Abstract
Pivotal response treatment (PRT) is an empirically validated behavioral treatment that has widespread positive effects on communication, behavior, and social skills in young children with autism spectrum disorder (ASD). For the first time, functional magnetic resonance imaging was used to identify the neural correlates of successful response to PRT in two young children with ASD. Baseline measures of social communication, adaptive behavior, eye tracking and neural response to social stimuli were taken prior to treatment and after 4 months of PRT. Both children showed striking gains on behavioral measures and also showed increased activation to social stimuli in brain regions utilized by typically developing children. These results suggest that neural systems supporting social perception are malleable through implementation of PRT.
Keywords: Pivotal response treatment, fMRI, Autism, Intervention, Outcome
Introduction
Pivotal response treatment (PRT) is an empirically validated behavioral treatment for children with autism spectrum disorder (ASD) that builds upon key techniques employed in Applied Behavioral Analysis (ABA). It was designed to improve social communication skills by addressing core deficits in social motivation, or pivotal responses. By working specifically with each child’s natural motivations, PRT focuses on functional communication rather than rote learning (Koegel and Koegel 2006). PRT was initially developed to improve verbal language acquisition for nonverbal children with autism (Koegel et al. 1987). Originally called the “Natural Language Teaching Paradigm”, this treatment differed from traditional analogue teaching (where a therapist presents instructions, prompts and reinforces correct responses only) in that it employed functional and varied stimuli, natural reinforcers, and reinforcement for communicative attempts, all within a natural interchange. Since its development, a parent component has been added (Laski et al. 1988), and PRT has been extended beyond basic language to target appropriate social behavior (Koegel and Frea 1993), symbolic play (Stahmer 1995), speech intelligibility (Koegel et al. 1998), and self-initiated queries (Koegel et al. 2003). The treatment has been repeatedly found to improve these and other pivotal behaviors in both nonverbal and verbal children with ASD.
Research validating PRT has relied on overt behavioral measures as outcome data. Though such measures demonstrate improvements in targeted skills, they provide minimal insight into underlying mechanisms of change. Recently, functional magnetic resonance imaging (fMRI) has been used as an outcome measure in a range of treatment research. A body of research focusing on the effects of cognitive therapy (CT) for depression has utilized this technique to highlight neural mechanisms targeted in therapy (for a review see DeRubeis et al. 2008). In addition, fMRI has shown potential for predicting treatment response in adults with uni-polar depression (Siegle et al. 2006), demonstrating that adults who exhibited a particular neural response to emotional stimuli at pre-treatment also displayed the most improvement after therapy. These findings support a strong translational connection between research and clinical practice, as they not only describe how a treatment is working, but also for whom it works best and why at a biological systems level. Given evidence that atypical development in ASD is evident in neural systems-level activity even when behavioral differences are not observed (e.g. Elsabbagh et al. 2012; Kaiser et al. 2010), measures of brain function may provide more effective predictors of treatment response and more sensitive measures of treatment outcome.
The current study evaluated the efficacy of PRT in two five-year-old children with ASD, using multiple modalities including fMRI, eye tracking, and behavioral outcome to assess treatment response. We anticipated that we would replicate the widespread positive effects of PRT on communication, behavior, and social skills (Koegel and Koegel 2006) in our two cases, as measured by standardized assessments of social and communicative function and adaptive behavior. In addition, we predicted, with successful treatment, an increased utilization of key nodes of the social brain previously implicated in processing socially salient visual stimuli. The social stimuli we utilized, comprised of point light displays of biological motion, have been repeatedly used to identify neural (Kaiser et al. 2010) and eye tracking (Klin et al. 2009) signatures of disruptions in social motivation in autism. Critically, using these stimuli, a recent fMRI study in our lab identified three categories of brain responses (State, Trait, and Compensatory) in a group of typical children (TD), unaffected siblings (US) and children with ASD. Regions reflecting the State of having ASD showed decreased activation in ASD compared to TD and US and were localized to the left ventrolateral prefrontal cortex, right amygdala, right posterior superior temporal sulcus, ventromedial prefrontal cortex, and bilateral fusiform gyri. Trait regions, reflecting an increased risk for developing ASD, showed decreased activation in children with ASD and US, and were localized to the bilateral fusiform gyrus, left dorsolateral prefrontal cortex, and right inferior temporal gyrus. Compensatory activity, unique to US, suggested a neural system–level mechanism by which US might compensate for an increased genetic risk for developing ASD (Kaiser et al. 2010) and was localized to the right posterior superior temporal sulcus and ventromedial prefrontal cortex. The fMRI component of the current study was designed to test whether these, a priori and independently identified brain regions were altered by PRT treatment. We hypothesized that treatment related changes in neural activation would be present in the previously identified State, Trait and Compensatory regions representing areas of atypical activation in children with ASD and unaffected siblings.
