Abstract
Background
miR-301a is frequently dysregulated and specific to human tumors, playing a critical role in tumorigenesis; however, the exact functions and regulatory mechanisms of miR-301a in glioma cells remain largely unknown. Herein, we show that miR-301a activated by the Wnt/β-catenin pathway promoted the invasion of glioma cells by directly targeting SEPT7.
Methods
Biochemical, luciferase reporter, and hromatin immunoprecipitation PCR assays characterized the function and regulatory mechanisms of miR-301a in glioma invasion.
Results
Initially, we detected the expression of miR-301a in glioma tissues and identified that miR-301a had increased, with ascending grades of the tumor. Furthermore, high levels of miR-301a were associated with a poorer prognosis in glioma patients. It is important to note that the Wnt/β-catenin/TCF4 pathway enhanced miR-301a expression by binding to the promoter region. To determine the oncogenic functions of miR-301a in glioma, SEPT7 was supported as the direct target gene. In addition, the Wnt/β-catenin pathway repressed SEPT7 expression, which was dependent on miR-301a in glioma cells. Finally, miR-301a was activated by Wnt/β-catenin and then promoted invasion of glioma cells by inhibiting the expression of SEPT7 in vitro and in vivo.
Conclusions
Our findings revealed the mechanism of action for miR-301a in tumor cell invasion. Moreover, the Wnt/miR-301a/SEPT7 signaling axis might be a novel target in treating glioma.
Keywords: glioma, invasion, miR-301a, SEPT7, Wnt/β-catenin signaling
Glioblastoma (GBM) is the most common primary brain tumor and is associated with poor prognosis due to its ability to infiltrate diffusely into the normal brain parenchyma. The prognosis for GBM has not seen any meaningful improvement despite advances in surgery and combined chemo-radiotherapy.1,2 Therefore, it is imperative to understand the detailed molecular pathogenesis of GBM and develop novel therapeutic strategies.
The Wnt/β-catenin signaling pathway is essential for regulating the growth, differentiation, and cell death pathways of normal epithelial cells. The Wnt/β-catenin signaling pathway is important for regulating calcium flux within cells, cytoskeletal changes, and gene transcription. The signaling is frequently dysregulated in malignant epithelial cells and represents a valid target in cancer drug development.3,4 This pathway is composed of a group of signal transduction elements that are involved in cancer initiation, cellular proliferation, differentiation, survival, apoptosis, and metastasis.
Previous studies have shown that multiple genes are currently being explored in Wnt/β-catenin downstream transcription that include Fra-1, c-myc, and Cyclin D.5 Several previously published reports suggest that the Wnt/β-catenin pathway plays a pivotal role in epithelial cancer progression, prognosis, and prediction of response to therapy.6,7 Accordingly, an association of a transcriptional relationship of cancer cells with Wnt/β-catenin signaling has generated significant interest in cancer treatment.
MicroRNAs (miRNAs) are evolutionarily conserved, small noncoding RNAs that contain 19–22 nucleotides. In general, miRNAs are abnormally dysregulated in a variety of cancers and are implicated in post-transcriptional regulation by targeting the 3′ untranslated region (3′-UTR) of a specific mRNA. miRNAs can act as oncogenes or tumor suppressors as protein-coding genes.8 Several reports have studied aberrant miRNA expression in human glioma. In previous studies, we have demonstrated that downregulation of serum miR-205 is a novel and valuable biomarker for the diagnosis of glioma and might serve as a prognostic factor for advanced pathological grades of cancer.9
MiR-144-3p plays an essential antitumor role in cellular resistance to temozolomide and radiation in GBM patients.10 MiR-301a acts as an oncogene in various tumors and does so by promoting proliferation, inhibiting apoptosis, and modulating chemical resistance. However, the status of miR-301a in glioma has not been reported. In the present study, therefore, we were committed to exploring the mechanisms of the Wnt/β-catenin pathway in regulating miR-301a by binding to the promoter region and its role in glioma cell invasion by its putative repression of SEPT7.
Materials and Methods
Cell Culture and Transfection
The human GBM cell lines U87, U251, LN229, LN18, and the low-grade glioma cell line H4 were obtained from the Institute of Biochemistry and Cell Biology of the Chinese Academy of Sciences.10 The cells were maintained in Dulbecco's modified Eagle's medium and incubated at 37°C in a fully humidified atmosphere containing 5% CO2 in filtered air.
We amplified full-length SEPT7 from U87 genomic DNA and cloned this into a pcDNA3 expression vector to construct the SEPT7 vector. An empty pcDNA3 vector was used as a control (referred to as “empty vector”). Chemical synthesis of miR-301a mimics (miR-301a), anti-sense miR-301a (AS-miR-301a), miR-301a negative control (miR-NC), siRNAs duplexes targeting human β-catenin (si-β-cat), and SEPT7 (si-SEPT7) was provided by GenePharma (Shanghai, China). siRNA duplexes with nonspecific sequences were used as siRNA negative controls (NCs). The cells were seeded into 6-well plates at a density of 3 × 105 cells/well using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. The double transfection method employs a first step of transfecting As-miR-301a for 48 hours, which is then followed by SEPT7 siRNA transfection.
Patients and Clinical Samples
This study was approved by the Tianjin Huanhu Hospital, Tianjin Hospital, Peking University Cancer Hospital and Institute, Laiwu City People's Hospital, and the P.L.A. Airforce General Hospital. All participants recruited for the study signed informed consent documents permitting us to use their donated tissue samples.
