Abstract
Newborns and infants communicate their needs and physiological states through crying and emotional facial expressions. Little is known about individual differences in responding to infant crying. Several theories suggest that people vary in their environmental sensitivity with some responding generally more and some generally less to environmental stimuli. Such differences in environmental sensitivity have been associated with personality traits, including neuroticism. This study investigated whether neuroticism impacts neuronal, physiological, and emotional responses to infant crying by investigating blood-oxygenation-level dependent (BOLD) responses using functional magnetic resonance imaging (fMRI) in a large sample of healthy women (N = 102) with simultaneous skin conductance recordings. Participants were repeatedly exposed to a video clip that showed crying infants and emotional responses (valence, arousal, and irritation) were assessed after every video clip presentation. Increased BOLD signal during the perception of crying infants was found in brain regions that are associated with emotional responding, the amygdala and anterior insula. Significant BOLD signal decrements (i.e., habituation) were found in the fusiform gyrus, middle temporal gyrus, superior temporal gyrus, Broca’s homologue on the right hemisphere, (laterobasal) amygdala, and hippocampus. Individuals with high neuroticism showed stronger activation in the amygdala and subgenual anterior cingulate cortex (sgACC) when exposed to infant crying compared to individuals with low neuroticism. In contrast to our prediction we found no evidence that neuroticism impacts fMRI-based measures of habituation. Individuals with high neuroticism showed elevated skin conductance responses, experienced more irritation, and perceived infant crying as more unpleasant. The results support the hypothesis that individuals high in neuroticism are more emotionally responsive, experience more negative emotions, and may show enhanced cognitive control during the exposure to infant distress, which may impact infant-directed behavior.
Introduction
Infant crying is considered to be a powerful communication signal ensuring infants`survival [1] and it includes both facial and vocal components [2]. Infant crying signals evoke strong emotional reactions, ranging from empathy to distress [3]. It has been shown that infant crying can also trigger child abuse such as neglect, slapping, and shaking [4], therefore, it is important to understand the emotional responses and the neural structures that mediate adults`emotional reactions to infant crying. Studies that investigated the neural responses to infant distress find circuits that include activation in the amygdala, anterior insular cortex, and inferior frontal gyrus [5–7]. However, little is known about individual differences in responding to infant crying. Several theories suggest that people vary in their environmental sensitivity with some responding generally more and some generally less to environmental stimuli [8–10]. Such differences in environmental sensitivity have been associated with a range of genetic, physiologic and behavioral factors, including the personality trait Sensory-Processing Sensitivity [11] that correlates with neuroticism (r = .40, see [12]), one of the five empirically derived main personality traits (“Big-5”). Neuroticism is characterized by the tendency to experience negative affect and distress [13] and hence, may be of particular interest in relation to how individuals respond to infant crying. Previous work has shown that individuals scoring high in neuroticism exhibit elevated skin conductance reactivity in response to emotionally distressing events when compared to emotionally more stable individuals [14]. High neuroticism appears to confer stress vulnerability when situations are perceived as threatening [15]. Neuroticism affects emotion regulation, which is the ability to control an emotion and the active attempt to modify a negative emotion towards a more positive emotional state [16]. Neuroticism has been associated with the amygdala and subgenual anterior cingulate cortex (sgACC) [17]. Research shows that these regions are functionally coupled [18] and that individuals with lesions in the sgACC region demonstrated abnormal autonomic responses during emotion processing [19]. More recently it was been shown that the sgACC is involved in overcoming a real-life stressful situation [20] and may thus play an important role when individuals with high neuroticism are repeatedly exposed to infant distress.
Previous studies underscores the importance of investigating temporal dynamics when studying affective processing using neuroimaging methods [21,22]. Studies in humans using various neuroimaging techniques such as functional magnetic resonance imaging (fMRI) [21,23] and magnetoencephalography (MEG) [24] have shown that brain areas habituate to repeated stimuli presentations—that is brain responses decline upon repeated stimulus presentations. Research has shown that habituation in response to repeatedly presented emotional stimuli include the (laterobasal) amygdala [21,23,25]
This study investigated whether neuroticism impacts neuronal, physiological and emotional responses to infant crying in healthy women using fMRI with simultaneous skin conductance recordings. This study repeatedly presented a video clip that showed crying infants. Participants`emotional responses were assessed by immediate ratings of valence, arousal, and irritation after every video clip presentation. Valence and arousal are considered to be two basic dimensions of emotional experience [26]. A study by Riem and colleagues found that infant crying also evokes feelings of irritation [27]. Furthermore, Seifritz and colleagues demonstrated that parents showed more amygdala activation than nonparents, suggesting that neuronal responses to infant crying may be modulated by parenting experiences [28]. It has been shown that sex differences exist in response to a baby crying [29]. Consequently, in order to account for such confounding influences, we recruited a large sample of healthy women (N = 102) that had no children of their own and no experience in professional child care, and investigated the following hypotheses: First, we expected that the exposure to infants crying evokes activation in brain regions that are associated with emotional responding. Second, we expected habituation of the blood-oxygenation-level dependent (BOLD) signal during the repeated exposure to infant crying in cortical and subcortical brain regions such as in the laterobasal amygdala. Third, we hypothesized that high-neuroticism individuals show during the repeated exposure to infant crying more activation in sgACC, which is involved in cognitive control during distressing emotions. Fourth, we expected that individuals with high neuroticism experience more negative emotions (assessed by valence and irritation ratings after every video presentation), exhibit greater skin conductance responses, and show greater amygdala activation during infant crying, because they are emotionally more responsive. Finally, we hypothesized that women high in neuroticism demonstrate less habituation on the neuronal, the physiological (measured by skin conductance recordings), as well as on the emotional level (assessed by valence, arousal, and emotional irritation ratings after every video presentation) in response to repeated infant crying.
Method
Participants
One-hundred-and-two healthy women were recruited from the University of Basel, Switzerland and participated in the fMRI experiment (mean age = 23.64, range = 18–35 years). Five participants were excluded from fMRI data analysis because of excessive motion (head movement exceeded 1.0 mm in any of the x, y, and z directions). Three participants were excluded from the analysis of skin conductance responses because of technical difficulties during recoding. Psychological measures and subjective affective ratings were analyzed from all individuals. Participants were screened for exclusion criteria using a checklist, comprising the following criteria: (1) Participants with children and experience in professional childcare at the time of investigation, (2) a history of seizure or head injury, (3) the use of medication that can influence the test results, (3) visual or auditory problems that cannot be corrected, (4) MRI incompatible implants, (5) pregnancy, (6) claustrophobia, (7) a history of psychiatric or neurological disorders. The ethics committee for medical research in Basel, Switzerland approved the study. Before participation, subjects gave their written informed consent.
Procedure
Participants were given 30 min to adjust to the laboratory setting. During this time they received instructions for the experiment. Subsequently, subjects were placed in the MR scanner and were asked to complete the state anxiety scale with 20 items [30] by using an MR-compatible mouse (please note: state-anxiety findings are reported elsewhere in relation to genetic polymorphisms [31]). Subsequently, 43 video clips were presented using stimulus presentation software (E-Prime 2.0, Psychology Software Tools, Sharpsburg, PA, USA). Video clips were presented via an LCD beamer that projected to a screen positioned behind the participants, which was visible over a mirror mounted on the head coil. Audio was presented via magnetic resonance compatible headphones (Resonance Technology, Los Angeles, USA). Subjects were instructed to attend to the video clips and to avoid any movement. The experiment had three phases: First, participants were familiarized with the experimental setting with a video clip that showed laughing infants (LI). That video clip was shown five times. Subsequently, a video clip with crying infants (CI) was presented 33 times including three video clip presentations that were varied for an attention-control task (please note: the findings related to that attention-control task are presented elsewhere [31]). This phase was used to assess habituation. At the end of the experiment we presented a video clip that showed laughing infants (i.e., another emotional category than during the habituation phase). This video clip was shown five times. This phase was used to distinguish habituation from sensory and motor fatigue [32]. Participants were asked to evaluate every video clip on a seven-point bipolar scale along the dimensions valence (ranging from –3 = unpleasant to 3 = pleasant), arousal (ranging from –3 = calming to 3 = arousing), and irritation (ranging from –3 = relaxed to 3 = irritated). Each evaluation period lasted 18 s. Participants conveyed what they felt by using a mouse that allowed them to move a white box on the visually presented scale leftwards or rightwards by pressing the left and right mouse buttons with their right hand. Each evaluation was followed by a baseline time window of 15 s duration (i.e., when subjects passively viewed a fixation cross) used as baseline for the fMRI. In total, there were 43 runs (each consisting of video presentation, evaluation, and baseline), each with a 60 s duration. After these 43 runs, the state-anxiety questionnaire was completed a second time in the scanner under the same conditions. The total duration of scanning was approximately 55 min per session. The experiment is illustrated in Fig A in S1 File.
