Abstract
This guideline provides recommendations for the use of PET imaging in gliomas. The review examines established clinical benefit in glioma patients of PET using glucose (18F-FDG) and amino acid tracers (11C-MET, 18F-FET, and 18F-FDOPA). An increasing number of studies have been published on PET imaging in the setting of diagnosis, biopsy, and resection as well radiotherapy planning, treatment monitoring, and response assessment. Recommendations are based on evidence generated from studies which validated PET findings by histology or clinical course. This guideline emphasizes the clinical value of PET imaging with superiority of amino acid PET over glucose PET and provides a framework for the use of PET to assist in the management of patients with gliomas.
Keywords: amino acid PET, glioma, guideline, PET imaging, recommendations
Gliomas are the second most common primary brain tumors, with an incidence of 4–5/100 000 individuals. Gliomas are the second leading cause of cancer mortality in adults under the age of 35, the fourth leading cause in those under the age of 54, and result in death in approximately 13 770 individuals per year in the United States.1 Median survival of glioblastoma, the most aggressive variant, is 16 months in patients treated with maximum safe resection, radiotherapy, and concurrent and adjuvant temozolomide in clinical trial populations.2–4
MRI is the mainstay of imaging of gliomas to monitor both treatment and response. T1-weighted MRI without and with contrast medium, T2-weighted as well as fluid-attenuated inversion recovery (FLAIR) MRI sequences are used for anatomic imaging. However, many brain tumors (particularly World Health Organization [WHO] grade II and a significant number of WHO grade III gliomas) do not enhance with contrast-agent administration, reducing the ability of contrast imaging to accurately quantify tumor burden. The challenge to accurately determine brain tumor response by MRI both in daily practice and in clinical trials has led to the introduction of updated guidelines by the Response Assessment in Neuro-Oncology (RANO) working group.5
Functional molecular imaging such as positron emission tomography (PET) uses various tracers to visualize biological processes such as cell proliferation, membrane biosynthesis, glucose consumption, and uptake of amino acid analogs.6 Hence, PET provides additional insight beyond MRI into the biology and treatment response of gliomas which may be used for noninvasive grading, differential diagnosis, delineation of tumor extent, surgical and radiotherapy treatment planning, posttreatment surveillance, and prognostication.
Analogous to the RANO effort regarding MRI use in gliomas, an initiative was undertaken by a group of clinicians and nuclear medicine physicians to similarly define standards of molecular imaging for gliomas using PET with respect to interpretation and validation as well as to define its role in clinical practice. In this paper, evidence-based recommendations are proposed for the use of PET imaging in the clinical management of glioma patients. Accordingly, the review discusses tracers which image glucose metabolism—18F-2-fluoro-2-deoxy-d-glucose (18F-FDG)—and amino acid transport ([11C-methyl]-methionine (11C-MET), O-(2-[18F]-fluoroethyl)-l-tyrosine (18F-FET) and 3,4-dihydroxy-6-[18F]-fluoro-l-phenylalanine (18F-FDOPA)), since these compounds have already entered clinical practice.
The current guidelines aim to serve medical professionals of all disciplines involved in the diagnosis and care of patients with gliomas. A separate procedural guideline focusing on the standardization of technical aspects of PET imaging for glioma will be the subject of another paper prepared by the EANM (European Association of Nuclear Medicine)/EANO (European Association of Neuro-Oncology)/RANO groups.
Levels of Validation and Clinical Evidence Search Strategy and Selection Criteria
The information retrieved from a PubMed search of the published literature with the combination of the search terms “glioma,” “glioblastoma,” “brain tumor,” “PET,” “FDG,” “FET,” “MET,” and “DOPA” until September 2015 as well as from articles identified through searches of the authors' own files was evaluated by the working group with respect to the level of evidence and the grade of validation of the PET studies examined.
Any study that correlated the PET findings with histopathology was considered to represent the highest degree of validation. Next, correlation with MRI (when applicable, according to RANO criteria) and with the patient's clinical course was used for the second level of validation. Only papers constituting levels 1–3 evidence according to the Oxford Centre for Evidence-based Medicine (OCEBM Levels of Evidence Working Group: “The Oxford 2011 Levels of Evidence”) were included.
General Recommendations
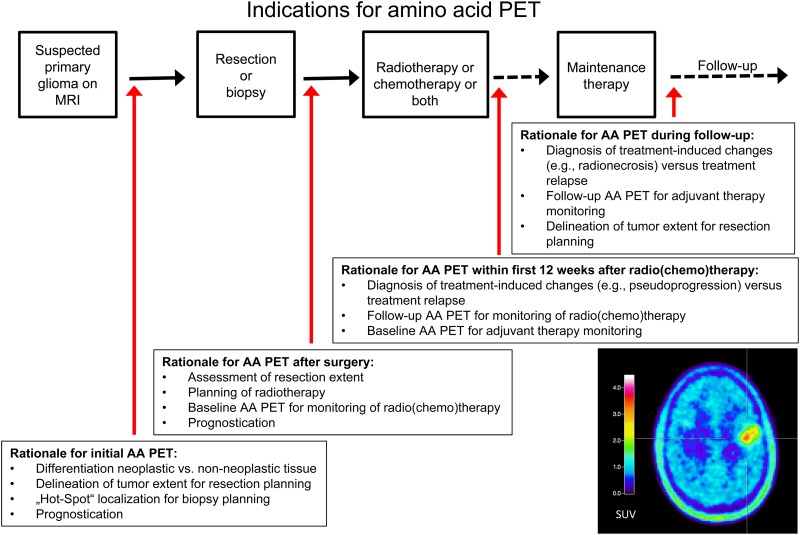
Recommendations for clinical use and diagnostic performance of differing PET tracers compared with MRI are presented in Tables 1 and 2 and in Fig. 1.
Table 1.
Diagnostic performance of different amino acid tracers compared with conventional and advanced MRI
| Clinical Problem | MET | FET | FDOPA |
|---|---|---|---|
| Differentiation of glioma from nonneoplastic lesions | Numerous studies,19 higher diagnostic accuracy than MRI alone | Higher diagnostic accuracy than MRI alone11,12,18 | Not available for the initial diagnosis |
| Glioma grading (including detection of anaplastic foci) | Higher diagnostic accuracy than MRI, but still limited accuracy due to high overlap between WHO grades19,96 | Higher diagnostic accuracy than MRI, in particular for dynamic PET14,26,93
High accuracy by combination of dynamic FET-PET and diffusion MRI97 |
No studies available comparing directly PET with MRI; in the pure PET studies, conflicting results reporting high38,98 and low28,99 performance |
| Delineation of glioma extent | Metabolically active tumor larger than contrast enhancement in LGG and HGG at diagnosis and recurrence100,101
Delineates metabolically active tumor in non-enhancing anaplastic glioma32,102 |
In newly diagnosed glioblastoma, metabolically active tumor larger than CE pre- and postoperatively46,103
In WHO grades II/IV gliomas metabolically active tumor larger than rCBV104 |
In glioma, metabolically active tumor larger than rCBV,105 ADC,106 and contrast enhancement34,36 |
| Differentiation of glioma recurrence from treatment-induced changes (eg, pseudoprogression, radionecrosis) | Higher diagnostic accuracy than MRI66 | Higher diagnostic accuracy than MRI74,81,107 | Higher diagnostic accuracy than MRI17,37,79,108 |
| Assessment of treatment response (including pseudoresponse) | Superior to MRI; metabolic response to TMZ predictive for survival70 | Superior to MRI; metabolic responses to TMZ,83 RT,69,71 and BEV76,78 occurred earlier and/or were associated with improved survival | Superior to MRI; metabolic response to BEV77 occurred earlier and was predictive of improved survival |
| Assessment of prognosis in gliomas | In contrast to pretreatment CE volumes, metabolically active tumor volumes are prognostic in HGG86,95 | Metabolically active tumor volume is prognostic in WHO grade IV glioma.46
Higher prognostic value of time-activity curves in dynamic PET than MRI within WHO grade II and WHO grades III/IV glioma.15,91,92 FET uptake in combination with specific MRI findings is prognostic94 for WHO grade II glioma |
Superior to MRI in WHO grade II glioma; maximum uptake is an independent predictor of progression109 |
Abbreviations: LGG, low-grade glioma; HGG, high-grade glioma; CE, contrast enhancement; rCBV, relative cerebral blood volume; ADC, apparent diffusion coefficient; TMZ, temozolomide; RT, radiotherapy; BEV, bevacizumab.
