The role of VEGFR signaling in glioblastoma cells was studied pharmacologically and genetically. Clonogenic survival was assessed by crystal violet staining for LTC or by spherogenicity for GIC. B20 and TB403 alone or in combination at 100 µg/mL had no effect in these assays in LNT-229 or ZH-161. Similarly, there was no effect of exogenous VEGF or PlGF. In contrast, cediranib reduced clonogenicity of LNT-229 and spherogenicity of ZH-161 with an EC50 of ∼3 µM. These concentrations had no significant effect on viability (Supplementary material, Fig. S9A and B, data not shown). For VEGFR1 gene silencing, we selected 4 cell lines (3 LTC (LNT-229, LN-308, and U87MG) and one GIC (ZH-161)), all possessing high VEGFR1 expression. Two LTC (LN-308, U87MG) and one GIC (T-325) with high VEGFR2 expression and further cell lines with lower VEGFR2 expression (LN-428, LNT-229, and ZH-161) were selected for VEGFR2 gene silencing. Successful gene silencing of VEGFR1 was confirmed by qRT- PCR (Supplementary material, Fig. S7C, E, G and K), immunoblot (Fig. 2A; Supplementary material, Fig. S10A), flow cytometry (Supplementary material, Fig. S2E), and total p-VEGFR1 ELISA (data not shown). VEGFR2 gene silencing was verified by qRT-PCR (Supplementary material, Fig. S7D, F, H, I, J, and L), immunoblot (Fig. 2B, Supplementary material, Fig. S10A), flow cytometry (Supplementary material, Fig. S2F) and total p-VEGFR2 ELISA (data not shown). Flow cytometry revealed a minor induction of apoptosis upon VEGFR1 depletion in LN-308 but not LNT-229. VEGFR2 depletion had no such effect in LN-308 but increased the G2/M fraction (Supplementary material, Fig. S9C, D and E). Accordingly, VEGFR1/2 depletion did not affect the doubling time of LNT-229 (25–30 h), where depletion of either receptor prolonged doubling times from ∼40–50 hours in LN-308 (data not shown). Further, VEGFR1 gene silencing decreased clonogenicity to 67% in LNT-229 and 14% in LN-308 and spherogenicity to 7% in ZH-161 (Fig. 3A). Similarly, VEGFR2 gene silencing reduced clonogenicity of U87MG, LN-308, and LN-428, although not in LNT-229, and spherogenicity in ZH-161 and T-325 (Fig. 3D and E). The specificity of the knockdown effects was confirmed by rescue experiments: clonogenicity was restored by CMV promoter-driven exogenously re-expressed VEGFR1 (P = .01) or VEGFR2 (P= .01) (Supplementary material, Fig. S10). At 72 hours VEGFR1 gene silencing inhibited invasion by 29% (P =.03) in LNT-229 (Fig. 3B and C). The invasiveness of the less invasive LN-308 cells was not suppressed by VEGFR1 depletion. Conversely, VEGFR2 depletion reduced invasiveness of LN-308 cells (Fig. 3F and G) to a similar extent as cediranib, but there was no effect on LNT-229 (data not shown).
Fig. 3.
Biological effects of vascular endothelial growth factor receptor (VEGFR) signaling inhibition in glioma cells. Effects of VEGFR1 gene silencing on (A) clonogenicity or spherogenicity and (B, C) invasion of LNT-229 were studied. (D, E) Effects of VEGFR2 gene silencing on clonogenicity and spherogenicity was evaluated. (F, G). Invasiveness of VEGFR2-depleted LN-308 cells was assessed by spheroid invasion assays. The data represent the average fold change in area of 3 spheroids ± standard deviation (*P < .05).