Questionnaires
To asses neuroticism, all participants completed the German version of the NEO Five Factor Inventory [13]. This questionnaire consists of 60 items and measures five different personality traits: neuroticism, extraversion, openness, agreeableness and conscientiousness Participants also completed the Center for Epidemiologic Studies Depression Scale (CES-D) [33] which assesses depressive symptoms. The CES-D is a 20-item measure that asks individuals how often over the past week they experienced symptoms associated with depression, such as restless sleep, poor appetite, and feeling lonely. Handedness was assessed with a questionnaire by Oldfield [34].
Acquisition of skin conductance responses (SCRs)
Skin conductance was continuously recorded from the thenar and hypothenar palm of the immobilized, left hand in parallel to the fMRI recording. Data were saved at a sampling rate of 200 Hz using the BIOPAC MP150 system skin conductance module (Biopac Systems, Inc., Goleta, California, USA) with MR-conditional, disposable electrodes (EL509) filled with isotonic gel (TD-246, Med Associates paste). Electrode cables were grounded and passed through an RF filter panel and stimulus timing information was recorded via TTL pulses from the MR scanner to synchronize fMRI and physiological data analysis.
Acquisition of fMRI data
Functional images were acquired on a 3T scanner (Siemens Magnetom Allegra MR 2004A, Erlangen, Germany). Image acquisition started with a localizer, a reference scan for the distortion correction, and a magnetization-prepared rapid-acquisition gradient echo (MPRAGE) sequence of 7-min duration (resolution: 1 mm x 1 mm x 1 mm, matrix: 256 * 256 * 176, TR: 2000 ms, TI: 1000 ms, 7° flip angle). Functional images were obtained using a multislice gradient echo planar imaging (EPI) method. Each volume consisted of 44 transversal slices (2.5 mm slice thickness with a 0.5-mm interslice distance, matrix: 96 * 96, field of view 240 mm * 240 mm resulting in 2.5 mm x 2.5 mm x 2.5 mm resolution, repetition time (TR) 3000 ms, echo time (TE) 35 ms, 90° flip angle). An accurate registration of the functional images was accomplished by online correction of the functional image data for geometric distortions [35]. The distortion field was derived from the local point-spread function (PSF) in each voxel as determined in a one-minute reference scan (see above). Prior to distortion correction, data were online motion-corrected by image realignment to the reference scan. A representative example of functional images showing the amygdala after application of the distortion correction algorithm is shown in the study by Ball et al., [36].
Data Analysis
Preprocessing and statistical analysis of fMRI data
Data analysis was performed using SPM5/12 (http://www.fil.ion.ucl.ac.uk/spm/). Preprocessing consisted of realignment, and normalization, and smoothing. All functional images were normalized into standard stereotaxic space of the Montreal Neurological Institute (MNI) template. The images were smoothed using a 9 mm full-width-at-half-maximum (FWHM) Gaussian kernel to minimize the effects of individual variations in anatomy and to improve the signal-to-noise ratio. A high-pass filter with a cut-off at 1/128 Hz was applied before parameter estimation. First level modeling: Regressors were the timing information of the video clips and the timing information of the evaluation period after every video clip presentation. Regressors were modeled with a boxcar function convolved with a canonical hemodynamic response function. The first model included the following regressors: 9 for the video clips (LI at the beginning and at the end, 6 for CI because the 30 crying-infant video clip presentations were grouped into 6 consecutive blocks, and 1 for the control film) and one for the evaluation period after each film clip. Additional regressors were included to model the six head-movement parameters obtained during realignment. For each individual a contrast image crying infants>baseline (that is the time periods during which subjects passively viewed the fixation cross without stimulus presentation) was analyzed. To analyze response decrements the 30 crying-infant video clip presentations were grouped into 6 consecutive blocks and habituation was modeled as a linear change across these groups (duration 35 min). The attention-control variant of the CI video was not included in the statistical analyses. Second level group analyses: Brain areas with significant response decrements (habituation) during crying-infant presentations were tested at p < 0.05, family wise error (FWE) corrected for multiple comparisons, cluster size ≥ 15 voxels. The contrast perception of crying infants > baseline was tested at p < 0.05, FWE corrected, cluster size ≥ 15 voxels. To test whether individuals with high neuroticism differ in comparison to individuals with low neuroticism in terms of amygdala and sgACC activation a two-sample t-test was applied and tested at p < 0.05, FWE small volume correction. A small volume correction was performed because the amygdala and sgACC were included in our a priori hypothesis based on previous research showing that both brain regions are associated with neuroticism [17]. The median (neuroticism score: 1.67) served as boundary between the high and the low neuroticism groups. A 10 mm radius sphere was placed in sgACC (MNI coordinate: 6/42/-16) and the amygdala (MNI coordinate: 22/-8/-12) based on previous findings that show the involvement of sgACC and amygdala in neuroticism [17].
Preprocessing of skin conductance responses
Offline data analysis of skin conductance responses (SCR) waveforms was conducted using ANSLAB software (http://www.sprweb.org). Recordings were visually inspected and periods of signal loss were manually excluded (approx. 1% of video epochs). For each video presentation, the level of SCR was assessed as the pre-film baseline (2 s) to peak difference for the largest deflection in the 0–15 s video window [37]; values > 0.005 μS were accepted as valid non-zero SCR responses). For between-subject standardization, SCRs exceeding 3 SD of a subject’s SCR mean were excluded and SCRs were then scaled relatively to a subject’s maximal SCR [38,39] resulting in a range of 0 to 1 for all SCR values.
Statistical analysis of skin conductance responses and psychological data
Statistical analyses were conducted using SPSS (Version 16.0, Chicago, Illinois, USA). For analysis of the time-course of SCR levels, as well as valence, arousal, and irritation ratings, values for five subsequent video clip presentations were averaged into one value. Thus, one mean value was obtained by averaging across the five video clip presentations with laughing infants at the beginning of the experiment (Laugh_1; Familiarization), six mean values (thirty video clips averaged across each of five) were obtained with crying children (Cry_1 to Cry_6; Habituation), and finally one mean value was obtained by averaging across five video clip presentations with laughing children presented at the end of the experiment (Laugh_2). The attention-control variant of the CI video was not included in the statistical analysis. All four outcome measures were analyzed using linear mixed models and each outcome measure was checked for normality using QQ-plots. SCR data were transformed using reciprocal transformation while the three ratings were left untransformed. The mixed model for testing habituation contained neuroticism as continuous between-subjects variable and time (Cry_1 to Cry_6) as within-subjects variable. The test for fatigue at the end of the experiment included neuroticism as continuous between-subjects variable and time with the levels Cry_6 versus Laugh_2. Interaction effects between neuroticism and time were also tested but were not significantly different from 0 for any outcome measure and therefore not considered further in the analysis of the SCR and rating data. The models also included a random intercept and, if significantly improving model fit, a random linear slope for time. To test whether at the end of the experiment (block Laugh_2) psychological (arousal, valence, irritation) and physiological (SCR) data were related to subjects’ neuroticism scores, we performed linear regression models with neuroticism as predictor and the respective psychological or physiological variable as outcome.
Results
Functional imaging data
FMRI results revealed increased BOLD signal during the perception of crying infants > baseline (i.e., when subjects passively viewed a fixation cross) in the Heschl’s gyrus, visual cortex, superior temporal gyrus (STG), insular cortex, fusiform gyrus, temporal pole, inferior frontal cortex (including Broca’s area and Broca’s homologue on the right hemisphere), supplementary motor area (SMA), cerebellum, amygdala, and hippocampus (p<0.05, FWE corrected, clustersize ≥ 15 voxels). All brain regions, MNI coordinates, and T-scores are provided in Table 1.
Table 1. Significant fMRI blood-oxygen-level dependent (BOLD) signal for the contrast perception of crying infants > baseline (p < 0.05 FWE corrected, clustersize clustersize ≥ 15 voxel).
Peak MNI-coordinates and T-values are given. Anatomical assignments were performed using a probabilistic anatomical atlas system [41,42].
