Abstract
On September 14–15, 2015, a meeting of clinicians and investigators in the fields of veterinary and human neuro-oncology, clinical trials, neuropathology, and drug development was convened at the National Institutes of Health campus in Bethesda, Maryland. This meeting served as the inaugural event launching a new consortium focused on improving the knowledge, development of, and access to naturally occurring canine brain cancer, specifically glioma, as a model for human disease. Within the meeting, a SWOT (strengths, weaknesses, opportunities, and threats) assessment was undertaken to critically evaluate the role that naturally occurring canine brain tumors could have in advancing this aspect of comparative oncology aimed at improving outcomes for dogs and human beings. A summary of this meeting and subsequent discussion are provided to inform the scientific and clinical community of the potential for this initiative. Canine and human comparisons represent an unprecedented opportunity to complement conventional brain tumor research paradigms, addressing a devastating disease for which innovative diagnostic and treatment strategies are clearly needed.
Keywords: comparative oncology, glioma, translational research
The past several decades have witnessed remarkable advances in our understanding of human cancers on a genomic level, and hence have provided new opportunities to implement the practice of personalized medicine. The pharmaceutical industry has invested substantial resources, in both capital and research effort, to identify new cancer drugs that might provide a major positive impact on cancer patient survival. Disappointingly, identification of new agents to improve cancer treatment remains elusive for many lethal cancers, with approximately 1 in 4 deaths in the United States being attributed to cancer annually (Cancer Facts & Figures 2015; www.cancer.org). This has been particularly true for malignant primary brain tumors, for which there have been only 2 new therapeutic agents approved over the last 2 decades.1 The pathway to successful cancer drug discovery remains difficult and expensive despite significant economic investment, and the development of breakthrough novel therapeutics to match the impact of compounds such as imatinib remains extremely infrequent. Given these challenges, there is potential for the pharmaceutical industry to become too risk averse and for the advancement of effective novel therapeutic discovery to ultimately slow. An improved drug development paradigm facilitated by the utilization of more predictive and accurate preclinical models would offer opportunities to mitigate some of the risk associated with drug development. Such an alternative strategy could be substantively enabled by comparative tumor oncology, pathology, and genomics through the inclusion of companion animals with naturally occurring cancer.2,3
Comparative oncology is a growing field of study centered around investigation of naturally occurring cancers in nonhuman species, such as pet dogs, as a parallel and complementary model for human cancer research efforts.4–6 Like humans, pet dogs naturally develop primary central nervous system neoplasms that encompass a wide variety of tumor histologies, including, but not limited to, glial tumors (astrocytomas, oligodendrogliomas), meningiomas, and pituitary gland tumors.7 At this time the pet dog is the most widely studied companion animal species, due primarily to the degree of genome similarity, shared environment, and the incidence of diagnosed spontaneous canine cancers reported each year in the US. The promise of comparative oncology and the specific role of the domestic dog formed the basis of a recent review in a special issue of the Royal Society's journal, and highlights the global opportunities for advancing medicine through the inclusion of naturally occurring canine disease models.8
The incidence of brain tumors in dogs is approximately 2%–4.5% based upon necropsy-dependent surveys and is likely an underestimation given the practical limitations of veterinary medical care.9,10 Most descriptive studies agree that approximately 50% of primary brain tumors are meningioma, with gliomas representing 30%–40% and the remaining comprising choroid plexus tumors, ependymoma, and primitive neuroectodermal tumors. Specific breeds are known to have significantly increased risk of primary intracranial tumor development, namely the Boxer, Boston Terrier, Golden Retriever, French Bulldog, Miniature Schnauzer, and Rat Terrier.10
Recent improvements in the diagnosis and management of canine brain tumors are largely due to the broad adoption of cross-sectional imaging techniques (CT and MRI) within the veterinary medical community, coupled with growing expertise and collective clinical experience among veterinary specialists in neurosurgical techniques, radiation therapy protocols, and use of chemotherapy. However, most therapeutic studies in dogs with brain tumors are descriptive in nature, and the conclusions are limited by retrospective study design and small patient numbers, along with a lack of standardization in tumor biopsy and/or imaging endpoints across studies. In the absence of prospective and rational clinical trial design, fundamental gaps in knowledge regarding canine brain tumor biology and therapeutic response persist within the veterinary scientific community, and advancement has been hindered by the challenges posed by limitations of the late-stage diagnosis of intracranial disease, the morbidity associated with intracranial procedures such as biopsy/surgical resection and external beam irradiation, and, not inconsequentially, pet owners' disposable income.9,11 Despite these limitations, available preliminary data suggest that core oncogenic pathways defined in human brain tumors may be implicated in dog tumors, supporting a more detailed and thorough evaluation of the model.12–18
Although much is known about the clinical outcomes, imaging characteristics, and breed predilection for certain intracranial malignancies, there is a paucity of data to enable accurate statistics to catalog true incidence and natural disease history or to establish histologic and molecular relevance to human brain tumors. Despite the expertise and training afforded by American College of Veterinary Internal Medicine specialists in oncology and neurology, as well as critically aligned fields and specialty colleges such as pathology, imaging, neurology, and surgery since the mid-1980s, much remains unknown regarding the molecular underpinnings of canine cancers to better optimize prevention and patient care. Therefore, there are recognized, but not insurmountable, limitations in being able to assess the true potential of including pet dogs with brain cancers as predictive models to advance therapies for human use. By organizing a focused initiative aimed at overcoming a number of these challenges, the potential inclusion of dogs with brain cancer as a sophisticated preclinical model of human patients offers several advantages for expediting drug discovery and development efforts.19
Comparative oncology is now being advanced as a research discipline by many diverse research institutions. The National Cancer Institute's (NCI) Comparative Oncology Program (COP) is a collaborative group, utilizing an extramural clinical trial consortium (Comparative Oncology Trials Consortium [COTC]) (Fig. 1) with a demonstrable track record in conducting high-quality scientifically robust clinical trials with a variety of sponsors to address drug development questions that would be difficult to study in the context of conventional animal models of cancer and/or to overcome developmental barrier issues not feasible within the human clinical trial setting.20,21 The COTC mechanism provides a facile method for recruiting dogs from multiple participating veterinary academic institutions. Funding for these trials comes from a variety of sources, including but not limited to the pharmaceutical industry, philanthropic organizations, and NCI. Study support provided to veterinarians caring for dogs enrolled in COTC trials includes clinical veterinary care, conduct of correlative assays on biologic samples, and in some cases financial support to owners for future veterinary care for their pet after the study concludes. This group has historically relied on veterinary medical oncologists as the institutional COTC investigators. The comparative aspects of canine brain tumors have not been previously studied within the context of a COTC clinical trial. Inconsistent availability of expertise in the field of veterinary neuro-oncology may be a reason that canine brain tumors have not been sufficiently explored as a model for human brain cancer. To address this challenge, we seek directed improvements in cooperation of veterinary neurologists, surgeons, and radiation oncologists who manage dogs with intracranial malignancies with other neuro-oncology investigators. Indeed, the somewhat “siloed” nature of research environments and reliance on single-institution efforts result in potentially biased incidence rates and sample populations and small numbers of samples and/or patients being reported in any given aspect of such research.