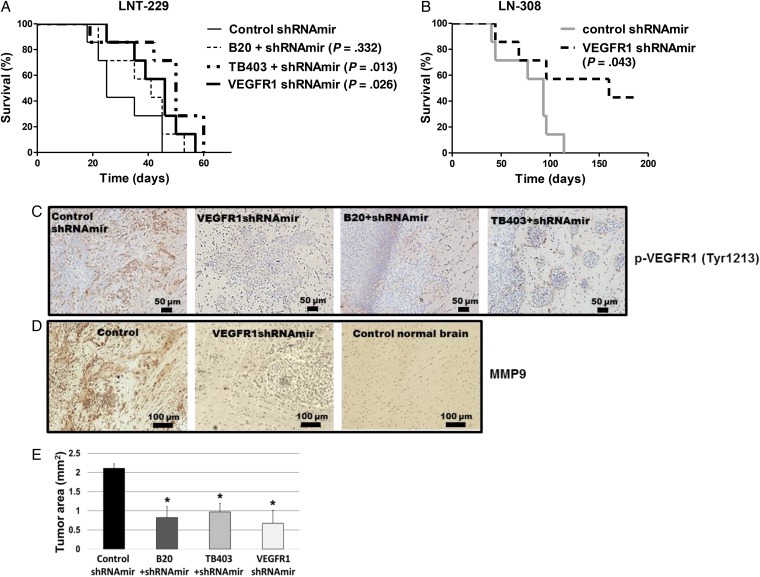
VEGFR1 and VEGFR2 Support Tumor Growth in Orthotopic Rodent Glioma Models
Finally, we investigated whether genetic or pharmacologic VEGFR inhibition affected tumor growth in vivo. Mice inoculated with VEGFR1-silenced LNT-229 (P = .026), LN-308 (P = .042), or U87MG (P = .003) cells experienced a significant survival benefit (Fig. 4A and B; Supplementary Material, Fig. S11B). In the LN-308 model, all control mice had to be euthanized because of tumor growth, whereas 3 mice in the shVEGFR1mir group were alive and free from major signs at day 190. Analysis of brain sections of these 3 surviving mice showed that only one mouse harbored a small tumor of 0.12 mm2. Appropriate control experiments confirmed that p-VEGFR1 levels were strongly suppressed in the VEGFR1-silenced tumors at days 22 (LNT-229), 27 (U87MG) or 44 (LN-308) in all models (Fig. 4C; Supplementary material, Fig. S11F and S12B). Furthermore, immunoreactivity of matrix metalloproteinase (MMP) 9 was strongly reduced in LNT-229 (Fig. 4D). A survival effect similar to VEGFR1 silencing was afforded by treatment of mice carrying LNT-229 control tumors with TB403 but not B20 (Fig. 4A). The latter was in part due to early onset of score 2 adverse events (≥15% weight loss) with B20 treatment. All interventions reduced areas involved by tumor at day 22 (Fig. 4E).
Fig. 4.
Genetic depletion of vascular endothelial growth factor receptor (VEGFR)1 or pharmaceutical neutralization of both VEGFR1 ligands, VEGF and placental growth factor (PlGF), delay tumor growth in vivo. (A) 75 000 nontargeting control shRNAmir-expressing human LNT-229 glioma cells were implanted into the brains of nude mice. The mice were treated either twice weekly with 20 mg/kg/day Xolair IgG control or 5 mg/kg/day B20 or 20 mg/kg/day TB403 by intraperitoneal injection. The treatment was initiated at the day of tumor implantation and maintained until the onset of clinical grade 2 symptoms. A parallel group of mice was transplanted with 75 000 VEGFR1-targeted shRNA expressing LNT-229 glioma cells. (B) 100 000 VEGFR1-silenced or corresponding control LN-308 cells were implanted into the brains of nude mice. Seven animals per group were used to monitor survival (Mantel-Cox test). (C) LNT-229 tumor specimens obtained per randomization list from animals sacrificed on the same day in each group when the first animal(s) became symptomatic were stained for p-VEGFR1Tyr1213 (brown color). Sections were counterstained with hematoxylin (blue). A higher magnification image shown in Fig. S12A confirmed subcellular localization. (D) LNT-229 tumor specimens or normal brain were stained for MMP9 immunoreactivity (brown color). Sections were counterstained with hematoxylin (blue). (E) LNT-229 tumor sizes were assessed on H&E-stained sections (n = 3; *P < .05, t test).
