Abstract
Background
p53 is a promising target in human cancer. p28 is a cell-penetrating peptide that preferentially enters cancer cells and binds to both wild-type and mutant p53 protein, inhibiting COP1-mediated ubiquitination and proteasomal degradation. This results in increased levels of p53, which induces cell cycle arrest at G2/M. We conducted a phase I study to determine the maximum-tolerated dose (MTD) and describe the dose-limiting toxicities (DLTs) and pharmacokinetics (PKs) of p28 in children.
Methods
Children aged 3–21 years with recurrent or progressive central nervous system tumors were eligible. Intravenous p28 was administered 3 times weekly for 4 consecutive weeks of a 6-week cycle at 4.16 mg/kg/dose (the adult recommended phase II dose) using a rolling-6 study design. Expression status of p53 was characterized by immunohistochemistry, and serum PK parameters were established on the second dose.
Results
Of the 18 eligible patients enrolled in the study, 12 completed the DLT monitoring period and were evaluable for toxicity. p28 was well-tolerated; 7 participants received ≥2 courses, and the most common adverse event attributed to the drug was transient grade 1 infusion-related reaction. PK analysis revealed a profile similar to adults; however, an increased area under the curve was observed in pediatric patients. High p53 expression in tumor cell nuclei was observed in 6 of 12 available tissue samples. There were no objective responses; 2 participants remained stable on the study for >4 cycles.
Conclusions
This phase I study demonstrated that p28 is well-tolerated in children with recurrent CNS malignancies at the adult recommended phase II dose.
Keywords: azurin, central nervous system tumors, p28, pediatric, phase I.
Survival rates for many types of pediatric central nevous system (CNS) tumors continue to improve. In contrast, patients with recurrent or progressive high-grade tumors generally have a poor prognosis despite current treatment regimens.1 The lack of long-term response to therapy has prompted detailed analyses of the molecular origins of adult and pediatric CNS tumors.2–6 Structural alterations in the tumor suppressor protein p53 are of fundamental importance to the pathogenesis and progression of both adult and pediatric CNS tumors.7,8 p53 is central to the regulation of the cell cycle, DNA repair, development, and programmed cell death (apoptosis) through a myriad of signaling pathways.9 The TP53 gene is mutated in ∼50% of all human solid tumors. These tumors can express constitutively high levels of mutant p53 due to a lack of feedback control of p53 protein levels.10–12 In malignant glioma, p53 mediates an initial response to conventional chemotherapy agents, and p53 regulation is also intimately involved in resistance to these agents.13–15 Overexpression of p53 in malignant gliomas during childhood is strongly associated with an adverse outcome, independent of clinical prognostic factors and histologic findings.16
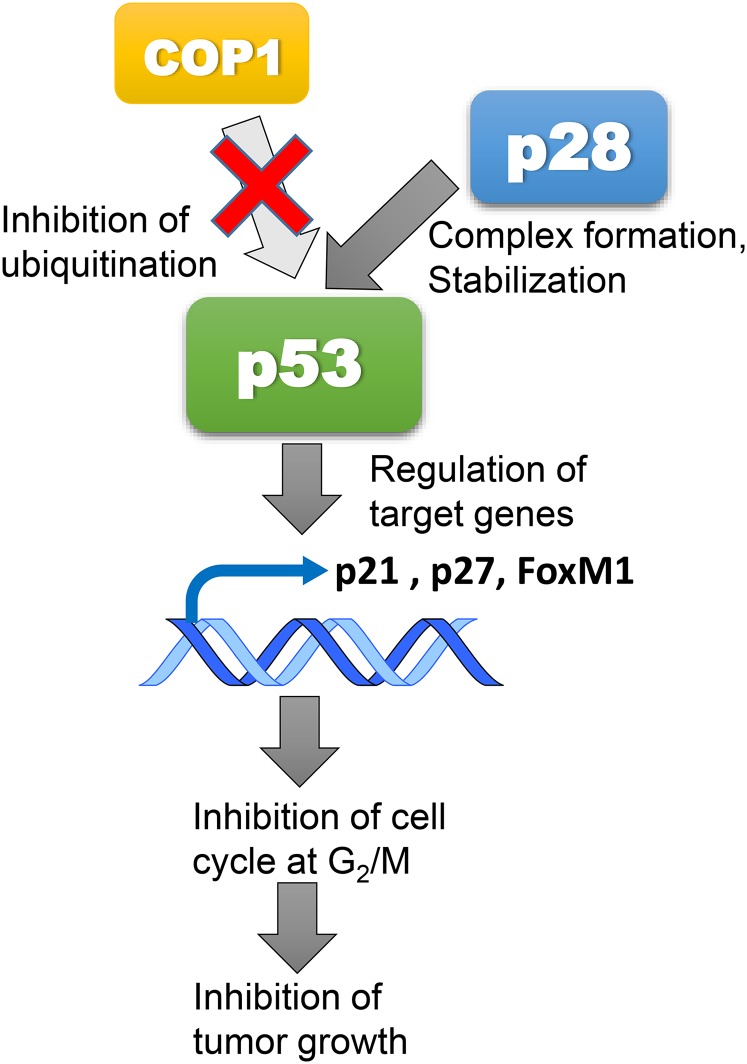