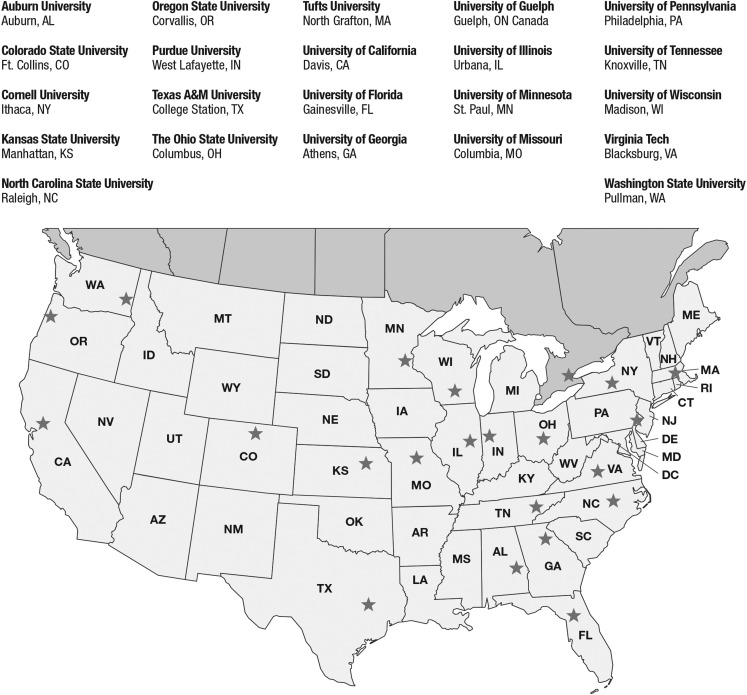
Fig. 1.
A map of the Comparative Oncology Trials Consortium (COTC).
Human brain tumor research would benefit from a similar need to improve our understanding of the molecular drivers of the disease. However, many novel strategies could potentially be borne out of a deeper understanding of tumor biology via The Cancer Genome Atlas and other efforts in molecular characterization which are supporting drug discovery.22,23 The limitations of current conventional animal models still impede forward progress. Murine models of brain cancer can be induced via cell line or patient-derived xenograft techniques or be the result of molecular manipulation, but do not recapitulate all facets of naturally occurring disease.24–27 One of the main obstacles for leveraging the comparative approach for brain tumor research is insufficient evidence that these mouse and rat models translate to human disease; this includes the lack of consensus among histopathology, molecular markers, genomics, and clinical outcome data.
Organized research groups such as the Collaborative Ependymoma Research Network (CERN) provide a roadmap to guide the organization and forward progress of the COP/COTC and other comparative initiatives.28–30 This initiative uses a working-group format that unifies multidisciplinary teams around central themes (pathology/molecular markers, tumor biology/immunology, drug discovery, clinical trials, and patient outcomes) to facilitate discussions and exchange of knowledge. CERN's mission and infrastructure were utilized as guiding principles for the discussions during this meeting.
The meeting convened at the National Institutes of Health had 3 main goals:
Establish a collaborative research network with an immediate goal of sharing cross-discipline experiences and knowledge.
Define a working group structure (Fig. 2) that could be populated with cross-discipline experts from the allied fields of veterinary medicine, human medicine, and basic research.
Define the goals of the consortium efforts in a project-based format for each working group.
Herein we present a consensus of the opportunities and challenges identified within the summary of each working group discussion and provide an agenda that can be used to advance this field for the bidirectional benefit of both humans and dogs with this challenging disease.
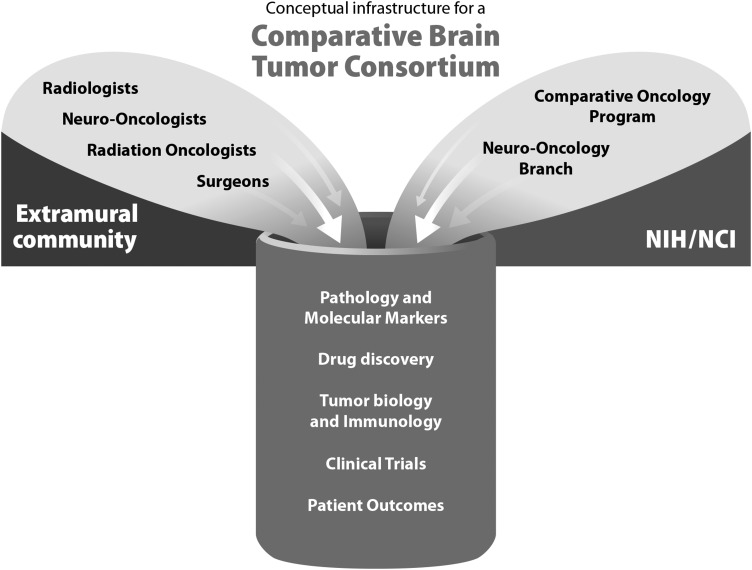
Fig. 2.