Table 2.
Overview of histologically validated amino acid PET studies in gliomas
| Clinical Problem | MET | FET | FDOPA |
|---|---|---|---|
| Differentiation of neoplastic from nonneoplastic lesions | Stereotactic biopsy110
Hot-spot guided resection111 |
Stereotactic biopsy and hot-spot guided resection11 | n.a. |
| Differentiation between WHO grades II and WHO grades III/IV glioma | In a subset of patients stereotactic biopsy112 | Stereotactic biopsy14,22,25 | In a subset of patients stereotactic biopsy and hot-spot guided resection38 |
| Delineation of glioma extent | Stereotactic biopsy39,43,113,114
Hot-spot guided resection101,115 |
Stereotactic biopsy41,116
Stereotactic biopsy and hot-spot guided resection117 |
In a subset of patients hot-spot guided resection36 |
| Differentiation of glioma recurrence from treatment-induced changes (eg, radionecrosis) | Stereotactic biopsy118 | Stereotactic biopsy119
Stereotactic biopsy and hot-spot guided resection81,120 |
In a subset of patients stereotactic biopsy17,108 |
| Detection of malignant tumor parts in MRI findings suggestive for WHO grade II glioma | Stereotactic biopsy and hot-spot guided resection96 | Stereotactic biopsy26
Stereotactic biopsy and hot-spot guided resection93 |
n.a. |
| Assessment of prognosis in untreated gliomas | Histological confirmation of glioma only95 (local comparison not necessary) | Histological confirmation of glioma only15,91 (local comparison not necessary) | Histological confirmation of glioma only109 (local comparison not necessary) |
Abbreviation: n.a., not available.
Fig. 1.
Overview of indications for amino acid PET.
Specific Recommendations
Primary Diagnosis/Differential Diagnosis
18F-FDG PET may provide useful information for distinguishing WHO grade III/IV gliomas from other malignant brain tumors, but its specificity is limited. Importantly, maximum standardized uptake values (SUVmax) were significantly higher in primary CNS lymphomas than in glioblastoma.7,8 However, corticosteroid medication may reduce uptake.
The differential diagnosis by 18F-FDG PET between WHO grades III/IV gliomas and brain metastases is limited, since considerable overlap of SUVmax exists between these tumor types.7 18F-FDG PET also has limited specificity for distinguishing glioma from other nonneoplastic lesions, such as brain abscesses, demyelinating tumefactive (“tumor-like”) lesions, fungal infections, and neurosarcoidosis9 due to increased 18F-FDG metabolism in inflammatory tissue.
Amino acid PET is useful for the noninvasive differentiation of tumor and nontumoral processes, as tumors have significantly higher uptake than nonneoplastic tissue.10,11 However, moderately increased uptake can also be seen in acute inflammatory lesions such as active multiple sclerosis and brain abscesses.12,13 Conversely, lack of 18F-FET uptake does not exclude a glioma, as approximately one-third of WHO grade II gliomas and most dysembryoplastic neuroepithelial tumors (WHO grade I) are 18F-FET negative.14 However, among WHO grades III and IV gliomas, the vast majority (>95%) show increased uptake,11,12,15 with a resultant high sensitivity for the detection of these tumors. A recent meta-analysis revealed that for brain tumor diagnosis, 18F-FET PET performed much better than 18F-FDG PET and consequently would be the preferred PET tracer when assessing patients with a newly diagnosed brain tumor.16 Furthermore, numerous studies validated by histology have demonstrated higher diagnostic accuracy of additional amino acid PET compared with anatomic MRI alone for the differentiation of gliomas from nonneoplastic lesions.11,12,17–19
In cases of diagnostic uncertainty, amino acid PET improves sensitivity, specificity, and accuracy and is markedly superior to 18F-FDG PET in differentiating between glioma and nonneoplastic tissue.
Tumor Grading
The value of 18F-FDG PET for grading of gliomas is hampered by the poor tumor-to-background contrast due to physiologically increased glucose uptake of cortical and subcortical (basal ganglia, thalamus) structures in brain and high variation of uptake and overlap of uptake values between tumors of different WHO grades, especially in oligodendroglial tumors.20,21 However, WHO grades III and IV gliomas generally have higher 18F-FDG values than WHO grade II gliomas, which often appear as a hypometabolic lesion, particularly when compared with the uptake in the cortex.16
Characteristically, amino acid uptake is higher in gliomas of WHO grades III/IV compared with WHO grade II gliomas. However, uptake intensities may vary, and tumor-to-brain ratios show a considerable overlap between different WHO grades as well as histological subtypes.11,12,22–24 For 18F-FET, accuracy for tumor grading can be markedly improved by evaluating dynamic (kinetic) PET data, which typically show steadily increasing time-activity curves in WHO grade II gliomas, as opposed to an early activity peak around 10–20 min after injection, followed by a decrease of 18F-FET uptake in WHO grades III/IV gliomas.22,25 This is particularly valuable in the clinical setting of patients with MRI non-contrast-enhancing gliomas suspected of harboring a WHO grade II glioma. In approximately 40% of such cases, an anaplastic focus is demonstrated.14,26 In these patients, kinetic analysis provides a higher sensitivity and specificity for the detection of WHO grades III/IV gliomas (95%).14 This method of kinetic analysis does not work for 11C-MET24; and for 18F-FDOPA, data are still controversial.27,28
Although 18F-FDG and amino acid uptake are usually higher in WHO grades III/IV gliomas than in WHO grade II gliomas, tumor grading is limited due to significant overlap in uptake values.
Dynamic analysis of 18F-FET PET uptake further improves differential diagnosis between WHO grade II and WHO grades III/IV gliomas.
Delineation of Glioma Extent
Multiple histopathological and postmortem series demonstrate the limitations of conventional MRI in defining the extent of glioma.29,30 Moreover, the usefulness of 18F-FDG PET in tumor delineation, given high uptake in normal brain cortex and low uptake in WHO grade II gliomas, is particularly limited for cortical or pericortical tumors, even when dual-timepoint images are performed.31 In contrast, amino acid PET imaging more accurately identifies infiltrating regions of tumor extending beyond the MRI contrast-enhancing lesion and often distinguishes among tumor, nontumoral edema, and normal brain.32 In addition, amino acid PET provides functional and metabolic information about the tumor and may identify tumor regions with different biological and clinical behavior. In both WHO grade II and WHO grades III/IV gliomas, amino acid PET complements conventional MRI by providing additional information about tumor extent and biology.