To date, strategies for restoration of p53 functions in tumors have focused on targeting wild-type p53 with the aim of protecting p53 from degradation by a major endogenous regulator, HDM2.17,18 p28 is a novel anticancer agent derived from azurin, a 128 amino acid cupredoxin, which is secreted by the opportunistic pathogen Pseudomonas aeruginosa and contains an amphipathic α-helical motif that is responsible for the preferential penetration of azurin and p28 into human cancer cells.15,19,20 As such, p28 acts as a cell-penetrating peptide that is processed into the nucleus and blocks the binding of constitutional morphogenic protein 1 (Cop1) to p53. The decrease in Cop1 through autodegradation results in an increase in intracellular levels of wild-type and mutant p53 and induces cell cycle arrest at G2/M.20–22 p28 is the lead agent in a series of cell-penetrating peptides that enhance the stability of p53 (Fig. 1).
Fig. 1.
p28 Mechanism of antitumor action. p28 binds with high affinity to the p53 DNA binding domain blocking COP1-mediated proteasomal degradation of p53. The posttranslational increase in the level and activity of p53 regulates the activity of the downstream genes, p21, p27 and FoxM1, leading to inhibition of the cancer cell cycle at G2/M and subsequent apoptosis.
p28 also enters endothelial cells, where it exerts a direct antiangiogenic effect halting tumor neoangiogenesis.23 p28 exerts this activity through a non-p53-mediated mechanism: a noncompetitive inhibition of the VEGFR2 and FGFR1 kinases, which in turn significantly reduces the phosphorylation of their downstream targets FAK and Akt, inhibiting endothelial cell motility and migration.23 Even more importantly, p28 transcends the endothelial cell, crossing the blood-brain barrier and saturating the brain parenchyma in a dose-related manner.24
In preclinical testing, the antitumor efficacy of p28 was assessed on human breast cancer, prostate cancer, and melanoma cells in vitro and resulted in dose-dependent reduced proteasomal degradation of p53 and induction of G2-M cell cycle arrest.19,20 Subsequently, a phase I trial in adults with metastatic solid tumors excluding CNS tumors with >10% p53 expression by immunohistochemistry (IHC) did not report any dose-limiting toxicities (DLTs) or significant adverse events in the 15 participants enrolled. The highest dose level (4.16 mg/kg/dose) was selected as the recommended phase II dose (RP2D). Best responses included 1 complete response (CR), 3 partial responses (PRs), and 7 patients with stable disease (SD). Three participants with melanoma or colon cancer were alive at 25, 32, and 36 months after therapy completion at the time of publication. Consistent with animal models, no immune response to the peptide was observed in any participant at any dose level.25
These promising data led to the development of this phase I trial within the Pediatric Brain Tumor Consortium (PBTC). The primary objectives of this study were to establish whether the adult R2PD of p28 was safe for children with recurrent or refractory CNS tumors and to characterize the serum PKs of p28 in children. Secondary objectives were to describe the antitumor activity of p28 in this patient population and characterize the level of p53 expression in available tumors.
Materials and Methods
Children aged 3–21 years with histologically confirmed progressive, recurrent, or refractory high-grade glioma, medulloblastoma, primitive neuroectodermal tumors, atypical teratoid rhabdoid tumor (AT/RT), diffuse intrinsic pontine glioma (DIPG), or choroid plexus carcinoma for whom no curative therapy existed were eligible. A histopathologic diagnosis was not required for participants with DIPG.