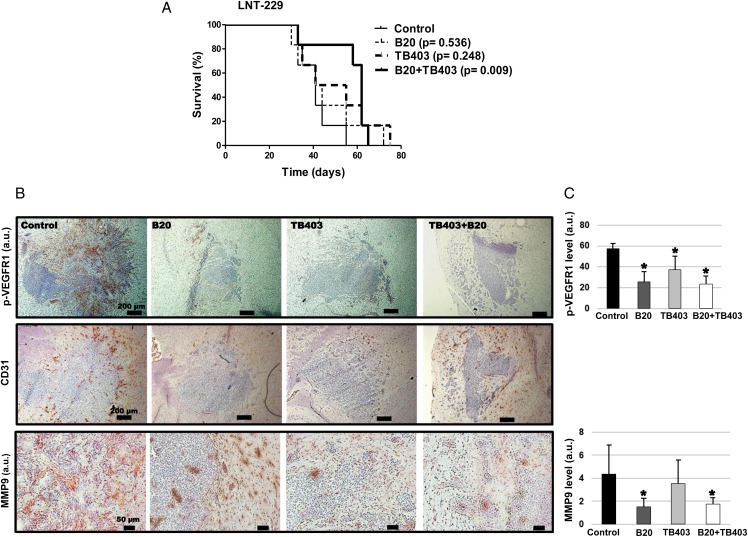
To determine possible synergy by inhibiting both growth factors, we allowed the tumors to establish for 15 days and then initiated treatment with either VEGF or PlGF antibody or both until progression. In this paradigm, compared with the control group, only cotreatment afforded a survival advantage (P = .009) (Fig. 5A). In control tumors, CD31 staining revealed a prominent signal, particularly at the tumor edges, which was associated with the invasion of glioma cells along the vessels. Invasive cells at the tumor periphery, as well as glioma cells in tumor satellites of control tumors, displayed strong p-VEGFR1 staining relative to the tumor core, indicating a role of VEGFR1 in tumor cell invasion in vivo. All interventions resulted in decreased p-VEGFR1 levels, decreased MMP9 immunoreactivity, and a trend towards decreased vessel density determined by CD31 staining and (Fig. 5B and C).
Fig. 5.
Synergistic growth inhibition by targeting both vascular endothelial growth factor receptor (VEGFR)1 ligands, VEGF and placental growth factor (PlGF) in vivo. (A) A similar experiment as in Fig. 4A was performed, but with the modification that antibody treatment was delayed until day 15 after tumor implantation and that another group of animals treated with both VEGF and PlGF antibody were included. (B) Tumor specimens obtained per randomization list from animals sacrificed on the same day in each group when the first animal(s) became symptomatic were stained for p-VEGFR1Tyr1213 (upper row, brown color), CD31 (middle row) or MMP9 (lower row). Sections were counterstained with hematoxylin (blue). (C) Quantification of immunoreactivity (n = 3; *P < .05, t test).
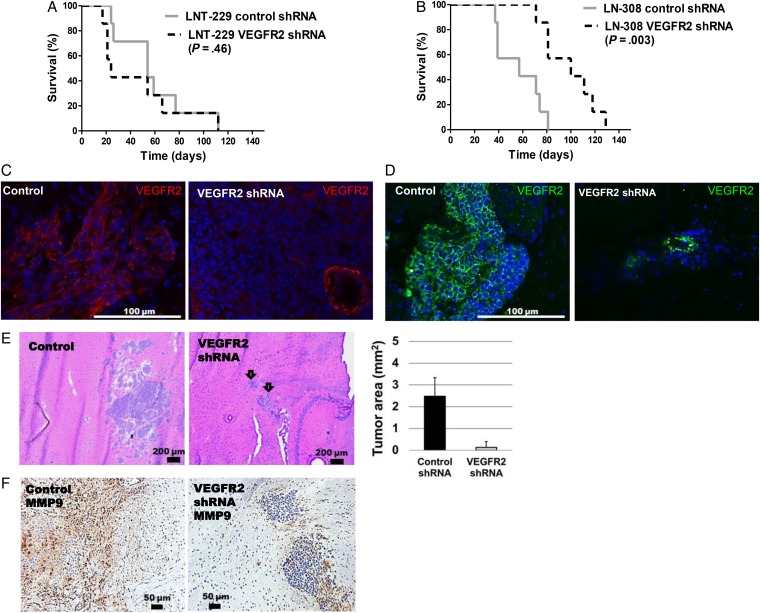
VEGFR2 depletion delayed tumor growth profoundly in LN-308 (P = .003) and U87MG (P = .009) but not in LNT-229 (Fig. 6A, B and E; Supplementary material, Fig. S11C and D), although gene silencing was confirmed to persist in all models (Fig. 6C and D; Supplementary material, Fig. S11F). Compared with the controls, tumor sizes and MMP9 protein levels in the tumor core and invasive area were strongly reduced by VEGFR2 gene silencing in LN-308 (Fig. 6E and F).