WHO grades III/IV glioma
Both the uptake and image contrast between tumor and normal tissue of amino acid tracers such as 11C-MET and 18F-FET are similar.33 PET-based tumor volumes have been shown to extend beyond the contrast-enhancing volume on conventional MRI by 2–3.5 cm for different tracers.34–37 In addition, amino acid PET identifies tumor extent within nonspecific regions of T2/FLAIR signal abnormality.34,36
WHO grade II glioma
Most WHO grade II gliomas are nonenhancing with infiltrating tumor borders that are difficult to delineate by conventional MRI. Several studies have demonstrated the usefulness of amino acid PET in defining tumor extent. This has been demonstrated in histology-validated series for 11C-MET, 18F-FET, and 18F-FDOPA PET.17,38–41
18F-FDG is not suitable for glioma volume delineation.
Delineation of tumor borders by amino acid PET is superior to standard MRI both in contrast-enhancing as well as non-contrast-enhancing gliomas.
Value for Treatment Planning: Biopsy and Resection
Implementation of PET into biopsy and resection planning is advantageous, as PET better delineates tumor extent compared with standard MRI and additionally identifies intratumoral heterogeneity, including malignant foci in non-contrast-enhancing gliomas.
Numerous studies have investigated the benefit of incorporating 18F-FDG or amino acid PET into biopsy target planning. The identification of malignant foci (“hot spots”) in MRI heterogeneous gliomas is essential for biopsy planning to ensure that the biologically most aggressive portion of the tumor, which determines the patient's prognosis as well as treatment, is not undersampled.26,42 There are several reports that illustrate the advantages of amino acid PET–based resection planning, of considerable importance whenever functional, eloquent areas may be involved,26,34,43 and which demonstrate a higher probability of detecting more highly malignant areas within an MRI heterogeneous glioma as well as decreased risk of incomplete resection.44,45,46
Integration of amino acid PET into surgical planning allows better delineation of the extent of resection beyond margins visible with standard MRI. This is of considerable importance whenever functional eloquent areas of brain are involved.
For biopsy planning, amino acid PET is particularly helpful in identifying malignant foci within non-contrast-enhancing gliomas.
Value for Treatment Planning: Radiation
Beyond MRI-based morphologic gross tumor volume delineation, a biological tumor volume may be defined by radiotracer uptake on amino acid PET that identifies tumor beyond the extent visible with standard MRI.47 In addition, the biologic and metabolic information provided by PET may identify subregions of tumor at higher risk of recurrence, which can be included in the radiation boost volume. The ability to better define tumor extent and biology may be used to improve the therapeutic ratio of radiation treatment. The current recommendations focus on the role of PET for radiation planning of WHO grades III/IV gliomas, as the role of PET imaging in irradiation of WHO grade II gliomas is not well established.
Small, prospective studies systematically comparing contrast MRI tumor volume (the “standard” radiation boost target) and 18F-FDG uptake abnormality generally demonstrate a smaller region of 18F-FDG uptake contained within the MRI abnormality, with only occasional extension outside of the MRI target.48,49 Although small studies have demonstrated the feasibility of radiation boost planning using 18F-FDG PET, its utility is limited by the low contrast between tumor and normal cortex.48
Studies analyzing patterns of failure following conventional chemoradiotherapy based on standard MRI-defined tumor volumes suggest that amino acid PET–defined tumor volumes may yield a more appropriate radiation target volume.50–52 In these small studies, a proportionate increase in marginal or noncentral tumor recurrences were seen when regions of 11C-MET and 18F-FET abnormality were not adequately covered by high-dose radiation. Prospective, single-arm studies evaluating the use of amino acid PET for radiation treatment planning of recurrent WHO grades III/IV glioma suggest the feasibility of this approach, and most studies suggest an improvement in outcome compared with radiation planning based on conventional MRI alone.53,54 However, the inclusion of amino acid PET–based tumor volumes in standard-dose radiation therapy and reirradiation protocols continue to demonstrate a predominance of in-field tumor recurrences, highlighting the need for more effective therapies.53–56
Amino acid PET may improve the delineation of a biological tumor volume beyond conventional MRI and identify aggressive tumor subregions that may be targeted by radiation therapy.
While 18F-FDG PET is of limited utility in radiation treatment planning of WHO grades III/IV gliomas, radiation planning using amino acid PET appears feasible, with preliminary evidence of potential benefit.
Follow-up: Treatment Response, Progression, Pseudoprogression, and Radionecrosis
To date, standard, structural MRI is the most important diagnostic tool for assessing treatment effects in patients with gliomas.4 The extent of contrast enhancement on MRI is usually considered an indicator of treatment response (eg, Macdonald criteria, RANO criteria),5,57 although its reliability in distinguishing tumor tissue from treatment effects, which can include blood–brain barrier breakdown, is limited.58 For example, transient blood–brain barrier alteration with contrast enhancement—such as after radiotherapy with or without concomitant temozolomide—can mimic tumor progression and is termed “pseudoprogression.”59,60 In addition, since the introduction of anti-angiogenic agents such as bevacizumab, the phenomenon of pseudoresponse complicates the assessment of treatment response using standard MRI alone.59,61
WHO grades III/IV glioma
Few 18F-FDG PET studies have measured the glucose metabolic rate following either radiotherapy, chemotherapy, or both: decrease of 18F-FDG uptake correlates with treatment response.62–64 18F-FDG PET has been found to be of only moderate additional value to MRI for differentiation between malignant glioma recurrence and radionecrosis, especially due to low specificity.65,66,67,68 However, there are several limitations: most studies were retrospective, jointly assessed gliomas of all WHO grades, used differing treatments, had varying assessment strategies, and utilized varying 18F-FDG thresholds of tumor to normal brain for image interpretation.
The feasibility and usefulness of amino acid PET such as 11C-MET, 18F-FET, or 18F-FDOPA PET for treatment assessment after chemoradiotherapy, stereotactic brachytherapy, chemotherapy, and other experimental approaches have been demonstrated in several studies, primarily in WHO grades III/IV gliomas. Current amino acid PET data suggest that a reduction of amino acid uptake and/or a decrease of the metabolically active tumor volume is a sign of treatment response associated with long-term outcome.69–73 Amino acid PET using 18F-FET may facilitate the diagnosis of pseudoprogression in glioblastoma patients within the first 12 weeks following completion of chemoradiotherapy.74
Furthermore, several studies suggest that treatment response and outcome in bevacizumab therapy can be assessed by amino acid PET using 18F-FET and 18F-FDOPA better than by MRI.75–78
Amino acid PET is useful for the differentiation between treatment-related changes and true progression with high sensitivity and specificity.37,79,80 A combination of static and dynamic 18F-FET PET parameters identified correctly progressive or recurrent glioma with higher accuracy (93%) than conventional MRI.81
WHO grade II glioma
In contrast to patients with WHO grades III/IV gliomas, the experience with amino acid PET for monitoring after treatment in patients with WHO grade II gliomas is limited, with only a few studies available in the literature.82,83 As these tumors are usually negative on 18FDG PET, the latter is not suitable for response evaluation.
Analysis of 18F-FDG uptake does not reliably distinguish between recurrence and radionecrosis.
A decrease in amino acid uptake and/or volume is associated with treatment response across gliomas of WHO grades III/IV.
Amino acid PET improves the assessment of pseudoprogression, radionecrosis, and pseudoresponse.