Other eligibility criteria included Karnofsky (for patients aged >16 y) or Lansky (for patients ≤16 y) performance status of ≥50, adequate renal, hepatic, and hematologic function, and recovery from prior therapy including myelosuppressive chemotherapy (3 weeks from the last dose and 6 weeks for nitrosureas), immunotherapy (3 weeks from the last dose), biological agents (≥7 days from the last dose), monoclonal antibody treatments (30 days or 3 half-lives from the last dose). Participants were required to be neurologically stable and on stable or decreasing doses of corticosteroids for at least one week before enrollment. For participants who had recently received radiation therapy, an interval of ≥3 months from craniospinal radiation, ≥8 weeks from local irradiation to the primary tumor, and ≥2 weeks from focal irradiation to symptomatic sites was required.
Patients were excluded if they were receiving other anticancer or experimental agents, required growth factors, or had uncontrolled infections, seizures, or other systemic illness. Women who were pregnant or lactating were also excluded. The institutional review board of each PBTC participating site approved the trial. Written informed consent was obtained from all participants or legal guardians, and assent was obtained from minor subjects according to institutional guidelines.
Drug Administration
p28 (NSC #745104) was supplied by CDG Therapeutics, Inc. as a sterile, preservative-free, lyophilized powder. The reconstituted solution was administered intravenously, followed by a 15–30 minute infusion of 50 mL normal saline or dextrose. Alternatively, p28 could be administered in 50 mL normal saline and infused over 15–30 minutes. p28 was given 3 times a week for 4 consecutive weeks followed by a 2-week rest (1 course = 6 weeks). It was generally administered in the outpatient setting. Participants were to receive up to 10 courses of therapy unless they experienced unacceptable toxicity or disease progression. The starting dose of p28 was 4.16 mg/kilogram/dose, which is the adult R2PD, considering that the drug was extremely well tolerated in the adult phase I study. Dose de-escalation was planned and governed by the rolling-6 design (to be implemented in the event that dose level 1 was found to be too toxic). All subsequent courses required a minimum of stable disease and organ function that met eligibility criteria prior to drug administration.
Monitoring
Toxicity monitoring included weekly history and physical examination, complete blood counts, metabolic profile, and serum pregnancy test for females of child-bearing potential during the first course. For subsequent courses, an interval history and physical assessment as well as a complete blood count and metabolic profile were required prior to receiving p28. Assessment of tumor status was performed by MRI of the brain (and spine, if applicable) at the end of courses 2, 4, 6, 8, and at the time of disease progression or end of therapy.
Trial Design
The rolling-6 phase I design was used to assess safety of the adult recommended dose (4.16 mg/kg/dose). Higher dose levels were not pursued due to solubility concerns. If dose de-escalation was needed, the MTD was to be defined as the highest dose level in which no more than 1 of 6 participants experienced DLTs. Patients who received <75% of the total dose of drug in the first course of protocol therapy for reasons other than toxicity were considered inevaluable for MTD evaluation and were replaced. Once the RP2D was achieved, there was a planned expansion to 12 participants.
Toxicities were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0 (CTCAE v4.0). DLT was defined as any of the following events occuring during the 6-week dose-finding period: grade 4 neutropenia or thrombocytopenia, any grade 4 nonhematologic toxicity at least possibly related to p28 persisting for ≥7 days, any grade 3 nonhematologic toxicity at least possibly related to p28 (except fever or infection <5 days duration, nausea and vomiting <3 days duration, electrolyte abnormalities responsive to supplementation, or elevation of transaminases that returned to baseline within 7 days of drug interruption and did not recur upon restarting therapy). A DLT was further defined as any drug-related toxicity resulting in the permanent cessation of therapy or that resulted in missing more than 3 consecutive doses of p28.
Standard 2-dimensional imaging criteria were used for response assessment. Response findings for stable disease must have been maintained for 24 weeks (4 courses).