| MNI-Coordinates (x/y/z) | T-score | Brain region | Probabilistic map | ||
|---|---|---|---|---|---|
| 10 | -85 | -9 | 22.48 | Visual cortex | Area 18: 90% (assigned) |
| -8 | -98 | 12 | 22.17 | Visual cortex | Area 17: 60% (assigned) |
| 43 | -50 | -24 | 22.09 | Right fusiform gyrus | No map |
| -13 | -75 | -27 | 21.23 | Left cerebellum | No map |
| 15 | -95 | 15 | 21.12 | Visual cortex | Area 18: 70% (assigned) |
| 48 | -78 | -6 | 20.80 | Right inferior occipital gyrus | No map |
| 18 | -95 | 21 | 20.71 | Visual cortex | Area 18: 50% (assigned) |
| 50 | -8 | -6 | 20.31 | Right Superior Temporal Gyrus | No map |
| -8 | -78 | -45 | 20.27 | Left cerebellum | No map |
| 40 | -80 | -15 | 19.38 | Right inferior occipital gyrus | hoC4v (V4): 60% (assigned) |
| -38 | -25 | 6 | 19.29 | Left Heschl’s gyrus | Te 1.1: 50% (assigned) |
| 25 | -95 | 21 | 19.19 | Right superior occipital gyrus | Area 18: 30% |
| 25 | -78 | -12 | 19.17 | Right fusiform gyrus | hOC3v (V3v): 50% (assigned) |
| -45 | -13 | -3 | 19.06 | Left superior temporal gyrus | No map |
| -50 | -78 | 3 | 18.94 | Left middle occipital gyrus | No map |
| 45 | -20 | 6 | 18.94 | Right Heschl’s gyrus | Te 1.1: 50% (assigned) |
| 55 | -13 | 3 | 18.82 | Right superior temporal gyrus | Te 1.0: 70% (assigned) |
| -43 | -50 | -24 | 18.50 | Left fusiform gyrus | No map |
| 0 | -90 | 3 | 18.47 | Visual cortex | Area 17: 100% (assigned) |
| 63 | -28 | 3 | 18.42 | Right superior temporal gyrus | No map |
| 48 | -65 | 3 | 18.42 | Right middle temporal gyrus | hOC5 (V5): 60% (assigned) |
| 68 | -35 | 9 | 18.20 | Right inferior parietal cortex | IPC (PF): 60% (assigned) |
| -38 | -63 | -27 | 17.68 | Left cerebellum | No map |
| 45 | 23 | 21 | 17.65 | Right inferior frontal gyrus | Area 45: 10% |
| 40 | -63 | -18 | 17.24 | Right fusiform gyrus | No map |
| 58 | -68 | 0 | 17.08 | Right middle temporal gyrus | hOC5 (V5): 20% |
| -55 | -18 | 3 | 17.04 | Left superior temporal gyrus | Te 1.2: 20% |
| -48 | 0 | -15 | 16.89 | Left middle temporal gyrus | No map |
| 30 | -10 | -15 | 16.71 | Right hippocampus | CA: 50% (assigned) |
| 18 | -3 | -21 | 16.11 | Right amygdala | SF: 70% (assigned) |
| -33 | -78 | -15 | 16.10 | Left fusiform gyrus | hOC4v (V4): 40% (assigned) |
| -48 | -68 | -30 | 16.02 | Left cerebellum | No map |
| -58 | -38 | 12 | 16.02 | Left inferior parietal cortex | IPC (PFcm): 20% |
| -18 | -83 | -12 | 15.86 | Left linual gyrus | hoC4v (V4): 50% (assigned) |
| -40 | -68 | -18 | 15.76 | Left fusiform gyrus | hoC4v (V4): 10% |
| 53 | -40 | 9 | 15.54 | Right middle temporal gyrus | No map |
| -45 | -70 | 9 | 15.45 | Left middle temporal gyrus | hOC5 (V5): 20% |
| 33 | 3 | -24 | 15.16 | Right amygdala | LB: 10% |
| 8 | -80 | -42 | 15.06 | Right cerebellum | No map |
| -58 | -28 | 6 | 14.69 | Left superior temporal gyrus | Te 1.1: 10% |
| 40 | -68 | -27 | 14.61 | Right cerebellum | No map |
| -18 | -5 | -21 | 14.53 | Left amygdala | SF: 100% (assigned) |
| 20 | -93 | 30 | 14.41 | Right superior occipital gyrus | Area 18: 20% |
| 50 | 5 | 51 | 14.11 | Right precentral gyrus | Area 6: 20% |
| 55 | 33 | 18 | 14.11 | Right inferior frontal gyrus | No map |
| 48 | 13 | 33 | 13.29 | Right inferior frontal gyrus | No map |
| -33 | -10 | -15 | 13.02 | Left Hippocampus | CA: 30% |
| 55 | 30 | -3 | 12.96 | Right inferior frontal gyrus | Area 45: 50% (assigned) |
| -10 | -88 | 42 | 12.46 | Left superior occipital gyrus | SPL (7P): 10% |
| 43 | 30 | -6 | 12.40 | Right inferior frontal gyrus | No map |
| 28 | -83 | 24 | 12.36 | Right superior occipital gyrus | No map |
| 8 | -90 | 30 | 12.23 | Right cuneus | Area 18: 20% |
| 15 | 8 | 9 | 12.21 | Right caudate nucleus | No map |
| -18 | -38 | -48 | 12.11 | Left cerebellum | No map |
| -28 | -70 | -48 | 11.75 | Left cerebellum | No map |
| 25 | -58 | -9 | 11.62 | Right linual gyrus | No map |
| -30 | 10 | -33 | 11.45 | Left temporal pole | No map |
| 40 | -88 | 12 | 11.44 | Right middle occipital gyrus | No map |
| -23 | 0 | -30 | 11.16 | Left amygdala | LB: 70% (assigned) |
| 28 | 35 | -18 | 11.04 | Right inferior frontal gyrus | No map |
| -55 | -58 | 9 | 10.78 | Left middle temporal gyrus | IPC (PGp): 10% |
| -65 | -20 | -6 | 10.78 | Left middle temporal gyrus | Te 3: 10% |
| 10 | -83 | 48 | 10.75 | Right superior parietal lobule | SPL (7P): 50% (assigned) |
| 33 | -78 | -24 | 10.70 | Right cerebellum | No map |
| -50 | -38 | 21 | 10.68 | Left inferior parietal cortex | IPC (PFcm): 40% (assigned) |
| 10 | -13 | 9 | 10.56 | Right thalamus | No map |
| -25 | -88 | -27 | 10.49 | Left cerebellum | No map |
| 20 | -43 | -12 | 10.27 | Right fusiform gyrus | No map |
| 0 | -40 | -3 | 9.83 | Cerebellar vermis | No map |
| 43 | 30 | -18 | 9.52 | Right inferior frontal gyrus | No map |
| 30 | -63 | -33 | 9.36 | Right cerebellum | No map |
| -28 | -53 | -9 | 9.35 | Left fusiform gyrus | Area 17: 10% |
| 65 | -38 | -12 | 9.20 | Right middle temporal gyrus | No map |
| -18 | -43 | -9 | 9.18 | Left linual gyrus | No map |
| 25 | -80 | 48 | 9.12 | Right superior parietal lobule | SPL (7P): 10% |
| 28 | -3 | -42 | 9.04 | Right hippocampus | EC: 60% (assigned) |
| -20 | -63 | -12 | 8.65 | Left linual gyrus | hoC4v (V4): 50% (assigned) |
| -13 | -53 | -42 | 8.37 | Left cerebellum | No map |
| 50 | -60 | -33 | 8.32 | Right cerebellum | No map |
| 40 | -13 | -33 | 7.56 | Right fusiform gyrus | No map |
| 23 | -50 | 0 | 7.50 | Right linual gyrus | Area 18: 50% (assigned) |
| 38 | 20 | 57 | 6.87 | Right middle frontal gyrus | No map |
| -43 | 13 | 27 | 10.98 | Left inferior frontal gyrus | Area 44: 60% (assigned) |
| -50 | 23 | 24 | 10.68 | Left inferior frontal gyrus | Area 45: 70% (assigned) |
| -43 | 3 | 39 | 8.54 | Left precentral gyrus | No map |
| -33 | 21 | -3 | 9.14 | Left insula | No map |
| 5 | 10 | 66 | 10.72 | Right motor area | Area 6: 60% (assigned) |
| 0 | 38 | 53 | 9.28 | Left superior medial gyrus | No map |
| 5 | 23 | 54 | 8.90 | Right supplementary motor area | Area 6: 20% |
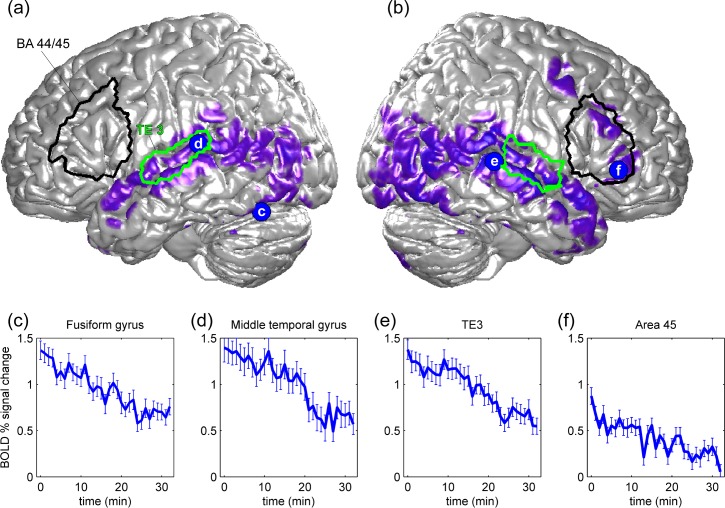
Significant fMRI BOLD signal decrements during crying infant presentations (35 min) were found in the fusiform gyrus, middle temporal gyrus, superior temporal gyrus (TE3 [40]), Broca’s homologue on the right hemisphere (BA 45), the (laterobasal) amygdala, hippocampus, and cerebellum (p<0.05, FWE corrected, clustersize ≥ 15 voxels, see Fig 1). Table 2 provides all brain regions, MNI-coordinates, and T-scores.
Fig 1. Illustrates significant fMRI blood-oxygen-level dependent (BOLD) signal decline during crying infant (CI) presentations.