Methods
Subject 1
MM was first diagnosed with Autistic Disorder at the age of 2 years by a developmental behavioral pediatrician. She was evaluated at the Yale Child Study Center at the age of 5 years, 1 month. Cognitive functioning was assessed using the Differential Abilities Scales, Second Edition (DAS-II; Elliott 2006), and her scores were in the above average range (GCA = 117; Verbal = 111; Nonverbal Reasoning = 117; Spatial = 114). Language functioning was assessed using the Clinical Evaluation of Language Fundamentals, Preschool Second Edition (CELF-P-2; Wiig et al. 2004), and her scores were in the average range (Core Language = 102; Receptive Language = 109; Expressive Language = 102; Language Content = 121; Language Structure = 92). Adaptive functioning, as measured by the Vineland Adaptive Behavior Scales, Second Edition (Vineland-II; Sparrow et al. 2005), was generally below average and impaired relative to cognitive abilities (Standard scores: Communication = 85, Daily Living Skills = 75, Socialization = 83). Diagnosis of ASD was made by direct clinical interactions using the Autism Diagnostic Observation Schedule (ADOS; Lord et al. 2002) (Module 3), and parent-report using the Autism Diagnostic Interview—Revised (ADI-R; Rutter et al. 2003), with final diagnosis confirmed by clinical judgment. Upon the initiation of the treatment program, goals were identified for MM. Goals included: (1) appropriate body positioning when speaking/interacting with a social partner, (2) appropriate voice modulation, and (3) initiations of social interactions.
Subject 2
CC was first diagnosed with Pervasive Developmental Disorder—Not Otherwise Specified (PDD-NOS) at 1 year, 9 months by a clinical psychologist. He was evaluated at the Yale Child Study Center at the age of 5 years, 5 months. Cognitive functioning was assessed using the DAS-II, and his scores ranged from the average to the very high range (GCA = 132; Verbal = 147; Nonverbal Reasoning = 107; Spatial = 123). Language functioning was assessed using the CELF-P-2, and his scores were in the above average range (Core Language = 123; Receptive Language = 119; Expressive Language = 123; Language Content = 131; Language Structure = 116). Adaptive functioning, as measured by the Vineland-II, was average but lower than his cognitive abilities (Standard scores: Communication = 106, Daily Living Skills = 111, Socialization = 90). Diagnosis of ASD was determined by using direct clinical interactions with the ADOS (Module 3) and parent-report using the ADI-R, with final diagnosis confirmed by clinical judgment. Upon the initiation of the treatment program, goals were identified for CC. Goals included: (1) develop narrative language skills, (2) improve perspective-taking skills, and (3) improve reciprocity within context of play and conversation.
Treatment Procedure
Baseline measures were taken immediately prior to treatment (t1), and post treatment measures were taken after 4 months of treatment (t2). Three clinicians trained on PRT implemented the treatment. Two were bachelor-level clinicians with several years experience working with children with ASD, and the lead clinician was a licensed psychologist with extensive experience in ASD. Therapy targeted pivotal areas of development, including motivation, social initiation and responsivity in order to improve social and language functioning in both participants. The idea behind targeting pivotal areas of development was that improvements in these areas should lead to more widespread and generalized improvements in multiple areas of development representing core changes in social motivation. A more detailed description of PRT can also be found in the original instruction manual (Koegel et al. 1989) as well as the more recent and user friendly “pocket guide” (Koegel and Koegel 2012). The treatment included individual work with the child as well as parent training and took place in both the clinic and home setting for 8–10 h per week for 4 months.
Instruments and Measures
Differential Ability Scales-II (DAS-II), Early Years Battery
The DAS-II is a standardized assessment of cognitive abilities that measures verbal, nonverbal and spatial reasoning abilities. The DAS-II was used in the current study for subject characterization at t1.
Autism Diagnostic Interview- Revised (ADI-R)
The ADI-R is a comprehensive diagnostic parent-report interview that focuses on language/communication, reciprocal social interactions and restricted, repetitive and stereotyped behaviors and interests. The ADI-R was administered to the mothers of both subjects at t1 for subject characterization.
Autism Diagnostic Observation Schedule (ADOS)
The ADOS is a semi-structured diagnostic assessment that allows clinicians to observe and assess social, communication and repetitive behaviors associated with ASD. The ADOS was performed on both subjects at t1 and t2 by expert clinicians with no involvement in the current study. The ADOS was used for subject characterization as well as for a measure of treatment outcome.
Vineland Adaptive Behavior Scales-II, Survey Form (Vineland-II)
The Vineland-II is an assessment of adaptive functioning and measures skills in four areas: communication, daily living, socialization and motor skills. Only three domains: communication, daily living and socialization were used in the current study. The mothers of the participating children were interviewed using the Vineland-II at t1 and t2 as an outcome measure.
Clinical Evaluation of Language Fundamentals-Preschool-2 (CELF-P-2) and Fourth Edition (CELF-4)
The CELF-P-2 is a standardized language assessment for children 3–6 years that evaluates receptive and expressive syntactic and semantic skills. It is useful for providing specific information regarding amounts and types of information the child understands and is capable of using. A speech-language pathologist with extensive experience in ASD administered the CELF-P-2 to both subjects at t1. Scores on the CELF-P-2 were used for subject characterization at t1. The Pragmatic Profile subtest of the CELF-4 (Semel et al. 2003) was also administered at t1 and t2 and was used as an outcome measure. The Pragmatic Profile subtest is used to evaluate a child’s language use. More specifically, it assesses pragmatic skills in the following areas: Rituals and Conversational Skills; Asking For, Giving and Responding to Information; and Nonverbal Communication Skills.
Behavioral Coding
Prior to and after treatment, both children were video taped interacting with their mother and also with a trained researcher. We collected data in an unstructured environment (free play) in which the participant’s mother was told to play with her child as she would in any other circumstance. We provided them with a standardized set of toys (Bubbles, plastic duck, foam shapes, Candyland, construction paper, animal book, play-doh, balloons, army men, plastic insects, foam gun, glitter pens, two stuffed animals). The interaction lasted 10 min. In addition we videotaped each participant interacting with a trained researcher, who had a set of four pre-established conversation probes (e.g., “I have a pet at home”).