Pharmacokinetics
During course 1, blood samples for pharmacokinetics (PK) were required preinfusion and 5, 10, 20, 30, and 60 minutes after the second dose of p28 in all participants. Blood samples were collected from the opposite limb or a site other than the site of administration. Samples were assayed for p28 and 2 major metabolites by fast liquid chromatography/tandem mass spectrometry as previously described.26,27 Individual participant serum concentration–time data for each dose of p28 were analyzed by standard noncompartmental methods, and dose- and time-related increases in the amount of each metabolite in serum were quantified as percentage of the total peak area of p28.26
Immunohistochemistry
For consenting participants for whom previous tumor tissue was available, representative tissue sections were stained for p53 status as previously described28 using a monoclonal antibody to mutant and wild-type p53 (DO-1, sk-126; Santa Cruz Biotechnology) and visualized by biotinylated secondary antibody and ABC kit (Vector). Sections were counterstained with hematoxylin to identify tumor morphology. Ten separate areas from each tumor slide were evaluated, and a minimum 1000 tumor cells were counted for statistical analysis. All slides were evaluated by 2 independent pathologists without prior knowledge of patient status. Only cells with nuclear staining for p53 were considered positive; a tumor was classified as p53-positive when ≥10% of cells analyzed were positive.
Results
A total of 18 eligible participants were enrolled from October 2013 to August 2014. Twelve of the 18 participants were fully evaluable for toxicity. Among the 6 participants who were determined to be inevaluable, 5 received <75% of study drug during the first course and came off treatment due to progressive disease, and 1 participant progressed prior to receiving any study medication. Patient characteristics are detailed in Table 1.
Table 1.
Patient characteristics
| Characteristic | No. of Patients |
|---|---|
| Total patients enrolled | 18 |
| Evaluable | 12 |
| Inevaluable | 6 |
| Diagnosis | |
| Atypical teratoid/rhabdoid tumor | 1 |
| Choroid plexus carcinoma | 2 |
| Diffuse intrinsic pontine glioma | 2 |
| High-grade glioma | |
| Anaplastic astrocytoma | 1 |
| Giant cell glioblastoma | 1 |
| Glioblastoma multiforme | 3 |
| Glioma, other | 4 |
| Medulloblastoma | 2 |
| Pineoblastoma | 2 |
| Sex | |
| Male | 11 |
| Female | 7 |
| Age, y | |
| Median | 11.8 |
| Range | 3–19 |
| Ethnicity | |
| Hispanic or Latino | 3 |
| Non-Hispanic | 15 |
| Race | |
| Black | 5 |
| Unknown | 1 |
| White, non-Hispanic | 12 |
Adverse Events
p28 was well-tolerated. Among the 12 evaluable participants, the most common adverse events attributed to the drug were transient grade 1 or 2 infusion-related reactions manifested as flushing, hot flashes, dizziness, headache, or changes in blood pressure. During the dose-finding period, 1 participant with metastatic pineoblastoma had 2 DLTs of grade 4 neutropenia and thrombocytopenia. Table 2 summarizes all grade 2 or higher adverse events that were at least possibly related to p28 among eligible participants on the study.
Table 2.
Summary of grade 2 and higher adverse events possibly, probably, or definitely related to p28 for 17 participants and a total of 32 courses
| Adverse Events [events (pts.)] | 2 | 3 | 4 |
|---|---|---|---|
| Platelet count decreased | 10 (2) | 10 (1) | 4 (1)a |
| Lymphocyte count decreased | 7 (4) | 2 (2) | |
| White blood cell decreased | 4 (4) | ||
| Anemia | 3 (2) | 1 (1) | |
| Neutrophil count decreased | 2 (2) | 1 (1)a | |
| Nausea | 1 (1) | ||
| Fatigue | 1 (1) | ||
| Hypoglycemia | 2 (2) | ||
| Constipation | 1 (1) | ||
| Hyperglycemia | 1 (1) | ||
| Abdominal pain | 1 (1) |
aAll grade 4 events were in a single participant.
