Abstract
Following local therapy for ductal carcinoma in situ (DCIS), tamoxifen reduces the risk of ipsilateral and contralateral breast cancer by 30%–50%. Studies of tamoxifen in women with resected DCIS have not shown any effect on overall or cancer-specific survival. The adverse event profile of tamoxifen is well characterized, and individual risks and benefits should be assessed to guide decision making. We review the results of the phase III trials of tamoxifen in DCIS as well as the emerging risk reduction therapies.
The prevalence of ductal carcinoma in situ (DCIS) has dramatically increased since the widespread adoption of screening mammography (1). Because DCIS is considered to be a potential precursor of invasive breast cancer, it has been treated aggressively with local therapy. The role of systemic endocrine therapy has also been evaluated. We review here the main findings of phase III trials using adjuvant tamoxifen as well as the rationale for ongoing trials of new agents.
Tamoxifen
The justification for using tamoxifen for the treatment of DCIS stems from the adjuvant treatment trials of invasive breast cancer as well as preclinical data. Adjuvant treatment trials of tamoxifen show that women of all ages with hormone receptor–positive breast cancer benefit from treatment with a 67% decrease in ipsilateral breast cancer recurrence and a 37% decrease in contralateral breast cancer (2). Additionally, animal studies show that tamoxifen prevents tumor initiation and growth (3). Taken together, these data support the concept that tamoxifen is active in preinvasive breast cancer, for example, DCIS. Two randomized phase III trials have been conducted to assess the efficacy of tamoxifen in addition to lumpectomy with or without radiation to reduce recurrence.
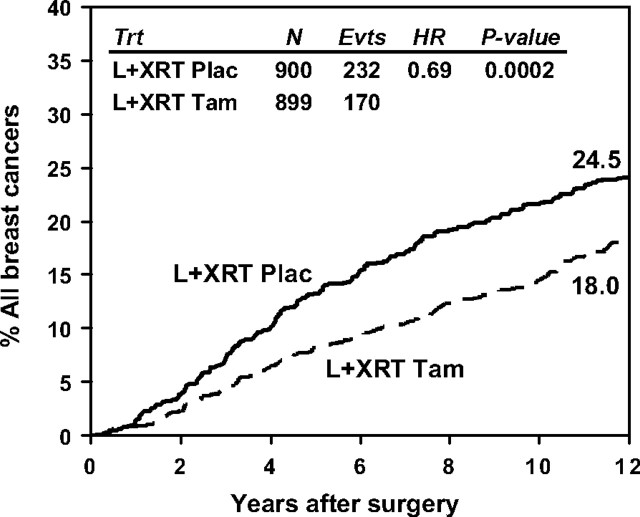
In the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-24 trial, 1804 women with DCIS were randomly assigned to 5 years of tamoxifen or placebo after local therapy. The trial opened to accrual in 1991, completed accrual in 1994, and was results were first published in 1999 (4,5). Eligible women had received lumpectomy and breast radiation for local control. Tumor involvement of surgical margins was allowed, and positive estrogen receptor (ER) status was not a prerequisite for treatment. Of note, one-third of the subjects were 49 years old or younger, and tumor size was <1 cm in more than 80%. Approximately 15% had positive surgical margins with an additional 9% where margin status was unknown. At 12 years of follow-up, tamoxifen use reduced the risk of all breast cancer recurrence by 31% with 170 events in 899 women on the tamoxifen arm compared with 232 events in 900 women on the placebo arm (hazard ratio [HR] = 0.69, P = .0002) (Figure 1). Ipsilateral invasive breast cancer was reduced by 31% with 59 events (6.6%) on tamoxifen compared with 81 events (9.0%) on placebo (HR = 0.69, P = .02). Noninvasive ipsilateral breast cancers were also fewer on tamoxifen, 60 (6.7%) vs 68 (7.6%), but did not reach statistical significance (HR = 0.83, P = .33) (Table 1). Contralateral breast cancer events were reduced by 43% with 44 events (4.9%) on tamoxifen and 73 events (8.1%) on placebo (HR = 0.57, P = .003). Additionally, benefit was seen in both younger and older cohorts. For women less than 50 years old, tamoxifen reduced the risk for all breast events by 29% with 77 events in 302 women on tamoxifen and 99 events in 299 women on placebo (HR = 0.71, P = .02). For women 50 years and older, a similar magnitude of benefit was seen with 93 events in 597 women on tamoxifen and 133 events in 601 women on placebo (HR = 0.67, P = .003). Overall survival did not differ between tamoxifen and placebo arms; 851 (94.7%) alive on tamoxifen vs 851 (94.6%) alive on placebo (HR = 0.86, P = .24). Hormone receptor status was not routinely analyzed in DCIS at the time of the B-24 trial; however, a retrospective analysis evaluating ER status was performed on 732 cases (368 on placebo and 364 on tamoxifen). ER status was determined either centrally or by treatment site, and 76% of cases were ER positive. Tamoxifen significantly reduced the risk of invasive breast cancer recurrence in ER-positive cases by 40% with 58 events in 284 women on tamoxifen and 84 events in 272 women on placebo (relative risk = 0.60, P = .003). There were a smaller number of ER-negative cases and respectively fewer recurrences, 20 events in 80 women on tamoxifen and 25 events in 96 women on placebo, such that a benefit cannot be excluded (relative risk = 0.88, P = .68) (Joseph P. Costantino, NSABP Biostatistical Center, personal communication).