Fig. 6.
Genetic depletion of vascular endothelial growth factor receptor (VEGFR)2 delays tumor growth in the LN-308 but not in the LN-229 glioma model. (A,B) 75 000 LNT-229 or 100 000 LN-308 depleted of VEGFR2 or their shRNA control cells were implanted intracranially and monitored for survival (n = 7). (C, D) VEGFR2 gene silencing was confirmed by immunofluoresence microscopy at days 21 (LNT-229) or 37 (LN-308). Preservation of blood vessel labeling after tumor-specific gene silencing serves as an internal control. (E) LN-308 tumor sizes were determined based on H&E-stained sections (n = 3). (F) IHC for MMP9 levels upon VEGFR2 silencing. The mean area (a.u.) of MMP9-positive segments was reduced by ∼86% relative to control values (n = 3, P < .05).
Discussion
The standard of care for patients with newly diagnosed glioblastoma includes resection or biopsy followed by radiotherapy and concomitant maintenance temozolomide.22,23 Many contemporary efforts to improve on this standard have explored the hypothesis that inhibition of angiogenesis will provide a survival benefit. In 2 randomized phase 3 trials, the most advanced antiangiogenic agent (ie, the VEGF antibody bevacizumab) has shown activity defined by an increased radiological response rate and prolonged progression-free survival, although not overall survival.24,25 In contrast, other VEGF-targeting agents such as cediranib or VEGF trap or non-VEGF-targeting antiangiogenic agents such as enzastaurin or cilengitide have failed.26 The search for and clinical validation of biomarkers that help select patients deriving benefit from antiangiogenic treatment continues.27,28
Of note, VEGF may also assume an angiogenesis-independent tumor-promoting function.29,30 Despite interest in the autocrine effects of VEGF on tumor cells, and specifically glioma cells,8,20 distinct biological functions and signaling pathways mediated by different VEGF-receptors in glioma cells have not been systemically analyzed. Here we have performed a comprehensive expression profiling of human glioma cells, including GIC, for VEGF family ligands and receptors. Most glioma cells coexpress various VEGF and PlGF species and their cognate receptors, however, at different levels (Supplementary Notes 5 and 6).
VEGFR1 expression was identified at the surface of almost all glioma cell lines by flow cytometry. The presence of an intracellular VEGFR1 pool was evidenced by the major shift of the flow cytometry signal in the permeabilized LNT-229 and LN-308 cells (Supplementary material, Fig S2). Only LNT-229 and LN-308 cells expressed soluble VEGFR1 (Supplementary material, Fig. S6B). VEGFR2 internalization and intracellular signaling have been described.31 VEGFR2 protein was only revealed by immunoblot and flow cytometry of prepermeabilized cells but not at the surface of nonpermeabilized cells, confirming intracellular localization (Supplementary material, Fig. S2F).
Most glioma cells exhibited autocrine VEGFR1 phosphorylation that is only slightly inducible by recombinant VEGF (Fig. 1A, C and F; Fig. 2A). VEGFR1 is phosphorylated at tyrosine Y1213 in response to both VEGF and PlGF on immunoblots. In contrast to cediranib, neutralizing antibodies to VEGF (B20) or PlGF (TB403) did not inhibit constitutive Tyr1213 phosphorylation. Yet, stimulation of VEGFR1Y1213 by recombinant VEGF or PlGF in LNT-229 was neutralized by B20 and less so by TB403, respectively (Fig. 1E and F).
Phosphorylation of VEGFR2Tyr1059; Tyr1175 , suggestive of autocrine signaling was also detected in some cell lines. Total VEGFR2 phosphorylation was not inducible by exogenously added VEGF in vitro, supporting the absence of VEGFR2 on the cellular surface (Fig. 1B and D; Fig. 2B).