Eye Tracking
Eye tracking data was collected using a Tobii T60 XL Eye Tracker, which utilizes the Pupil Center Corneal Reflection (PCCR) technique. The system tracks both eyes, to a rated accuracy of 0.5°, sampled at 60 Hz. Raw eye movement data points are collected every 16.7 ms and are identified by a timestamp and a Cartesian plane coordinate. These data points are aggregated into fixations in Tobii Studio 2.1. The ClearView Fixation Filter was applied to all raw data in analyzing fixations. The fixation radius, or smallest distance that can separate fixations, was 35 pixels. The eye tracker was calibrated for each participant using 5-point calibration. Prior to calibration, participants were told they were going to be looking at pictures of faces and houses on a large computer screen. Participants were told that their job was to look at the pictures and to stay still. If a child was becoming distracted, the child was quietly asked, “what do you see?” in order to redirect their attention to the computer screen. Eye tracking data was collected from each subject at t1 and t2.
The stimuli presented during eye tracking sessions included 14 adult faces from the NimStim Face Stimulus Set (each shown three times: expressing a happy, fearful and neutral expression) and 14 houses, and were presented on a 24-inch monitor at a distance of 60 cm from the participant. The task consisted of two runs, each 4 min 13 s in length, made up of randomly ordered faces and houses with staggered fixations (lasting 2 or 3 s, consisting of a cross in one of the four corners of the screen). Areas of interest (AOIs) were delineated for the eyes and the mouth on each face, and were applied to every face in the stimulus set. An additional AOI was created for the total image.
We computed the Total Fixation Duration (TFD), a measure of total duration (in seconds) of all the fixations within a given AOI. The three AOIs utilized in all analyses were Eyes, Mouth and Total Image. In order to take into account the fact that every child does not look at the actual face image for the same amount of time, we computed an additional measure we call percent of looking time (POLT). POLT was calculated for the Eyes and Mouth AOIs by dividing TFD for each AOI by Total Image (i.e. TFD Eyes/TFD Total Image = POLT Eyes).
fMRI
The task for the fMRI study task was taken from a recent study in our lab (Kaiser et al. 2010) used to probe neural processing of biological motion, a basic form of social information. This was a passive viewing task in which the participants saw either coherent or scrambled point-light displays of biological motion created from motion capture data. The coherent biological motion displays featured an adult male actor performing various actions while the scrambled motion displays were created by randomly selecting 16 points from the biological motion displays and repositioning their trajectories. Thus, both conditions contained the same local motion information, but only the coherent displays contained the configuration of a person (Bertenthal 1994).
During the MRI scan, the stimuli were presented using E-Prime 2.0 software (Psychological Software Tools) without audio. The experiment began and ended with a 20 s fixation period and consisted of six biological motion clips and six scrambled motion clips presented in an alternating-block design (time per block, 24 s). The participants were instructed to watch the videos and to remain still. The paradigm lasted a total of 328 s and was shown to both subjects at t1 and t2. In order to avoid a multiple-comparison problem, activity in pre-specified, independently identified regions of interest, the State, Trait, and Compensatory regions identified by Kaiser et al. (2010), about which we had a priori hypotheses, was used as outcome data.
Images were collected on a Siemens 3 Tesla Tim Trio scanner located in the Yale University Magnetic Resonance Research Center. High-resolution T1-weighted anatomical images were acquired using an MPRAGE sequence (TR = 1,230 ms; TE = 1.73 ms; FOV = 256 mm; image matrix 2562; 1 mm × 1 mm × 1 mm). Whole-brain functional images were acquired using a single-shot, gradient-recalled echo planar pulse sequence (TR = 2,000 ms; TE = 25 ms; flip angle = 60°; FOV = 220 mm; image matrix = 642; voxel size = 3.4 mm × 3.4 mm × 4.0 mm; 34 slices) sensitive to blood oxygenation level dependent (BOLD) contrast. Runs consisted of the acquisition of 164 successive brain volumes.
The fMRI datasets were preprocessed and analyzed using the Brain Voyager QX 2.0.08 software package (Brain Innovation, Maastricht, The Netherlands). Prior to preprocessing, the first 10 volumes were discarded to allow for scanner equilibrium. Preprocessing of the functional data included slice time correction (using sinc interpolation), 3-dimensional rigid-body motion correction (using trilinear-sinc interpolation), spatial smoothing with a FWHM 4-mm Gaussian kernel, linear trend removal, and temporal high-pass filtering (GLM with Fourier basis set, using 2 cycles/time course). Functional datasets were co-registered to within-session, T-1 weighted anatomical images, which were in turn normalized to Talairach space (Talairach and Tournoux 1988). Estimated motion plots and cine loops of the raw activation data were examined for each participant. A qualitative analysis of motion revealed that for both subjects, significant motion corresponding to movement greater than 3 mm of translation or 3 degrees of rotation from head position at the first volume of acquisition were present in t1 functional data sets. In order to remove this motion, we removed three blocks of each type of motion (biological and scrambled), which constituted the first six experimental blocks for CC and the last six experimental blocks for MM. Thus, for each participant, three blocks of each biological and scrambled motion remained for analysis. After removing the raw data from these blocks, preprocessing was repeated as described above. In order to perform a balanced comparison between time points, the same experimental blocks were removed from t2 data in each participant. The resulting motion maxima were under 3 mm or 3° for both participants at t1 and t2. General linear model (GLM)-based analyses were conducted for each participant to assess task-related BOLD responses. Regressors were defined as boxcar functions with values of 1 during each condition and 0 otherwise, convolved with a double-gamma hemodynamic response function (HRF). Predictors depicting motion in all six parameters were included as predictors of no interest.