A schematic representation of the participating groups within the Comparative Brain Tumor Consortium.
Strengths
The wide variety of brain tumor histologies that dogs spontaneously develop, including but not limited to glial tumors (astrocytomas, oligodendrogliomas), meningiomas, and pituitary gland tumors, are an obvious strength of the comparative approach. A focus on the dog as a model for human gliomas specifically (Fig. 3), as a first priority for tissue-based projects, was consistently identified among all 5 working groups as most immediately impactful for improving both human and dog outcomes. A noteworthy advantage of the companion dog model is that spontaneous disease in an immune-competent host is critical for developing immunomodulatory therapies.31,32 Similar physical size and vascular biology should facilitate investigation of the role of the blood–brain barrier and how it limits drug therapies, along with comparable pharmacokinetics (PK) (and potentially pharmacodynamics [PD]) to facilitate dose finding in early clinical studies. Realistic assessments of novel physical delivery/therapeutic strategies have already been demonstrated across several different approaches using dog tumors as a model platform.33–36 Finally and most importantly, disease heterogeneity, particularly genomic and epigenomic heterogeneity, is a critical attribute of canine and human brain cancers that cannot be accurately modeled in conventional animal model approaches to date.
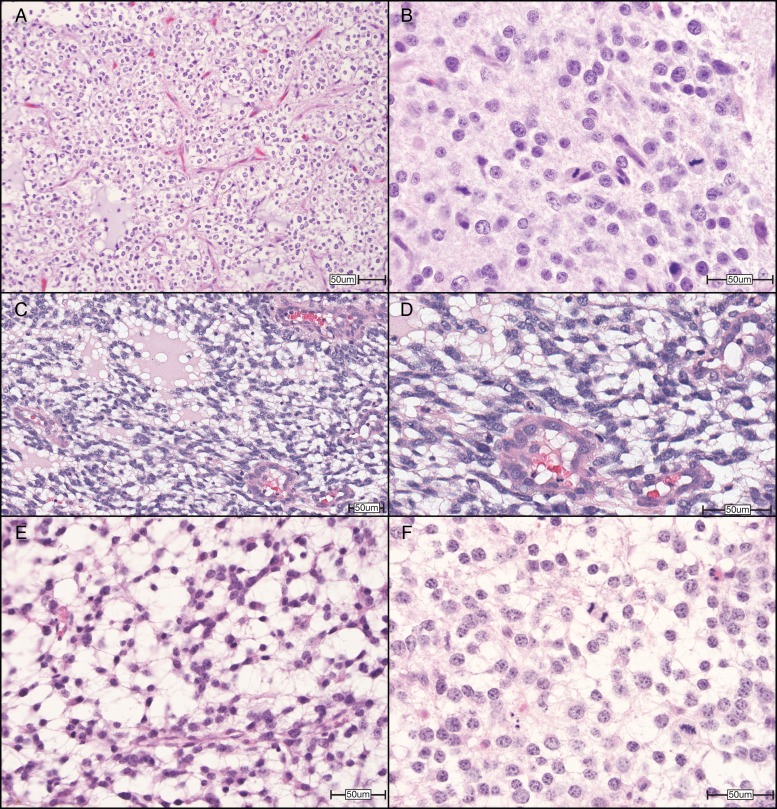
Fig. 3.
Representative photomicrographs of high-grade (WHO grade III) cerebral oligodendrogliomas in humans, dogs, and mice. (A) Human high-grade oligodendroglioma contains a delicate “chicken wire” vasculature and mucin-rich microcystic spaces and is composed of neoplastic cells with oval, hyperchromatic nuclei, and clear cytoplasm. (B) Higher magnification of a human high-grade oligodendroglioma shows tumor nuclei with crisp nuclear borders and abundant mitoses. (C) A surgical biopsy of a high-grade (grade III) cerebral oligodendroglioma in a Boston Terrier, demonstrating clear cytoplasm and distinct cell membranes, with ovoid hyperchromatic nuclei. (D) Higher magnification highlights endothelial hyperplasia of this lesion. (E) A high-grade oligodendroglioma allograft from a mouse stereotactically injected with oligodendrocyte progenitor cells harboring deletion mutations in transformation related protein 53 and neurofibromatosis type 1 (Ref PMID 25246577) shows similar features, including (F) the delicate vasculature and microcystic spaces and nuclei with crisp nuclear membranes and abundant mitoses. All sections were stained with hematoxylin and eosin. Original magnifications: 200× (A), 600× (B), 200× (C), 400× (D), 400× (E), 600× (F). Scale bars = 50 µm. Human and murine images courtesy of Dr C. Ryan Miller, University of North Carolina School of Medicine. Canine images courtesy of Dr R. Timothy Bentley, Purdue University College of Veterinary Medicine.
From a clinical trial perspective, one particular strength/opportunity of the canine brain tumor model in drug development is the initiation of phase 0 studies—small-cohort studies in which the primary endpoints of investigation are (i) target modulation: confirmed and serially evaluated (diagnosis, surgery, necropsy), (ii) outcome assessment: radiological or clinical metrics of drug effect, and (iii) biodistribution, PK, and PD endpoints to enable identification of “effective exposure” correlated to target modulation while also supporting discovery and validation of biomarkers representative of outcome effect. Several examples of such studies in other canine cancers exist in the scientific literature and form a basis for such endeavors in brain tumor drug development.37–40
Clinical trials in pet dogs with cancer and a variety of other diseases are routinely carried out at veterinary academic centers that evaluate investigational therapies under the care and direction of veterinary specialists in a wide range of disciplines. Clinical care is provided within the veterinary teaching hospital by highly trained teams of veterinary clinicians and technicians according to a standardized protocol. There are specific opportunities for dogs with brain tumors to participate in a clinical trial offered alongside standard treatments, such as surgical resection and external beam radiotherapy. In this situation, owners have the ability to choose the best option for their pet based upon their financial resources and goals and expectations for their pet's outcome. Funding for such clinical trials is obtained through grants and/or research contracts via philanthropic agencies or pharmaceutical companies. Dogs are recruited from the community by participating sites using a variety of social networking, mail, and web-based advertising techniques. The conduct of COTC trials is centrally managed by the NCI-COP, with input from a Data Safety Monitoring Board convened for each trial. Data from these trials are presented and published in the proceedings of national and international meetings as well as in the biomedical research literature.