Figure 1.
Cumulative incidence of all breast cancer events in National Surgical Adjuvant Breast and Bowel Project B-24. L+XRT plac = lumpectomy and radiation therapy followed by placebo; L+XRT tam = lumpectomy and radiation therapy followed by tamoxifen.
Table 1.
National Surgical Adjuvant Breast and Bowel Project B-24 results with 12-year follow-up
| L+XRT + placebo, N (%) | L+XRT + tamoxifen, N (%) | |
| Ipsilateral breast tumor | 149 (16.6) | 119 (13.2) |
| Invasive | 81 (9.0) | 59 (6.6) |
| Noninvasive | 68 (7.6) | 60 (6.7) |
| Contralateral breast tumor | 73 (8.1) | 44 (4.9) |
| Invasive | 48 (5.3) | 30 (3.3) |
| Noninvasive | 25 (2.8) | 14 (1.6) |
| Other local, regional, or distant recurrence | 10 (1.1) | 7 (0.8) |
| Second cancer, except opposite breast | 64 (7.1) | 71 (7.9) |
| Dead, NED | 49 (5.4) | 48 (5.3) |
| Total first events | 345 (38.3) | 289 (32.1) |
| Alive, event-free | 555 (61.7) | 610 (67.9) |
| Total patients | 900 (100.0) | 899 (100.0) |
L+XRT= lumpectomy and radiation therapy; NED = no evidence of disease.
In the adjuvant trial from the United Kingdom/Australia/New Zealand (UK/ANZ), 1701 patients with DCIS were enrolled between 1990 and 1998 (6). Microinvasive disease was permitted (3% in the total cohort), and surgical margins were required to be free of disease. The study design was complex in that subjects could chose whether to enter into a 4-way randomization (radiation and tamoxifen, tamoxifen only, radiation only, or no adjuvant treatment) or one of two 2-way randomizations. This design allowed 12 distinct treatment regimens. To evaluate the benefits of tamoxifen, the investigators grouped the same treatments together regardless of whether aspects of the treatment were chosen or were due to randomization within the study. With 52 months median follow-up in the tamoxifen/no tamoxifen comparison, there was a total of 251 breast events in 1576 women, 114 (14%) in the tamoxifen group, and 137 (18%) in the patients not taking tamoxifen (HR = 0.83, P = .13). Statistically significant findings included a 32% reduction in ipsilateral and contralateral noninvasive cancers, 58 (7%) on tamoxifen vs 84 (11%) not on tamoxifen (HR = 0.68, P = .03). The incidence of death was too rare to allow analysis of this endpoint. In women who were randomized to tamoxifen and then received radiation (N = 523), no statistically significant differences were seen in invasive or noninvasive breast cancers. In women who did not receive radiation and were randomized to tamoxifen or not (N = 1053), a 32% decrease in total DCIS was the only significant outcome, 51 (6%) events occurred on tamoxifen vs 75 (10%) not on tamoxifen (HR = 0.68, 95% confidence interval = 0.47 to 0.97, P = .03).
There are a number of differences between the NSABP B-24 and the UK/ANZ trials, which may explain the inconsistency in results. First, the populations differed in the distribution of age. UK/ANZ had a smaller proportion of patients younger than 50 years compared with NSABP B-24 (9.5% and 33.5%, respectively). Tamoxifen therapy may be more beneficial in the population younger than 50 years. Analysis of NSABP B-24 at 5 years showed that tamoxifen therapy resulted in a 38% reduction in ipsilateral events in patients younger than 50 years compared with the 22% reduction in ipsilateral events that tamoxifen produced in women 50 years and older (4). In a subgroup analysis stratified by age, the hazard ratios for breast events in the UK/ANZ trial were similar to those observed in the B-24 trial. Second, the design and analysis of the UK/ANZ trial make it difficult to make comparisons with B-24 because only 523 subjects were randomized to tamoxifen or not following radiation, and the potential bias of allowing the patient and/or physician to select some components of treatment may confound the results.
