Abstract
Background
Cancer is currently among the three leading causes of death after solid organ transplantation and its incidence is increasing. Non-melanoma skin cancer - squamous cell carcinoma and basal cell carcinoma - is the most common malignancy found in kidney transplant recipients (KTRs). The incidence of non-melanoma skin cancer in KTRs has not been extensively studied in Portugal.
Objectives
To determine the incidence of non-melanoma skin cancer in KTRs from the largest Portuguese kidney transplant unit; and to study risk factors for non-melanoma skin cancer.
Methods
Retrospective analysis of clinical records of KTRs referred for the first time for a dermatology consultation between 2004 and 2013. A case-control study was performed on KTRs with and without non-melanoma skin cancer.
Results
We included 288 KTRs with a median age at transplantation of 47 years, a male gender predominance (66%) and a median transplant duration of 3.67 years. One fourth (n=71) of KTRs developed 131 non-melanoma skin cancers, including 69 (53%) squamous cell carcinomas and 62 (47%) basal cell carcinomas (ratio squamous cell carcinoma: basal cell carcinoma 1.11), with a mean of 1.85 neoplasms per patient. Forty percent of invasive squamous cell carcinomas involved at least two clinical or histological high-risk features. The following factors were associated with a higher risk of non-melanoma skin cancer: an older age at transplantation and at the first consultation, a longer transplant duration and the presence of actinic keratosis. KTRs treated with azathioprine were 2.85 times more likely to develop non-melanoma skin cancer (p=0.01).
Conclusion
Non-melanoma skin cancer was a common reason for dermatology consultation in Portuguese KTRs. It is imperative for KTRs to have access to specialized dermatology consultation for early referral and treatment of skin malignancies.
Keywords: Carcinoma, Basal Cell; Carcinoma, Squamous Cell; Kidney Transplantation; Survival Analysis; Incidence; Risk Factors
INTRODUCTION
Cancer is currently one of the three major causes of death among solid organ transplant recipients and its incidence has grown in recent decades.1,2
The two most common types of non-melanoma skin cancer (NMSC) - squamous cell carcinoma (SCC) and basal cell carcinoma (BCC) - account for 95% of skin cancers and 40% of all neoplasms in solid organ transplant recipients. These skin tumors cause greater morbidity and mortality than in the general population.3
The risk of SCC and BCC in KTRs is, respectively, 65-250 times and 10 times higher than in the general population.3,4
The oncogenesis of NMSC is multifactorial but immune surveillance reduction caused by immunosuppressive drugs, along with the direct carcinogenic effect of these agents, is enhanced by cumulative ultraviolet radiation exposure.5,6
The following factors also have an impact on the risk of NMSC: Fitzpatrick skin types I-III, an older age at transplantation, being Caucasian, geographic locations situated at lower latitudes, as well as infections by oncogenic viruses.3,7
Kidney transplantation occupies an intermediate position in the associated risk of NMSC, being lower than heart or lung transplantation, but higher than liver transplantation.3,5,8
In 2013, Portugal was ranked 8th in the world in terms of the numbers of kidney transplantations from deceased donors, with 38.3 transplants per 1 million inhabitants.9
The incidence of NMSC in KTRs has been poorly evaluated in Portugal. The most recently published studies evaluated small samples of patients from kidney transplant units in the country's southern region and reported a limited number of NMSCs.10,11
OBJECTIVES
Our objectives were to determine the incidence and characterize the NMSCs in a series of patients from the largest Portuguese kidney transplant unit (in Coimbra); and to study the risk factors for NMSC in this population.
METHODS
We performed an observational retrospective study using (non-probability) purposive sampling - we included all KTRs followed up at post-transplant consultation in the Centro Hospitalar e Universitário de Coimbra, who were referred for the first time for a dermatological evaluation between January 2004 and December 2013. Medical records were analyzed from the time of transplantation until the last dermatology consultation or death.
Patients who underwent double transplants (e.g. heart, liver, lung) and patients with personal history of skin cancer were excluded.
The primary endpoint was the occurrence of NMSC (SCC or BCC) during the follow-up. We considered two groups for comparison: KTRs who were diagnosed with NMSC (case group) and KTRs without NMSC (control group). Skin cancers were characterized according to their number, histological subtype, anatomic location and time for occurrence (after transplantation). In SCCs, clinical and histological high-risk features were also assessed. Chart 1 presents the comparison of the risk variables for the two groups.
Chart 1.