All analyses were limited to voxels within regions of interest, including all State, Trait and Compensatory regions independently identified in our prior fMRI study of individuals with ASD, US and TD individuals with no familial history of ASD (Kaiser et al. 2010). A mask including these three kinds of regions was created by interpolating all voxels within the ROIs into 3 mm × 3 mm × 3 mm functional space. Analyses within this functional mask were conducted using single-participant GLM-based analyses. After the contrast of interest (biological > scrambled) was applied to the functional data, beta maps (comprised of beta values; a scaling factor in the linear model which correspond to the magnitude of activation) were created for each participant at each time point. T1 beta maps were subtracted from t2 beta maps in order to identify changes in processing of socially relevant stimuli after treatment. Any voxel where the difference in beta values between t1 and t2 exceeded 0.5 was included for further analyses. A cluster threshold of 4 contiguous functional voxels was additionally applied. Regions of interest (ROIs) were created based on these difference maps and labeled as State, Trait or Compensatory based on overlap with these a priori regions of interest (Kaiser et al. 2010).
Results
To assess effects of treatment for each child and keeping in line with the case series design, data were examined on an individual-participant basis. Individual changes in social communication, adaptive behavior and neural response to social stimuli are discussed below.
Social Communication Skills
The ADOS was administered to both children at t1 and t2 by clinicians independent from the project. While both children made improvements in terms of their individualized social-communication goals, only CC showed improvements on his ADOS scores from t1 to t2. CC’s total algorithm score on the ADOS met the cut-off for an ASD diagnosis at t1, but not at t2. ADOS scores from both subject’s at t1 and t2 are depicted in Table 1.
Table 1.
Behavioral data
| Pre (t1) | Post (t2) | |
|---|---|---|
| ADOS algorithm total score (autism total score cut-off = 9) | ||
| CC | 12 | 6 |
| MM | 14 | 16 |
| Pragmatics profile subtest score (typical range > 120) | ||
| CC | 86 | 132 |
| MM | 49 | 74 |
| Vineland age equivalent (months) |
CC |
MM |
||
|---|---|---|---|---|
| Pre (t1) | Post (t2) | Pre (t1) | Post (t2) | |
| Receptive | 78 | 90a | 26 | 59a |
| Expressive | 66 | 53 | 38 | 67a |
| Written | 73 | 82a | 68 | 84a |
| Personal | 90 | 78 | 38 | 42 |
| Domestic | 66 | 91a | 18 | 35a |
| Community | 74 | 71 | 47 | 56a |
| Interpersonal relationships | 54 | 31 | 46 | 46 |
| Play and leisure time | 70 | 78a | 46 | 67a |
| Coping skills | 27 | 55a | 29 | 47a |
Increase greater than 4 months
Pragmatic language was assessed at t1 and t2 for both participants using the Pragmatic Profile subtest of the CELF-4. An experienced speech-language pathologist uninvolved in the study completed both assessments. The Pragmatic Profile subtest uses a criterion-based scoring, with a cut-off point for scores falling in the impaired/typical range. Both CC and MM showed improvement after treatment on this measure. CC received a pragmatic language score in the typical range after treatment. Scores are depicted in Table 1.
Adaptive Behavior Skills
The mothers of both children were interviewed using the Vineland-II at t1 and t2. MM showed increases on her age equivalent scores in the following subdomains: Receptive, Expressive, Written, Domestic, Community, Play and Leisure Time and Coping Skills, all of which increased greater than would be expected based on chronological development (4 months), with an average of 18.4 months of gains. Her Interpersonal Relationship and Personal Daily Living scores remained stable. CC showed increases on his age equivalent scores in the following subdomains: Receptive, Written, Domestic, Play and Leisure Time and Coping Skills; all of which increased greater than expected based on chronological development, with an average increase of 16.4 months. His scores on Expressive, Personal and Interpersonal Relationship subdomains were lower after treatment. His Community score remained stable. Table 1 depicts these changes.
Behavioral Coding
All videos were coded based on the predetermined goals set for each child. MM’s coded behaviors were: “appropriate voice modulation” (i.e., typical prosody: no ‘silly’ sing-song quality), “questions”, “comments” and “requests”. CC’s coded behaviors were “on-topic comments”, “questions”, “conversations” (e.g. volleys with at least 4 turns) and “total narrative details” (e.g. number of details included in a narrative description). A trained experimenter completed the coding. MM increased her number of instances in every category except for “requests”- which remained at zero after treatment during the conversation probes. During free play MM increased instances of “appropriate voice modulation” and “comments”, but remained stable on number of “requests” and showed a decrease in number of “questions.” Results are depicted in Table 2.
Table 2.
MM Behavioral Coding
| MM | Appropriate voice modulation |
Questions | Comments | Requests |
|---|---|---|---|---|
| Conversation probes | ||||
| Pre (t1) | 6 | 6 | 1 | 0 |
| Post (t2) | 14a | 7a | 9a | 0 |
| Free play | ||||
| Pre (t1) | 23 | 6 | 23 | 2 |
| Post (t2) | 25a | 3 | 26a | 2 |
Increased from t1 to t2
CC increased instances of “on-topic comments”, and “total narrative details”, but remained constant on number of “conversations” and decreased his number of “questions”. During the free play, CC increased appropriate instances of three out of his four coded behaviors (“questions”, “conversations”, and “total narrative details”) but remained stable on number of “on topic comments”. Results can be found in Table 3.
Table 3.