Controls of normal brain tissues were obtained from patients with severe traumatic brain injury. Thirty-two patients identified with glioma were recruited into the current study and included 10 pilocytic astrocytomas (WHO I), 10 diffuse astrocytomas (WHO II), 25 anaplastc astrocytomas (WHO III), and 30 primary GBMs (WHO IV) between October 2011 and July 2012. All specimens were handled and made anonymous according to local ethical and legal standards.
Luciferase Assays
TOP and FOP-FLASH (Millipore) reporter constructs were designed to detect the transcriptional activity of Wnt/β-catenin. Cells were cultured in 96-well plates and transfected with pGL3-WT-miR-301-promoter-Luc reporter or pGL3-MUT-miR-301-promoter-Luc reporter constructs after treatment with LiCl or β-catenin siRNA. Cells were co–transfected with wild-type or mutated SEPT7 3′UTR luciferase reporter vector and miR-301a mimics or AS-miR-301a. Following 48 hours of incubation, luciferase activity was measured using a dual-luciferase reporter system (Promega).11
Chromatin Immunoprecipitation PCR
Chromatin immunoprecipitation (ChIP) PCR was conducted using the ChIP assay kit (Millipore) according to the manufacturer's protocol. Glioma cells were cross-linked with 1% formaldehyde for 10 minutes at 37°C. The cells were then washed with cold phosphate-buffered saline containing protease inhibitors at 4°C for 10 minutes each. The ChIP incubation buffers were used to suspend cells that were then kept on ice. The solution was sonicated at 5 W for 12 pulses of 10 seconds each using a VirSonic sonicator. Cellular lysates were centrifuged for 15 minutes and incubated for 12 hours at 4°C to obtain DNA fragments with an average size of 500 bp, which were then immunoprecipitated with either anti-TCF4, anti-RNA Polymerase II antibodies, or nonimmune IgG as control at 1 µg of antibody per 106 cells with protein G beads. The beads were successively washed twice with low-salt wash buffer 1, one wash in high-salt wash buffer 2, followed by one wash in LiCi wash buffer 3, and 2 washes in Tris-EDTA buffer 4 for 5 minutes at 4°C. Purified DNA was subjected to PCR using primers that amplified a region before the transcription start site.
Real-Time qPCR and Western Blot
Trizol Reagent (Invitrogen) was applied to the extract for the isolation of total RNA. M-MLV Reverse Transcriptase (Promega) was used to reverse-transcribe the RNA according to the manufacturer's protocol. Real-time PCR was performed with the QuantiTect SYBR Green PCR mixture (Invitrogen) as previously described.10
Total protein lysates were separated from both parental and transfected cells by SDS-PAGE and transferred to PVDF membranes. The blot was incubated with primary antibody to enable the detection of SEPT7 and β-catenin (Santa Cruz; 1:1000 dilution), and β-actin (Santa Cruz, 1:1000 dilution) was used as control.
In vitro Matrigel Invasion Assay
The Matrigel invasion assay was designed in a 24-well invasion chamber system (BD Biosciences) with a polycarbonic membrane (diameter 6.5 mm and pore size 8 μm) according to the manufacturer's protocol. Cells were counted under a light microscope in 4 random fields (magnification ×100) after staining. Assays were done in triplicate for every experiment, and each experiment was repeated 3 times.
Establishment of Subcutaneous Tumor-bearing LN229 Cells in a Nude Mouse Model
All animal experiment procedures were carried out according to the regulations and internal biosafety and bioethical guidelines of Tianjin Municipal Science and Technology Commission. BALB/c-A nude mice were purchased from the Cancer Institute of the Chinese Academy of Sciences. A subcutaneous LN229 xenograft model was established in the right flanks of mice. Forty mice were randomized into 5 groups (ie, control, β-catenin siRNA, As-miR-301a, β-catenin siRNA + miR-301a mimics, and As-miR-301a + SEPT7 siRNA) when tumors reached a mass of approximately 200 mm3 in size. Lipofectamine 2000 with synthetic reagents was injected subcutaneously once every 3 days. At the end of 21 days, all mice were euthanized, and subcutaneous tumors were collected for further experiments.
Statistical Analysis
All statistical analyses were assessed with a commercially available software program (SPSS version 13.0; SPSS). The analysis of variance (ANOVA) was performed to evaluate the statistical differences among groups. The Kaplan-Meier method was applied to calculate data from the survival curves. Cox regression analysis was used to evaluate survival-related predictors. Statistical significance was determined at an alpha value of P < .01 (*).