Risk variables studied in KTRs
| Risk variables |
|---|
| Age at transplantation |
| Age at first consultation |
| Gender (male/female) |
| Pre-transplant induction therapy (Yes/No) |
| Donor type (Deceased/Living) |
| Presence of actinic keratosis (Yes/No) |
| Immunosuppressive drugs (prednisolone, mycophe-nolate, cyclosporine, tacrolimus, azathioprine, sirolimus and everolimus) |
| Initial immunosuppressive regimen |
In order to assess the immunosuppressive regimens associated with a higher risk of NMSC, we divided the KTRs into four groups in accordance with the initial prescribed regimens: regimen A - mycophenolate, tacrolimus and prednisolone; regimen B - mycophenolate, cyclosporine and prednisolone; regimen C - azathioprine, cyclosporine and prednisolone; regimen D - mycophenolate, sirolimus and prednisolone. The comparison was adjusted for age at transplantation, age at first consultation and gender.
We used the IBM SPSS® software, version 21.0 (Armonk, NY: IBM Corp.) to perform: the Mann-Whitney U and X2 tests, which compare, respectively, quantitative and nominal variables between groups; the odds ratio (OR) to measure intensity of association of nominal variables; logistic regression for adjusted comparison between groups; and the Kaplan-Meier method to estimate the cumulative incidence (CI) of NMSC over the time post-transplant. The significance level was set at p< 0.05.
RESULTS
i) Population studied
This study included 288 patients, predominantly males (n=189, 66%). The median age was 47 years at the time of transplantation and 54 years at the time of the first consultation. The patients' clinical characteristics are listed in table 1. Most (52.4%) underwent transplantations between 2000 and 2009. The median time period between the transplant and the first dermatology consultation was 3.67 years. Ninety-four percent (n=271) of patients received kidneys from deceased donors. Only 4% (n=12) of KTRs had undergone previous kidney transplantation.
Table 1.
Characterization of KTRs with and without NMSC according to the variables studied
| Variables | Total no. of cases (288) | With NMSC (n=71) | Without NMSC (n=217) |
|---|---|---|---|
| Age at transplantation (median) | 47 (8-73) | 54.5 (24-73) | 41.7 (8-70) |
| Age at first consultation (median) | 54 (12.5-78.5) | 61.9 (33.30-75-40) | 47.5 (12.49-78.52) |
| Duration of transplant (median) | 3.67 (0.15-25.27) | 5.35 (0.1-24.35) | 2.97 (0.1-25.57) |
| Gender | |||
| Male | 189 (66%) | 50 (71%) | 139 (64.1%) |
| Female | 99 (33%) | 21 (29%) | 78 (35.9%) |
| Donor type | |||
| Deceased | 271 (94%) | 66 (92.6%) | 205 (94.5%) |
| Living | 17 (6%) | 5 (7.04%) | 12 (5.5%) |
| Pre-transplant induction therapy | |||
| Yes | 138 (47.9%) | 29 (40.8%) | 99 (45.6%) |
| No | 150 (52.1%) | 42 (59.2%) | 118 (54.4%) |
| Actinic keratosis | |||
| Yes | 54 (18.8%) | 31 (43.7%) | 23 (10.6%) |
| No | 234 (81.2%) | 40 (56.3%) | 194 (89.4%) |
| Immunosuppressive drugs | |||
| Prednisolone | 288 (100%) | 71 (100%) | 217 (100%) |
| Mycophenolate | 205 (71.2%) | 48 (67.6%) | 157 (72.4%) |
| Cyclosporine | 149 (51.2%) | 38 (53.5%) | 111 (51.2%) |
| Tacrolimus | 112 (38.9%) | 28 (39.4%) | 84 (38.7%) |
| Azathioprine | 68 (23.6%) | 17 (23.9%) | 51 (23.5) |
| Sirolimus | 34 (11.8%) | 7 (9.9%) | 27 (12.4%) |
| Everolimus | 4 (1.3%) | 2 (2.8%) | 2 (0.9%) |
| Immunosuppressive regimen | |||
| A | 106 (36.8%) | 27 (38.0%) | 79 (36.4%) |
| B | 76 (26.4%) | 18 (25.4%) | 58 (26.7%) |
| C | 63 (21.9%) | 15 (21.1%) | 48 (22.1%) |
| D | 23 (8%) | 3 (4.2%) | 20(9.2%) |
| Other regimens | 20 (6.9%) | 8 (11.3%) | 12 (5.5%) |
ii) Diagnosed neoplasms
In this population of KTRs, the overall incidence of NMSC was 24.7% (n=71).