Regarding drug adherence and side effects, in the UK/ANZ trial, 11% of the population did not complete their course of tamoxifen, and B-24 does not report compliance. In prospective quality-of-life evaluations, tamoxifen was well tolerated in healthy at-risk women (7). Tamoxifen’s adverse event profile is well described and serious adverse events (thromboembolic disease, endometrial cancer) are rare and age related (8). Common side effects on tamoxifen vs placebo included hot flashes (69.6% vs 59.0%), fluid retention (32.7% vs 27.9%), and vaginal discharge (32.4% vs 20.0%) (4). However, in a retrospective review of tamoxifen in the setting of DCIS, Yen et al. (9) found that of the 166 women offered tamoxifen, only 90 (54%) accepted; moreover, on follow-up, 21% of subjects had discontinued the drug. These findings highlight the need to better identify who will benefit from systemic therapy. Adequate selection of patients will increase efficacy of treatments.
Aromatase Inhibitors
Aromatase inhibitors reduce the risk of contralateral breast cancer by more than 50% when compared with tamoxifen in adjuvant breast cancer trials (10–12). The NSABP B-35 trial and the International Breast Cancer Intervention Study-II (IBIS-II) are currently evaluating the role of anastrazole as adjuvant therapy in patients with DCIS. In NSABP B-35, postmenopausal women with ER-positive and/or progesterone receptor–positive DCIS are treated with lumpectomy and radiotherapy and then randomized to anastrazole and placebo or tamoxifen and placebo. The planned accrual of 3000 patients has been completed (13,14). In a similar design, the investigators of the IBIS-II trial are evaluating the same drugs, but the radiation therapy is offered at the discretion of the attending physician. IBIS-II plans to accrue 4000 patients (15). The use of exemestane in postmenopausal patients at high risk of developing breast cancer is currently under evaluation in the NCIC-CTG MAP-3 Study, which plans to recruit 4560 women (16). Patients with a prior diagnosis of DCIS treated with mastectomy, but not with tamoxifen, are eligible for this trial, as well as women at high risk due to Gail model risk or high-risk pathological lesions. The primary endpoint is to compare the incidence of invasive breast cancer between women randomized to exemestane or placebo for 5 years. To investigate neoadjuvant therapy in DCIS, investigators (17) have initiated a pilot study of tamoxifen or letrozole in 40 women with ER-positive DCIS. Hormonal therapy is offered during the 3 months before surgery. Response is evaluated through mammography, magnetic resonance imaging, and tissue biomarkers (18). These evaluations will yield information on which DCIS tumors will benefit from each intervention.
Other Targeted Agents Under Study
A number of other molecular targets are of interest in DCIS. Compared with invasive ductal cancer, pure DCIS more often overexpresses HER2/neu (22% vs 56%) (19). NSABP B-43 is a phase III trial of adjuvant trastuzumab for patients with HER2-positive DCIS and negative margins after breast-conserving surgery. Patients will be randomly assigned to receive 6 weeks of whole-breast irradiation with or without concurrent trastuzumab for two cycles (20). The planned accrual is 2000 patients, with the primary endpoint of ipsilateral breast cancer events (invasive or noninvasive). Trastuzumab may also be an effective neoadjuvant therapy for DCIS. The MD Anderson Cancer Center is completing a trial of neoadjuvant trastuzumab for HER2-positive DCIS, where a single dose of trastuzumab (8 mg/kg) is given 2 weeks before surgery (21). The objective is to determine the effect of trastuzumab on the proliferation and apoptotic rates of these lesions. Investigators at the Baylor College of Medicine have begun a multicenter trial of neoadjuvant lapatinib in three different doses (750 mg, 1000 mg, and 1500 mg) compared with placebo for patients with either HER2-positive or epidermal growth factor receptor–positive DCIS (22).