Significant response decrements (p < 0.05, FWE-corrected) during CI presentations are shown in purple and rendered on a standard brain surface, (a) the left and (b) right hemisphere. The outline of brain-surface projections of the areas BA44/45 and TE3 from a probabilistic atlas system [41,42] are indicated by black and green lines. (c) to (f): Significant BOLD signal decline (i.e., habituation) was found in the fusiform gyrus, middle temporal gyrus, TE3 (superior temporal gyrus [40]), and in the right BA 45. Median percentage of BOLD signal change over the 30 CI presentations for the four example peaks shown in (a) and (b). Error bars indicate standard errors.
Table 2. Significant fMRI blood-oxygen-level dependent (BOLD) signal decline during crying infant presentations.
(p < 0.05 FWE corrected, clustersize clustersize ≥ 15 voxel). IPC = inferior parietal cortex, hOC = human occipital lobe, Te = auditory cortex, EC = entorhinal cortex, SUB = subicular complex, LB = laterobasal amygdala, area 45 = Broca’s homologue, and area 6 = premotor cortex. Peak MNI-coordinates and T-values are given. Anatomical assignments were performed using a probabilistic anatomical atlas system [41,42]
| MNI-Coordinates (x/y/z) | T-score | Brain region | Probabilistic map | ||
|---|---|---|---|---|---|
| 45 | -40 | -21 | 11 | Inferior temporal gyrus | No map |
| 60 | -32.5 | 0 | 10.8 | Middle temporal gyrus | No map |
| 65 | -30 | 9 | 10.2 | Inferior parietal cortex | IPC (PF): 40% (assigned) |
| 57.5 | -15 | -6 | 9.9 | Superior temporal gyrus | No map |
| 67.5 | -35 | 18 | 9.6 | Inferior parietal cortex | IPC (PF): 80% (assigned) |
| 35 | -55 | -18 | 9.6 | Fusiform gyrus | No map |
| 47.5 | -20 | -9 | 9.5 | Middle temporal gyrus | No map |
| 40 | -50 | -21 | 9.3 | Right fusiform gyrus | No map |
| 50 | -25 | -3 | 9.2 | Superior temporal gyrus | No map |
| 55 | -67.5 | 0 | 9.2 | Middle temporal gyrus | hOC5 (V5): 30% |
| 52.5 | -75 | 0 | 9.1 | Middle temporal gyrus | hOC5 (V5): 10% |
| 57.5 | -2.5 | -12 | 9 | Superior temporal gyrus | No map |
| 45 | -50 | -24 | 9 | Fusiform gyrus | No map |
| 55 | 12.5 | -15 | 9 | Temporal pole | No map |
| 47.5 | -72.5 | -15 | 8.9 | Inferior occipital gyrus | No map |
| 50 | -30 | 0 | 8.7 | Superior temporal gyrus | No map |
| 45 | -65 | 9 | 8.6 | Middle temporal gyrus | hOC5 (V5): 30% |
| 20 | -2.5 | -24 | 8.5 | Hippocampus | EC: 50% (assigned) |
| 50 | 7.5 | -21 | 8.5 | Temporal pole | No map |
| 40 | -62.5 | -15 | 8.4 | Fusiform gyrus | No map |
| 67.5 | -25 | -3 | 8.4 | Middle temporal gyrus | Te 3: 40% (assigned) |
| 40 | -50 | -15 | 8.4 | Fusiform gyrus | No map |
| 55 | -67.5 | 9 | 8.3 | Middle temporal gyrus | hOC5 (V5): 20% |
| 52.5 | 0 | -18 | 8.2 | Middle temporal gyrus | No map |
| 50 | -57.5 | -18 | 8.1 | Inferior temporal gyrus | No map |
| 65 | -52.5 | 9 | 7.9 | Middle temporal gyrus | IPC (PGp): 10% |
| 50 | -57.5 | 9 | 7.9 | Middle temporal gyrus | IPC (PGp): 10% |
| 30 | 0 | -27 | 7.9 | Amygdala | LB: 70% (assigned) |
| 47.5 | -72.5 | -3 | 7.8 | Inferior temporal gyrus | No map |
| 55 | -27.5 | 9 | 7.8 | Superior temporal gyrus | No map |
| 50 | -40 | 12 | 7.8 | Superior temporal gyrus | No map |
| 52.5 | -70 | -9 | 7.8 | Inferior temporal gyrus | hOC5 (V5): 10% |
| 32.5 | -50 | -12 | 7.8 | Fusiform gyrus | No map |
| 45 | -80 | -9 | 7.5 | Inferior occipital gyrus | hOC4v (V4): 10% |
| 40 | -77.5 | 6 | 7.4 | Middle occipital gyrus | No map |
| 60 | -52.5 | 3 | 7.4 | Middle temporal gyrus | IPC (PGa): 10% |
| 47.5 | -32.5 | 12 | 7.3 | Superior temporal gyrus | IPC (PFcm): 10% |
| 25 | -82.5 | -12 | 7.3 | Fusiform gyrus | hOC3v (V3): 80% (assigned) |
| 50 | 15 | -30 | 7.3 | Medial temporal pole | No map |
| 27.5 | -65 | -12 | 7.2 | Fusiform gyrus | hOC4v (V4): 40% (assigned) |
| 27.5 | -80 | -6 | 7 | Fusiform gyrus | hOC3v (V3v): 40% (assigned) |
| 42.5 | -30 | 15 | 7 | Superior temporal gyrus | OP 1: 40% (assigned) |
| 25 | -45 | -15 | 7 | Fusiform gyrus | No map |
| 50 | -47.5 | 9 | 6.9 | Middle temporal gyrus | IPC (PGa): 10% |
| 40 | -57.5 | 3 | 6.7 | Middle temporal region | No map |
| 45 | 17.5 | -33 | 6.7 | Medial temporal pole | No map |
| 45 | -82.5 | 9 | 6.7 | Right middle occipital gyrus | IPC (PGp): 20% |
| 37.5 | -85 | 18 | 6.5 | Middle occipital gyrus | No map |
| 37.5 | 0 | -21 | 6.4 | Temporal pole region | No map |
| 25 | 10 | -27 | 6.3 | Parahippocampal gyrus | No map |
| 30 | -90 | 27 | 6.3 | Superior occipital gyrus | Area 18: 10% |
| 32.5 | 10 | -33 | 6.2 | Medial temporal pole | No map |
| 37.5 | 7.5 | -27 | 6.1 | Temporal pole | No map |
| 35 | -75 | -15 | 5.8 | Fusiform gyrus | hOC4v (V4): 40% (assigned) |
| 40 | -72.5 | 24 | 5.7 | Middle occipital gyrus | No map |
| 17.5 | -42.5 | -6 | 5.6 | Parahippocampal gyrus | SUB: 20% |
| 27.5 | 2.5 | -36 | 5.3 | Hippocampus | EC: 80% (assigned) |
| -37.5 | -52.5 | -21 | 8.9 | Fusiform gyrus | No map |
| -42.5 | -45 | -21 | 8.6 | Fusiform gyrus | No map |
| -40 | -40 | -24 | 8.4 | Fusiform gyrus | No map |
| -35 | -65 | -18 | 8.4 | Fusiform gyrus | No map |
| -42.5 | -57.5 | -18 | 7.9 | Fusiform gyrus | No map |
| -20 | -57.5 | -12 | 6.9 | Linual gyrus | hOC4v (V4): 20% |
| -42.5 | -80 | -15 | 6.7 | Fusiform gyrus | hOC4v (V4): 30% |
| -45 | -80 | -6 | 6.3 | Inferior occipital gyrus | hOC4v (V4): 10% |
| -25 | -45 | -12 | 6.3 | Fusiform gyrus | No map |
| -27.5 | -50 | -9 | 6 | Fusiform gyrus | No map |
| -20 | -37.5 | -15 | 5.7 | Fusiform gyrus | No map |
| -62.5 | -40 | 6 | 8.6 | Middle temporal gyrus | Te 3: 20% |
| -62.5 | -32.5 | 12 | 8.3 | Superior temporal gyrus | Te 3: 40% (assigned) |
| -57.5 | -30 | 12 | 8.2 | Superior temporal gyrus | OP 1: 40% (assigned) |
| -50 | -77.5 | 9 | 8 | Middle occipital gyrus | IPC (PGp): 20% |
| -50 | -37.5 | 9 | 7.6 | Middle temporal gyrus | No map |
| -60 | -27.5 | 0 | 7.6 | Middle temporal gyrus | No map |
| -57.5 | -15 | -9 | 7.5 | Middle temporal gyrus | Te 3: 10% |
| -47.5 | -35 | 21 | 7.3 | Inferior parietal cortex | IPC (PFcm): 70% (assigned) |
| -52.5 | -37.5 | 3 | 7.3 | Middle temporal gyrus | No map |
| -62.5 | -17.5 | -6 | 7.3 | Middle temporal gyrus | No map |
| -52.5 | 5 | -21 | 7.2 | Middle temporal gyrus | No map |
| -57.5 | -2.5 | -6 | 7.2 | Superior temporal gyrus | No map |
| -57.5 | -12.5 | 3 | 7 | Superior temporal gyrus | Te 1.2: 40% (assigned) |
| -47.5 | -40 | 3 | 7 | Middle temporal gyrus | No map |