The cost/benefit rationale for performing comparative studies in tumor-bearing pet dogs prior to or in parallel with human clinical trials was recently discussed at a National Academies workshop entitled “The Role of Clinical Studies for Pets with Naturally Occurring Tumors in Translational Cancer Research” (http://iom.nationalacademies.org/Activities/Disease/NCPF/2015-JUN-08.aspx).41 Of the many advantages to studying cancer in dogs for the purposes of human cancer drug development, one significant benefit is the ability to evaluate novel therapies in treatment-naïve disease, particularly when dogs' owners are not able or interested in pursuing conventional treatments for their pet. This allows interrogation of relationships between drug target and drug exposure within a naturally occurring tumor that has not been extensively pretreated with other therapies and thus may provide a more accurate assessment of that drug's potential for human use. The value of the comparative approach is highlighted by the unique opportunities to explore PK/PD relationships over a range of doses, including serial tissue and biologic sample collections, and the ability to make changes to the study protocol in response to data in real time.42
Imaging agent and device development can also uniquely benefit from the physical size of dogs' brains, and several examples demonstrate the opportunities to evaluate prototype devices (eg, convection-enhanced delivery catheters, stereotactic radiosurgery) utilizing the same or similar clinical technologies that are routinely used in human cancer treatment. To facilitate such efforts, most veterinary medical centers that routinely diagnose and treat dogs with brain tumors maintain state-of-the-art imaging and therapeutic technologies, such as CT and/or high-strength MRI for brain imaging and stereotactic brain biopsy, interventional radiology suites, and linear accelerators for radiotherapy (Fig. 4).43–50
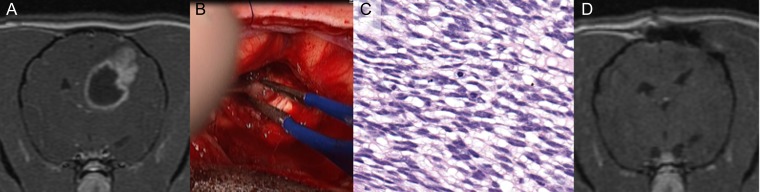
Fig. 4.
High-grade (grade III) cerebral oligodendroglioma in a Boxer dog. (A) T1-weighted post-contrast transverse MRI. An intra-axial mass lesion displays mixed contrast enhancement and partial ring enhancement. (B) Intraoperative image. A gray lesion has indistinct margins with the surrounding cerebrum. (C) Photomicrograph, surgical biopsy, hematoxylin and eosin. Neoplastic cells have ovoid, hyperchromatic nuclei and clear cytoplasm. Nuclei are ovoid and hyperchromatic. (D) Postoperative T1-weighted post-contrast MRI. There is no evidence of the previous mass. The adjacent lateral ventricle is displaced toward the resection cavity. Note the bone defect associated with previous craniectomy. Images courtesy of Dr R. Timothy Bentley, Purdue University College of Veterinary Medicine.
Weaknesses: Where Are the Critical Gaps in This Field?
Limitations include a paucity of knowledge about the natural history of disease progression and its impact on clinical diagnosis, which can be addressed by a robust survey of veterinarians involved in the diagnosis, management, and treatment of such pets. Most diagnoses of brain cancer in dogs come when significant neurologic deficits and/or seizures are noted, thus tumors are often large and responses even to aggressive therapy are poor.9,51,52 The advanced stage of these tumors may actually lend itself to a new era of collaboration between veterinary and physician neurologists and neurosurgeons, particularly in the area of complex neurosurgical approaches wherein achieving a gross total resection may be of high prognostic value for the canine patient based upon the human literature.53–55
The lack of standardized methods to evaluate response to therapy limits investigation of patterns of recurrence. This too can be addressed via consensus documents generated within the veterinary community. The lack of robust incidence and prevalence estimates, which may be intensely biased by patterns of care, can be addressed in a similar fashion. Some unique features of veterinary medicine are that many patients remain untreated, as dog owners have an option for early election of humane euthanasia, and the overriding impact of finance-driven decisions influences selection biases in treatment. These issues can skew estimates of the true incidence of disease among pet dogs, as well as effectiveness of therapies and outcomes data.
The current lack of understanding of molecular underpinnings that may drive genetics of tumorigenesis and progression in both dogs and humans leaves open the possibility that these may differ between dogs and humans in substantial ways. This area, including an improved understanding of the molecular basis for brain cancer development in dogs, should receive major emphasis in future research. Successful inclusion of pet dogs with brain cancer into the drug discovery and development pathway will require additional resources and research to confirm the suitability of the pet dog as a reliable preclinical model of human cancer patients. This also would require the thorough characterization and annotation of the canine immune system and its response to neoplasia, as well as confirmation that brain cancers that arise naturally in dogs possess similar or overlapping histopathological, molecular, and genetic signatures as those in human cancer patients. Some data have been generated in the field to address these critical gaps, but larger studies are clearly needed.12,15,24,56–59
Opportunities: How to Address the Weaknesses and Advocate for the Strengths of the Canine Cancer Model
Each working group generated a robust list of concepts and specific projects in direct response to the acknowledged strengths and weaknesses of studying and utilizing the canine brain cancer model. Creation of a central tissue registry, a central catalog of veterinary institutions highlighting their individual fixed technology/infrastructure, expertise, resources in support of clinical trials, and development of consensus protocols were all considered key to rapid advancement of the field with a critical mass of investigators, knowledge, and materials. The largest opportunity facing this initiative is to substantiate valid translation to human disease, and then to leverage this unique model for funding activities to support large-scale investigations.