Results
MiR-301a Overexpression in Glioma Tissues Confers Poor Prognosis
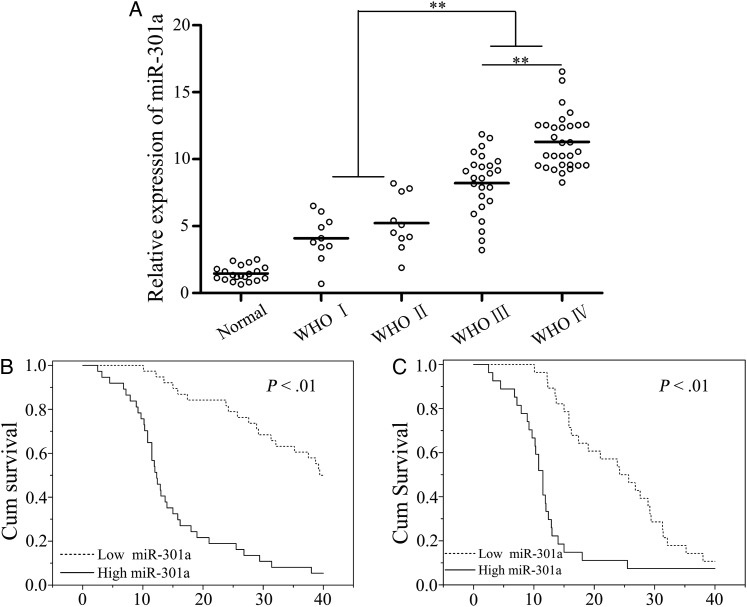
MiR-301a exhibited a relatively low level of expression in nonneoplastic brain tissues, whereas its expression was distinctly increased in glioma tissues (Fig. 1A). Importantly, a significant difference existed in the expression of miR-301a among low-grade (I-II), grade III, and grade IV glioma tissues (P < .01; Fig. 1A).
Fig. 1.
Analysis of miR-301a expression and the effect on glioma patient survival. (A) miR-301a expression in glioma and normal brain tissues as detected by quantitative PCR. (B) Kaplan-Meier (KM) survival curves analyzed glioma patients who had high or low expression of miR-301a. (C) KM survival curves analyzed high-grade glioma patients (III–IV) with high or low expression of miR-301a. *P < .01 as compared with normal brain tissues.
Furthermore, we investigated the association of miR-301a with various clinicopathological characteristics of human glioma. All partcipants were distributed into 2 groups with a median concentration of miR-301a (9.16). One way ANOVA demonstrated that overexpressed miR-301a was associated with a higher pathological grade and lower KPS (P < .01). However, there was no statistical significance seen between the level of miR-301a and other clinicopathological parameters such as sex and diagnostic age (Supplementary Material, Table S1).
We also evaluated the prognostic value of miR-301a expression in overall survival (OS) of glioma patients. Kaplan-Meier survival analysis demonstrated that patients with a higher than median level of miR-301a had a poorer survival rate in contrast to those with lower expression of miR-301a (P < .01; Fig. 1B). We then demonstrated that overexpression of miR-301a (more than a median concentration of 9.58) correlated with poorer survival in higher grades of glioma (grade III-IV) (P < .01; Fig. 1C). Finally, Cox regression analyses revealed a significant association between the presence of miR-301a and OS in glioma patients (P < 0.01; hazard ratio, 8.1; 95% CI, 4.8–17.4; Supplementary Material, Table S2). Taken together, miR-301a was shown to be enhanced in glioma tissues and might transpire to be a novel and valuable biomarker in the prognosis of human glioma.
MiR-301a Is Activated by the Wnt/β-catenin Pathway by Direct Binding to the Promoter Region
The mRNA level of β-catenin was detected in similar tissues and was to be upregulated along with an increased state of malignancy (Supplementary Material, Fig. S1A). Immunohistochemical staining analysis revealed that protein levels of β-catenin were also overexpressed in high-grade glioma (Supplementary Material, Fig. S1B). Moreover, β-catenin mRNA was positively correlated with miR-301a in glioma tissues (R2 = 0.647, P < .01; Supplementary Material, Fig S1C). Next, we examined the expression of β-catenin protein in different glioma cell lines. The levels of β-catenin in total, nuclear, and cytoplasmic proteins were lower in H4 as compared with other cell lines (Supplementary Material, Fig. S1D). Similarly, the relation of the Wnt/β-catenin activity and miR-301a expression was also paralleled in glioma cell lines. MiR-301a expression was accompanied with that of Wnt/β-catenin activity as detected by TOP/FOP reporter constructs (Supplementary Material, Fig. S1E and F).
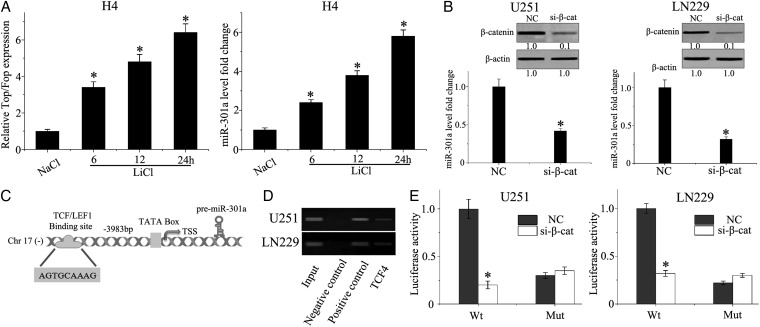
To determine the correlation between the Wnt/β-catenin signaling pathway and miR-301a, H4 glioma cells were treated with LiCl, which is an activator of the Wnt/β-catenin pathway by its effect on inhibiting glycogen synthase kinase (GSK)-3β and subsequent stability of β-catenin.11 As displayed in Fig. 2A, the expression of miR-301a was increased in a time-dependent manner following LiCl treatment when compared with NaCl control-treated cells. Moreover, we transfected U251 and LN229 cells with siRNA specific for β-catenin and quantified the expression of miR-301a by qPCR. Knockdown of β-catenin was associated with a significant decrease in miR-301a expression in U251 and LN229 cell lines (Fig. 2B). To characterize the transcriptional factor binding sites within the miR-301a promoter, human mature miR-301a sequences were identified by miRBase Sequences Database. Searching the Transcription Element Search System database (found at http://www.cbil.upenn.edu/cgi-bin/tess/tess), suggested a TCF4 binding site at 3983 bp from the transcription start site of the miR-301a promoter region and identified a putative consensus binding sequence of TCF4 is AGTGCAAAG (Fig. 2C).