Pharmacokinetics of p28
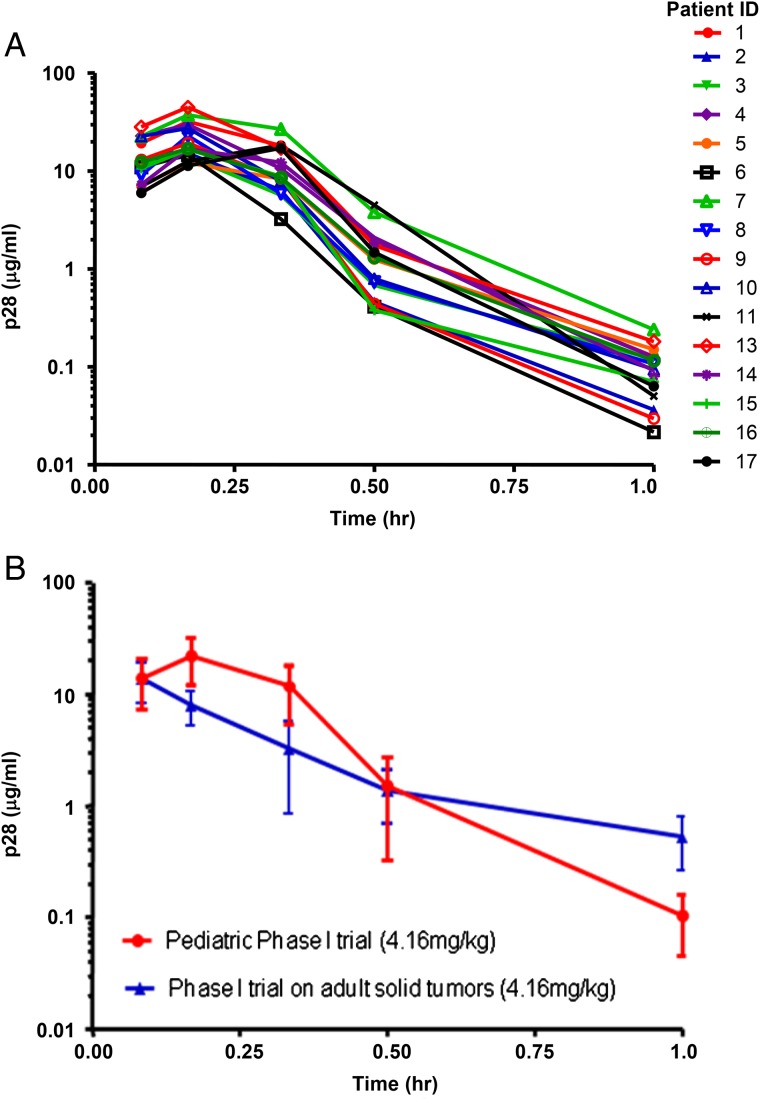
PK analysis was performed on 16 participants. The overall concentration of p28 with time after administration was similar among the 16 participants as shown in Fig. 2A. The time to reach maximum serum concentration (Tmax) was 11.3 ± 0.8 minutes, and half-life was 0.12 ± 0.02 hours (Fig. 2B). An increased area under the curve (AUC) was observed in pediatric participants when compared with adult participants, which is likely a result of a higher Cmax concentration (pediatric patients 22.6 µg/mL; adult patients 13.7 µg/mL), a prolonged half-life of β-phase (elimination), and shorter γ-phase. PK parameters are summarized in Table 3.
Fig. 2.
(A) Individual participant p28 serum concentration. Plots of p28 concentration versus time in pediatric patients receiving 4.16 mg/kg of p28. Serum samples were applied to liquid chromatography-tandem mass spectrometry and p28 concentration was determined from the elution profiles. (B) Concentration profiles of p28. p28 concentration versus time profiles of 16 pediatric patients (red) and 7 adult patients (blue) receiving 4.16 mg/kg dose.
Table 3.
Summary of pharmacokinetic parameters for 16 pediatric participants receiving p28
| p28 dose (mg/kg) | 4.16 |
| Cmax (μg/mL) | 22.6 ± 2.2 |
| Tmax (min) | 11.3 ± 0.8 |
| t1/2 (h) | 0.12 ± 0.02 |
| t1/2α (h) | 0.01 ± 0.002 |
| t1/2β (h) | 0.07 ± 0.01 |
| t1/2γ (h) | 0.43 ± 0.1 |
| AUClast (h-μg/mL) | 6.4 ± 0.6 |
| Cl (mL/kg/r) | 743 ± 64 |
| Vdss (mL/kg) | 168 ± 13 |
Abbreviations: h, hour; min, minute.