| -60 | -7.5 | -6 | 7 | Middle temporal gyrus | Te 3: 10% |
| -55 | -37.5 | 18 | 6.9 | Inferior parietal cortex | IPC (PFcm): 40% (assigned) |
| -55 | -62.5 | 12 | 6.7 | Middle temporal gyrus | IPC (PGa): 10% |
| -47.5 | -55 | 9 | 6.6 | Middle temporal gyrus | No map |
| -50 | 12.5 | -21 | 6.5 | Temporal pole | No map |
| -55 | 7.5 | -6 | 6.5 | Temporal pole | No map |
| -55 | -50 | 9 | 6.4 | Middle temporal gyrus | No map |
| -45 | -50 | 3 | 6.4 | Middle temporal gyrus | No map |
| -52.5 | -60 | 6 | 6.3 | Middle temporal gyrus | No map |
| -50 | -67.5 | 15 | 6.3 | Middle temporal gyrus | IPC (PGp): 30% |
| -57.5 | -40 | 24 | 6 | Inferior parietal cortex | IPC (PF): 50% (assigned) |
| -55 | -47.5 | 0 | 5.8 | Middle temporal gyrus | No map |
| -62.5 | -47.5 | 15 | 5.8 | Superior temporal gyrus | IPC (PFm): 20% |
| -42.5 | -75 | 15 | 5.7 | Middle occipital gyrus | No map |
| -50 | -5 | -15 | 5.5 | Middle temporal gyrus | No map |
| -42.5 | -67.5 | 9 | 5.5 | Middle temporal gyrus | hOC5 (V5): 10% |
| -47.5 | -80 | 21 | 5.3 | Inferior parietal cortex | IPC (PGp): 70% (assigned) |
| 45 | 20 | 21 | 8.3 | Inferior frontal gyrus | Area 45: 10% |
| -12.5 | -75 | -45 | 7.5 | Cerebellum | No map |
| -17.5 | -77.5 | -39 | 7.4 | Cerebellum | No map |
| 55 | 32.5 | 0 | 7.2 | Inferior frontal gyrus | Area 45: 50% (assigned) |
| 50 | 35 | 9 | 6 | Inferior frontal gyrus | Area 45: 40% (assigned) |
| 55 | 27.5 | -6 | 5.4 | Inferior frontal gyrus | Area 45: 50% (assigned) |
| 50 | 5 | 48 | 7 | Precentral gyrus | Area 6: 10% |
| -22.5 | -7.5 | -18 | 6.9 | Amygdala | LB: 90% (assigned) |
| -15 | -7.5 | -27 | 5.6 | Hippocampus | EC: 90% (assigned) |
| 12.5 | -27.5 | -3 | 6.8 | Dorsal midbrain | No map |
| 17.5 | -20 | -12 | 5.9 | Hippocampus | SUB: 10% |
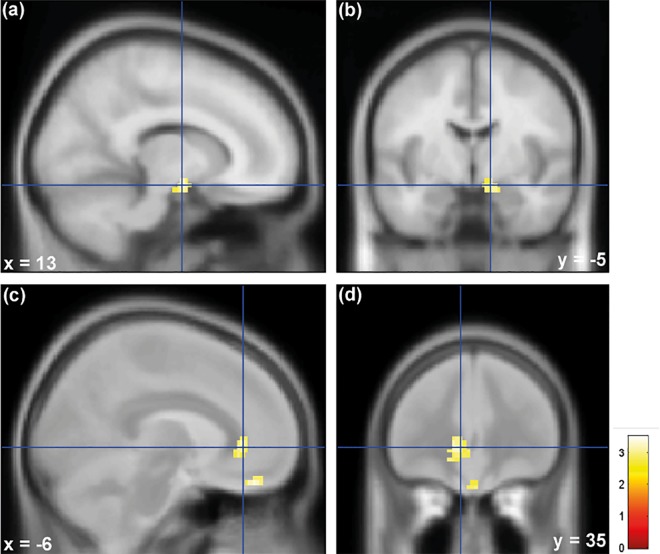
Individuals with high neuroticism levels showed stronger activation in the amygdala and sgACC when exposed to infant crying compared to individuals with lower neuroticism levels (p < 0.05, FWE small volume correction, see Fig 2). Anatomical assignments were performed using a probabilistic anatomical atlas system [41,42].
Fig 2.
Shows that Individuals with high neuroticism showed stronger activation in the (a)(b) amygdala and (c)(d) subgenual anterior cingulate cortex (sgACC) when exposed to infant crying compared to individuals with lower neuroticism levels (p < 0.05, FWE small volume corrected). The median (neuroticism score: 1.67) served as the boundary between the high and the low neuroticism groups. A 10 mm radius sphere was placed in sgACC (MNI coordinate: 6/42/-16) and the amygdala (MNI coordinate: 22/-8/-12) based on previous findings that show the involvement of sgACC and amygdala in neuroticism [17]. The results are superimposed on the MNI-152 standard brain (SPM12).
Psychological data
Neuroticism scores (M = 1.76, SD = 0.62) were in a normal range [43]. All subjects were right-handed according to the Edinburgh handedness questionnaire [34] (mean = 84.11,% SD = 12.69). Across the six blocks showing the video clip with crying infants, arousal decreased (b = –0.083, SE = 0.020, p<0.001), and irritation increased (b = 0.089, SE = 0.035, p = 0.012), whereas no significant trend was observed for valence (b = –0.003, SE = 0.018, p = 0.868). Individuals with increased neuroticism experienced more irritation (b = 0.460, SE = 0.218, p = 0.038, for linear relationship) and perceived infant crying as more unpleasant (valence ratings were more negative; b = –.281, SE = 0.121, p = 0.022) in comparison to individuals with lower neuroticism scores. Arousal was not related to neuroticism (b = .197, SE = 0.130, p = 0.132). We did not detect any interaction effects between time and neuroticism (p>.05 for all three outcome measures). To test for fatigue we presented at the end of the experiment a video clip that showed laughing infants (i.e., participants perceived another emotional category than during the habituation phase). We compared Cry_6 with Laugh_2 and found significant differences for all three psychological variables, with arousal and irritation exhibiting strongly reduced values (arousal, b = –0.871, SE = 0.177, p<0.001; irritation, b = –1.586, SE = 0.155, p<0.001), and valence exhibiting strongly increased values (b = 3.172, SE = 0.126, p<0.001) when showing a video clip with laughing rather than crying children. At the end of the experiment (block Laugh_2) psychological values for arousal and valence were not related to neuroticism (arousal: b = 0.069, SE = 0.227, t = 0.303, p = .763; valence: b = -0.243, SE = 0.139, t = -1.748, p = .083). Psychological values for irritation were significantly higher in subjects with higher neuroticism scores (b = 0.675, SE = 0.194, t = 3.475, p < .001).
Physiological data
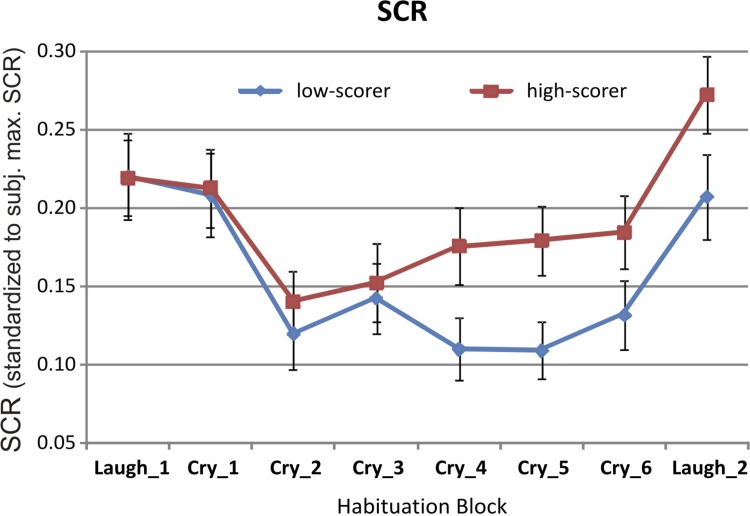
SCR showed a curvilinear temporal trend over the six blocks of repeated presentation of crying children: SCR values first strongly decreased from the first to the second block but then remained more or less at the same level towards block 6 (linear time trend, b = –0.005, SE = 0.003, p = .118; quadratic time trend, b = 0.006, SE = 0.002, p < .001; Fig 3). Averaged across temporal blocks, SCR values showed a curvilinear relationship with participants’ neuroticism: SCR values increased from low- to relatively high neuroticism scores, but declined again to average values for highest neuroticism scores (linear trend, b = 0.022, SE = 0.014, p = 0.104; quadratic trend, b = –0.040, SE = 0.019, p = 0.034). Participants with the highest neuroticism values also displayed high CES-D values (see Fig B in S1 File). When adjusting the model for CES-D values, the relationship between neuroticism and SCR was still somewhat curvilinear but less so for women with high neuroticism values (p = 0.014 for linear trend, p = 0.047 for quadratic trend). CES-D values themselves had little effect on SCR values (p = 0.063 for linear relationship). The test for fatigue at the end of the experiment (comparing block Cry_6 with block Laugh_2) showed that SCRs were strongly increased when displaying a video clip with laughing rather than crying children at the end of the experiment (b = 0.069, SE = 0.016, p<0.001, Fig 3). At the end of the experiment (block Laugh_2) SCR values were not related to neuroticism (SCR: b = 0.025, SE = 0.023, t = 1.094, p = .277, see Fig 3).