Moderate to high levels of cyclooxygenase (COX)-2 expression have been detected in invasive breast cancer (43%), in DCIS (63%) (23), and in breast cancers overexpressing HER2 (93%) (24). A randomized phase I trial is studying the effects of sulindac, thought to act on enzymes COX-1 and COX-2 in breast cancer prevention. Women at high risk, including those with a history of DCIS, are randomly assigned to sulindac once daily or twice daily for 6 weeks (25). The primary endpoint is evaluating sulindac and sulindac metabolite levels in nipple aspirate fluid. Of note, a phase II trial of celecoxib, a selective COX-2 inhibitor, in high-risk premenopausal women did not result in favorable modulation of Ki-67, ER, or COX-2 expression in breast epithelial cells or mammographic density (26).
Fenretinide, a synthetic derivative of all-trans retinoic acid, showed activity in inhibiting mammary carcinogenesis in an animal model (27). An Italian study of 2972 women with surgically removed stage I breast cancer or DCIS (only 35 cases) who were randomly assigned to either fenretinide or no treatment did not find benefit in the use of adjuvant fenretinide. However, a subgroup analysis detected a possible benefit in premenopausal women (contralateral breast cancer: HR = 0.66, P = .045; ipsilateral breast cancer: HR = 0.65, P = .045) (28). Possible interactions between retinoid- and estrogen-induced signaling have been demonstrated (29–31). In this scenario, a combination of an estrogen antagonist and a retinoid could be effective. A pilot study evaluated the tolerability of fenretinide combined with tamoxifen in a group of women at high risk for breast cancer (32) and demonstrated acceptable toxicity (33). A randomized phase II trial is ongoing to evaluate the effectiveness of fenretinide and tamoxifen given for a short course before surgery in women with either stage T1 breast cancer or DCIS (33), although a biomarker-based prevention trial in premenopausal high-risk women did not find that the combination of fenretinide and tamoxifen reduced breast cancer events more than either agent alone (34).
Systemic therapy with tamoxifen reduces ipsilateral and contralateral breast cancer events in women with DCIS and is the only Food and Drug Administration–approved systemic therapy for this disease (35). Ongoing studies are evaluating newer endocrine agents and other targeted therapy. Because some DCIS may never progress to invasive breast cancer and therapeutic interventions are never risk free, patient selection for these agents needs to be more specific. Expression profiling of DCIS may help either in the selection of lesions more likely to progress to invasive disease or in identification of specific targets responsive to treatment. Multiple trials are currently evaluating these strategies.
Funding
This study was supported by Public Health Service grants U10-CA-37377, U10-CA-69974, U10-CA-12027, U10-CA-69651, and CA-25224 from the National Cancer Institute, National Institutes of Health, Department of Health and Human Services. Auxiliary support was provided by sanofi-aventis.
Note
The authors acknowledge Bernard Fisher, MD, who designed the National Surgical Adjuvant Breast and Bowel Project B-24 study and presented the original report.
References
- 1.Ernster VL, Ballard-Barbash R, Barlow WE, et al. Detection of ductal carcinoma in situ in women undergoing screening mammography. J Natl Cancer Inst. 2002;94(20):1546–1554. doi: 10.1093/jnci/94.20.1546. [DOI] [PubMed] [Google Scholar]
- 2.Fisher B, Dignam J, Bryant J, et al. Five versus more than five years of tamoxifen therapy for breast cancer patients with negative lymph nodes and estrogen receptor-positive tumors. J Natl Cancer Inst. 1996;88(21):1529–1542. doi: 10.1093/jnci/88.21.1529. [DOI] [PubMed] [Google Scholar]
- 3.Jordan VC, Allen KE. Evaluation of the antitumour activity of the non-steroidal antioestrogen monohydroxytamoxifen in the DMBA-induced rat mammary carcinoma model. Eur J Cancer. 1980;16(2):239–251. doi: 10.1016/0014-2964(80)90156-5. [DOI] [PubMed] [Google Scholar]