Table 2 provides a characterization of NMSC cases according to: the type of neoplasm, the proportion of SCCs and BCC, as well as the distribution of cases with multiple neoplasms.
Table 2.
Characterization of NMSC cases according to the type and number of NMSCs
| Total no. of NMSC cases | n=71 | 100% |
| Only SCC | 30 | 42.25% |
| Only BCC | 27 | 38.3% |
| Combined SCC and BCC | 12 | 16.90% |
| Combined NMSC and melanoma | 2 | 2.82% |
| Cases with >1 NMSC | n=29 | 41% |
| 2 NMSC | 16 | 22.54% |
| 3 NMSC | 3 | 4.23% |
| 4 NMSC | 5 | 7,04% |
| 5 NMSC | 3 | 4.23% |
| 6 NMSC | 1 | 1.41% |
| 7 NMSC | 0 | 0% |
| 8 NMSC | 1 | 1.41% |
| Total no. of neoplasms | n=131 | 100% |
| BCC | 62 | 46% |
| SCC | 69 | 53% |
| Invasive | 43 | 62% |
| In situ | 26 | 38% |
The topographic distribution of the NMSCs is displayed in table 3. The mean number of tumours identified per patient was 1.85. We observed a slight predominance of SCCs over BCCs, with a ratio of 1.11:1. Only 2 out of 42 SCC cases (5%) developed nodal metastasis. No deaths were attributable to NMSC. Most NMSCs appeared in sun-exposed areas, mainly on the face.
Table 3.
Anatomical distribution of SCCs and BCCs
| Localization | SCC=69 | BCC=62 |
| Face * | 39 | 46 |
| Front | 3 | 5 |
| Eyelids | 4 | 5 |
| Lips | 3 | 3 |
| Nose | 4 | 20 |
| Malar | 12 | 4 |
| Mandibular | 1 | 1 |
| Nasolabial | 1 | 3 |
| Ear | 15 | 5 |
| Scalp | 2 | 3 |
| Neck * | 3 | 5 |
| Anterior chest wall | 4 | 2 |
| Abdomen | 1 | |
| Pelvis | 1 | 0 |
| Dorsum | 1 | 1 |
| Upper limb Shoulder | 16 1 | 4 |
| Arm | 1 | 1 |
| Forearm* | 4 | 3 |