Fig 3. Illustrates time course of skin conductance responses (SCR, group means).
For this illustration the median (neuroticism score: 1.67) served as the boundary between the high and the low neuroticism groups (please note: in the statistical model neuroticism was used as continuous variable). Five video clip presentations were averaged into one block, i.e. the first block summarizes five video clip presentations that showed laughing infants at the beginning of the fMRI experiment, the following six blocks summarize each five video clip presentations that showed crying children, and finally the last block summarizes five video clip presentations that showed laughing children at the end of the fMRI experiment. Women scoring higher on neuroticism showed overall stronger skin conductance responses to the ongoing exposure to film clips of crying infants. Error bars indicate standard errors.
Discussion
At the neural level, this study found that infant crying activates the left and right inferior frontal gyrus (including Broca's area and Broca's homologue on the right hemisphere), the left anterior insula, left and right fusiform gyrus, hippocampus, amygdala, and superior temporal gyrus (all brain regions are listed in Table 1). Research shows that infant crying activates the amygdala in nonparental women [44]. Riem and colleagues found that women without children with insecure attachment showed increased amygdala activation when exposed to infant crying compared to women with secure attachment [27]. Recently, it has been shown that the administration of oxytocin (a neuropeptide that facilitates mother-infant bonding) to nonparental women reduced activation in amygdala and increased activation in the insula and in the inferior frontal gyrus [5]. The amygdala and the anterior insula are brain structures that have been associated with emotional responding [36,45]. Previous functional imaging studies have shown that the anterior insula plays are role in empathy [46] as well as during the perception of infant vocalizations [47]. Research shows that the amygdala is involved in face processing [48] and responsive to infant vocalizations [49]. A recent study found, the amygdala responds to human and computer-generated faces (avatar faces) similar and that the fusiform gyrus showed a greater response to human faces, suggesting that that fusiform gyrus may help the human brain to distinguish computer-generated faces from real faces [50]. The right inferior frontal gyrus may be involved in decoding emotional facial expressions [51,52]. A recent study suggests that the inferior frontal gyrus and superior temporal gyrus may play a role in affective prosody comprehension [53]. Furthermore, significant BOLD signal decrements (i.e., habituation) in response to a repeatedly presented video clip that showed crying infants were found in the fusiform gyrus, middle temporal gyrus, superior temporal gyrus, Broca’s homologue on the right hemisphere, (laterobasal) amygdala, and hippocampus (all brain regions are listed in Table 2). The left inferior frontal gyrus (including Broca`s area) did not show significant habituation (see Fig 1). Previous research shows that the (laterobasal) amygdala is preferentially activated in response to emotionally valenced stimuli and habituates in response to repeatedly presented human faces [23] and music [21]. A study by Britton and colleagues demonstrates that both the amygdala and fusiform gyrus habituate over time in response to emotional facial expressions [54]. Habituation has been considered to be a simple form of learning that allows humans to filter out irrelevant and focus selectively on important stimuli [32]. Researchers believe that habituation might be a prerequisite for other forms of learning [32].
Our results suggest that individual differences in neural processing of infant distress are related to differences in neuroticism. Individuals with high neuroticism levels showed stronger activation in the amygdala and in sgACC when exposed to infant crying compared to individuals with low neuroticism. Stronger activation in sgACC may indicate enhanced cognitive control in women scoring high in neuroticism when repeatedly exposed to infant distress. A recent study found that sgACC correlates positively with fear levels when participant chose to bring a live snake closer to the scanner. These findings suggest that sgACC plays an important role in cognitive control during the process of successfully overcoming a real-life stressful situation [20]. A study by Haas and colleagues found that neuroticism correlated positively with sgACC and amygdala activation during trials of high emotional conflict, compared with trials of low emotional conflict [17], which may reflect increased cognitive control in individuals with high neuroticism during an emotionally distressing task. Based on several theories suggesting people differ substantially in environmental sensitivity (for an integrative overview see reference [9]), we hypothesized that high-neuroticism individuals are more emotionally responsive and experience more negative emotions during the exposure to infant distress. In agreement with these hypotheses, we found that women with high neuroticism showed stronger amygdala activation and experienced more irritation, and perceived infant crying as more unpleasant in comparison to individuals with lower neuroticism scores. Arousal ratings, however, were not affected by neuroticism. Furthermore, individuals with high neuroticism demonstrated significantly greater SCRs in response to infant crying. These findings are consistent with recent research showing that elevated neuroticism is associated with increased physiological reactivity (e.g., electrodermal activity) during distress [14,55]. Taken together, individuals with high neuroticism showed stronger emotions during the repeated exposure to infant crying which is reflected by the fact that they showed stronger amygdala activation, greater SCRs, experienced more irritation, and perceived infant crying as more unpleasant in comparison to individuals with lower neuroticism scores. We argue that individuals with high neuroticism therefore exercise more cognitive control, which is reflected by the fact that they also displayed stronger sgACC activation. As mentioned above this brain region plays an important role in cognitive control during the process of successfully overcoming a stressful situation.
In contrast to our prediction we found no evidence that neuroticism impacts habituation on the neuronal, peripheral-physiological or affective-behavioral level.
Lin and McFatter suggested that infant crying may evoke two different emotional responses: distress and empathy [3]. In this study we found that infant crying evoked in all participants activation in the inferior frontal gyrus and the anterior insula. Riem and colleagues suggested that those brain regions may underlie empathy in response to infant crying [5]. A future study, however, is required to examine whether the activations we found in the inferior frontal gyrus and anterior insula in response to infant crying are associated with empathy. Our study found in agreement with Lin and Mc Fatter`s suggestion that individuals with high neuroticism experienced more negative emotions during the exposure to infant crying, indicating that they felt more distressed than individuals with low neuroticism. During the perception of infant laughter at the end of the experiment SCR values and psychological values for arousal and valence were not related to neuroticism, yet, psychological values for irritation were higher in subjects with higher neuroticism scores. This finding might be in agreement with previous research showing that increased neuroticism goes along with stronger emotional responses during “negative events” such as infant crying and also with heightened emotional responses during “positive events” such as infant laughter [8,15].
Some participants with high neuroticism scores who also obtained high scores on the CES-D [33], which assesses depressive symptoms, displayed relatively low skin conductance responses (see Fig B in S1 File). It has been previously shown that individuals with depressive symptoms may demonstrate suppressed SCR responses [56]. This has also been found in individuals with subsyndromal depression [57].
In the following paragraph potential limitations of this study are discussed and suggestions are made for future research. We tested for fatigue by presenting at the end of the experiment stimuli from a different emotion category (i.e., a video clip that showed laughing infants). This study found highly significant differences for all three variables of emotional reactivity (valence, arousal and irritation) as well as for the level of SCRs between the laughing and crying conditions. These findings are in agreement with the definition of habituation according to which response decrement results from repeated stimulation and does not involve sensory and motor fatigue [32]. A future study should investigate habitation effects during the repeated exposure to infant laughter in order to clarify whether the habituation effects we observed in this study during infant crying are specific or a more general habitation effect.
To reduce sources of variance not directly related to the study aims this sample was confined to healthy women. Women have been found to score higher than men on neuroticism [58] and show compared to men differences in brain activity in response to infant cries [29], underlining that generalization of our findings to men is an important topic for future research. Neuroticism scores in this study spanned the normal range [43] and hence results do not inform about clinical neuroticism. Whether the results generalize to clinical levels of neuroticism needs to be examined in future research. Furthermore, future studies may use the Highly Sensitive Person (HSP) scale by Aron & Aron [12] to test for individual differences in environmental sensitivity. The hypothesis for differences in environmental sensitivity was developed after data collection was completed. Hence, this study relied on neuroticism as a marker of environmental sensitivity (in [12], HSP was significantly associated with neuroticism with r = .41). As mentioned, research suggests that neuronal responses to infant crying may be modulated by parenting experiences [28]. Future studies may consider investigating neuronal, physiological, and affective habituation patterns in response to infant crying in mothers and fathers because neuroticism may impact parenting [59]. An interesting area for future research is (similarly to a study by Riem et al., [60]) to investigate amygdala-connectivity during the perception of infant crying.
In conclusion, the findings of this study show that individuals high in neuroticism are more emotionally responsive, experience more negative emotions, and may show enhanced cognitive control during the exposure to infant distress, which may impact infant-directed behavior. This study presents a valuable approach to investigate neuronal, physiological and emotional responses to infant crying by using simultaneous fMRI and SCR recordings and the assessment of emotional experience.