CC Behavioral Coding
| CC | On-Topic comments |
Questions | Conversations | Total narrative details |
|---|---|---|---|---|
| Conversation probes | ||||
| Pre (t1) | 11 | 13 | 4 | 3 |
| Post (t2) | 20a | 7 | 4 | 16a |
| Free play | ||||
| Pre (t1) | 23 | 2 | 3 | 0 |
| Post (t2) | 23 | 6a | 4a | 1a |
Increased from t1 to t2
fMRI
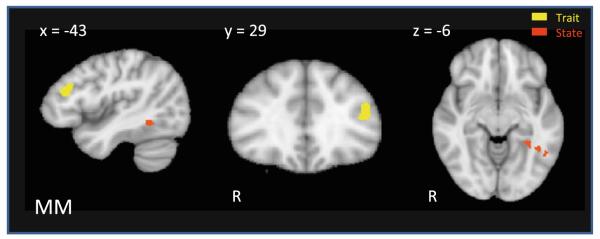
Single-participant GLM analyses were limited to our regions of interest about which we could formulate a priori hypotheses (confined to State, Trait and Compensatory regions functionally defined using the same task by Kaiser et al. (2010)) and conducted for Biological > Scrambled motion for both participants at t1 and t2. Doing so allowed us to create individual beta maps for MM and CC at each time point and compare activation within these regions after treatment. As illustrated in Fig. 1, MM demonstrated greater activation in three regions after treatment; two distinct regions of the State-defined left fusiform gyrus (FG) and a portion of the Trait-defined left dorsolateral prefrontal cortex (dlPFC).
Fig. 1.
MM fMRI results. Results of t2–t1 beta maps (biological > scrambled) for MM (beta difference >0.5, k = 4) limited to State, Trait and Compensatory regions as defined by Kaiser et al. (2010). After treatment, MM demonstrated increased activation to biological motion in Trait-defined left dorsolateral prefrontal cortex (dlPFC) and two distinct regions of the State-defined left fusiform gyrus (FG)
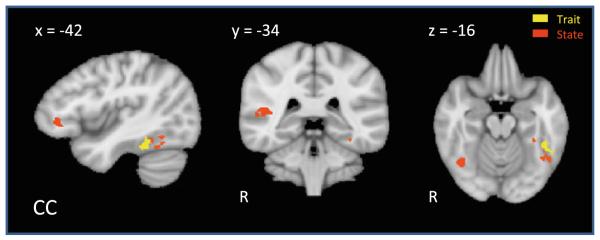
CC demonstrated greater activation in four regions after treatment; a portion of the State-defined right posterior superior temporal sulcus (pSTS), State-defined left ventrolateral prefrontal cortex (vlPFC), State-defined right FG and State/Trait-defined left FG, as depicted in Fig. 2.
Fig. 2.
CC fMRI results. Results of t2–t1 beta maps (Biological > Scrambled) for MM (beta difference >0.5, k = 4) limited to State, Trait and Compensatory regions as defined by Kaiser et al. (2010). After treatment, CC demonstrated greater activation in a portion of the State-defined right posterior superior temporal sulcus (pSTS), State-defined left ventrolateral prefrontal cortex (vlPFC), State-defined right fusiform gyrus (FG) and State/Trait-defined left FG
Eye Tracking
Table 4 depicts the raw TFD data for all three AOIs for MM and CC, respectively.
Table 4.
Eye tracking data
| Pre (t1) | Post (t2) | |
|---|---|---|
| Total fixation duration (TFD) | ||
| CC | ||
| Eyes | 14.38 | 16.58 |
| Mouth | 21.03 | 16.69 |
| Total image | 56.66 | 56.71 |
| Eyes:mouth ratio | 0.68 | 0.99 |
| MM | ||
| Eyes | 15.92 | 4.97 |
| Mouth | 13.78 | 5.73 |
| Total image | 39.69 | 26.16 |
| Eyes:mouth ratio | 1.15 | 0.87 |
| Percent of looking time (POLT) | ||
| CC | ||
| Eyes | 25.39 | 29.30 |
| Mouth | 37.08 | 29.37 |
| Eyes:mouth ratio | 0.68 | 1.00 |
| MM | ||
| Eyes | 39.80 | 24.90 |
| Mouth | 34.86 | 25.71 |
| Eyes:mouth ratio | 1.14 | 0.97 |
MM looked less overall at Total Image at t2 (13.52 s) compared to t1, and thus looked less at both the eye and mouth AOIs. Her ratio of eye to mouth looking went from 1.15 to 0.87. In contrast, CC looked equivalently at the Total Image at both time points (t1 = 56.66, t2 = 56.71) and showed an increased ratio of looking at eyes after treatment (t1 = 0.68, t2 = 0.99). Table II also depicts the POLT data for Eyes and Mouth for MM and CC, respectively, at t1 and t2. While MM appeared to spend less time looking at both AOIs at t2, her eye gaze pattern remained stable (POLT Eyes : POLT Mouth at t1 = 1.14, t2 = 0.97). While CC looked for a greater percentage of time at the Mouth than the Eyes AOI at t1, he appeared to move towards normalization at t2 (POLT Eyes: POLT Mouth at t1 = 0.68, t2 = 1.00).