We confirm that exogenous VEGF or PlGF and anti-VEGF or PlGF neutralizing antibodies have little or no effect on glioblastoma cell growth in vitro32 (Supplementary material, Fig. S9A and B). Ligand interaction with VEGFR1 and VEGFR2 may be sterically protected from antibody interference but still targeted by intracellularly acting agents such as cediranib (Fig. 1E and F). Accordingly, VEGFA- or PlGF-deficient glioma cells had reduced basal p-VEGFR1Tyr1213, confirming endogenous ligand-dependent receptor phosphorylation (Fig. 1G).
To better delineate autocrine signaling and deduce the biological role of VEGF family receptors, cell lines with different levels of VEGFR1 or VEGFR2 expression and activation were subjected to receptor-specific gene silencing by lentivirus-delivered shRNA. VEGFR2 gene silencing had major effects in cell lines with increased intracellular VEGFR2 levels lacking detectable VEGFR2 at the cell surface and indicating that autocrine VEGFR2 signaling is regulated at the level of cytoplasmic intracellular receptor cycling. Silencing of either receptor resulted in distinct changes in downstream signaling that may be interpreted as a stress response and point to potential escape strategies that might be exploited therapeutically: phosphorylation of MAPK in response to VEGFR1 depletion and of AKT and MAPK in response to VEGFR2 depletion (Fig. 2).
VEGFR-depleted glioma cells showed a strong phenotype at the level of clonogenicity, spherogenicity, and invasiveness in a cell line- and receptor type-dependent manner (Fig. 3). For example, clonogenic growth and invasion of LNT-229 cells were unaffected by VEGFR2 depletion, consistent with low-level VEGFR2 expression and phosphorylation, whereas VEGFR1 depletion led to a significant decrease of both clonogenic and motogenic potential demonstrating that VEGFR2 signaling is dispensable in some glioma cell lines. In contrast, the clonogenic survival of VEGFR1 and VEGFR2 high-expressing LN-308 cells was strongly affected by the silencing of both receptors. Unlike LNT-229, downregulation of VEGFR2 significantly inhibited invasion, whereas VEGFR1 gene silencing hardly affected invasion in LN-308. Rescue experiments further confirmed the specific biological functions of both receptors (Supplementary material, Fig. S10).
These observations were expanded and confirmed by in vivo studies in nude mice. VEGFR1 phosphorylation was unevenly distributed throughout LNT-229 tumors with predominant staining localized to the infiltrating tumor edge. Either shRNAmir-mediated suppression of VEGFR1 or the early exposure of mice to neutralizing antibodies to VEGF or PlGF inhibited VEGFR1 phosphorylation and reduced tumor growth (Fig. 4A). Using a paradigm of pre-established tumors, the combination of both antibodies was superior to administration of either antibody alone and conferred a significant survival benefit in the LNT-229 model (Fig. 5A). The inhibition of VEGFR1 expression or activity was uniformly associated with loss of MMP9 levels in the tumors. Among various MMP, only the transcriptional and enzymatic levels of MMP9 correlated with tumor grade in gliomas.33 Correlation analyses using gene expression data from TCGA-540 database (http://hgserver1.amc.nl/cgi-bin/r2/main.cgi#) confirmed that MMP9 expression correlated with VEGFR1 expression (r = 0.26, 8e-10) and VEGFR2 expression (r = 0.22, P = 1.4e-7) (data not shown).
A role for VEGFR1 signaling in tumor cells in promoting tumor growth has been reported in different tumor models,3,29,34–36 however, not yet in glioblastoma. Although the addition of TB403 to bevacizumab did not generate a signal of enhanced activity in a phase 1 study in human patients with recurrent glioblastoma,37 the patient number was small, and no effort was made to preselect patients based on VEGFR1 phosphorylation. Thus, a biomarker-driven clinical trial focusing on p-VEGFR1 levels and PlGF expression in glioblastoma might still represent an effective strategy to define a role for PlGF targeting in glioblastoma or other cancers. Similarly, one might speculate that an enrichment of glioblastomas dependent on VEGFR signaling might have helped to define a role for cediranib in subsets of glioblastoma patients.38 VEGFR2 gene silencing resulted in profound growth inhibition associated with reduced MMP9 immunoreactivity in the LN-308 model, further delineating an important tumor-promoting function of VEGFR2 in selected gliomas (Fig. 6). The potential role of VEGFR1/2 in tumor growth was confirmed in the U87MG model (Supplementary material, Fig. S11).