Supporting Information
Fig A. Illustration of the experiment design. (A) State-anxiety was assessed in the scanner before (STAI before) and after the experiment (STAI after). (B) Females were familiarized with the experimental setting and a video clip showing laughing infants (LI) was presented five times. Subsequently, in order to assess habituation, a video clip that showed crying infants (CI) was presented 30 times. A crontrol movie was presented three times. In order to distinguish habituation from sensory and motor fatigue we tested for fatigue at the end of the experiment by presenting the video clip with laughing infants five times. Individuals rated valence, arousal, and irritation after every video clip presentation. (C) Each video clip consisted of a sequence of five laughing or five crying children with a total length of 15 s. Fig B. Some participants with high neuroticism scores who obtained high scores on the Center for Epidemiologic Studies Depression Scale displayed low skin conductance responses. The highest neuroticism scores are shown in squares. X-axis: Shows scores on the Center for Epidemiologic Studies Depression Scale (CES-D). Y-axis: Shows skin conductance responses (SCR, standardized to subject`s max SCR) during the exposure to infant crying.
(DOCX)
Acknowledgments
We gratefully acknowledge the support from Peter Peyk and Fabrizio Esposito.
Data Availability
Our data are available upon request because of an ethical restriction. Herewith, we confirm that data will be available upon request to all interested researchers. Interested researchers should contact: Dr. Isabella Mutschler, University of Basel, Missionsstrasse 60/62, 4055 Basel, Switzerland. Email: isabella.mutschler@unibas.ch (please see Cover Letter) Please note: The authors have declared that no competing interests exist. (please see Cover Letter)
Funding Statement
This work was supported by the Swiss National Science Foundation (SNF): Grant 51A240-104890 to FHW and ES, and the Swiss National Science Foundation (SNF): Grant PA00P1_145418 to IM and the Freiwillige Akademische Gesellschaft to IM. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Arnal LH, Flinker A, Kleinschmidt A, Giraud AL, Poeppel D (2015) Human screams occupy a privileged niche in the communication soundscape. Curr Biol 25: 2051–2056. 10.1016/j.cub.2015.06.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Piallini G, De Palo F, Simonelli A (2015) Parental brain: cerebral areas activated by infant cries and faces. A comparison between different populations of parents and not. Front Psychol 6: 1625 10.3389/fpsyg.2015.01625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin HC, McFatter R (2012) Empathy and distress: two distinct but related emotions in response to infant crying. Infant Behav Dev 35: 887–897. 10.1016/j.infbeh.2012.08.001 [DOI] [PubMed] [Google Scholar]
- 4.Reijneveld SA, van der Wal MF, Brugman E, Sing RA, Verloove-Vanhorick SP (2004) Infant crying and abuse. Lancet 364: 1340–1342. [DOI] [PubMed] [Google Scholar]
- 5.Riem MM, Bakermans-Kranenburg MJ, Pieper S, Tops M, Boksem MA, Vermeiren RRJM, et al. (2011) Oxytocin modulates amygdala, insula, and inferior frontal gyrus responses to infant crying: a randomized controlled trial. Biol Psychiatry 70: 291–297. 10.1016/j.biopsych.2011.02.006 [DOI] [PubMed] [Google Scholar]
- 6.Lorberbaum JP, Newman JD, Horwitz AR, Dubno JR, Lydiard RB, Hamner MB, et al. (2002) A potential role for thalamocingulate circuitry in human maternal behavior. Biol Psychiatry 51: 431–445. [DOI] [PubMed] [Google Scholar]
- 7.Musser ED, Kaiser-Laurent H, Ablow JC (2012) The neural correlates of maternal sensitivity: an fMRI study. Dev Cogn Neurosci 2: 428–436. 10.1016/j.dcn.2012.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Belsky J, Pluess M (2009) Beyond diathesis stress: differential susceptibility to environmental influences. Psychol Bull 135: 885–908. 10.1037/a0017376 [DOI] [PubMed] [Google Scholar]
- 9.Pluess M (2015) Individual Differences in Environmental Sensitivity. Child Development Perspectives 9: 138–143. [Google Scholar]
- 10.Ellis BJ, Boyce WT, Belsky J, Bakermans-Kranenburg MJ, van IJzendoorn MH (2011) Differential susceptibility to the environment: an evolutionary—neurodevelopmental theory. Development and Psychopathology 23: 7–28. 10.1017/S0954579410000611 [DOI] [PubMed] [Google Scholar]
- 11.Aron EN, Aron A, Jagiellowicz J (2012) Sensory processing sensitivity: a review in the light of the evolution of biological responsivity. Personality and Social Psychology Review 16: 262–282. 10.1177/1088868311434213 [DOI] [PubMed] [Google Scholar]
- 12.Aron EN, Aron A (1997) Sensory-processing sensitivity and its relation to introversion and emotionality. J Pers Soc Psychol 73: 345–368. [DOI] [PubMed] [Google Scholar]
- 13.McCrae RR, Costa F (1991) The NEO Personality Inventory: Using the Five-Factor Model in Counceling. Journal of Consulting and Development 69: 367–372. [Google Scholar]
- 14.Norris CJ, Larsen JT, Cacioppo JT (2007) Neuroticism is associated with larger and more prolonged electrodermal responses to emotionally evocative pictures. Psychophysiology 44: 823–826. [DOI] [PubMed] [Google Scholar]
- 15.Ormel J, Bastiaansen A, Riese H, Bos EH, Servaas M, Ellenbogen M, et al. (2013) The biological and psychological basis of neuroticism: current status and future directions. Neurosci Biobehav Rev 37: 59–72. 10.1016/j.neubiorev.2012.09.004 [DOI] [PubMed] [Google Scholar]
- 16.Kokkonen M, Pulkkinen L (2001) Extraversion and neuroticism as antecedents of emotion regulation and dysregulation in adulthood. European Journal of Personality 15: 407–424. [Google Scholar]
- 17.Haas BW, Omura K, Constable RT, Canli T (2007) Emotional conflict and neuroticism: personality-dependent activation in the amygdala and subgenual anterior cingulate. Behav Neurosci 121: 249–256. [DOI] [PubMed] [Google Scholar]
- 18.Stein JL, Wiedholz LM, Bassett DS, Weinberger DR, Zink CF, Mattay VS, et al. (2007) A validated network of effective amygdala connectivity. Neuroimage 36: 736–745. [DOI] [PubMed] [Google Scholar]
- 19.Bechara A, Damasio AR, Damasio H, Anderson SW (1994) Insensitivity to future consequences following damage to human prefrontal cortex. Cognition 50: 7–15. [DOI] [PubMed] [Google Scholar]
- 20.Nili U, Goldberg H, Weizman A, Dudai Y (2010) Fear thou not: activity of frontal and temporal circuits in moments of real-life courage. Neuron 66: 949–962. 10.1016/j.neuron.2010.06.009 [DOI] [PubMed] [Google Scholar]
- 21.Mutschler I, Wieckhorst B, Speck O, Schulze-Bonhage A, Hennig J, Seifritz E, et al. (2010) Time scales of auditory habituation in the amygdala and cerebral cortex. Cereb Cortex 20: 2531–2539. 10.1093/cercor/bhq001 [DOI] [PubMed] [Google Scholar]
- 22.Schuyler BS, Kral TR, Jacquart J, Burghy CA, Weng HY, Perlman DM, et al. (2014) Temporal dynamics of emotional responding: amygdala recovery predicts emotional traits. Soc Cogn Affect Neurosci 9: 176–181. 10.1093/scan/nss131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Breiter HC, Etcoff NL, Whalen PJ, Kennedy WA, Rauch SL, Buckner RL et al. (1996) Response and habituation of the human amygdala during visual processing of facial expression. Neuron 17: 875–887. [DOI] [PubMed] [Google Scholar]
- 24.Weiland BJ, Boutros NN, Moran JM, Tepley N, Bowyer SM (2008) Evidence for a frontal cortex role in both auditory and somatosensory habituation: a MEG study. Neuroimage 42: 827–835. 10.1016/j.neuroimage.2008.05.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fischer H, Wright CI, Whalen PJ, McInerney SC, Shin LM, Rauch SL (2003) Brain habituation during repeated exposure to fearful and neutral faces: a functional MRI study. Brain Res Bull 59: 387–392. [DOI] [PubMed] [Google Scholar]
- 26.Russell JA (1980) A Circumplex Model of Affect. Journal of Personality and Social Psychology 39: 1161–1178. [DOI] [PubMed] [Google Scholar]
- 27.Riem MM, Bakermans-Kranenburg MJ, van IMH, Out D, Rombouts SA (2012) Attachment in the brain: adult attachment representations predict amygdala and behavioral responses to infant crying. Attach Hum Dev 14: 533–551. 10.1080/14616734.2012.727252 [DOI] [PubMed] [Google Scholar]
- 28.Seifritz E, Esposito F, Neuhoff JG, Luthi A, Mustovic H, Dammann G, et al. (2003) Differential sex-independent amygdala response to infant crying and laughing in parents versus nonparents. Biol Psychiatry 54: 1367–1375. [DOI] [PubMed] [Google Scholar]
- 29.De Pisapia N, Bornstein MH, Rigo P, Esposito G, De Falco S, Venuti P (2013) Sex differences in directional brain responses to infant hunger cries. Neuroreport 24: 142–146. 10.1097/WNR.0b013e32835df4fa [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spielberger CD (1983) Manual for the State-Trait Anxiety Inventory. Palo Alto, Ca, USA: Consulting Psychologists Press. [Google Scholar]