Discussion
The perception of human motion is central to effective social interaction (Blake and Shiffrar 2007). Evidence for this comes from that fact that a preference for such visual input is early emerging, evident in children as young as 2 days (Simion et al. 2008). This preference is also present in other species, including monkeys (Oram and Perrett 1996) and birds (Omori and Watanabe 1996), indicating that biological motion perception is an evolutionarily well-conserved mechanism. We are able to detect biological motion even when it is at its most basic (Neri et al. 1998), as in point-light displays, demonstrating the robust nature of this ability. Taken together, these aspects of biological motion perception “suggest ready benefits for adaptive interaction with other living beings: following the movements of a conspecific; looking at others to entreat or avoid interaction; learning by imitation; or directing preferential attention to cues that build on biological motion [such as facial expression and gaze direction]” (Klin et al. 2009). Disrupted biological motion perception has been documented in children with ASD (Kaiser and Shiffrar 2009; Kaiser et al. 2010) as well as toddlers with ASD (Klin et al. 2009). We utilized the aforementioned paradigm to assess whether PRT would affect the way in which children with ASD process biological motion. We employed a well-established biological motion fMRI paradigm (Kaiser et al. 2010) to assess the neural mechanisms supporting social perception. We were thus able to constrain our analyses to a priori regions of interest (State, Trait and Compensatory regions). Both children showed increased activation in portions of State and Trait, but not Compensatory regions after treatment. Thus, PRT resulted in increased activation in regions recruited by typically developing children during social perception. These findings demonstrate that the neural systems supporting social perception are malleable with treatments for ASD that target pivotal behaviors and contingently reward children with naturalistic reinforcers.
State regions are defined as having reduced differential activation to biological versus scrambled motion in children with ASD relative to US and TD children. In the original study (Kaiser et al. 2010), activity in the State-defined right pSTS was found to negatively correlate with severity of social deficits in individuals with ASD. Activity in the State-defined vlPFC was found to negatively correlate with level of social responsiveness in TD children. CC demonstrated increased activation in both of these regions after treatment. These changes tracked with CC’s decreased severity of social deficits, as indicated by his improved ADOS and pragmatic language scores. These results combined with those of our prior study provide evidence for a tight coupling of social behavior and neural mechanisms for social perception. Interestingly, MM did not show increased activation in these regions. This maps on to the fact that she was working on lower level social skills, such as social orienting, rather than higher-level skills, such as improving perspective taking (one of CC’s goals). Both the pSTS and vlPFC have been implicated in higher level social processing (i.e. right pSTS in not only biological motion, but also how another person’s motion is related to his or her intentions (Vander wyk et al. 2009) and the vlPFC in top-down processing of social stimuli (Pinkham et al. 2008). Since MM was not at this level, she did not show activation in regions involved in this type of advanced social processing.
Both children demonstrated increased activation in the State-defined FG after treatment. This may reflect an increased ability to discriminate biological versus nonbiological motion, as Grossman and Blake (2002) reported that the lateral fusiform gyrus (FG) contains neural signals capable of differentiating the two types of motion. Additionally, the FG is engaged in social cognition with non-face stimuli, as measured by the Social Attribution Task (Schultz et al. 2003). This and other research demonstrating the role of the FG in non-face social cognition (Castelli et al. 2000), suggest that increased activation in this area to biological motion may represent a post-treatment halo effect, in which MM and CC are now able to generalize social salience to more abstract social stimuli.
Multiple studies have also demonstrated FG hypoactivation during face processing in children with ASD (e.g., Pelphrey et al. 2007; Pierce et al. 2001; Schultz et al. 2000). Our preliminary results demonstrate that the FG is not irretrievably “broken” in children with ASD; instead, it is simply not being effectively recruited in the processing of biological motion. This is consistent with our prior findings demonstrating that experimental reorientation of ASD participants’ eye fixations to the eye region of faces increases activation in the FG (Perlman et al. 2011). These results are extremely informative for the development of treatment approaches, as they emphasize the importance of creating salience around social entities or situations. In thinking about ASD as a neurodevelopmental disorder, the earlier we are able to create this salience for a child with ASD, the earlier his or her brain is able to differentiate between social and nonsocial, increasing proficiency in processing such stimuli and potentially providing cascading effects in other areas of development.
Trait regions are defined as showing reduced differential activation to biological versus scrambled motion in children with ASD and US relative to TD. In our original study (Kaiser et al. 2010), activity in Trait-defined dlPFC correlated with social responsiveness in TD children. Interestingly, MM showed increased activation in this region after treatment. This may reflect improvements in her social responsivity, as this was a major goal of her treatment plan. Since Trait regions are defined as showing reduced differential activation in both children with ASD and US, hypoactivation in these regions may reflect more subtle deficits in social perception.
Eye tracking was employed to investigate the influence of PRT on the way in which children with ASD observe faces. Results were highly variable. One child seemed to move towards a more typical visual scanning pattern, as he demonstrated a preference for the mouth region pretreatment and no such preference after treatment. The other child seemed to stay fairly stable in her gaze pattern. Note that qualitatively, the child who showed no change was very distracted during her post-treatment eye tracking session. This highlights the difficulty of collecting data with young children in general, and young children with ASD more specifically. These results highlight the need for short, sensitive eye tracking tasks in order to combat variability in this modality as a measure of treatment response. They also emphasize the importance of utilizing measures of brain function, which are more robust and stable than many behavioral measures, including eye tracking, as a means of elucidating underlying mechanisms targeted by treatment.
As predicted, both children made clinically significant gains in skills related to core vulnerabilities in ASD. Both children made the most notable gains in adaptive skills. These results are consistent with the nature of PRT, namely that the treatment is inherently naturalistic and generalization is central to the approach. Notably, however, both children’s scores in adaptive interpersonal relationships, as well as in personal daily living skills either remained stable or reportedly declined. These changes were likely not due to true regression of skill but instead, the parent training component of PRT may have made parents more aware of their child’s delays. It is also important to note that interpersonal skills, as measured by the Vineland-II, are not likely to show changes over such a short period of time. This domain is related to friendships (i.e. “Has a best friend or shows preference for certain friends”) and reciprocal relationships (i.e. “Recognizes the likes and dislikes of others”), which take time to develop, even after social skills are gained and/or strengthened through treatment.