Fig. 2.
miR-301a is activated by the Wnt/β-catenin pathway by direct binding to the promoter region. (A) TOP/FOP-FLASH and quantitative PCR were used to detect Wnt/β-catenin activity and miR-301a expression in H4 cells incubated with LiCl or NaCl at 20 mM for 24 hours. (B) LN229 and U251 cells were transfected with β-catenin siRNA or scrambled siRNA using lipofectamine 2000. The expression of β-catenin and miR-301a was detected by Western blot and qPCR, respectively. (C) TCF/LEF1 binding to the promoter region of miR-301a. (D) Predicted TCF4 binding site in the promoter region of miR-301a was validated by chromatin immunoprecipitation (ChIP) PCR. The negative control was incubated with nonimmune IgG, and the positive control was incubated with anti-RNA polymerase II). (E) The pGL3-WT-miR-301a-promoter-Luc reporter or pGL3-MUT-miR-301a-promoter-Luc reporter was transfected into LN229 or U251 cells, which were then treated with β-catenin siRNA. *P < .01 as compared with the control group.
To further validate the results, we performed ChIP assay using an anti-TCF4 antibody. ChIP-PCR bands were observed using primers, which were specific for the miR-301a promoter region, that covered the putative TCF/LEF-binding site to the control against irrelevant IgG (Fig. 2D). Finally, we constructed the pGL3-WT-miR-301a-promoter-Luc reporter and the pGL3-MUT-miR-301a-promoter-Luc reporter to investigate the functional transcriptional regulation of miR-301a by Wnt/β-catenin/TCF4 signaling. The WT-miR-301a reporter demonstrated low luciferase activity after dampening the functional expression of β-catenin in LN229 and U251 cells as compared with the empty vector control (P < .001; Fig. 2E). These data indicated that miR-301a is a direct target of the Wnt/β-catenin/TCF4 pathway.
MiR-301a Directly Targets the 3′-UTR of SEPT7 and Represses its Expression
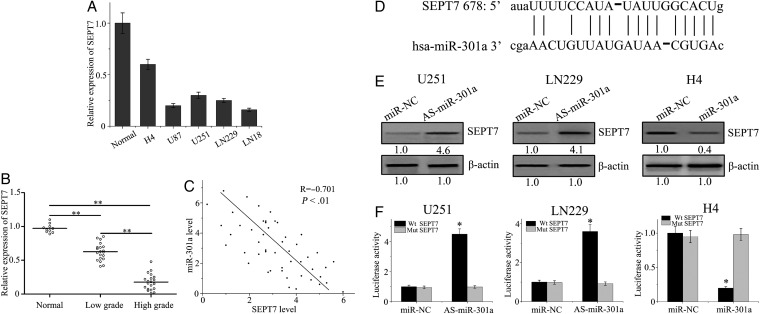
To explore the underlying mechanism of miR-301a in glioma, we predicted the target genes of miR-301a using the web-based software program, Targetscan. SEPT7, which is a member of the septin family and functions as an important tumor suppressor, is implicated in exocytosis, apoptosis, synaptogenesis, and tumorigenesis in malignant glioma. SEPT7 was predicted to be a possible target gene of miR-301a. Initially, we detected expression of SEPT7 protein in various kinds of GBM cells (Fig. 3A). To further explore the exact role of miR-301a in SEPT7 regulation, qPCR was performed in glioma and normal brain specimens to analyze SEPT7 expression. As shown in Fig. 3B, the expression of SEPT7 in glioma tissues was lower than normal tissue counterparts and decreased with ascending pathological grade. The level of SEPT7 was inversely correlated with the expression levels of miR-301a (Fig. 3C).
Fig. 3.
SEPT7 is a direct target gene of the action of miR-301a. (A) SEPT7 was analyzed by quantitative PCR (qPCR) in glioma cell lines. (B) The expression of SEPT7 mRNA in glioma and normal brain tissues was detected by qPCR. (C) The correlation between the levels of miR-301a and SEPT7 in glioma tissues was analyzed by the Pearson rank correlation test. (D) Sequence alignment between miR-301a and the 3′UTR of SEPT7. (E)Western blot analysis was performed to evaluate the expression levels of SEPT7 that were transfected with miR-301a mimics or AS-miR-301a. (F) pGL3-WT-SEPT7-3′UTR-Luc and pGL3-MUT-SEPT7-3′UTR-Luc reporter constructs were transfected with miR-301a mimics or AS-miR-301a in glioma cells. *P < .01 as compared with the control group.
Interestingly, SEPT7 3′UTR contains highly conserved target sites of miR-301a (Fig. 3D). To validate whether SEPT7 was regulated by miR-301a, we further examined the expression of SEPT7 in GBM cells that were transfected with miR-301a mimics or As-miR-301a. Our data showed that miR-301a expression dramatically changed the protein levels of SEPT7 (Fig 3E). To obtain direct evidence that miR-301a targets SEPT7 mRNA on the 3′UTR, the luciferase reporter assay was designed, whereby the 3′UTR of SEPT7 was closed. As demonstrated in Fig. 3F, the luciferase activity in Luc-SEPT7-UTR-transfected cells was significantly disrupted by miR-301a as compared with the SEPT7 3′UTR mutant or the NC group. Collectively, miR-301a directly targeted SEPT7 and actively repressed SEPT7 expression in glioma cells.