| Hand * | 11 | 0 |
| Lower limb | ||
| Leg | 1 | 1 |
| * Sun-exposed areas | 57 (83%) | 54 (87%) |
In most cases, KTRs who started with a histologic type of NMSC developed only subsequent neoplasms of the same type. When the first tumour was a SCC, 61.5% (n=8) of patients ultimately developed new SCCs. In cases whose first NMSC was BCC, 66% (n=9) developed only successive BCCs.
The median time period from the date of transplant until the diagnosis of the first NMSC was 5.35 years (0.4-25). The median time period from the first tumour until the subsequent neoplasm was 15.5 months. Approximately 80% of cases with more than one NMSC developed a second tumour 2 years after the first.
iii) Cumulative incidence of NMSC
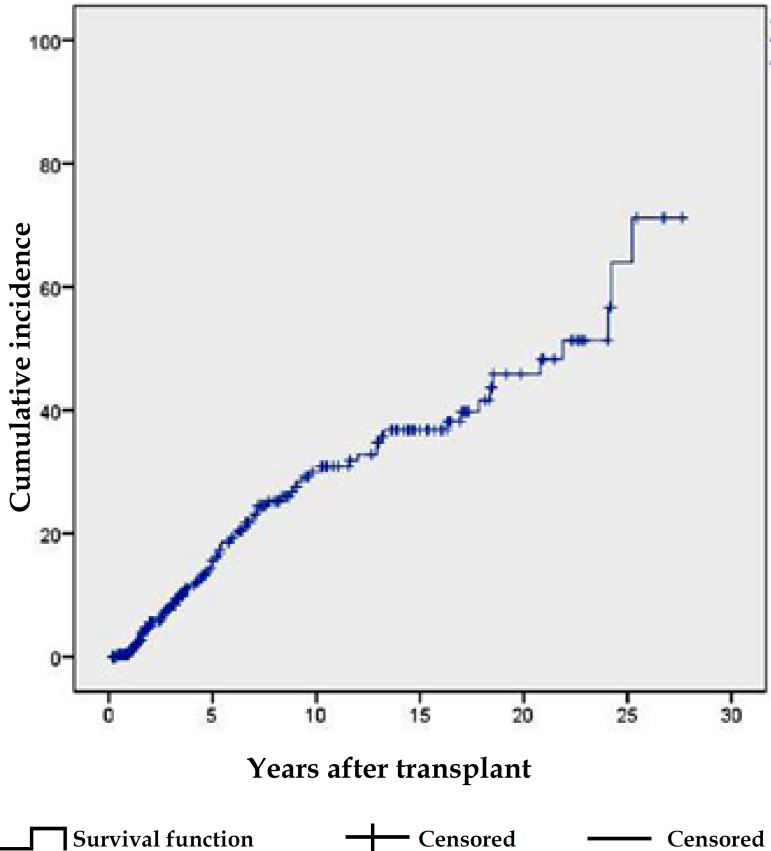
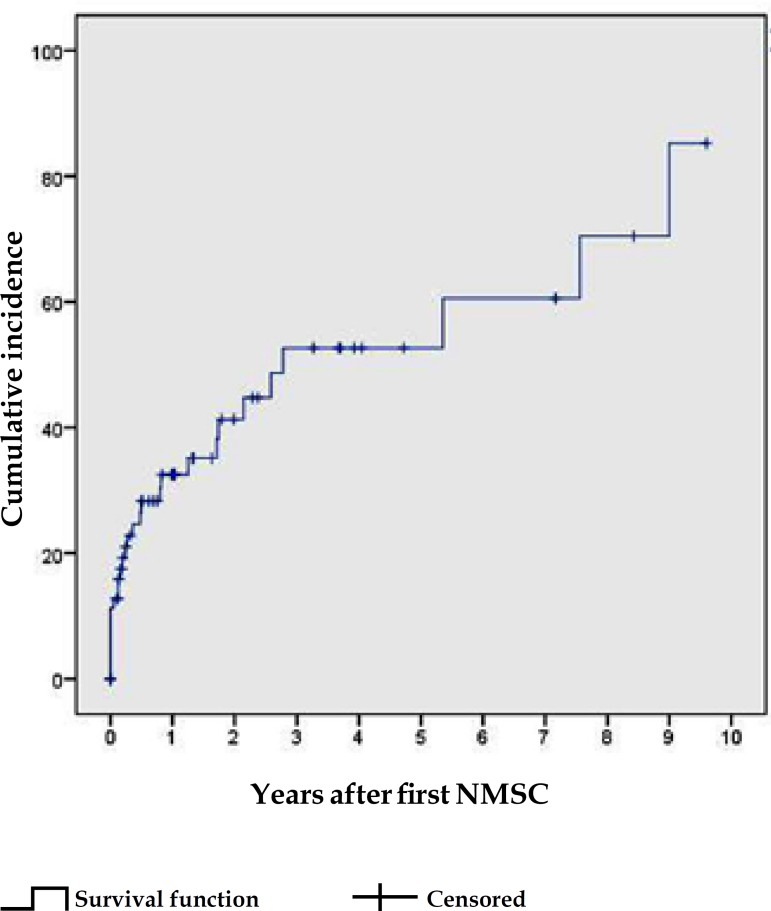
The estimated probability of the first NMSC, based on the Kaplan-Meier method, increased from 16% at 5 years post-transplant, to 31%, 39% and 49%, respectively, at 10, 15 and 20 years (Graph 1). The cumulative incidence of the second NMSC increased from 33% in the first year, to 42% in the second and 53% in the third year, following the first NMSC (Graph 2).
Graph 1.
Cumulative incidence of first NMSC in KTRs, derived from the Kaplan-Meier method
Graph 2.
Cumulative incidence of subsequent NMSC in KTRs, derived from the Kaplan-Meier method
iv) Clinical and histological high-risk features of SCC
The frequency of clinical and histological features of SCC associated with increased risk of recurrence, metastasis, and death (high-risk features), is outlined in table 4.
Table 4.