Prognostication
The prognostic value of 18F-FDG uptake in gliomas has been suggested by several studies.84–87 Additionally, pretreatment 18F-FDG PET has been reported to correlate with survival in patients with newly diagnosed glioblastoma88 or recurrent high-grade gliomas receiving bevacizumab.89
The prognostic value of amino acid PET has been increasingly explored.15,90–92 At diagnosis, dynamic 18F-FET PET identified highly aggressive astrocytomas within the same WHO grade—for instance, WHO grade II gliomas with decreasing time-activity curves manifested earlier tumor progression, malignant transformation, as well as shorter survival.91,93 Similarly dynamic 18F-FET PET identified anaplastic astrocytomas with a very early decrease of time-activity curves—and consequently short time-to-peak—as having a comparably poor outcome.15
To date, the association of glioma 18F-FET uptake with survival has remained controversial. Some groups have reported a better outcome of patients with absent or only low tumoral amino acid uptake.86,90,94 In contrast, a larger study of 18F-FET-negative glioma patients did not reveal an association with improved outcome, as neither time to transformation, which was proven upon histological evaluation, nor overall survival differed from that of FET-positive glioma patients.15
A prospective multicenter trial investigating the role of pretreatment 18F-FET PET in newly diagnosed glioblastoma found biological tumor volume prior to chemoradiotherapy to be highly prognostic for outcome.46 This is in accordance with results of previous studies investigating amino acid PET in malignant glioma prior to therapy.69,95
Uptake of 18F-FDG and amino acid tracer is associated with outcome in WHO grades III/IV glioma both in a pretreatment setting and following therapy.
Biological tumor volume in amino acid PET is associated with survival following therapy in glioblastoma.
Dynamic analysis of 18F-FET uptake provides prognostic information within all grades of glioma prior to treatment.
Current Limitations
While 18F-FDG is used at all PET sites, only a few centers offer amino acid PET so far. However, due to the 18F labeling of FET and FDOPA, the radiotracer can be delivered in the same way as 18F-FDG, facilitating the availability of amino acid PET. Only for 11C-MET is an on-site cyclotron required. The major obstacle for the widespread use of amino acid PET in glioma patients is to date the limited reimbursement by health insurance companies/institutions, despite the fact that current data clearly favor amino acid over 18F-FDG PET.
Across all tracers, numerous studies differed in terms of methodology, which limits comparability of data and might eventually jeopardize acceptance in the clinical setting. However, this guideline collected convincing support that PET imaging is of additional value beyond MRI in glioma management.
Outlook Perspective
Future clinical studies should consider the use of amino acid PET as an imaging modality for gliomas complementary to MRI. Standardized technical guidelines for PET imaging procedures and recommendations by the EANM/EANO/RANO group will be published separately.
Funding
This work has not been funded.
Conflict of interests statement. M.W. has received research grants from Acceleron, Actelion, Alpinia Institute, Bayer, Isarna, MSD, Merck & Co, Novocure, PIQUR, and Roche and honoraria for lectures or advisory board participation or consulting from Celldex, Immunocellular Therapeutics, Isarna, Magforce, MSD, Merck & Co, Northwest Biotherapeutics, Novocure, Pfizer, Roche, and Teva. M.M.K. has received research funding from EpicentRx, Inc. C.l.F. has received personal fees from Bayer and GE Healthcare, advisory board fees from Bayer, Endocyte, and GE Healthcare, grants from GE Healthcare, Pierre Fabre, and Siemens Healthcare. W.P. is consultant for Celldex Therapeutics, Imaging Endpoints, LLC, and Tocagen. J.A. has received financial research support from Siemens and Avid-Lilly and honoraria as a speaker or advisory board member from Bayer, Advanced Accelerator Applications, Lilly, Piramal, and General Electric. M.A.V. is an officer with intellectual property, equity, and royalty interests at Infuseon Therapeutics, is a member of DSMB Neuralstem, and is a consultant for Pharmaco-Kinesis. B.M.E. is a consultant for MedQIA, LLC and is a consultant for and has received grants from or participated on the advisory board of Siemens Healthcare, Roche/Genentech, Agios Pharmaceuticals, Bristol-Meyers Squibb, and Merck. J.C.T. is a consultant/advisory board member for Merck Serono, Roche, and Celldex. He received speakers bureau honoraria from Merck Serono, Roche, Brainlab, and Siemens. N.L.A., B.S., N.G., R.S., I.L., and M.C. report no conflicts of interest.
References
- 1. Ostrom QT, Gittleman H, Liao P et al. . CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2007–2011. Neuro Oncol. 2014;16 (Suppl 4):iv1–iv63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stupp R, Mason WP, van den Bent MJ et al. . Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352 (10):987–996. [DOI] [PubMed] [Google Scholar]
- 3. Chinot OL, Wick W, Mason W et al. . Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014;370 (8):709–722. [DOI] [PubMed] [Google Scholar]
- 4. Weller M, van den Bent M, Hopkins K et al. . EANO guideline for the diagnosis and treatment of anaplastic gliomas and glioblastoma. Lancet Oncol. 2014;15 (9): e395–e403. [DOI] [PubMed] [Google Scholar]
- 5. Wen PY, Macdonald DR, Reardon DA et al. . Updated response assessment criteria for high-grade gliomas: Response Assessment in Neuro-Oncology working group. J Clin Oncol. 2010;28 (11):1963–1972. [DOI] [PubMed] [Google Scholar]
- 6. la Fougere C, Suchorska B, Bartenstein P et al. . Molecular imaging of gliomas with PET: opportunities and limitations. Neuro Oncol. 2011;13 (8):806–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kosaka N, Tsuchida T, Uematsu H et al. . 18F-FDG PET of common enhancing malignant brain tumors. AJR Am J Roentgenol. 2008;190 (6):W365–W369. [DOI] [PubMed] [Google Scholar]
- 8. Yamashita K, Yoshiura T, Hiwatashi A et al. . Differentiating primary CNS lymphoma from glioblastoma multiforme: assessment using arterial spin labeling, diffusion-weighted imaging, and (1)(8)F-fluorodeoxyglucose positron emission tomography. Neuroradiology. 2013;55 (2):135–143. [DOI] [PubMed] [Google Scholar]
- 9. Omuro AM, Leite CC, Mokhtari K et al. . Pitfalls in the diagnosis of brain tumours. Lancet Neurol. 2006;5 (11):937–948. [DOI] [PubMed] [Google Scholar]