Pharmacokinetics parameters (C max = p28 maximum concentration in serum, T max = time to C max, t 1 / 2 = terminal half-life of p28, t1 / 2 α = rapid distribution half-life, t1 / 2β = slow distribution half-life, t1 / 2 γ = elimination half-life, AUClast = area-under curve, Cl = total clearance and Vdss = volume distribution at steady state) were calculated from the p28 concentrations in serum versus postinjection time. The concentration at 0 min is defined as 0 ng mL−1.
p53 Expression in Tumor Tissue
p53 expression in formalin-fixed, paraffin-embedded tumor slides of 12 eligible participants was evaluated by IHC. Six tumors tested positive for p53 (10% to 87%), representing 1 AT/RT and 5 malignant gliomas.
Clinical Outcomes
Seven participants received ≥2 courses of p28 (median 2; range, 2–7). No complete or partial responses were observed. Two participants (both with malignant glioma) with stable disease in course 2 and course 7 came off study without disease progression because of a lack of drug supply. Duration of therapy and best response for all participants who received at least 1 dose of p28 (n = 17) are detailed in Table 4. Within the limitation of this phase I study, there was no correlation between response to p28 and p53 expression evaluated by IHC.
Table 4.
Clinical outcomes and p53 expression by immunohistochemistry for participants on PBTC-041
| Patient ID | Diagnosis | Days of Treatment | Total Courses | Best Response | IHC % p53 |
|---|---|---|---|---|---|
| 1 | CPC | 80 | 2 | PD | 7% |
| 2 | DIPG | 84 | 2 | PD | N.A. |
| 3 | AT/RT | 83 | 2 | PD | 23% |
| 4 | AA | 7 | <1 | PD | 30% |
| 5 | Malignant glioma | 36 | 1 | PD | 8% |
| 6 | GBM | 4 | <1 | PD | >75% |
| 7 | Medulloblastoma | 34 | 1 | PD | 5% |
| 8 | Pineoblastoma | 44 | 1 | PD | 6% |
| 9 | DIPG | 9 | <1 | PD | N.A. |
| 10 | Malignant glioma | 7 | <1 | PD | 5% |
| 11 | Pineoblastoma | 209 | 5 | SD | N.A. |
| 12 | GBM | 14 | <1 | PD | 87% |
| 13 | CPC | 75 | 2 | PD | < 1% |
| 14 | GBM | 303 | 7 | SD | 10% |
| 15 | Medulloblastoma | 37 | 1 | PD | N.A. |
| 16 | GBM | 36 | 1 | PD | 64% |
| 17 | Malignant Glioma | 65 | 2 | SD | N.A. |
Abbreviations: AA, anaplastic astrocytoma; CPC, choroid plexus carcinoma; DIPG, diffuse intrinsic pontine glioma; GBM, glioblastoma; IHC, immunohistochemistry; SD, stable disease; PD, progressive disease.
Discussion
The search for novel treatments for recurrent and progressive pediatric CNS tumors has prompted investigation of agents targeting multiple oncogenic pathways. p53 is central to the regulation of the cell cycle, DNA repair, development, and a myriad of signaling pathways. Dysregulation of p53 has been found in virtually all malignancies including pediatric CNS tumors. In this study, we present results from a pediatric phase I trial of p28, a novel cell-penetrating peptide targeting the p53 pathway. Past studies have utilized compounds primarily focused on protecting p53 from degradation by the endogenous regulator HDM2 in hopes of suppressing the transcriptional activity of p53.29 In contrast, p28 blocks the binding of constitutional morphogenic protein 1 (Cop1) to the DNA binding domain of p53.22,30 As an E3 ubiquitin ligase, Cop1, like MDM2, is a known major negative regulator of p53 activity in many cancers. p28 exerts its antitumor affect by inducing cell-cycle arrest at G2/M. Furthermore, p28 also has an antiangiogenic effect that is independent of p53 status. Thus, p28 represents a novel and potentially promising agent for anticancer therapy.
p28 was well-tolerated in this population of heavily pretreated children. Given the favorable safety profile in adult patients, we evaluated and demonstrated the tolerance of the adult R2PD in the participants on our study. The most common adverse event related to p28 was grade 1 or 2 infusion-related reactions, which were short and rarely required intervention. A single participant with extraneural metastatic pineoblastoma with pre-existing bone marrow metastases experienced 2 DLTs of neutropenia and thrombocytopenia during course 1.