- 4.Fisher B, Dignam J, Wolmark N, et al. Tamoxifen in treatment of intraductal breast cancer: National Surgical Adjuvant Breast and Bowel Project B-24 randomised controlled trial. Lancet. 1999;353(9169):1993–2000. doi: 10.1016/S0140-6736(99)05036-9. [DOI] [PubMed] [Google Scholar]
- 5.Fisher B, Land S, Mamounas E, Dignam J, Fisher ER, Wolmark N. Prevention of invasive breast cancer in women with ductal carcinoma in situ: an update of the National Surgical Adjuvant Breast and Bowel Project experience. Semin Oncol. 2001;28(4):400–418. doi: 10.1016/s0093-7754(01)90133-2. [DOI] [PubMed] [Google Scholar]
- 6.Houghton J, George WD, Cuzick J, Duggan C, Fentiman IS, Spittle M. Radiotherapy and tamoxifen in women with completely excised ductal carcinoma in situ of the breast in the UK, Australia, and New Zealand: randomised controlled trial. Lancet. 2003;362(9378):95–102. doi: 10.1016/s0140-6736(03)13859-7. [DOI] [PubMed] [Google Scholar]
- 7.Day R, Ganz PA, Costantino JP, Cronin WM, Wickerham DL, Fisher B. Health-related quality of life and tamoxifen in breast cancer prevention: a report from the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Clin Oncol. 1999;17(9):2659–2669. doi: 10.1200/JCO.1999.17.9.2659. [DOI] [PubMed] [Google Scholar]
- 8.Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 2005;97(22):1652–1662. doi: 10.1093/jnci/dji372. [DOI] [PubMed] [Google Scholar]
- 9.Yen TWF, Hunt KK, Mirza NQ, et al. Physician recommendations regarding tamoxifen and patient utilization of tamoxifen after surgery for ductal carcinoma in situ. Cancer. 2004;100(5):942–949. doi: 10.1002/cncr.20085. [DOI] [PubMed] [Google Scholar]
- 10.Baum M, Budzar AU, Cuzick J, et al. Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early breast cancer: first results of the ATAC randomised trial. Lancet. 2002;359(9324):2131–2139. doi: 10.1016/s0140-6736(02)09088-8. [DOI] [PubMed] [Google Scholar]
- 11.Coombes RC, Hall E, Gibson LJ, et al. A randomized trial of exemestane after two to three years of tamoxifen therapy in postmenopausal women with primary breast cancer. N Engl J Med. 2004;350(11):1081–1092. doi: 10.1056/NEJMoa040331. [DOI] [PubMed] [Google Scholar]
- 12.Goss PE, Ingle JN, Martino S, et al. A randomized trial of letrozole in postmenopausal women after five years of tamoxifen therapy for early-stage breast cancer. N Engl J Med. 2003;349(19):1793–1802. doi: 10.1056/NEJMoa032312. [DOI] [PubMed] [Google Scholar]
- 13.Vogel VG, Costantino JP, Wickerham DL, Cronin WM. National Surgical Adjuvant Breast and Bowel Project update: prevention trials and endocrine therapy of ductal carcinoma in situ. Clin Cancer Res. 2003;9(1, pt 2):495S–501S. [PubMed] [Google Scholar]
- 14.Clinical trial: A clinical trial comparing anastrozole with tamoxifen in postmenopausal patients with ductal carcinoma in situ (DCIS) undergoing lumpectomy with radiation therapy. Bethesda, MD: National Institutes of Health: http://www.clinicaltrials.gov/ct2/show/NCT00053898?term=NSABP+B-35&rank=1. Accessed December 17, 2009. [Google Scholar]
- 15.Cuzick J. Aromatase inhibitors for breast cancer prevention. J Clin Oncol. 2005;23(8):1636–1643. doi: 10.1200/JCO.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 16.Richardson H, Johnston D, Pater J, Goss P. The National Cancer Institute of Canada Clinical Trials Group MAP.3 trial: an international breast cancer prevention trial. Curr Oncol. 2007;14(3):89–96. doi: 10.3747/co.2007.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hwang ES, Esserman L. Neoadjuvant hormonal therapy for ductal carcinoma in situ: trial design and preliminary results. Ann Surg Oncol. 2004;11(1) suppl:37S–43S. doi: 10.1007/BF02524794. [DOI] [PubMed] [Google Scholar]
- 18.Clinical trial: Tamoxifen or letrozole in treating women with ductal carcinoma in situ. Bethesda, MD: National Institutes of Health: http://www.clinicaltrials.gov/ct2/show/NCT00290745. Accessed December 15, 2009. [Google Scholar]
- 19.Allred DC, Clark GM, Molina R, et al. Overexpression of HER-2/neu and its relationship with other prognostic factors change during the progression of in situ to invasive breast cancer. Hum Pathol. 1992;23(9):974–979. doi: 10.1016/0046-8177(92)90257-4. [DOI] [PubMed] [Google Scholar]