Both children also displayed gains in social-communication skills based on the scores on the pragmatic language subtest of the CELF-4 as well as on their own individualized goals as measured using behavioral coding of standardized scenarios and probes. Strikingly, one child’s (CC) score even reflected a “typical” level of skill on the CELF-4 pragmatic language subtest at end of treatment. Social-communication skills, as assessed using the ADOS, however, were more variable. One child (CC) made impressive gains, and his score at the end of treatment was below the cut-off for ASD classification, while the other child’s score remained stable. Differences were likely due to the nature of the goals, as the child who showed more improvement on ADOS scores (CC) was less impaired pre-treatment and worked on high-order goals, such as narrative language and perspective taking. In contrast, MM entered the study with a more pronounced social disability and her goals were more fundamental (e.g. appropriate body positioning) and perhaps ones not as sensitive to change based on ADOS scores. It is important to note as well that the ADOS is a diagnostic instrument, and was not designed as an outcome measure to assess changes in skill level. However, given that it does assess social-communication skills, which corresponded to the goals of treatment, we thought it to be a valuable addition to the exploratory analyses.
There are, of course, inherent limitations for case studies. We are currently conducting a full-scale study of 60 children with a waitlist control group so as to demonstrate that any behavioral or neural changes are due to effects of treatment rather than 4 months of development. In our ongoing work, we are also incorporating event-related potentials (ERPs), as a complementary neuroimaging method to provide increased temporal resolution; in this way, we will be able to monitor changes in strength and efficiency of brain regions involved in social perception.
As each child with ASD presents with distinct patterns of social and communicative skills, the strength of using each child as his/her own control cannot be overlooked. As such, while both children in the current study were diagnosed with ASD and received the same type of treatment, results were not homogeneous. ASD is a heterogeneous disorder, and research aimed at understanding treatment must address this heterogeneity. In the current study, our two children started treatment at different baselines with different profiles of skills, and although both made progress, their degree of progress and level of skills at the conclusion of treatment were distinct. Of note, both children did show some overlapping improvements in adaptive skills as well as in their neural response to biological motion in the State-defined left FG. We see this work as a first step in a novel approach to treatment planning, integrating behavioral and brain-based measures as predictors of outcome in order to transition from generic treatment applications to individually tailored interventions customized to behavioral and neural characteristics of the child.
Acknowledgements
Funding for this study came from the Harris Professorship at the Yale Child Study Center given to Kevin Archer Pelphrey, Allied World and NIMH grant K23MH086785.
References
- Bertenthal B, Pinto J. Global processing of biological motions. Psychological Science. 1994;5:221–224. [Google Scholar]
- Blake R, Shiffrar M. Perception of human motion. Annual Review of Psychology. 2007;58:47–73. doi: 10.1146/annurev.psych.57.102904.190152. [DOI] [PubMed] [Google Scholar]
- Castelli F, Happe F, Frith U, Frith C. Movement and mind: A functional imaging study of perception and interpretation of complex intentional movement patterns. NeuroImage. 2000;12:314–325. doi: 10.1006/nimg.2000.0612. [DOI] [PubMed] [Google Scholar]
- DeRubeis RJ, Siegle GJ, Hollon SD. Cognitive therapy versus medication for depression: Treatment outcomes and neural mechanisms. Nature Reviews Neuroscience. 2008;9:788–796. doi: 10.1038/nrn2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott CD. Differential ability scales. Second Edition (DAS-II) Psychological Corporation; San Antonio, TX: 2006. [Google Scholar]
- Elsabbagh M, Mercure E, Hudry K, Chandler S, Pasco G, Charman T, et al. Infant neural sensitivity to dynamic eye gaze is associated with later emerging autism. Current Biology. 2012;22(4):338–342. doi: 10.1016/j.cub.2011.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman ED, Blake R. Brain areas active during perception of biological motion. Neuron. 2002;35(6):1167–1175. doi: 10.1016/s0896-6273(02)00897-8. [DOI] [PubMed] [Google Scholar]
- Kaiser MD, Hudac CM, Shultz S, Lee SM, Cheung C, Berken AM, et al. Neural signatures of autism. Proceedings of the National Academy of Sciences. 2010;107(49):21223. doi: 10.1073/pnas.1010412107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser MD, Shiffrar M. The visual perception of motion by observers with autism spectrum disorder: A review and synthesis. Psychonomic Bulletin & Review. 2009;16(5):761–777. doi: 10.3758/PBR.16.5.761. [DOI] [PubMed] [Google Scholar]
- Klin A, Lin DJ, Gorrindo P, Ramsay G, Jones W. Two-year-olds with autism orient to non-social contingencies rather than biological motion. Nature. 2009;459:257–261. doi: 10.1038/nature07868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koegel RL, Camarata S, Koegel LK, Ben-Tall A, Smith AE. Increasing speech intelligibility in children with autism. Journal of Autism and Developmental Disorders. 1998;28(3):241–251. doi: 10.1023/a:1026073522897. [DOI] [PubMed] [Google Scholar]