- 10. Chung JK, Kim YK, Kim SK et al. . Usefulness of 11C-methionine PET in the evaluation of brain lesions that are hypo- or isometabolic on 18F-FDG PET. Eur J Nucl Med Mol Imaging. 2002;29 (2):176–182. [DOI] [PubMed] [Google Scholar]
- 11. Rapp M, Heinzel A, Galldiks N et al. . Diagnostic performance of 18F-FET PET in newly diagnosed cerebral lesions suggestive of glioma. J Nucl Med. 2013;54 (2):229–235. [DOI] [PubMed] [Google Scholar]
- 12. Hutterer M, Nowosielski M, Putzer D et al. . [18F]-fluoro-ethyl-L-tyrosine PET: a valuable diagnostic tool in neuro-oncology, but not all that glitters is glioma. Neuro Oncol. 2013;15 (3):341–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Floeth FW, Pauleit D, Sabel M et al. . 18F-FET PET differentiation of ring-enhancing brain lesions. J Nucl Med. 2006;47 (5):776–782. [PubMed] [Google Scholar]
- 14. Jansen NL, Graute V, Armbruster L et al. . MRI-suspected low-grade glioma: is there a need to perform dynamic FET PET? Eur J Nucl Med Mol Imaging. 2012;39 (6):1021–1029. [DOI] [PubMed] [Google Scholar]
- 15. Jansen NL, Suchorska B, Wenter V et al. . Prognostic significance of dynamic 18F-FET PET in newly diagnosed astrocytic high-grade glioma. J Nucl Med. 2015;56 (1):9–15. [DOI] [PubMed] [Google Scholar]
- 16. Dunet V, Pomoni A, Hottinger A et al. . Performance of 18F-FET versus 18F-FDG-PET for the diagnosis and grading of brain tumors: systematic review and meta-analysis. Neuro Oncol. 2016;18 3:426–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Karunanithi S, Sharma P, Kumar A et al. . Comparative diagnostic accuracy of contrast-enhanced MRI and (18)F-FDOPA PET-CT in recurrent glioma. Eur Radiol. 2013;23 (9):2628–2635. [DOI] [PubMed] [Google Scholar]
- 18. Dunet V, Rossier C, Buck A et al. . Performance of 18F-fluoro-ethyl-tyrosine (18F-FET) PET for the differential diagnosis of primary brain tumor: a systematic review and Metaanalysis. J Nucl Med. 2012;53 (2):207–214. [DOI] [PubMed] [Google Scholar]
- 19. Glaudemans AW, Enting RH, Heesters MA et al. . Value of 11C-methionine PET in imaging brain tumours and metastases. Eur J Nucl Med Mol Imaging. 2013;40 (4):615–635. [DOI] [PubMed] [Google Scholar]
- 20. Giammarile F, Cinotti LE, Jouvet A et al. . High and low grade oligodendrogliomas (ODG): correlation of amino-acid and glucose uptakes using PET and histological classifications. J Neurooncol. 2004;68 (3):263–274. [DOI] [PubMed] [Google Scholar]
- 21. Manabe O, Hattori N, Yamaguchi S et al. . Oligodendroglial component complicates the prediction of tumour grading with metabolic imaging. Eur J Nucl Med Mol Imaging. 2015;42 (6):896–904. [DOI] [PubMed] [Google Scholar]
- 22. Popperl G, Kreth FW, Mehrkens JH et al. . FET PET for the evaluation of untreated gliomas: correlation of FET uptake and uptake kinetics with tumour grading. Eur J Nucl Med Mol Imaging. 2007;34 (12):1933–1942. [DOI] [PubMed] [Google Scholar]
- 23. Jansen NL, Schwartz C, Graute V et al. . Prediction of oligodendroglial histology and LOH 1p/19q using dynamic [(18)F]FET-PET imaging in intracranial WHO grade II and III gliomas. Neuro Oncol. 2012;14 (12):1473–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Moulin-Romsee G, D'Hondt E, de Groot T et al. . Non-invasive grading of brain tumours using dynamic amino acid PET imaging: does it work for 11C-methionine? Eur J Nucl Med Mol Imaging. 2007;34 (12):2082–2087. [DOI] [PubMed] [Google Scholar]
- 25. Lohmann P, Herzog H, Rota Kops E et al. . Dual-time-point O-(2-[(18)F]fluoroethyl)-L-tyrosine PET for grading of cerebral gliomas. Eur Radiol. 2015;25 (10):3017–3024. [DOI] [PubMed] [Google Scholar]
- 26. Kunz M, Thon N, Eigenbrod S et al. . Hot spots in dynamic (18)FET-PET delineate malignant tumor parts within suspected WHO grade II gliomas. Neuro Oncol. 2011;13 (3):307–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schiepers C, Chen W, Cloughesy T et al. . 18F-FDOPA kinetics in brain tumors. J Nucl Med. 2007;48 (10):1651–1661. [DOI] [PubMed] [Google Scholar]
- 28. Kratochwil C, Combs SE, Leotta K et al. . Intra-individual comparison of (1)(8)F-FET and (1)(8)F-DOPA in PET imaging of recurrent brain tumors. Neuro Oncol. 2014;16 (3):434–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Watanabe M, Tanaka R, Takeda N. Magnetic resonance imaging and histopathology of cerebral gliomas. Neuroradiology. 1992;34 (6):463–469. [DOI] [PubMed] [Google Scholar]
- 30. Johnson PC, Hunt SJ, Drayer BP. Human cerebral gliomas: correlation of postmortem MR imaging and neuropathologic findings. Radiology. 1989;170 (1 Pt 1):211–217. [DOI] [PubMed] [Google Scholar]
- 31. Prieto E, Marti-Climent JM, Dominguez-Prado I et al. . Voxel-based analysis of dual-time-point 18F-FDG PET images for brain tumor identification and delineation. J Nucl Med. 2011;52 (6):865–872. [DOI] [PubMed] [Google Scholar]
- 32. Arbizu J, Tejada S, Marti-Climent JM et al. . Quantitative volumetric analysis of gliomas with sequential MRI and (1)(1)C-methionine PET assessment: patterns of integration in therapy planning. Eur J Nucl Med Mol Imaging. 2012;39 (5):771–781. [DOI] [PubMed] [Google Scholar]
- 33. Weber WA, Wester HJ, Grosu AL et al. . O-(2-[18F]fluoroethyl)-L-tyrosine and L-[methyl-11C]methionine uptake in brain tumours: initial results of a comparative study. Eur J Nucl Med. 2000;27 (5):542–549. [DOI] [PubMed] [Google Scholar]
- 34. Pafundi DH, Laack NN, Youland RS et al. . Biopsy validation of 18F-DOPA PET and biodistribution in gliomas for neurosurgical planning and radiotherapy target delineation: results of a prospective pilot study. Neuro Oncol. 2013;15 (8):1058–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Becherer A, Karanikas G, Szabo M et al. . Brain tumour imaging with PET: a comparison between [18F]fluorodopa and [11C]methionine. Eur J Nucl Med Mol Imaging. 2003;30 (11):1561–1567. [DOI] [PubMed] [Google Scholar]
- 36. Ledezma CJ, Chen W, Sai V et al. . 18F-FDOPA PET/MRI fusion in patients with primary/recurrent gliomas: initial experience. Eur J Radiol. 2009;71 (2):242–248. [DOI] [PubMed] [Google Scholar]
- 37. Chen W, Silverman DH, Delaloye S et al. . 18F-FDOPA PET imaging of brain tumors: comparison study with 18F-FDG PET and evaluation of diagnostic accuracy. J Nucl Med. 2006;47 (6):904–911. [PubMed] [Google Scholar]
- 38. Fueger BJ, Czernin J, Cloughesy T et al. . Correlation of 6-18F-fluoro-L-dopa PET uptake with proliferation and tumor grade in newly diagnosed and recurrent gliomas. J Nucl Med. 2010;51 (10):1532–1538. [DOI] [PubMed] [Google Scholar]