PK parameters of p28 in children closely correlated with the adult experience. PK analysis of 16 participants revealed an overall t1/2 and t1/2α similar to those in adults. An increased AUC was observed in pediatric participants as a result of a higher Cmax and longer t1/2αβ. The PK parameters identified here as well as the prolonged intranuclear half-life of p28 may suggest evaluating the drug under a less intensive dosing schedule.
As expected, approximately one-half of the tumor specimens available were positive for p53 by IHC. All positive samples were either AT/RT or malignant glioma. The expression of p53 as determined by IHC was not correlated with best response to p28 mirroring the adult experience, in which p28 demonstrated antitumor activity independent of p53 status.32
As a single cytostatic agent, p28 is not likely to be effective against pediatric CNS tumors. However, combinatorial strategies may prove more promising. Preliminary data have shown additive cell kill with agents such as dacarbazine and temozolomide in a number of high-grade glioma cell lines including U87 (p53wt) and LN229 (p53mut). (T. Yamada et al, unpublished). Further combination strategies are being explored preclinically.
In conclusion, the results of this trial have established that p28 is safe and well-tolerated in children with progressive CNS malignancies. The further development of this agent in combination with other agents is currently being explored.
Funding
This work was supported in part by NIH grant U01 CA81457 for the Pediatric Brain Tumor Consortium and the American Lebanese Syrian Associated Charities grant to St. Jude Children's Research Hospital.
Acknowledgments
The authors thank Ms. Emily Carps and Ms. Stacye Richardson from the Pediatric Brain Tumor Consortium for clinical research support. We thank T.K. Das Gupta, CDG Therapeutics Inc., for providing the p28.
Conflict of interests statement. T.Y. and C.W.B. are Director of Drug Development and Chief Scientific Officer, respectively, of CDG Therapeutics Inc.
References
- 1. Chintagumpala M, Gajjar A. Brain tumors. Pediatr Clin North Am. 2015;62 (1):167–178. [DOI] [PubMed] [Google Scholar]
- 2. Jones DT, Jager N, Kool M et al. . Dissecting the genomic complexity underlying medulloblastoma. Nature. 2012;488 (7409):100–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Northcott PA, Shih DJ, Peacock J et al. . Subgroup-specific structural variation across 1,000 medulloblastoma genomes. Nature. 2012;488 (7409):49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Paugh BS, Qu C, Jones C et al. . Integrated molecular genetic profiling of pediatric high-grade gliomas reveals key differences with the adult disease. J Clin Oncol. 2010;28 (18):3061–3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pugh TJ, Weeraratne SD, Archer TC et al. . Medulloblastoma exome sequencing uncovers subtype-specific somatic mutations. Nature. 2012;488 (7409):106–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Robinson G, Parker M, Kranenburg TA et al. . Novel mutations target distinct subgroups of medulloblastoma. Nature. 2012;488 (7409):43–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Maire CL, Ligon KL. Glioma models: new GEMMs add “class” with genomic and expression correlations. Cancer Cell. 2011;19 (3):295–297. [DOI] [PubMed] [Google Scholar]
- 8. Pollack IF, Hamilton RL, Finkelstein SD et al. . The relationship between TP53 mutations and overexpression of p53 and prognosis in malignant gliomas of childhood. Cancer Res. 1997;57 (2):304–309. [PubMed] [Google Scholar]
- 9. Lane DP. Cancer. p53, guardian of the genome. Nature. 1992;358 (6381):15–16. [DOI] [PubMed] [Google Scholar]
- 10. Midgley CA, Lane DP. p53 protein stability in tumour cells is not determined by mutation but is dependent on Mdm2 binding. Oncogene. 1997;15 (10):1179–1189. [DOI] [PubMed] [Google Scholar]
- 11. Olivier M, Eeles R, Hollstein M, Khan MA, Harris CC, Hainaut P. The IARC TP53 database: new online mutation analysis and recommendations to users. Hum Mutat. 2002;19 (6):607–614. [DOI] [PubMed] [Google Scholar]