Opportunities to expand upon the existing, albeit limited, knowledge of canine brain tumors begin with the fact that many veterinary institutions have existing tumor banks in various forms (formalin-fixed paraffin-embedded/frozen) which could support a multiplatform integrative molecular analysis analogous to that performed by The Cancer Genome Atlas on retrospective cohorts, thus helping to establish canine tumor molecular genetic landscapes for comparison with humans.60,61 This could include single nucleotide variant and copy number alterations, expression and methylation profiles, comprehensive histopathological characterization to establish diagnostic criteria with comparisons with analogous human tumors, and characterization of tumor immunology via immunohistochemistry and related expression profiles.
Such an effort would begin with the development of an updated classification and grading scheme for canine gliomas. The pathology and molecular markers working group felt that developing such a scheme would promote uniformity in diagnosis across institutions and improve understanding in making comparisons with human adult and pediatric glial tumors. This initiative was envisioned to take place in 2 distinct phases: first, a retrospective pathologic assessment of approximately 200 hematoxylin and eosin stained glass slides of treatment-naïve canine gliomas of all subtypes and grades by both veterinary and physician neuropathologists from multiple participating institutions. High-resolution images will be centrally scanned and hosted digitally, and a consensus panel of a minimum of 6 of each physician and veterinary pathologists would develop a consensus on clear, concise criteria for classification and grading; these should be based whenever possible on the current human World Health Organization system to allow for comparison but should incorporate species-specific features as identified and agreed upon. A subgroup of tumors (formalin-fixed paraffin-embedded tissue) that were optimally stored and characterized for this review would then be selected for expression of pertinent molecular markers (such as glial fibrillary acidic protein, vimentin, Olig2, CNPase, synaptophysin, neurofilament M, neuronal nuclei/beta-III tubulin/neurofilament, and Ki67).62 This will be performed using immunohistochemistry to allow further uniformity in diagnosis and comparisons between human adult and pediatric glial tumors. Development and optimization of the appropriate species-specific reagents to interrogate additional markers of molecular pathogenesis, such as protein 53, alpha thalassemia/mental retardation syndrome X-linked, beta-catenin, and integrase interactor 1, would be valuable in this context and could also be assessed in the setting of RNA sequencing or other techniques.
Second, this group proposed genomic analyses and expression profiling of 50 canine high-grade gliomas meeting these newly revised classification criteria to allow for comparison with human adult and pediatric glial tumors, and to generate potential pharmacologic targets for canine patients. This aspect of the project will be both retrospective and prospective in nature. For the retrospective portion, snap-frozen tissue from institutions with paired banked formalin-fixed paraffin-embedded tissue from glial tumors which have been histopathology-confirmed within the newly proposed grading and classification scheme will be subjected to whole-exome and RNA sequencing. For the prospective portion, kits and detailed standard operating procedures (SOPs) will be distributed to selected sites, both academic institutions and private practices, for the collection of tumor specimens. Samples will be snap-frozen tissue or tissue in RNAlater and formalin-fixed tissue. Samples will be stored at a central biobank location and subjected to histopathological confirmation, whole-exome sequencing, and RNA sequencing, as funding is available. Sequence information will be subjected to informatics, processed, and shared publicly as soon as publication ready. Collection of tissues and sharing of data for public dissemination to advance these goals was felt to be optimally facilitated by the NCI-COP. The NCI is ideally positioned to take a leadership role in this effort, as demonstrated within other comparative oncology initiatives.63
If successful, the above effort could be used to create infrastructure that would facilitate establishing a multi-institutional collaboration for prospective tissue banking in order to establish best practices for tissue collection and establish SOPs for analysis and public dissemination of data. This could be followed by formation of a multi-institutional cooperative group for generating patient-derived xenografts and cell lines to facilitate in vitro and in vivo studies alongside a multi-institutional cooperative clinical trials group to establish standards of diagnosis (pathology and imaging), treatment (surgery, radiation and chemotherapy), and definition of outcomes (pathology and clinical care). Additionally, there is the opportunity to leverage the existing NCI-COTC infrastructure, currently comprising 22 active veterinary academic centers, many of which represent the key leaders in veterinary neurology and oncology. The existing COTC membership and agreements for study conduct and data management could be expanded and/or modified to include additional collaborators.
The Patient Outcomes working group defined a goal of the formulation and validation of scales for subjective outcome data that include quality of life measures, functional scales, pain scoring, seizure frequency, mental and cognitive ability, and the influence of extent of tumor burden. Measures must be sensitive to disease status and should be validated for interobserver variation, as well as be evaluated against natural progression of disease. Such measures can then be applied as consensus for assessing treatment response in canine brain tumor trials. Alongside this document, this working group suggested the ongoing development and refinement of a consensus statement on brain MRI protocol for veterinary facilities, following a human consensus statement as a model to include data on instrument brand and strength, sequences and parameters, and timing of posttreatment and longer-term follow-up scans.64,65 This type of consensus will directly support the comparison of dog data with those of humans for comparative studies within multi-institutional clinical research and identify those scan protocols and MRI sequences that are most useful in patient management.
Threats to Advancement of the Field
In order for the scientific community to assess the potential value of diagnoses of brain cancer in pet dogs as a valuable and predictive preclinical model for drug discovery and development, several key opportunities must be seized and acted upon by leaders in the field as described above. The meeting participants widely acknowledged that a critical first step needs to be the establishment of a consortium of scientific and clinical investigators working toward well-defined and common goals. These will be identified as areas of priority within the brain cancer community, and as such will help to galvanize these investigators to engage in continued scientific collaborations, requiring ongoing attention and leadership within a designated neutral third party. The NCI naturally fulfills this role with its commitment to support of the extramural community while leveraging unique resources and acting as a stimulus to convene cross-discipline audiences around topics such as comparative oncology.
Perhaps the biggest risk facing this new consortium is the possibility that the model of the molecular landscape of canines is not widely translatable to the human disease and/or is limited to the study of a small subset of human tumors. If comparative overlap is narrow, the momentum behind the consortium may be lost and a lack of buy-in from other researchers will result in continuation of small independent projects with lesser impact. Also acknowledged during this meeting was the need for increased understanding by physicians of the status of veterinary knowledge and expertise in canine brain tumors, realizing that there is an ongoing need to overcome preconceptions within the human cancer research community.