Altogether, this systematic analysis of VEGF receptors using different glioma models indicates differential biologic functions of VEGFR1 and VEGFR2 that may be context-dependent. Such in-depth studies may also resolve some apparently contradictory research findings (eg, PlGF has been shown to promote tumor growth and local invasiveness in subcutaneous melanoma, orthotopic pancreatic syngeneic tumors and GL-261 rodent glioma models,7,39 or to inhibit tumor growth in lung, colon, and U87MG glioma models).40 Similarly, VEGFR2 has been reported to promote glioma cell viability and invasion;6,8,19 however, blocking VEGFR2 may also trigger invasiveness of some glioma cells by activating the c-MET pathway.20
Although VEGF antagonism has been shown to limit glioma growth in rodent models in vivo,41 this effect has commonly been attributed to antiangiogenesis. We now provide firm evidence that intrinsic VEGFR signaling in glioma cells sustains glioma growth at least in certain models: VEGFR1 suppression induced a major delay of tumor growth in the LNT-229, LN-308, and U87MG models (Fig. 4 and 5; Supplementary material, Fig. S11 and S12), whereas VEGFR2 decreased growth in LN-308 and U87MG (Fig. 6; Supplementary material, Fig. S11).
Translating VEGFR expression into a prognostic or predictive biomarker may remain challenging and require careful consideration of the type and level of intratumoral VEGFR phosphorylation, tumoral versus endothelial expression, intratumoral heterogeneity, alternatively spliced VEGFR variants, and soluble, proteolytically cleaved, truncated VEGFR1 and VEGFR2 variants.
Supplementary Material
Funding
Swiss National Science Foundation (SNF) (to M.W.).
Supplementary Material
Acknowledgments
The authors would like to express their sincere appreciation to Konrad Honold (Roche Innovation Center Penzberg, Pharma Research and Early Development) for his support. The authors thank Silvia Dolski and Julia Friesen for expert technical assistance.
Conflict of interest statement. E.S. was a recipient of a Postdoctoral Fellowship from Roche (Basel, Switzerland). H.S. and E.R. report no conflicts of interest. K.S. has received honoraria for advisory board participation from Roche. F.H. and K.M.W. are employed by Roche. M.W. has received research grants from Acceleron, Actelion, Alpinia Institute, Bayer, Isarna, M.S.D., Merck & Co, Novocure, PIQUR and Roche and honoraria for lectures or advisory board participation or consulting from Celldex, Immunocellular Therapeutics, Isarna, Magforce, M.S.D., Merck & Co, Northwest Biotherapeutics, Novocure, Pfizer, Roche, and Teva.
References
- 1. Folkins C, Shked Y, Man S et al. . Glioma tumor stem-like cells promote tumor angiogenesis and vasculogenesis via vascular endothelial growth factor and stromal-derived factor 1. Cancer Res. 2009;69 (18):7243–7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Plate KH, Breier G, Weich HA et al. . Vascular endothelial growth-factor and glioma angiogenesis - coordinate induction of VEGF receptors, distribution of VEGF protein and possible in-vivo regulatory mechanisms. Int J Cancer. 1994;59 (4):520–529. [DOI] [PubMed] [Google Scholar]
- 3. Yao J, Wu XM, Zhuang GL et al. . Expression of a functional VEGFR-1 in tumor cells is a major determinant of anti-PlGF antibodies efficacy. Proc Natl Acad Sci U S A. 2011;108 (28):11590–11595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nomura M, Yamagishi S, Harada S et al. . Placenta growth factor (PlGF) mRNA expression in brain tumors. J Neurooncol. 1998;40 (2):123–130. [DOI] [PubMed] [Google Scholar]
- 5. Carmeliet P, Moons L, Luttun A et al. . Synergism between vascular endothelial growth factor and placental growth factor contributes to angiogenesis and plasma extravasation in pathological conditions. Nat Med. 2001;7 (5):575–583. [DOI] [PubMed] [Google Scholar]