- 39. Kracht LW, Miletic H, Busch S et al. . Delineation of brain tumor extent with [11C]L-methionine positron emission tomography: local comparison with stereotactic histopathology. Clin Cancer Res. 2004;10 (21):7163–7170. [DOI] [PubMed] [Google Scholar]
- 40. Tripathi M, Sharma R, D'Souza M et al. . Comparative evaluation of F-18 FDOPA, F-18 FDG, and F-18 FLT-PET/CT for metabolic imaging of low grade gliomas. Clin Nucl Med. 2009;34 (12):878–883. [DOI] [PubMed] [Google Scholar]
- 41. Pauleit D, Floeth F, Hamacher K et al. . O-(2-[18F]fluoroethyl)-L-tyrosine PET combined with MRI improves the diagnostic assessment of cerebral gliomas. Brain. 2005;128 (Pt 3):678–687. [DOI] [PubMed] [Google Scholar]
- 42. Pirotte B, Goldman S, Brucher JM et al. . PET in stereotactic conditions increases the diagnostic yield of brain biopsy. Stereotact Funct Neurosurg. 1994;63 (1–4):144–149. [DOI] [PubMed] [Google Scholar]
- 43. Pirotte B, Goldman S, Massager N et al. . Comparison of 18F-FDG and 11C-methionine for PET-guided stereotactic brain biopsy of gliomas. J Nucl Med. 2004;45 (8):1293–1298. [PubMed] [Google Scholar]
- 44. Tanaka Y, Nariai T, Momose T et al. . Glioma surgery using a multimodal navigation system with integrated metabolic images. J Neurosurg. 2009;110 (1):163–172. [DOI] [PubMed] [Google Scholar]
- 45. Ewelt C, Floeth FW, Felsberg J et al. . Finding the anaplastic focus in diffuse gliomas: the value of Gd-DTPA enhanced MRI, FET-PET, and intraoperative, ALA-derived tissue fluorescence. Clin Neurol Neurosurg. 2011;113 (7):541–547. [DOI] [PubMed] [Google Scholar]
- 46. Suchorska B, Jansen NL, Linn J et al. . Biological tumor volume in 18FET-PET before radiochemotherapy correlates with survival in GBM. Neurology. 2015;84 (7):710–719. [DOI] [PubMed] [Google Scholar]
- 47. Grosu AL, Weber WA, Riedel E et al. . L-(methyl-11C) methionine positron emission tomography for target delineation in resected high-grade gliomas before radiotherapy. Int J Radiat Oncol Biol Phys. 2005;63 (1):64–74. [DOI] [PubMed] [Google Scholar]
- 48. Tralins KS, Douglas JG, Stelzer KJ et al. . Volumetric analysis of 18F-FDG PET in glioblastoma multiforme: prognostic information and possible role in definition of target volumes in radiation dose escalation. J Nucl Med. 2002;43 (12):1667–1673. [PubMed] [Google Scholar]
- 49. Gross MW, Weber WA, Feldmann HJ et al. . The value of F-18-fluorodeoxyglucose PET for the 3-D radiation treatment planning of malignant gliomas. Int J Radiat Oncol Biol Phys. 1998;41 (5):989–995. [DOI] [PubMed] [Google Scholar]
- 50. Lee IH, Piert M, Gomez-Hassan D et al. . Association of 11C-methionine PET uptake with site of failure after concurrent temozolomide and radiation for primary glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 2009;73 (2):479–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Niyazi M, Schnell O, Suchorska B et al. . FET-PET assessed recurrence pattern after radio-chemotherapy in newly diagnosed patients with glioblastoma is influenced by MGMT methylation status. Radiother Oncol. 2012;104 (1):78–82. [DOI] [PubMed] [Google Scholar]
- 52. Mahasittiwat P, Mizoe JE, Hasegawa A et al. . l-[METHYL-(11)C] methionine positron emission tomography for target delineation in malignant gliomas: impact on results of carbon ion radiotherapy. Int J Radiat Oncol Biol Phys. 2008;70 (2):515–522. [DOI] [PubMed] [Google Scholar]
- 53. Grosu AL, Weber WA, Franz M et al. . Reirradiation of recurrent high-grade gliomas using amino acid PET (SPECT)/CT/MRI image fusion to determine gross tumor volume for stereotactic fractionated radiotherapy. Int J Radiat Oncol Biol Phys. 2005;63 (2):511–519. [DOI] [PubMed] [Google Scholar]
- 54. Miwa K, Matsuo M, Ogawa S et al. . Re-irradiation of recurrent glioblastoma multiforme using 11C-methionine PET/CT/MRI image fusion for hypofractionated stereotactic radiotherapy by intensity modulated radiation therapy. Radiat Oncol. 2014;9:181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Weber DC, Casanova N, Zilli T et al. . Recurrence pattern after [(18)F]fluoroethyltyrosine-positron emission tomography-guided radiotherapy for high-grade glioma: a prospective study. Radiother Oncol. 2009;93 (3):586–592. [DOI] [PubMed] [Google Scholar]
- 56. Piroth MD, Pinkawa M, Holy R et al. . Integrated boost IMRT with FET-PET-adapted local dose escalation in glioblastomas. Results of a prospective phase II study. Strahlenther Onkol. 2012;188 (4):334–339. [DOI] [PubMed] [Google Scholar]
- 57. Macdonald DR, Cascino TL, Schold SC Jr. et al. . Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8:1277–1280. [DOI] [PubMed] [Google Scholar]
- 58. Kumar AJ, Leeds NE, Fuller GN et al. . Malignant gliomas: MR imaging spectrum of radiation therapy- and chemotherapy-induced necrosis of the brain after treatment. Radiology. 2000;217:377–384. [DOI] [PubMed] [Google Scholar]
- 59. Brandsma D, Stalpers L, Taal W et al. . Clinical features, mechanisms, and management of pseudoprogression in malignant gliomas. Lancet Oncol. 2008;9:453–461. [DOI] [PubMed] [Google Scholar]
- 60. Radbruch A, Fladt J, Kickingereder P et al. . Pseudoprogression in patients with glioblastoma: clinical relevance despite low incidence. Neuro Oncol. 2015;17 1:151–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Brandsma D, van den Bent MJ. Pseudoprogression and pseudoresponse in the treatment of gliomas. Curr Opin Neurol. 2009;22 (6):633–638. [DOI] [PubMed] [Google Scholar]
- 62. Spence AM, Muzi M, Graham MM et al. . 2-[(18)F]Fluoro-2-deoxyglucose and glucose uptake in malignant gliomas before and after radiotherapy: correlation with outcome. Clin Cancer Res. 2002;8 (4):971–979. [PubMed] [Google Scholar]
- 63. Rozental JM, Levine RL, Mehta MP et al. . Early changes in tumor metabolism after treatment: the effects of stereotactic radiotherapy. Int J Radiat Oncol Biol Phys. 1991;20 (5):1053–1060. [DOI] [PubMed] [Google Scholar]
- 64. Charnley N, West CM, Barnett CM et al. . Early change in glucose metabolic rate measured using FDG-PET in patients with high-grade glioma predicts response to temozolomide but not temozolomide plus radiotherapy. Int J Radiat Oncol Biol Phys. 2006;66 (2):331–338. [DOI] [PubMed] [Google Scholar]
- 65. Caroline I, Rosenthal MA. Imaging modalities in high-grade gliomas: pseudoprogression, recurrence, or necrosis? J Clin Neurosci. 2012;19 (5):633–637. [DOI] [PubMed] [Google Scholar]