We believe that it is critical to define the comparative areas most likely to provide informative data for cross-species therapeutic development, whether these are pathway targeted therapies, cell targeting therapies, gross delivery/interventional strategies, or assessment of immunomodulatory strategies. Early goals must be attainable and informative to maintain consortium momentum with a focus on defining model strengths, which we contend are “barrier” questions that are difficult to answer in rodent models or human trials but which could be answered in the validated dog model with relatively small numbers of cases. Care should be taken not to oversell the potential for therapeutic outcome trials as the primary or only long-term goal for this consortium, particularly given the logistical, financial, and technical limitations inherent in the veterinary neuro-oncology field. Similarly, it could be imprudent to assign go/no-go decisions for the continuation of consortium activities that are linked to the outcomes of experiments that have yet to be defined. The National Academies workshop on comparative oncology highlighted a number of critical gaps in the field, most notably characterization of the molecular landscape of canine cancers. We agree that definition of the critical similarities and differences that may exist in histopathological characterization and key molecular pathways is an early and important priority. However, there are still several important facets of canine brain cancer that make this approach a valuable complement to traditional animal models. A significant body of published work indicates where successes exist in the application of the comparative approach to brain tumor research for the purposes of drug development and delivery techniques.31–36 An ongoing priority will be the advocacy and dissemination of such data to the larger brain tumor community to maintain momentum of this consortium.
In summary, a commitment to collaboration has been established among veterinarians involved in canine brain tumor patient management and physician investigators. Translational and basic research is clearly needed for an improved understanding of natural disease progression and how clinical observations can be leveraged for the development of novel treatment strategies for both dogs and humans. Further, acknowledgment and appreciation of the unique strengths and opportunities that exist between species and among veterinarians and physicians are keys to establishing a consensus of what comparative aspects are most important to the field.
Funding
This research was supported in part by the Intramural Research Program of the National Institutes of Health (NIH) , the NCI, and the Center for Cancer Research. The results and interpretations do not reflect the views of the US government. K.C. is a fellow in the NIH Comparative Biomedical Scientist Training Program supported by the National Institute of Neurological Disorders and Stroke, the NCI, and North Carolina State University.
References
- 1. Kang JH, Adamson C. Novel chemotherapeutics and other therapies for treating high-grade glioma. Expert Opin Investig Drugs. 2015;24 (10):1361–1379. [DOI] [PubMed] [Google Scholar]
- 2. Gordon IK, Khanna C. Modeling opportunities in comparative oncology for drug development. ILAR J. 2010;51 (3):214–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gordon I, Paoloni M, Mazcko C et al. The Comparative Oncology Trials Consortium: using spontaneously occurring cancers in dogs to inform the cancer drug development pathway. PLoS Med. 2009;6 (10):e1000161 10.1371/journal.pmed.1000161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vail DM, MacEwen EG. Spontaneously occurring tumors of companion animals as models for human cancer. Cancer Invest. 2000;18:781–792. [DOI] [PubMed] [Google Scholar]
- 5. Lairmore MD, Khanna C. Naturally occurring diseases in animals: contributions to translational medicine. ILAR J. 2014;55 (1):1–3. [DOI] [PubMed] [Google Scholar]
- 6. Khanna C, London C, Vail D et al. Guiding the optimal translation of new cancer treatments from canine to human cancer patients. Clin Cancer Res. 2009;15 (18):5671–5677. 10.1158/1078-0432.CCR-09-0719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hicks J, Platt S, Kent M et al. Canine brain tumours: a model for the human disease? Vet Comp Oncol. 2015. 10.1111/vco.12152. [DOI] [PubMed] [Google Scholar]
- 8. Schiffman JD, Breen M. Comparative oncology: what dogs and other species can teach us about humans with cancer. Philos Trans R Soc Lond B Biol Sci. 2015;370 (1673) pii: 20140231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Song RB, Vite CH, Bradley CW et al. Postmortem evaluation of 435 cases of intracranial neoplasia in dogs and relationship of neoplasm with breed, age, and body weight. J Vet Intern Med. 2013;27 (5):1143–1152. 10.1111/jvim.12136 [DOI] [PubMed] [Google Scholar]
- 10. Dickinson PJ. Advances in diagnostic and treatment modalities for intracranial tumors. J Vet Intern Med. 2014;28 (4):1165–1185. 10.1111/jvim.12370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hu H, Barker A, Harcourt-Brown T et al. Systematic review of brain tumor treatment in dogs. J Vet Intern Med. 2015;29 (6):1456–1463. 10.1111/jvim.13617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Boudreau CE, York D, Higgins RJ et al. Molecular signaling pathways in canine gliomas. Vet Comp Oncol. 2015. [Epub ahead of print] PubMed PMID: 25808605. doi: 10.1111/vco.12147. [DOI] [PubMed] [Google Scholar]
- 13. Debinski W, Dickinson P, Rossmeisl JH et al. New agents for targeting of IL-13RA2 expressed in primary human and canine brain tumors. PLoS One. 2013;8 (10):e77719 10.1371/journal.pone.0077719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dickinson PJ, Roberts BN, Higgins RJ et al. Expression of receptor tyrosine kinases VEGFR-1 (FLT-1), VEGFR-2 (KDR), EGFR-1, PDGFRalpha and c-Met in canine primary brain tumours. Vet Comp Oncol. 2006;4:132–140. [DOI] [PubMed] [Google Scholar]