- 6. Hamerlik P, Lathia JD, Rasmussen R et al. . Autocrine VEGF-VEGFR2-Neuropilin-1 signaling promotes glioma stem-like cell viability and tumor growth. J Exp Med. 2012;209 (3):507–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kerber M, Reiss Y, Wickersheim A et al. . Flt-1 signaling in macrophages promotes glioma growth in vivo. Cancer Res. 2008;68 (18):7342–7351. [DOI] [PubMed] [Google Scholar]
- 8. Knizetova P, Ehrmann J, Hlobilkova A et al. . Autocrine regulation of glioblastoma cell cycle progression, viability and radioresistance through the VEGF-VEGFR2 (KDR) interplay. Cell Cycle. 2008;7 (16):2553–2561. [DOI] [PubMed] [Google Scholar]
- 9. Autiero M, Waltenberger J, Communi D et al. . Role of PIGF in the intra- and intermolecular cross talk between the VEGF receptors Flt1 and Flk1. Nat Med. 2003;9 (7):936–943. [DOI] [PubMed] [Google Scholar]
- 10. Tchaikovski V, Fellbrich G, Waltenberger J. The molecular basis of VEGFR-1 signal transduction pathways in primary human monocytes. Arterioscl Throm Vas. 2008;28 (2):322–328. [DOI] [PubMed] [Google Scholar]
- 11. Cao YH, Chen H, Zhou L et al. . Heterodimers of placenta growth factor vascular endothelial growth factor - Endothelial activity, tumor cell expression, and high affinity binding to Flk-1/KDR. J Biol Chem. 1996;271 (6):3154–3162. [DOI] [PubMed] [Google Scholar]
- 12. Cudmore MJ, Hewett PW, Ahmad S et al. . The role of heterodimerization between VEGFR-1 and VEGFR-2 in the regulation of endothelial cell homeostasis. Nat Commun. 2012;3:1–12. [DOI] [PubMed] [Google Scholar]
- 13. Dixelius J, Makinen T, Wirzenius M et al. . Ligand-induced vascular endothelial growth factor receptor-3 (VEGFR-3) heterodimerization with VEGFR-2 in primary lymphatic endothelial cells regulates tyrosine phosphorylation sites. J Biol Chem. 2003;278 (42):40973–40979. [DOI] [PubMed] [Google Scholar]
- 14. Favier B, Alam A, Barron P et al. . Neuropilin-2 interacts with VEGFR-2 and VEGFR-3 and promotes human endothelial cell survival and migration. Blood. 2006;108 (4):1243–1250. [DOI] [PubMed] [Google Scholar]
- 15. Zhang LQ, Zhou F, Han WC et al. . VEGFR-3 ligand-binding and kinase activity are required for lymphangiogenesis but not for angiogenesis. Cell Res. 2010;20 (12):1319–1331. [DOI] [PubMed] [Google Scholar]
- 16. Gee MFW, Tsuchida R, Eichler-Jonsson C et al. . Vascular endothelial growth factor acts in an autocrine manner in rhabdomyosarcoma cell lines and can be inhibited with all-trans-retinoic acid. Oncogene. 2005;24 (54):8025–8037. [DOI] [PubMed] [Google Scholar]
- 17. Soker S, Takashima S, Miao HQ et al. . Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell. 1998;92 (6):735–745. [DOI] [PubMed] [Google Scholar]
- 18. Tanno S, Ohsaki Y, Nakanishi K et al. . Human small cell lung cancer cells express functional VEGF receptors, VEGFR-2 and VEGFR-3. Lung Cancer. 2004;46 (1):11–19. [DOI] [PubMed] [Google Scholar]
- 19. Kil WJ, Tofilon PJ, Camphausen K. Post-radiation increase in VEGF enhances glioma cell motility in vitro. Radiat Oncol. 2012;7:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lu KV, Chang JP, Parachoniak CA et al. . VEGF inhibits tumor cell invasion and mesenchymal transition through a MET/VEGFR2 complex. Cancer Cell. 2012;22 (1):21–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pan Q, Chathery Y, Wu Y et al. . Neuropilin-1 binds to VEGF(121) and regulates endothelial cell migration and sprouting. J Biol Chem. 2007;282 (33):24049–24056. [DOI] [PubMed] [Google Scholar]
- 22. Stupp R, Mason WP, van den Bent MJ et al. . Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. New Engl J Med. 2005;352 (10):987–996. [DOI] [PubMed] [Google Scholar]
- 23. Weller M, van den Bent M, Hopkins K et al. . EANO guideline for the diagnosis and treatment of anaplastic gliomas and glioblastoma. Lancet Oncol. 2014;15 (9):E395–E403. [DOI] [PubMed] [Google Scholar]
- 24. Chinot OL, Wick W, Mason W et al. . Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. New Engl J Med. 2014;370 (8):709–722. [DOI] [PubMed] [Google Scholar]