- 31.Mutschler I, Wieckhorst B, Meyer AH, Schweizer T, Klarhofer M, Wilhelm FH, et al. (2014) Who gets afraid in the MRI-scanner? Neurogenetics of state-anxiety changes during an fMRI experiment. Neurosci Lett 583: 81–86. 10.1016/j.neulet.2014.09.021 [DOI] [PubMed] [Google Scholar]
- 32.Rankin CH, Abrams T, Barry RJ, Bhatnagar S, Clayton DF, Colombo J, et al. (2009) Habituation revisited: an updated and revised description of the behavioral characteristics of habituation. Neurobiol Learn Mem 92: 135–138. 10.1016/j.nlm.2008.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Radloff LS (1977) The CES-D scale: A self report depression scale for research in the general population’. Applied Psychological Measurement 1: 385–401. [Google Scholar]
- 34.Oldfield RC (1971) The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9: 97–113. [DOI] [PubMed] [Google Scholar]
- 35.Zaitsev M, Hennig J, Speck O (2004) Point spread function mapping with parallel imaging techniques and high acceleration factors: fast, robust, and flexible method for echo-planar imaging distortion correction. Magn Reson Med 52: 1156–1166. [DOI] [PubMed] [Google Scholar]
- 36.Ball T, Rahm B, Eickhoff SB, Schulze-Bonhage A, Speck O, Mutschler I (2007) Response properties of human amygdala subregions: evidence based on functional MRI combined with probabilistic anatomical maps. PLoS One 2: e307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boucsein W, Fowles DC, Grimnes S, Ben-Shakhar G, Roth WT, Dawson ME, et al. (2012) Publication recommendations for electrodermal measurements. Psychophysiology 49: 1017–1034. 10.1111/j.1469-8986.2012.01384.x [DOI] [PubMed] [Google Scholar]
- 38.Bush LK, Hess U, Wolford G (1993) Transformations for within-subject designs: a Monte Carlo investigation. Psychol Bull 113: 566–579. [DOI] [PubMed] [Google Scholar]
- 39.Lykken DT (1972) Range correction applied to heart rate and to GSR data. Psychophysiology 9: 373–379. [DOI] [PubMed] [Google Scholar]
- 40.Morosan P, Schleicher A, Amunts K, Zilles K (2005) Multimodal architectonic mapping of human superior temporal gyrus. Anat Embryol (Berl) 210: 401–406. [DOI] [PubMed] [Google Scholar]
- 41.Eickhoff SB, Paus T, Caspers S, Grosbras MH, Evans AC, Zilles K, et al. (2007) Assignment of functional activations to probabilistic cytoarchitectonic areas revisited. Neuroimage 36: 511–521. [DOI] [PubMed] [Google Scholar]
- 42.Amunts K, Zilles K (2001) Advances in cytoarchitectonic mapping of the human cerebral cortex. Neuroimaging Clin N Am 11: 151–169, vii. [PubMed] [Google Scholar]
- 43.Körner A, Drapeau M, Albani C, Geyer M, Schmutzer G, Brähler E (2008) Deutsche Normierung des NEO-Fünf-Faktoren-Inventars (NEO-FFI). Z Med Psychol 17: 133–144. [DOI] [PubMed] [Google Scholar]
- 44.Sander K, Frome Y, Scheich H (2007) FMRI activations of amygdala, cingulate cortex, and auditory cortex by infant laughing and crying. Hum Brain Mapp 28: 1007–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mutschler I, Ball T, Wankerl J, Strigo IA (2012) Pain and emotion in the insular cortex: evidence for functional reorganization in major depression. Neurosci Lett 520: 204–209. 10.1016/j.neulet.2012.03.095 [DOI] [PubMed] [Google Scholar]
- 46.Mutschler I, Reinbold C, Wankerl J, Seifritz E, Ball T (2013) Structural basis of empathy and the domain general region in the anterior insular cortex. Front Hum Neurosci 7: 177 10.3389/fnhum.2013.00177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mutschler I, Wieckhorst B, Kowalevski S, Derix J, Wentlandt J, Schulze-Bonhage A, et al. (2009) Functional organization of the human anterior insular cortex. Neurosci Lett 457: 66–70. 10.1016/j.neulet.2009.03.101 [DOI] [PubMed] [Google Scholar]
- 48.Ball T, Derix J, Wentlandt J, Wieckhorst B, Speck O, Schulze-Bonhage A, et al. (2009) Anatomical specificity of functional amygdala imaging of responses to stimuli with positive and negative emotional valence. J Neurosci Methods 180: 57–70. 10.1016/j.jneumeth.2009.02.022 [DOI] [PubMed] [Google Scholar]
- 49.Sander K, Scheich H (2005) Left auditory cortex and amygdala, but right insula dominance for human laughing and crying. J Cogn Neurosci 17: 1519–1531. [DOI] [PubMed] [Google Scholar]
- 50.Moser E, Derntl B, Robinson S, Fink B, Gur RC, Grammer K (2007) Amygdala activation at 3T in response to human and avatar facial expressions of emotions. J Neurosci Methods 161: 126–133. [DOI] [PubMed] [Google Scholar]
- 51.Adolphs R (2002) Neural systems for recognizing emotion. Curr Opin Neurobiol 12: 169–177. [DOI] [PubMed] [Google Scholar]
- 52.Nakamura K, Kawashima R, Ito K, Sugiura M, Kato T, Nakamura A, et al. (1999) Activation of the right inferior frontal cortex during assessment of facial emotion. J Neurophysiol 82: 1610–1614. [DOI] [PubMed] [Google Scholar]
- 53.Leitman DI, Wolf DH, Ragland JD, Laukka P, Loughead J, Valdez JN, et al. (2010) "It's Not What You Say, But How You Say it": A Reciprocal Temporo-frontal Network for Affective Prosody. Front Hum Neurosci 4: 19 10.3389/fnhum.2010.00019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Britton JC, Shin LM, Barrett LF, Rauch SL, Wright CI (2008) Amygdala and fusiform gyrus temporal dynamics: responses to negative facial expressions. BMC Neurosci 9: 44 10.1186/1471-2202-9-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reynaud E, El Khoury-Malhame M, Rossier J, Blin O, Khalfa S (2012) Neuroticism modifies psychophysiological responses to fearful films. PLoS One 7: e32413 10.1371/journal.pone.0032413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Iacono WG, Lykken DT, Peloquin LJ, Lumry AE, Valentine RH, Tuason VB (1983) Electrodermal activity in euthymic unipolar and bipolar affective disorders. A possible marker for depression. Arch Gen Psychiatry 40: 557–565. [DOI] [PubMed] [Google Scholar]
- 57.Lenhart RE (1985) Lowered skin conductance in a subsyndromal high-risk depressive sample: response amplitudes versus tonic levels. J Abnorm Psychol 94: 649–652. [DOI] [PubMed] [Google Scholar]
- 58.Weisberg YJ, Deyoung CG, Hirsh JB (2011) Gender Differences in Personality across the Ten Aspects of the Big Five. Front Psychol 2: 178 10.3389/fpsyg.2011.00178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Koenig JL, Barry RA, Kochanska G (2010) Rearing Difficult Children: Parents' Personality and Children's Proneness to Anger as Predictors of Future Parenting. Parent Sci Pract 10: 258–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Riem MM, van IMH, Tops M, Boksem MA, Rombouts SA, Bakermans-Kranenburg MJ (2012) No laughing matter: intranasal oxytocin administration changes functional brain connectivity during exposure to infant laughter. Neuropsychopharmacology 37: 1257–1266. 10.1038/npp.2011.313 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig A. Illustration of the experiment design. (A) State-anxiety was assessed in the scanner before (STAI before) and after the experiment (STAI after). (B) Females were familiarized with the experimental setting and a video clip showing laughing infants (LI) was presented five times. Subsequently, in order to assess habituation, a video clip that showed crying infants (CI) was presented 30 times. A crontrol movie was presented three times. In order to distinguish habituation from sensory and motor fatigue we tested for fatigue at the end of the experiment by presenting the video clip with laughing infants five times. Individuals rated valence, arousal, and irritation after every video clip presentation. (C) Each video clip consisted of a sequence of five laughing or five crying children with a total length of 15 s. Fig B. Some participants with high neuroticism scores who obtained high scores on the Center for Epidemiologic Studies Depression Scale displayed low skin conductance responses. The highest neuroticism scores are shown in squares. X-axis: Shows scores on the Center for Epidemiologic Studies Depression Scale (CES-D). Y-axis: Shows skin conductance responses (SCR, standardized to subject`s max SCR) during the exposure to infant crying.
(DOCX)
Data Availability Statement
Our data are available upon request because of an ethical restriction. Herewith, we confirm that data will be available upon request to all interested researchers. Interested researchers should contact: Dr. Isabella Mutschler, University of Basel, Missionsstrasse 60/62, 4055 Basel, Switzerland. Email: isabella.mutschler@unibas.ch (please see Cover Letter) Please note: The authors have declared that no competing interests exist. (please see Cover Letter)