Wnt/β-catenin Pathway Represses SEPT7 Expression Through miR-301a in Glioma Cells
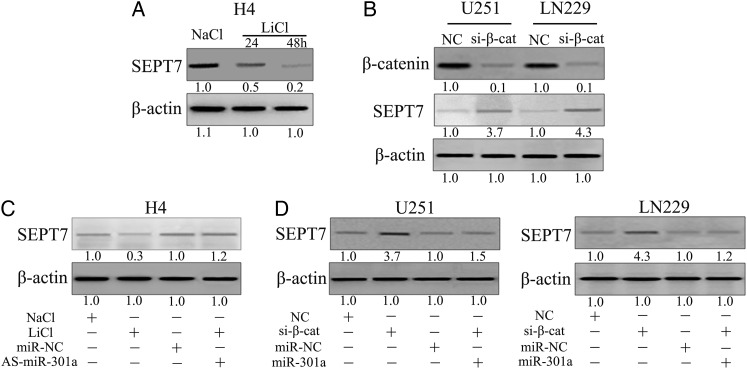
To determine the effect of the Wnt/β-catenin signaling pathway and disruption of SEPT7 expression, H4 cells were treated with LiCl to activate Wnt/β-catenin signaling, following which SEPT7 expression diminished in a time-dependent manner (Fig. 4A). Next, β-catenin siRNA was transfected into U251 and LN229 cells. As shown in Fig 4B, inhibition of β-catenin clearly augmented SEPT7 expression in both cells. We treated H4 cells with LiCl together with As-miR-301a and then examined the expression of SEPT7 to clarify whether the Wnt/β-catenin pathway repressed SEPT7 expression in a manner that depended on miR-301a. Expression of SEPT7 was reduced by LiCl treatment as compared with the control; however, downregulation of miR-301a rescued the repressive effect of the Wnt/β-catenin signaling pathway (Fig. 4C). Finally, we transfected β-catenin siRNAs into U251 and LN229 cells with or without co-transfecting miR-301a and found that miR-301a recovered the functional abilities of β-catenin siRNAs on SEPT7 regulation (Fig. 4D). Collectively, the above results demonstrated that the Wnt/β-catenin pathway diminished SEPT7 expression, which was dependent on miR-301a.
Fig. 4.
Wnt/β-catenin pathway represses SEPT7 expression through miR-301a in glioma cells. (A) H4 cells were treated with 20 mM LiCl or NaCl for the indicated periods of time, and expression of SEPT7 was detected by Western blot. (B) U87 and LN229 cells were transfected with β-catenin siRNA, and the expression of SEPT7 was detected by Western blot. (C) H4 cells were treated with LiCl and AS-miR-301a, and SEPT7 expression was detected by Western blot. (D) U251 and LN229 cells were transfected with β-catenin siRNA and miR-301a mimics, and SEPT7 expression were detected by Western blot.
MiR-301a Activated by Wnt Signaling Promotes Glioma Cell Invasion Mediated by SEPT7
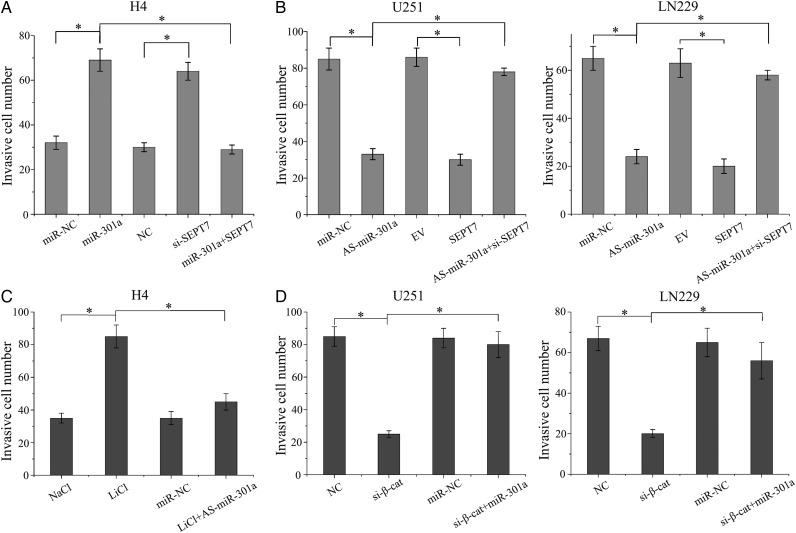
Transwell assay was performed to analyze the role of miR-301a in GBM cell invasion and the interactive mechanisms of the Wnt/β-catenin pathway and SEPT7. We transfected miR-301a mimics, SEPT7 siRNA, or miR-301a mimics with pcDNA3-SEPT7 into H4 cells. Both miR-301a mimics and SEPT7 siRNA significantly promoted the invasion of cells as compared with the negative control group; however, restoration of SEPT7 rescued the effects of miR-301a (Fig. 5A). In addition, we performed reverse experiments to detect the invasive ability when treated with As-miR-301a, pcDNA3-SEPT7, or As-miR-301a plus SEPT7 siRNA. As-miR-301a and pcDNA3-SEPT7 dramatically inhibited the invasion of GBM cells; nevertheless, the effect of miR-301a on cell invasion was rescued by SEPT7 inhibition (Fig. 5B).