- 66. Nihashi T, Dahabreh IJ, Terasawa T. Diagnostic accuracy of PET for recurrent glioma diagnosis: a meta-analysis. AJNR Am J Neuroradiol. 2013;34 (5):944–950. S1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Van Laere K, Ceyssens S, Van Calenbergh F et al. . Direct comparison of 18F-FDG and 11C-methionine PET in suspected recurrence of glioma: sensitivity, inter-observer variability and prognostic value. Eur J Nucl Med Mol Imaging. 2005;32 (1):39–51. [DOI] [PubMed] [Google Scholar]
- 68. Chao ST, Suh JH, Raja S et al. . The sensitivity and specificity of FDG PET in distinguishing recurrent brain tumor from radionecrosis in patients treated with stereotactic radiosurgery. Int J Cancer. 2001;96 (3):191–197. [DOI] [PubMed] [Google Scholar]
- 69. Galldiks N, Langen K, Holy R et al. . Assessment of treatment response in patients with glioblastoma using [18F]fluoroethyl-l-tyrosine PET in comparison to MRI. J Nucl Med. 2012;53:1048–1057. [DOI] [PubMed] [Google Scholar]
- 70. Galldiks N, Kracht LW, Burghaus L et al. . Use of 11C-methionine PET to monitor the effects of temozolomide chemotherapy in malignant gliomas. Eur J Nucl Med Mol Imaging. 2006;33 (5):516–524. [DOI] [PubMed] [Google Scholar]
- 71. Jansen NL, Suchorska B, Schwarz SB et al. . [18F]fluoroethyltyrosine-positron emission tomography-based therapy monitoring after stereotactic iodine-125 brachytherapy in patients with recurrent high-grade glioma. Mol Imaging. 2013;12:137–147. [PubMed] [Google Scholar]
- 72. Pöpperl G, Goldbrunner R, Gildehaus FJ et al. . O-(2-[18F]fluoroethyl)-L-tyrosine PET for monitoring the effects of convection-enhanced delivery of paclitaxel in patients with recurrent glioblastoma. Eur J Nucl Med Mol Imaging. 2005;32:1018–1025. [DOI] [PubMed] [Google Scholar]
- 73. Pöpperl G, Götz C, Rachinger W et al. . Serial O-(2-[(18)F]fluoroethyl)-L:-tyrosine PET for monitoring the effects of intracavitary radioimmunotherapy in patients with malignant glioma. Eur J Nucl Med Mol Imaging. 2006;33:792–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Galldiks N, Dunkl V, Stoffels G et al. . Diagnosis of pseudoprogression in patients with glioblastoma using O-(2-[18F]fluoroethyl)-L-tyrosine PET. Eur J Nucl Med Mol Imaging. 2015;42 (5):685–695. [DOI] [PubMed] [Google Scholar]
- 75. Harris RJ, Cloughesy TF, Pope WB et al. . 18F-FDOPA and 18F-FLT positron emission tomography parametric response maps predict response in recurrent malignant gliomas treated with bevacizumab. Neuro Oncol. 2012;14 (8):1079–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Galldiks N, Rapp M, Stoffels G et al. . Response assessment of bevacizumab in patients with recurrent malignant glioma using [18F]fluoroethyl-L-tyrosine PET in comparison to MRI. Eur J Nucl Med Mol Imaging. 2013;40:22–33. [DOI] [PubMed] [Google Scholar]
- 77. Schwarzenberg J, Czernin J, Cloughesy TF et al. . Treatment response evaluation using 18F-FDOPA PET in patients with recurrent malignant glioma on bevacizumab therapy. Clin Cancer Res. 2014;20:3550–3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Hutterer M, Nowosielski M, Putzer D et al. . O-(2-18F-fluoroethyl)-L-tyrosine PET predicts failure of antiangiogenic treatment in patients with recurrent high-grade glioma. J Nucl Med. 2011;52 (6):856–864. [DOI] [PubMed] [Google Scholar]
- 79. Herrmann K, Czernin J, Cloughesy T et al. . Comparison of visual and semiquantitative analysis of 18F-FDOPA-PET/CT for recurrence detection in glioblastoma patients. Neuro Oncol. 2014;16 (4):603–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Walter F, Cloughesy T, Walter MA et al. . Impact of 3,4-dihydroxy-6-18F-fluoro-L-phenylalanine PET/CT on managing patients with brain tumors: the referring physician's perspective. J Nucl Med. 2012;53 (3):393–398. [DOI] [PubMed] [Google Scholar]
- 81. Galldiks N, Stoffels G, Filss C et al. . The use of dynamic O-(2-18F-fluoroethyl)-l-tyrosine PET in the diagnosis of patients with progressive and recurrent glioma. Neuro Oncol. 2015;17 (9):1293–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Voges J, Herholz K, Holzer T et al. . 11C-methionine and 18F-2-fluorodeoxyglucose positron emission tomography: a tool for diagnosis of cerebral glioma and monitoring after brachytherapy with 125I seeds. Stereotact Funct Neurosurg. 1997;69 (1–4 Pt 2):129–135. [DOI] [PubMed] [Google Scholar]
- 83. Wyss M, Hofer S, Bruehlmeier M et al. . Early metabolic responses in temozolomide treated low-grade glioma patients. J Neurooncol. 2009;95 (1):87–93. [DOI] [PubMed] [Google Scholar]
- 84. Patronas NJ, Di Chiro G, Kufta C et al. . Prediction of survival in glioma patients by means of positron emission tomography. J Neurosurg. 1985;62 (6):816–822. [DOI] [PubMed] [Google Scholar]
- 85. De Witte O, Lefranc F, Levivier M et al. . FDG-PET as a prognostic factor in high-grade astrocytoma. J Neurooncol. 2000;49 (2):157–163. [DOI] [PubMed] [Google Scholar]
- 86. Singhal T, Narayanan TK, Jacobs MP et al. . 11C-methionine PET for grading and prognostication in gliomas: a comparison study with 18F-FDG PET and contrast enhancement on MRI. J Nucl Med. 2012;53 (11):1709–1715. [DOI] [PubMed] [Google Scholar]
- 87. Colavolpe C, Metellus P, Mancini J et al. . Independent prognostic value of pre-treatment 18-FDG-PET in high-grade gliomas. J Neurooncol. 2012;107 (3):527–535. [DOI] [PubMed] [Google Scholar]
- 88. Omuro A, Beal K, Gutin P et al. . Phase II study of bevacizumab, temozolomide, and hypofractionated stereotactic radiotherapy for newly diagnosed glioblastoma. Clin Cancer Res. 2014;20 (19):5023–5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Colavolpe C, Chinot O, Metellus P et al. . FDG-PET predicts survival in recurrent high-grade gliomas treated with bevacizumab and irinotecan. Neuro Oncol. 2012;14 (5):649–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Floeth FW, Sabel M, Stoffels G et al. . Prognostic value of 18F-fluoroethyl-L-tyrosine PET and MRI in small nonspecific incidental brain lesions. J Nucl Med. 2008;49 (5):730–737. [DOI] [PubMed] [Google Scholar]
- 91. Jansen NL, Suchorska B, Wenter V et al. . Dynamic 18F-FET PET in newly diagnosed astrocytic low-grade glioma identifies high-risk patients. J Nucl Med. 2014;55 (2):198–203. [DOI] [PubMed] [Google Scholar]
- 92. Thon N, Kunz M, Lemke L et al. . Dynamic F-FET PET in suspected WHO grade II gliomas defines distinct biological subgroups with different clinical courses. Int J Cancer. 2015;136 9:2132–2145. [DOI] [PubMed] [Google Scholar]
- 93. Galldiks N, Stoffels G, Ruge MI et al. . Role of O-(2-18F-fluoroethyl)-L-tyrosine PET as a diagnostic tool for detection of malignant progression in patients with low-grade glioma. J Nucl Med. 2013;54 (12):2046–2054. [DOI] [PubMed] [Google Scholar]