- Koegel LK, Carter CM, Koegel RL. Teaching children with autism self-initiations as a pivotal response. Topics in Language Disorders. 2003;23(2):134–145. [Google Scholar]
- Koegel RL, Frea WD. Treatment of social behavior in autism through the modification of pivotal social skills. Journal of Applied Behavior Analysis. 1993;26(3):369–377. doi: 10.1901/jaba.1993.26-369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koegel RL, Koegel L. Pivotal response treatments for autism: Communication, social, and academic development. Brookes; Baltimore, MD: 2006. [Google Scholar]
- Koegel RL, Koegel L. The PRT pocket guide. Brookes; Baltimore, MD: 2012. [Google Scholar]
- Koegel RL, O’Dell MC, Koegel LK. A natural language paradigm for nonverbal autistic children. Journal of Autism and Developmental Disorders. 1987;17(2):187–200. doi: 10.1007/BF01495055. [DOI] [PubMed] [Google Scholar]
- Koegel RL, Schreibman L, Good A, Cemiglia L, Murphy C, Koegel LK. How to teach pivotal behaviors to children with autism: A training manual. University of California, Santa Barbara; Santa Barbara, CA: 1989. [Google Scholar]
- Laski KE, Charlop MH, Schreibman L. Training parents to use the natural language paradigm to increase their autistic children’s speech. Journal of Applied Behavior Analysis. 1988;21(4):391–400. doi: 10.1901/jaba.1988.21-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore PC, Risi S. Autism diagnostic observation schedule—WPS (ADOS-WPS) Western Psychological Services; Los Angeles, CA: 2002. [Google Scholar]
- Neri P, Morrone MC, Burr DC. Seeing biological motion. Nature. 1998;395(6705):894–895. doi: 10.1038/27661. [DOI] [PubMed] [Google Scholar]
- Oram MW, Perrett DI. Integration of form and motion in the anterior superior temporal polysensory area (STPa) of the macaque monkey. Journal of neurophysiology. 1996;76(1):109–129. doi: 10.1152/jn.1996.76.1.109. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Morris JP, McCarthy G, LaBar KS. Perception of dynamic changes in facial affect and identity in autism. Social Cognitive and Affective Neuroscience. 2007;2(2):140–149. doi: 10.1093/scan/nsm010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman SB, Hudac CM, Pegors T, Minshew NJ, Pelphrey KA. Experimental manipulation of face-evoked activity in the fusiform gyrus of individuals with autism. Social Neuroscience. 2011;6(1):22–30. doi: 10.1080/17470911003683185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce K, Muller R-A, Ambrose J, Allen G, Courchesne E. Face processing occurs outside the fusiform ‘face area’ in autism: evidence from functional MRI. Brain. 2001;124(10):2059–2073. doi: 10.1093/brain/124.10.2059. [DOI] [PubMed] [Google Scholar]
- Pinkham AE, Hopfinger JB, Pelphrey KA, Piven J, Penn DL. Neural bases for impaired social cognition in schizophrenia and autism spectrum disorders. Schizophrenia Research. 2008;99(1–3):164–175. doi: 10.1016/j.schres.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M, Le Couter A, Lord C. ADI-R: Autism diagnostic interview-revised. Western Psychological Services; Los Angeles, CA: 2003. [Google Scholar]
- Schultz RT, Gauthier I, Klin A, Fulbright RK, Anderson AW, Volkmar F, et al. Abnormal ventral temporal cortical activity during face discrimination among individuals with autism and Asperger syndrome. Archives of General Psychiatry. 2000;57(4):331–340. doi: 10.1001/archpsyc.57.4.331. [DOI] [PubMed] [Google Scholar]
- Schultz RT, Grelotti DJ, Klin A, Kleinman J, Van der Gaag C, Marois R, et al. The role of the fusiform face area in social cognition: Implications for the pathobiology of autism. Philosophical Transactions of the Royal Society B- Biological Science. 2003;358:415–427. doi: 10.1098/rstb.2002.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semel E, Wiig E, Secord WA. Clinical evaluation of language fundamentals 4 (CELF-4) The Psychological Corporation; San Antonio, TX: 2003. [Google Scholar]
- Siegle GJ, Carter SC, Thase ME. Use of fMRI to predict recovery from unipolar depression with cognitive behavior therapy. The American Journal of Psychiatry. 2006;163(4):735–738. doi: 10.1176/ajp.2006.163.4.735. [DOI] [PubMed] [Google Scholar]
- Simion F, Regolin L, Bulf H. A predisposition for biological motion in the newborn baby. Proceedings of the National Academy of Sciences. 2008;105(2):809–813. doi: 10.1073/pnas.0707021105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparrow SS, Cicchetti DV, Balla DA. Vineland adaptive behavior scales: Second edition (Vineland II), Survey interview form/caregiver rating form. Pearson Assessments; Livonia, MN: 2005. [Google Scholar]
- Stahmer AC. Teaching symbolic play skills to children with autism using pivotal response training. Journal of Autism and Developmental Disorders. 1995;25(2):123–141. doi: 10.1007/BF02178500. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain: 3-dimensional proportional system: An approach to cerebral imaging. Thieme Medical; New York: 1988. [Google Scholar]
- Vander Wyk BC, Hudac CM, Carter EJ, Sobel DM, Pelphrey KA. Action understanding in the superior temporal sulcus region. Psychological Science. 2009;20(6):771–777. doi: 10.1111/j.1467-9280.2009.02359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiig EH, Secord WA, Semel E. CELF Preschool 2: Clinical evaluation of language fundamentals, Preschool. 2nd ed Harcourt Assessment; San Antonio, TX, USA: 2004. [Google Scholar]