- 25. Gilbert MR, Dignam JJ, Armstrong TS et al. . A randomized trial of bevacizumab for newly diagnosed glioblastoma. New Engl J Med. 2014;370 (8):699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Batchelor TT, Reardon DA, de Groot JF et al. . Antiangiogenic therapy for glioblastoma: current status and future prospects. Clin Cancer Res. 2014;20 (22):5612–5619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sandmann T, Bourgon R, Garcia J et al. . Patients with proneural glioblastoma may derive overall survival benefit from the addition of bevacizumab to first-line radiotherapy and temozolomide: retrospective analysis of the AVAglio trial. J Clin Oncol. 2015;33 (25):2735–U2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tabouret E, Boudouresque F, Farina P et al. . MMP2 and MMP9 as candidate biomarkers to monitor bevacizumab therapy in high-grade glioma. Neuro Oncol. 2015;17 (8):1174–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lee TH, Seng S, Sekine M et al. . Vascular endothelial growth factor mediates intracrine survival in human breast carcinoma cells through internally expressed VEGFR1/FLT1. Plos Med. 2007;4 (6):1101–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cao Y, E GQ, Wang EF et al. . VEGF exerts an angiogenesis-independent function in cancer cells to promote their malignant progression. Cancer Res. 2012;72 (16):3912–3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lampugnani MG, Orsenigo F, Gagliani MC et al. . Vascular endothelial cadherin controls VEGFR-2 internalization and signaling from intracellular compartments. J Cell Biol. 2006;174 (4):593–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bais C, Wu X, Yao J et al. . PlGF blockade does not inhibit angiogenesis during primary tumor growth. Cell. 2010;141 (1):166–177. [DOI] [PubMed] [Google Scholar]
- 33. Forsyth PA, Wong H, Laing TD et al. . Gelatinase-A (MMP-2), gelatinase-B (MMP-9) and membrane type matrix metalloproteinase-1 (MT1-MMP) are involved in different aspects of the pathophysiology of malignant gliomas. Brit J Cancer. 1999;79 (11–12):1828–1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Frank NY, Schatton T, Kim S et al. . VEGFR-1 expressed by malignant melanoma-initiating cells is required for tumor growth. Cancer Res. 2011;71 (4):1474–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lesslie DP, Summy JM, Parikh NU et al. . Vascular endothelial growth factor receptor-1 mediates migration of human colorectal carcinoma cells by activation of Src family kinases. Brit J Cancer. 2006;94 (11):1710–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mezquita B, Mezquita P, Pau M et al. . Unlocking doors without keys: activation of Src by truncated C-terminal intracellular receptor tyrosine kinases lacking tyrosine kinase activity. Cells. 2014;3 (1):92–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lassen U, Chinot OL, McBain C et al. . Phase 1 dose-escalation study of the antiplacental growth factor monoclonal antibody RO5323441 combined with bevacizumab in patients with recurrent glioblastoma. Neuro Oncol. 2015;17 (7):1007-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Batchelor TT, Mulholland P, Neyns B et al. . III randomized trial comparing the efficacy of cediranib as monotherapy, and in combination with lomustine, versus lomustine alone in patients with recurrent glioblastoma. J Clin Oncol. 2013;31 (26):3212–3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fischer C, Jonckx B, Mazzone M et al. . Anti-PIGF inhibits growth of VEGF(R)-inhibitor-resistant tumors without affecting healthy vessels. Cell. 2007;131 (3):463–475. [DOI] [PubMed] [Google Scholar]
- 40. Xu L, Cochran DM, Tong RT et al. . Placenta growth factor overexpression inhibits tumor growth, angiogenesis, and metastasis by depleting vascular endothelial growth factor homodimers in orthotopic mouse models. Cancer Res. 2006;66 (8):3971–3977. [DOI] [PubMed] [Google Scholar]
- 41. Cheng SY, Huang HJS, Nagane M et al. . Suppression of glioblastoma angiogenicity and tumorigenicity by inhibition of endogenous expression of vascular endothelial growth factor. Proc Natl Acad Sci U S A. 1996;93 (16):8502–8507. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.