- 94. Floeth FW, Pauleit D, Sabel M et al. . Prognostic value of O-(2-18F-fluoroethyl)-L-tyrosine PET and MRI in low-grade glioma. J Nucl Med. 2007;48 (4):519–527. [DOI] [PubMed] [Google Scholar]
- 95. Galldiks N, Dunkl V, Kracht LW et al. . Volumetry of [(1)(1)C]-methionine positron emission tomographic uptake as a prognostic marker before treatment of patients with malignant glioma. Mol Imaging. 2012;11 (6):516–527. [PubMed] [Google Scholar]
- 96. Ullrich RT, Kracht L, Brunn A et al. . Methyl-L-11C-methionine PET as a diagnostic marker for malignant progression in patients with glioma. J Nucl Med. 2009;50 (12):1962–1968. [DOI] [PubMed] [Google Scholar]
- 97. Dunet V, Maeder P, Nicod-Lalonde M et al. . Combination of MRI and dynamic FET PET for initial glioma grading. Nuklearmedizin. 2014;53 (4):155–161. [DOI] [PubMed] [Google Scholar]
- 98. Nioche C, Soret M, Gontier E et al. . Evaluation of quantitative criteria for glioma grading with static and dynamic 18F-FDopa PET/CT. Clin Nucl Med. 2013;38 (2):81–87. [DOI] [PubMed] [Google Scholar]
- 99. Chen W, Cloughesy T, Kamdar N et al. . Imaging proliferation in brain tumors with 18F-FLT PET: comparison with 18F-FDG. J Nucl Med. 2005;46 (6):945–952. [PubMed] [Google Scholar]
- 100. Galldiks N, Ullrich R, Schroeter M et al. . Volumetry of [(11)C]-methionine PET uptake and MRI contrast enhancement in patients with recurrent glioblastoma multiforme. Eur J Nucl Med Mol Imaging. 2010;37 (1):84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Pirotte B, Goldman S, Dewitte O et al. . Integrated positron emission tomography and magnetic resonance imaging-guided resection of brain tumors: a report of 103 consecutive procedures. J Neurosurg. 2006;104 (2):238–253. [DOI] [PubMed] [Google Scholar]
- 102. Galldiks N, Kracht LW, Dunkl V et al. . Imaging of non- or very subtle contrast-enhancing malignant gliomas with [(1)(1)C]-methionine positron emission tomography. Mol Imaging. 2011;10 (6):453–459. [PubMed] [Google Scholar]
- 103. Piroth MD, Holy R, Pinkawa M et al. . Prognostic impact of postoperative, pre-irradiation (18)F-fluoroethyl-l-tyrosine uptake in glioblastoma patients treated with radiochemotherapy. Radiother Oncol. 2011;99 (2):218–224. [DOI] [PubMed] [Google Scholar]
- 104. Filss CP, Galldiks N, Stoffels G et al. . Comparison of 18F-FET PET and perfusion-weighted MR imaging: a PET/MR imaging hybrid study in patients with brain tumors. J Nucl Med. 2014;55 (4):540–545. [DOI] [PubMed] [Google Scholar]
- 105. Cicone F, Filss CP, Minniti G et al. . Volumetric assessment of recurrent or progressive gliomas: comparison between F-DOPA PET and perfusion-weighted MRI. Eur J Nucl Med Mol Imaging. 2015;42 (6):905–915. [DOI] [PubMed] [Google Scholar]
- 106. Rose S, Fay M, Thomas P et al. . Correlation of MRI-derived apparent diffusion coefficients in newly diagnosed gliomas with [18F]-fluoro-L-dopa PET: what are we really measuring with minimum ADC? AJNR Am J Neuroradiol. 2013;34 (4):758–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Popperl G, Gotz C, Rachinger W et al. . Value of O-(2-[18F]fluoroethyl)-L-tyrosine PET for the diagnosis of recurrent glioma. Eur J Nucl Med Mol Imaging. 2004;31 (11):1464–1470. [DOI] [PubMed] [Google Scholar]
- 108. Karunanithi S, Sharma P, Kumar A et al. . 18F-FDOPA PET/CT for detection of recurrence in patients with glioma: prospective comparison with 18F-FDG PET/CT. Eur J Nucl Med Mol Imaging. 2013;40 (7):1025–1035. [DOI] [PubMed] [Google Scholar]
- 109. Villani V, Carapella CM, Chiaravalloti A et al. . The Role of PET [18F]FDOPA in Evaluating Low-grade Glioma. Anticancer Res. 2015;35 (9):5117–5122. [PubMed] [Google Scholar]
- 110. Massager N, David P, Goldman S et al. . Combined magnetic resonance imaging- and positron emission tomography-guided stereotactic biopsy in brainstem mass lesions: diagnostic yield in a series of 30 patients. J Neurosurg. 2000;93 (6):951–957. [DOI] [PubMed] [Google Scholar]
- 111. Braun V, Dempf S, Weller R et al. . Cranial neuronavigation with direct integration of (11)C methionine positron emission tomography (PET) data—results of a pilot study in 32 surgical cases. Acta Neurochir. 2002;144 (8):777–782. [DOI] [PubMed] [Google Scholar]
- 112. Sadeghi N, Salmon I, Tang BN et al. . Correlation between dynamic susceptibility contrast perfusion MRI and methionine metabolism in brain gliomas: preliminary results. J Magn Reson Imaging. 2006;24 (5):989–994. [DOI] [PubMed] [Google Scholar]
- 113. Goldman S, Levivier M, Pirotte B et al. . Regional methionine and glucose uptake in high-grade gliomas: a comparative study on PET-guided stereotactic biopsy. J Nucl Med. 1997;38 (9):1459–1462. [PubMed] [Google Scholar]
- 114. Pirotte BJ, Lubansu A, Massager N et al. . Results of positron emission tomography guidance and reassessment of the utility of and indications for stereotactic biopsy in children with infiltrative brainstem tumors. J Neurosurg. 2007;107 (5 Suppl):392–399. [DOI] [PubMed] [Google Scholar]
- 115. Pirotte BJ, Levivier M, Goldman S et al. . Positron emission tomography-guided volumetric resection of supratentorial high-grade gliomas: a survival analysis in 66 consecutive patients. Neurosurgery. 2009; 64 (3):471–481. [DOI] [PubMed] [Google Scholar]
- 116. Stockhammer F, Plotkin M, Amthauer H et al. . Correlation of F-18-fluoro-ethyl-tyrosin uptake with vascular and cell density in non-contrast-enhancing gliomas. J Neurooncol. 2008;88 (2):205–210. [DOI] [PubMed] [Google Scholar]
- 117. Misch M, Guggemos A, Driever PH et al. . (18)F-FET-PET guided surgical biopsy and resection in children and adolescence with brain tumors. Childs Nerv Syst. 2015;31 (2):261–267. [DOI] [PubMed] [Google Scholar]
- 118. Tsuyuguchi N, Takami T, Sunada I et al. . Methionine positron emission tomography for differentiation of recurrent brain tumor and radiation necrosis after stereotactic radiosurgery—in malignant glioma. Ann Nucl Med. 2004;18 (4):291–296. [DOI] [PubMed] [Google Scholar]
- 119. Mehrkens JH, Popperl G, Rachinger W et al. . The positive predictive value of O-(2-[18F]fluoroethyl)-L-tyrosine (FET) PET in the diagnosis of a glioma recurrence after multimodal treatment. J Neurooncol. 2008;88 (1):27–35. [DOI] [PubMed] [Google Scholar]
- 120. Rachinger W, Goetz C, Popperl G et al. . Positron emission tomography with O-(2-[18F]fluoroethyl)-L-tyrosine versus magnetic resonance imaging in the diagnosis of recurrent gliomas. Neurosurgery. 2005;57 (3):505–511. [DOI] [PubMed] [Google Scholar]