Fig. 5.
miR-301a that was activated by Wnt signaling promoted glioma cell invasion through SEPT7. (A) H4 cells were transfected with miR-301a, SEPT7 siRNA, or miR-301a and pcDNA3-SEPT7, and in vitro cell invasion assays were studied. (B) The effects of AS-miR-301a, SEPT7 siRNA, or miR-301a and pcDNA3-SEPT7 on the invasive ability of U251 and LN229 cells are shown. (C) Transwell assays were set up for H4 cells that were treated with LiCl or As-miR-301a. (D) Transwell assays also set up for U251 and LN229 cells that were treated with β-catenin siRNA and miR-301a mimics. *P < .01 as compared with the control group.
Activated Wnt signaling enhanced the invasion of H4 cells, and the effect was overridden by downregulation of miR-301a (Fig. 5C). Likewise, downregulated β-catenin expression significantly reduced GBM cell invasive capacity, and overexpression of miR-301a could rescue the inhibited role of β-catenin siRNA in U251 and LN229 cell lines (Fig. 5D). Therefore, miR-301a activated by Wnt signaling promoted invasion of glioma cells in a mechanism that was dependent on the expression of SEPT7.
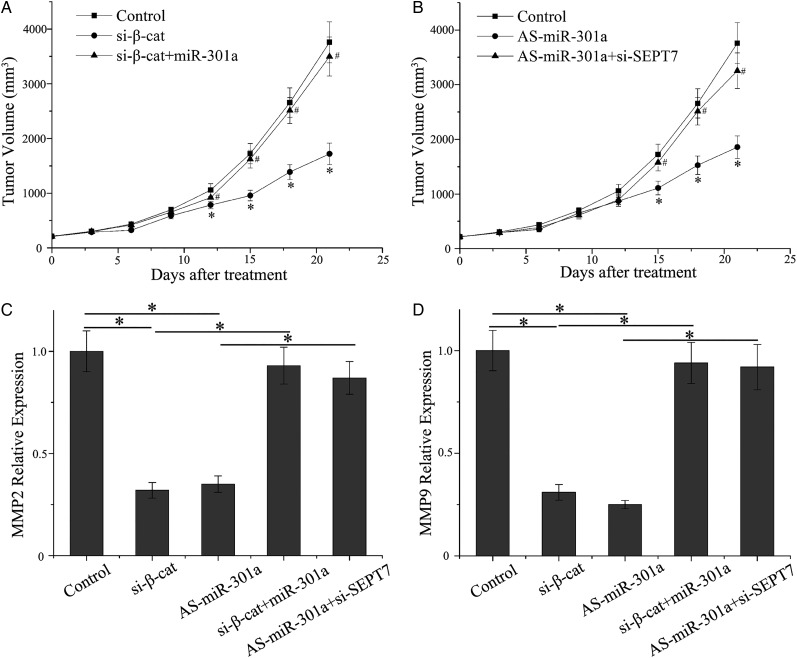
miR-301a Sppresses Invasion of Glioblastoma Cells in Vivo
To facilitate in vitro experiments, subcutaneous tumors were established by using the LN229 cell line to further assess the invasive effects of miR-301a in vivo. As shown in Fig. 6A, downregulation of β-catenin significantly reduced tumor invasive growth as compared with the control group, and the effects of tumor inhibition were overridden by miR-301a overexpression as observed by in vitro detection analyses (P < .01). Because miR-301a decreased tumor growth very effectively, the differences in tumor mass were still marked as compared with the control. Knockdown of SEPT7 counteracted the function of AS-miR-301a (Fig. 6B). Western blot assay was performed to examine the level of MMP2/9 proteins, which represented the invasive capacity of tumor cells. Moreover, β-catenin siRNA and As-miR-301a both inhibited the expression of MMP2/9. However, there are no statistical differences when cells were co-transfected with β-catenin siRNA and miR-301a mimics or As-miR-301a and SEPT7 siRNA (Fig. 6C and D). Therefore, the Wnt/β-catenin/miR-301a/SEPT7 axis could interfere in the invasiveness of glioma cells in vivo.
Fig. 6.
miR-301a suppresses invasion of GBM cells in vivo. (A) Tumor growth was measured after treatment with β-catenin siRNA or β-catenin siRNA plus miR-301a mimics. (B) Tumor growth was measured after treatment with As-miR-301a or As-miR-301a plus SEPT7 siRNA. (C, D) Western blot revealed MMP2 and MMP9 levels in different groups.
Discussion
The invasive ability of GBM is an essential contributing factor leading to an aggressive clinical course and poor patient outcome. Owing to the fact that invasive growth is the most important feature of malignant glioma, targeting the involved process is a rational approach in an attempt to find a cure for glioma.4,12 Accumulating evidence suggests that miRNAs play crucial roles in carcinogenesis and malignant progression such as invasion.