- 15. Higgins RJ, Dickinson PJ, LeCouteur RA et al. Spontaneous canine gliomas: overexpression of EGFR, PDGFRalpha and IGFBP2 demonstrated by tissue microarray immunophenotyping. J Neurooncol. 2010;98 (1):49–55. 10.1007/s11060-009-0072-5 [DOI] [PubMed] [Google Scholar]
- 16. Stoica G, Kim HT, Hall DG et al. Morphology, immunohistochemistry, and genetic alterations in dog astrocytomas. Vet Pathol. 2004;41:10–19. [DOI] [PubMed] [Google Scholar]
- 17. Lipsitz D, Higgins RJ, Kortz GD et al. Glioblastoma multiforme: clinical findings, magnetic resonance imaging, and pathology in five dogs. Vet Pathol. 2003;40:659–669. [DOI] [PubMed] [Google Scholar]
- 18. Dickinson PJ, Sturges BK, Higgins RJ et al. Vascular endothelial growth factor mRNA expression and peritumoral edema in canine primary central nervous system tumors. Vet Pathol. 2008;45:131–139. [DOI] [PubMed] [Google Scholar]
- 19. Kimmelman J, Nalbantoglu J. Faithful companions: a proposal for neurooncology trials in pet dogs. Cancer Res. 2007;67 (10):4541–4544. [DOI] [PubMed] [Google Scholar]
- 20. Paoloni MC, Tandle A, Mazcko C et al. Launching a novel preclinical infrastructure: Comparative Oncology Trials Consortium directed therapeutic targeting of TNFalpha to cancer vasculature. PLoS One. 2009;4:e4972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Paoloni M, Mazcko C, Selting K et al. Defining the pharmacodynamic profile and therapeutic index of NHS-IL12 immunocytokine in dogs with malignant melanoma. PLoS One. 2015;10 (6):e0129954 10.1371/journal.pone.0129954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kim YW, Koul D, Kim SH et al. Identification of prognostic gene signatures of glioblastoma: a study based on TCGA data analysis. Neuro Oncol. 2013;15 (7):829–839. 10.1093/neuonc/not024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Doucette T, Rao G, Rao A et al. Immune heterogeneity of glioblastoma subtypes: extrapolation from The Cancer Genome Atlas. Cancer Immunol Res. 2013;1 (2):112–122 10.1158/2326-6066.CIR-13-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Candolfi M, Curtin JF, Nichols SW et al. Intracranial glioblastoma models in preclinical neuro-oncology: neuropathological characterization and tumor progression. J Neurooncol. 2007;85 (2):133–148. 10.1007/s11060-007-9400-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lim SK, Llaguno SR, McKay RM et al. Glioblastoma multiforme: a perspective on recent findings in human cancer and mouse models. BMB Rep. 2011;44 (3):158–164. 10.5483/BMBRep.2011.44.3.158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. El Meskini R, Iacovelli AJ, Kulaga A et al. A preclinical orthotopic model for glioblastoma recapitulates key features of human tumors and demonstrates sensitivity to a combination of MEK and PI3K pathway inhibitors. Dis Model Mech. 2015;8 (1):45–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McNeill RS, Vitucci M, Wu J et al. Contemporary murine models in preclinical astrocytoma drug development. Neuro Oncol. 2015;17 (1):12–28. 10.1093/neuonc/nou288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. DeWire M, Fouladi M, Turner DC et al. An open-label, two-stage, phase II study of bevacizumab and lapatinib in children with recurrent or refractory ependymoma: a Collaborative Ependymoma Research Network study (CERN). J Neurooncol. 2015;123 (1):85–91. 10.1007/s11060-015-1764-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Raghunathan A, Wani K, Armstrong TS et al. Histological predictors of outcome in ependymoma are dependent on anatomic site within the central nervous system. Brain Pathol. 2013;23 (5):584–594. 10.1111/bpa.12050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wani K, Armstrong TS, Vera-Bolanos E et al. A prognostic gene expression signature in infratentorial ependymoma. Acta Neuropathol. 2012;123 (5):727–738. 10.1007/s00401-012-0941-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Olin MR, Pluhar GE, Andersen BM et al. Victory and defeat in the induction of a therapeutic response through vaccine therapy for human and canine brain tumors: a review of the state of the art. Crit Rev Immunol. 2014;34 (5):399–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Andersen BM, Pluhar GE, Seiler CE et al. Vaccination for invasive canine meningioma induces in situ production of antibodies capable of antibody-dependent cell-mediated cytotoxicity. Cancer Res. 2013;73 (10):2987–2997. 10.1158/0008-5472.CAN-12-3366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Platt S, Nduom E, Kent M et al. Canine model of convection-enhanced delivery of cetuximab-conjugated iron-oxide nanoparticles monitored with magnetic resonance imaging. Clin Neurosurg. 2012;59:107–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dickinson PJ, LeCouteur RA, Higgins RJ et al. Canine spontaneous glioma: a translational model system for convection-enhanced delivery. Neuro Oncol. 2010;12 (9):928–940. 10.1093/neuonc/noq046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dickinson PJ, LeCouteur RA, Higgins RJ et al. Canine model of convection-enhanced delivery of liposomes containing CPT-11 monitored with real-time magnetic resonance imaging: laboratory investigation. J Neurosurg. 2008;108 (5):989–998. 10.3171/JNS/2008/108/5/0989 [DOI] [PubMed] [Google Scholar]
- 36. Packer RA, Freeman LJ, Miller MA et al. Evaluation of minimally invasive excisional brain biopsy and intracranial brachytherapy catheter placement in dogs. Am J Vet Res. 2011;72:109–121. [DOI] [PubMed] [Google Scholar]