- 12. Martin AC, Facchiano AM, Cuff AL et al. . Integrating mutation data and structural analysis of the TP53 tumor-suppressor protein. Hum Mutat. 2002;19 (2):149–164. [DOI] [PubMed] [Google Scholar]
- 13. Park CM, Park MJ, Kwak HJ et al. . Induction of p53-mediated apoptosis and recovery of chemosensitivity through p53 transduction in human glioblastoma cells by cisplatin. Int J Oncol. 2006;28 (1):119–125. [PubMed] [Google Scholar]
- 14. Janouskova H, Maglott A, Leger DY et al. . Integrin alpha5beta1 plays a critical role in resistance to temozolomide by interfering with the p53 pathway in high-grade glioma. Cancer Res. 2012;72 (14):3463–3470. [DOI] [PubMed] [Google Scholar]
- 15. Jin G, Cook S, Cui B et al. . HDMX regulates p53 activity and confers chemoresistance to 3-bis(2-chloroethyl)-1-nitrosourea. Neuro Oncol. 2010;12 (9):956–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pollack IF, Finkelstein SD, Burnham J et al. . Age and TP53 mutation frequency in childhood malignant gliomas: results in a multi-institutional cohort. Cancer Res. 2001;61 (20):7404–7407. [PubMed] [Google Scholar]
- 17. Vassilev LT, Vu BT, Graves B et al. . In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science. 2004;303 (5659):844–848. [DOI] [PubMed] [Google Scholar]
- 18. Yamada T, Goto M, Punj V et al. . Bacterial redox protein azurin, tumor suppressor protein p53, and regression of cancer. Proc Natl Acad Sci USA. 2002;99 (22):14098–14103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Taylor BN, Mehta RR, Yamada T et al. . Noncationic peptides obtained from azurin preferentially enter cancer cells. Cancer Res. 2009;69 (2):537–546. [DOI] [PubMed] [Google Scholar]
- 20. Yamada T, Mehta RR, Lekmine F et al. . A peptide fragment of azurin induces a p53-mediated cell cycle arrest in human breast cancer cells. Mol Cancer Ther. 2009;8 (10):2947–2958. [DOI] [PubMed] [Google Scholar]
- 21. Yamada T, Christov K, Shilkaitis A et al. . p28, a first in class peptide inhibitor of cop1 binding to p53. Br J Cancer. 2013;108 (12):2495–2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dornan D, Shimizu H, Mah A et al. . ATM engages autodegradation of the E3 ubiquitin ligase COP1 after DNA damage. Science. 2006;313 (5790):1122–1126. [DOI] [PubMed] [Google Scholar]
- 23. Mehta RR, Yamada T, Taylor BN et al. . A cell penetrating peptide derived from azurin inhibits angiogenesis and tumor growth by inhibiting phosphorylation of VEGFR-2, FAK and Akt. Angiogenesis. 2011;14 (3):355–369. [DOI] [PubMed] [Google Scholar]
- 24. Hong CS, Yamada T, Fialho AM, Das Gupta TK, Chakrabarty AM, inventors; The Board of Trustees of the University of Illinois, assignee. Transport agents for crossing the blood-brain barrier and into brain cancer cells, and methods of use thereof. US patent 8,188,251. October 4, 2010.
- 25. Warso MA, Richards JM, Mehta D et al. . A first-in-class, first-in-human, phase I trial of p28, a non-HDM2-mediated peptide inhibitor of p53 ubiquitination in patients with advanced solid tumours. Br J Cancer. 2013;108 (5):1061–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gorman GS, Coward LU, Freeman L, Noker PE, Beattie CW, Jia L. A novel and rapid LC/MS/MS assay for bioanalysis of Azurin p28 in serum and its pharmacokinetics in mice. J Pharm Biomed Anal. 2010;53 (4):991–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jia L, Gorman GS, Coward LU et al. . Preclinical pharmacokinetics, metabolism, and toxicity of azurin-p28 (NSC745104) a peptide inhibitor of p53 ubiquitination. Cancer Chemother Pharmacol. 2011;68 (2):513–524. [DOI] [PubMed] [Google Scholar]
- 28. Pollack IF, Finkelstein SD, Woods J et al. . Expression of p53 and prognosis in children with malignant gliomas. N Engl J Med. 2002;346 (6):420–427. [DOI] [PubMed] [Google Scholar]
- 29. Wang Z, Sun Y. Targeting p53 for novel anticancer therapy. Transl Oncol. 2010;3 (1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yamada T, Das Gupta TK, Beattie CW. p28, an anionic cell-penetrating peptide, increases the activity of wild type and mutated p53 without altering its conformation. Mol Pharm. 2013;10 (9):3375–3383. [DOI] [PubMed] [Google Scholar]