MiR-940 promotes tumor cell invasion and metastasis by directly targeting ZNF24 in gastric cancer.13 MiR-494 inhibits cell proliferation and invasion of chondrosarcoma cells by regulating SOX9 expression both in vivo and in vitro.14 MiR-106b induces apoptosis and suppresses invasion of thyroid cancer by mediating inhibition of C1orf24 expression.15 MiR-542-3p acts as a negative regulator and suppresses tumor cell invasion by targeting the AKT pathway in astrocytoma progression.16 MiR-30a-5p is regulated by the Wnt/β-catenin pathway and promotes glioma cell invasion by repressing NCAM.17
MiR-301a is identified as a candidate oncogene that is controversial in different types of cancers. As reported, miR-301a functions as a novel oncogene in pancreatic cancer, and the oncogenic activity may involve its negative regulation of the target gene, SMAD4.18 MiR-301a augments the metastasis of prostate cancer by modulating AR/TGF-β1/Smad/MMP9 signals.19 MiR-301a is a potential biomarker for doxorubicin resistance in osteosarcoma and modulates doxorubicin resistance by targeting AMP-activated protein kinase alpha 1.20 Overexpression of miR-301a correlates with a poor prognosis in triple-negative breast cancer.21 Upregulated miR-301a promotes tumor metastasis by targeting PTEN and activating Wnt/β-catenin signaling in breast cancer.22 However, the role of miR-301a in the occurrence and development of GBM and its transcriptional regulation is unclear.
In this work, we further clarified that miR-301a was induced by the Wnt/β-catenin pathway and the detailed functions of the invasive ability of tumors by identifying SEPT7 as a novel target gene, which are necessary for the invasive function of glioma cells.
It is now clear that Wnt/β-catenin signaling regulates cell proliferation, differentiation, adhesion, migration, and stem cell self-renewal in various types of human cancers.23 The central event of Wnt/β-catenin signaling is β-catenin accumulating, translocating, interacting, and activating TCF/LEF DNA binding proteins, thus forming a complex of transcription activators.24
Recently, many researchers have reported that miRNA interferes with the Wnt/β-catenin signaling pathway by regulating the post-transcription of targeted genes.25 However, there are a few studies concerning the roles of miRNAs in mediating the Wnt/β-catenin pathway. In the previous study, we analyzed TCF4 binding sites of the miRNA promoter element in genome-wide scanning and demonstrated that miR-21 was a direct target of the Wnt/β-catenin/TCF4 pathway in GBM cells.11
Here, we explored the function of miR-301a in human glioma and clarified its relationship with the Wnt/β-catenin pathway. We initially detected the expression of miR-301a in glioma tissues and identified that miR-301a increased with ascending grades. Furthermore, high levels of miR-301a were associated with poorer prognoses for glioma patients. It is important to note that our studies indicated that miR-301a expression is positively correlated with that of β-catenin in glioma tissues. We show for the first time that the Wnt/β-catenin/TCF4 upregulates miR-301a expression by binding directly to the promoter region. To determine the exact oncogenic functions of miR-301a in glioma, SEPT7 was supported as the direct target gene of miR-301.
It has been previously reported that SEPT7 plays a suppressive role and inhibits the migratory and invasive abilities of glioma cells. SEPT7 can enhance the expression of TIMP1 and TIMP2, which can markedly reduce the expression of MMP2, MMP9, MT1-MMP, and integrin αvβ3 can also finally alter the structure of α-tubulin.26–29 In the present study, we clarified that SEPT7 could be repressed by Wnt/β-catenin signaling and the important mediating gene is miR-301a. Finally, we showed that under conditions where miR-301a was stimulated by Wnt signaling, this promoted invasion of glioma cells by repressing the expression of SEPT7 both in vitro and in vivo.
In conclusion, these results suggest that the Wnt/β-catenin pathway can serve as a key transcriptional regulator for manipulating miR-301a expression and that miR-301a promotes glioma invasion by repressing SEPT7 expression. Our findings revealed the putative mechanism for miR-301a in tumor cell invasion. We also revealed that the Wnt/miR-301a/SEPT7 signaling axis may be a novel target for the treatment of glioma.
Supplementary Material
Funding
This work was supported by the China National Natural Scientific Fund (Grant No. 81201973 and No. 81502654).
Conflict of interest statement. None.