- 37. Paoloni MC, Mazcko C, Fox E et al. Rapamycin pharmacokinetic and pharmacodynamic relationships in osteosarcoma: a comparative oncology study in dogs. PLoS One. 2010;5 (6):e11013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. London CA, Bear MD, McCleese J et al. Phase I evaluation of STA-1474, a prodrug of the novel HSP90 inhibitor ganetespib, in dogs with spontaneous cancer. PLoSOne. 2011;6 (11):e27018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. London CA, Bernabe LF, Barnard S et al. Evaluation of the novel, orally bioavailable selective inhibitor of nuclear export (SINE) KPT-335 in spontaneous canine cancer: results of a phase I study. PLoSOne. 2014;9 (2):e87585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Honigberg LA, Smith AM, Sirisawad M et al. The Bruton tyrosine kinase inhibitor PCI-32765 blocks B-cell activation and is efficacious in models of autoimmune disease and B-cell malignancy. Proc Natl Acad Sci U S A. 2010;107 (29):13075–13080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. LeBlanc AK, Mazcko CN, Khanna C. Defining the value of a comparative approach to cancer drug development. Clin Cancer Res. 2015. pii: clincanres.2347.2015. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. LeBlanc AK, Breen M, Choyke P et al. Perspectives from man's best friend: National Academy of Medicine's workshop on comparative oncology. Sci Transl Med. 2016;8 (324) [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rossmeisl JH Jr, Garcia PA, Pancotto TE et al. Safety and feasibility of the NanoKnife system for irreversible electroporation ablative treatment of canine spontaneous intracranial gliomas. J Neurosurg. 2015;123 (4):1008–1025 10.3171/2014.12.JNS141768. [DOI] [PubMed] [Google Scholar]
- 44. Zwingenberger AL, Pollard RE, Kent MS. Measuring response of brain tumors to stereotactic radiosurgery: interim results. Vet Radiol Ultrasound. 2010;51:577. [Google Scholar]
- 45. Mariani CL, Schubert TA, House RA et al. Frameless stereotactic radiosurgery for the treatment of primary intracranial tumours in dogs. Vet Comp Oncol. 2015;13 (4):409–423. [DOI] [PubMed] [Google Scholar]
- 46. Taylor AR, Cohen ND, Fletcher S et al. Application and machine accuracy of a new frameless computed tomography-guided stereotactic brain biopsy system in dogs. Vet Radiol Ultrasound. 2013;54:332–342. [DOI] [PubMed] [Google Scholar]
- 47. Chen AV, Winniger FA, Frey S et al. Description and validation of a magnetic resonance imaging-guided stereotactic brain biopsy device in the dog. Vet Radiol Ultrasound. 2012;53:150–156. [DOI] [PubMed] [Google Scholar]
- 48. Bentley RT, Ober CP, Anderson KL et al. Canine intracranial gliomas: relationship between magnetic resonance imaging criteria and tumor type and grade. Vet J. 2013;198:463–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Troxel MT, Vite CH. CT-guided stereotactic brain biopsy using the Kopf stereotactic system. Vet Radiol Ultrasound. 2008;49:438–443. [DOI] [PubMed] [Google Scholar]
- 50. Young BD, Levine JM, Porter BF et al. Magnetic resonance imaging features of intracranial astrocytomas and oligodendrogliomas in dogs. Vet Radiol Ultrasound. 2011;52:132–141. [DOI] [PubMed] [Google Scholar]
- 51. Rossmeisl JH Jr, Jones JC, Zimmerman KL et al. Survival time following hospital discharge in dogs with palliatively treated brain tumors. J Am Vet Med Assoc. 2013;242:193–198. [DOI] [PubMed] [Google Scholar]
- 52. Van Meervenne S, Verhoeven PS, de Vos J et al. Comparison between symptomatic treatment and lomustine supplementation in 71 dogs with intracranial, space-occupying lesions. Vet Comp Oncol. 2012;12:67–77. [DOI] [PubMed] [Google Scholar]
- 53. Almeida JP, Chaichana KL, Rincon-Torroella J et al. The value of extent of resection of glioblastomas: clinical evidence and current approach. Curr Neurol Neurosci Rep. 2015;15 (2):517 10.1007/s11910-014-0517-x [DOI] [PubMed] [Google Scholar]
- 54. D'Amico RS, Kennedy BC, Bruce JN. Neurosurgical oncology: advances in operative technologies and adjuncts. J Neurooncol. 2014;119:451–463. [DOI] [PubMed] [Google Scholar]
- 55. Chaichana KL, Jusue-Torres I, Navarro-Ramirez R et al. Establishing percent resection and residual volume thresholds affecting survival and recurrence for patients with newly diagnosed intracranial glioblastoma. Neuro Oncol. 2014;16 (1):113–122. 10.1093/neuonc/not137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Thomas R, Duke SE, Wang HJ et al. ‘Putting our heads together’: insights into genomic conservation between human and canine intracranial tumors. J Neurooncol. 2009;94 (3):333–349 10.1007/s11060-009-9877-5. Epub 2009 Mar 31. PubMed PMID: 19333554; PubMed Central PMCID: PMC3225023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Reitman ZJ, Olby NJ, Mariani CL et al. IDH1 and IDH2 hotspot mutations are not found in canine glioma. Int J Cancer. 2010;127:245–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sloma EA, Creneti CT, Erb HN et al. Characterization of inflammatory changes associated with canine oligodendroglioma. J Comp Pathol. 2015;153 (2–3):92–100 10.1016/j.jcpa.2015.05.003. Epub 2015 Jul 3. [DOI] [PubMed] [Google Scholar]
- 59. Cancer Genome Atlas Research Network. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455 (7216):1061–1068. 10.1038/nature07385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Cancer Genome Atlas Research Network, Brat DJ, Verhaak RG et al. Comprehensive, integrative genomic analysis of diffuse lower-grade gliomas. N Engl J Med. 2015;372 (26):2481–2498. 10.1056/NEJMoa1402121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Verhaak RG, Hoadley KA, Purdom E et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17 (1):98–110. 10.1016/j.ccr.2009.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Johnson GC, Coates JR, Wininger F. Diagnostic immunohistochemistry of canine and feline intracalvarial tumors in the age of brain biopsies. Vet Pathol. 2014;51 (1):146–160. 10.1177/0300985813509387 [DOI] [PubMed] [Google Scholar]
- 63. Simpson RM, Bastian BC, Michael HT et al. Sporadic naturally occurring melanoma in dogs as a preclinical model for human melanoma. Pigment Cell Melanoma Res. 2014;27 (1):37–47. 10.1111/pcmr.12185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Rossmeisl JH Jr, Garcia PA, Daniel GB et al. Invited review—neuroimaging response assessment criteria for brain tumors in veterinary patients. Vet Radiol Ultrasound. 2014;55 (2):115–132. 10.1111/vru.12118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ellingson BM, Bendszus M, Boxerman J et al. Consensus recommendations for a standardized brain tumor imaging protocol in clinical trials. Neuro Oncol. 2015;17 (9):1188–1198. 10.1093/neuonc/nov095 [DOI] [PMC free article] [PubMed] [Google Scholar]