- 20.Clinical trial: Radiation therapy with or without trastuzumab in treating women with ductal carcinoma in situ who have undergone lumpectomy. Bethesda, MD: National Institutes of Health: http://clinicaltrials.gov/ct2/show/NCT00769379. Accessed December 15, 2009. [Google Scholar]
- 21.Clinical trial: Neoadjuvant herceptin for ductal carcinoma in situ of the breast. Bethesda, MD: National Institutes of Health: http://clinicaltrials.gov/ct2/show/NCT00496808. Accessed December 15, 2009. [Google Scholar]
- 22.Clinical trial: Lapatinib in treating women with ductal carcinoma in situ of the breast. Bethesda, MD: National Institutes of Health: http://clinicaltrials.gov/ct2/show/NCT00570453. Accessed December 15, 2009. [Google Scholar]
- 23.Half E, Tang XM, Gwyn K, Sahin A, Wathen K, Sinicrope FA. Cyclooxygenase-2 expression in human breast cancers and adjacent ductal carcinoma in situ. Cancer Res. 2002;62(6):1676–1681. [PubMed] [Google Scholar]
- 24.Subbaramaiah K, Norton L, Gerald W, Dannenberg AJ. Cyclooxygenase-2 is overexpressed in HER-2/neu-positive breast cancer: evidence for involvement of AP-1 and PEA3. J Biol Chem. 2002;277(21):18649–18657. doi: 10.1074/jbc.M111415200. [DOI] [PubMed] [Google Scholar]
- 25.Clinical trial: Sulindac in preventing breast cancer in women at high risk for breast cancer. Bethesda, MD: National Institutes of Health: http://clinicaltrials.gov/ct2/show/NCT00245024. Accessed December 15, 2009. [Google Scholar]
- 26.Fabian CKB, Mayo MS, Zalles CM, et al. San Antonio Breast Cancer Symposium. San Antonio TX: Sprinter; 2007. Phase II tissue-based biomarker prevention trial of celecoxib in premenopausal women at high risk for development of breast cancer; p. S179. Abstract 4040. [Google Scholar]
- 27.Moon RC, Thompson HJ, Becci PJ, et al. N-(4-Hydroxyphenyl)retinamide, a new retinoid for prevention of breast cancer in the rat. Cancer Res. 1979;39(4):1339–1346. [PubMed] [Google Scholar]
- 28.Veronesi U, De Palo G, Marubini E, et al. Randomized trial of fenretinide to prevent second breast malignancy in women with early breast cancer. J Natl Cancer Inst. 1999;91(21):1847–1856. doi: 10.1093/jnci/91.21.1847. [DOI] [PubMed] [Google Scholar]
- 29.Fontana JA. Interaction of retinoids and tamoxifen on the inhibition of human mammary carcinoma cell proliferation. Exp Cell Biol. 1987;55(3):136–144. doi: 10.1159/000163409. [DOI] [PubMed] [Google Scholar]
- 30.Sheikh MS, Shao ZM, Li XS, et al. Retinoid-resistant estrogen receptor-negative human breast carcinoma cells transfected with retinoic acid receptor-alpha acquire sensitivity to growth inhibition by retinoids. J Biol Chem. 1994;269(34):21440–21447. [PubMed] [Google Scholar]
- 31.Rubin M, Fenig E, Rosenauer A, et al. 9- cis retinoic acid inhibits growth of breast cancer cells and down-regulates estrogen receptor RNA and protein. Cancer Res. 1994;54(24):6549–6556. [PubMed] [Google Scholar]
- 32.Conley B, O’Shaughnessy J, Prindiville S, et al. Pilot trial of the safety, tolerability, and retinoid levels of N-(4-hydroxyphenyl) retinamide in combination with tamoxifen in patients at high risk for developing invasive breast cancer. J Clin Oncol. 2000;18(2):275–283. doi: 10.1200/JCO.2000.18.2.275. [DOI] [PubMed] [Google Scholar]
- 33.Clinical trial: Chemoprevention therapy plus surgery in treating women with breast cancer. Bethesda, MD: National Institutes of Health: http://www.clinicaltrials.gov/ct2/show/NCT00003099. Accessed December 15, 2009. [Google Scholar]
- 34.Decensi A, Robertson C, Guerrieri-Gonzaga A, et al. Randomized double-blind 2 × 2 trial of low-dose tamoxifen and fenretinide for breast cancer prevention in high-risk premenopausal women. J Clin Oncol. 2009;27(23):3749–3756. doi: 10.1200/JCO.2008.19.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nolvadex tamoxifen citrate. NDA 17-970/S-049. Bethesda, MD: National Institutes of Health: 2002. http://www.accessdata.fda.gov/drugsatfda_docs/label/2002/17970s37s44s49lbl.pdf. Accessed December 15, 2009. [Google Scholar]