Supplementary Material
References
- 1. Wakimoto H, Tanaka S, Curry WT et al. . Targetable signaling pathway mutations are associated with malignant phenotype in IDH-mutant gliomas. Clin Cancer Res. 2014;20 (11):2898–2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hutterer M, Hattingen E, Palm C, Proescholdt MA, Hau P. Current standards and new concepts in MRI and PET response assessment of antiangiogenic therapies in high-grade glioma patients. Neuro Oncol. 2015;17 (6):784–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Paul I, Bhattacharya S, Chatterjee A, Ghosh MK. Current understanding on EGFR and Wnt/beta-Catenin signaling in glioma and their possible crosstalk. Genes Cancer. 2013;4 (11–12):427–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Xie Q, Mittal S, Berens ME. Targeting adaptive glioblastoma: an overview of proliferation and invasion. Neuro Oncol. 2014;16 (12):1575–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gu X, Yao L, Ma G et al. . TCTP promotes glioma cell proliferation in vitro and in vivo via enhanced beta-catenin/TCF-4 transcription. Neuro Oncol. 2014;16 (2):217–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Madan B, Virshup DM. Targeting Wnts at the source–new mechanisms, new biomarkers, new drugs. Mol Cancer Ther. 2015;14 (5):1087–1094. [DOI] [PubMed] [Google Scholar]
- 7. Blagodatski A, Poteryaev D, Katanaev VL. Targeting the Wnt pathways for therapies. Mol Cell Ther. 2014;2:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Djuranovic S, Nahvi A, Green R. A parsimonious model for gene regulation by miRNAs. Science. 2011;331 (6017):550–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yue X, Lan F, Hu M, Pan Q, Wang Q, Wang J. Downregulation of serum microRNA-205 as a potential diagnostic and prognostic biomarker for human glioma. J Neurosurg. 2016;124 1:122–128. [DOI] [PubMed] [Google Scholar]
- 10. Lan F, Yu H, Hu M, Xia T, Yue X. miR-144-3p exerts anti-tumor effects in glioblastoma by targeting c-Met. J Neurochem. 2015;135 2:274–286. [DOI] [PubMed] [Google Scholar]
- 11. Lan F, Pan Q, Yu H, Yue X. Sulforaphane enhances temozolomide-induced apoptosis because of down-regulation of miR-21 via Wnt/beta-catenin signaling in glioblastoma. J Neurochem. 2015;134 (5):811–818. [DOI] [PubMed] [Google Scholar]
- 12. Paw I, Carpenter RC, Watabe K, Debinski W, Lo HW. Mechanisms regulating glioma invasion. Cancer Lett. 2015;362 (1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liu X, Ge X, Zhang Z et al. . MicroRNA-940 promotes tumor cell invasion and metastasis by downregulating ZNF24 in gastric cancer. Oncotarget. 2015;6 28:25418–25428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li J, Wang L, Liu Z et al. . MicroRNA-494 inhibits cell proliferation and invasion of chondrosarcoma cells in vivo and in vitro by directly targeting SOX9. Oncotarget. 2015;6 28:26216–26229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Carvalheira G, Nozima BH, Cerutti JM. microRNA-106b-mediated down-regulation of C1orf24 expression induces apoptosis and suppresses invasion of thyroid cancer. Oncotarget. 2015;6 29:28357–28370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cai J, Zhao J, Zhang N et al. . MicroRNA-542-3p suppresses tumor cell invasion via targeting AKT pathway in human astrocytoma. J Biol Chem. 2015;290 41:24678–24688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang Z, Dai X, Chen Y et al. . MiR-30a-5p is induced by Wnt/beta-catenin pathway and promotes glioma cell invasion by repressing NCAM. Biochem Biophys Res Commun. 2015;465 (3):374–380. [DOI] [PubMed] [Google Scholar]
- 18. Xia X, Zhang K, Cen G et al. . MicroRNA-301a-3p promotes pancreatic cancer progression via negative regulation of SMAD4. Oncotarget. 2015;6 (25):21046–21063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Xie H, Li L, Zhu G et al. . Infiltrated pre-adipocytes increase prostate cancer metastasis via modulation of the miR-301a/androgen receptor (AR)/TGF-beta1/Smad/MMP9 signals. Oncotarget. 2015;6 (14):12326–12339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang Y, Duan G, Feng S. MicroRNA-301a modulates doxorubicin resistance in osteosarcoma cells by targeting AMP-activated protein kinase alpha 1. Biochem Biophys Res Commun. 2015;459 (3):367–373. [DOI] [PubMed] [Google Scholar]
- 21. Yu H, Li H, Qian H et al. . Upregulation of miR-301a correlates with poor prognosis in triple-negative breast cancer. Med Oncol. 2014;31 (11):283. [DOI] [PubMed] [Google Scholar]
- 22. Ma F, Zhang J, Zhong L et al. . Upregulated microRNA-301a in breast cancer promotes tumor metastasis by targeting PTEN and activating Wnt/beta-catenin signaling. Gene. 2014;535 (2):191–197. [DOI] [PubMed] [Google Scholar]
- 23. Hoffmeyer K, Raggioli A, Rudloff S et al. . Wnt/beta-catenin signaling regulates telomerase in stem cells and cancer cells. Science. 2012;336 (6088):1549–1554. [DOI] [PubMed] [Google Scholar]
- 24. Wang Y, Krivtsov AV, Sinha AU et al. . The Wnt/beta-catenin pathway is required for the development of leukemia stem cells in AML. Science. 2010;327 (5973):1650–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ghahhari NM, Babashah S. Interplay between microRNAs and WNT/beta-catenin signalling pathway regulates epithelial-mesenchymal transition in cancer. Eur J Cancer. 2015;51 (12):1638–1649. [DOI] [PubMed] [Google Scholar]
- 26. Xu S, Jia ZF, Kang C et al. . Upregulation of SEPT7 gene inhibits invasion of human glioma cells. Cancer Invest. 2010;28 (3):248–258. [DOI] [PubMed] [Google Scholar]
- 27. Jia ZF, Huang Q, Kang CS et al. . Overexpression of septin 7 suppresses glioma cell growth. J Neurooncol. 2010;98 (3):329–340. [DOI] [PubMed] [Google Scholar]
- 28. Jia Z, Wang K, Wang G, Zhang A, Pu P. MiR-30a-5p antisense oligonucleotide suppresses glioma cell growth by targeting SEPT7. PLoS One. 2013;8 (1):e55008. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 29. Jiang H, Hua D, Zhang J et al. . MicroRNA-127-3p promotes glioblastoma cell migration and invasion by targeting the tumor-suppressor gene SEPT7. Oncol Rep. 2014;31 (5):2261–2269. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.