Prevalence of clinical and histological high-risk features in invasive SCCs
| High-risk features | Invasive SCC n=43 | Frequency |
|---|---|---|
| Location: lower lip, ear, scar, anogenital | 13 | 30.23% |
| Depth >4mm | 13 | 30.23% |
| Desmoplasia/infitration | 10 | 23.26% |
| Ulceration | 8 | 18.60% |
| Poor differentiation | 7 | 16.28% |
| Clark level ≥ 4 | 5 | 11.63% |
| Diameter >2cm | 3 | 6.98% |
| Perineural infiltration | 1 | 2.33% |
| > 1 high-risk feature | 18 | 41.86% |
| > 1 high-risk feature | 17 | 40% |
| (other than diameter>2cm) |
v) Risk factors for NMSC
NMSC was significantly associated with an older age at transplantation (p<0.001) and at the first consultation (p<0.001), as well as a longer transplant duration (p=0.02). There was no difference in NMSC risk in terms of: gender (X2 0.96, p=0.33), history of pre-transplant induction therapy (X2 0.323, p=0.57), donor type (X2 0.22, p=0.64) or history of previous kidney transplantation (X2 1.795, p=0.18). In patients presenting actinic keratosis, the risk of NMSC was 6.54 times higher (OR 3.46-12.37).
With the exception of prednisolone - used in all patients - mycophenolate was the most prescribed drug and azathioprine was the least common antimetabolite. In the multivariate analysis, we verified that patients treated with mycophenolate presented a significantly lower risk of NMSC compared with those who were not treated with it (OR 0.288, p<0.001) (Table 5). Inversely, patients treated with azathioprine had a higher risk of NMSC (OR 2.85, p=0.01). The vast majority of KTRs (93.1%) were initially prescribed with one of the four previously mentioned immunosuppressive regimens. Importantly, regimen C - including azathioprine and cyclosporine - was associated with a 3.85 times higher risk of NMSC than regimen A, containing the more recent antimetabolite and calcineurin inhibitor - mycophenolate and tacrolimus. There were no statistically significant differences between other regimens (Table 6).
Table 5.
Adjusted risk (odds ratio) of NMSC for each type of immunosuppressive drug
| Immunosuppressive drug | Adjusted odds ratio of NMSC (Logistic regression) | p |
|---|---|---|
| Mycophenolate* | 0.288 | 0.001* |
| Cyclosporine | 1.49 | 0.18 |
| Tacrolimus | 0.59 | 0.1 |
| Azathioprine* | 2.85 | 0.01* |
| Sirolimus | 1.25 | 0.64 |
| Everolimus | 3.20 | 0.27 |
Table 6.
Adjusted risk (odds ratio) of NMSC according to the initial immunosuppressive regimen
| Immunosuppressive regimen | Adjusted odds ratio of NMSC Logistic regression | p |
|---|---|---|
| Regimen A | Reference | - |
| Regimen B | 0.98 | p=0.98 |
| Regimen C | 3.84 | p=0.01** |
| Regimen D | 1.69 | p=0.51 |
Regimen A: mycophenolate-tacrolimus-prednisolone; Regimen B: mycophenolate cyclosporine-prednisolone; Regimen C: azathioprine-cyclosporine-prednisolone: Regimen D: mycophenolate-sirolimus-prednisolone.
DISCUSSION
Kidney transplantation started routinely in Portugal in 1980, reaching the highest number of annual procedures in 2009, with 56 transplants per million inhabitants. Along with the increase in the KTR population, the number of diagnosed NMSCs has been growing.12
i) Time evolution of NMSC incidence
In this study, NMSC was diagnosed in about one fourth of KTRs over a decade. At the beginning of the 1990s, the incidence of malignant and premalignant skin lesions in a prospective study conducted in this same kidney transplant unit was considerably lower (13.4%).13
ii) NMSC incidence in other series
The overall incidence found was similar to that reported by García et al. in a recent Spanish series of 289 patients (25.2%), but higher than the rates detected in two other studies previously conducted in Lisbon, Portugal - 11.7% (48/410 KTRs) and 16% (20/127 KTRs).10,11,14 Although the threshold for dermatology consultation referral is low, the incidence observed may be underestimated.
iii) Cumulative incidence of first NMSC
Transplant duration (that correlates with the duration of immunosuppressive treatment) is a major risk factor for NMSC and it is translated in the increased cumulative incidence (CI) of the first and subsequent NMSCs over the post-trasnplant period, though at different magnitudes.3 Regarding the first NMSC, CI increased almost linearly over that period, doubling between the 5th and 10th years. Several international series confirm this tendency, with increments in CI from 1.6% to 7.8% between the 5th and 10th years in Sweden, 3.3% to 8.8% in Italy, 8.4% to 9.3% in the United Kingdom and 20.78% to 37.35% in Spain.14-16 Australia has one of the highest reported CIs, reaching 24.8% at 5 years.16 As occurs in the general population, the CI of NMSC is higher with lower latitudes.5,16
iv) Cumulative incidence of subsequent NMSC
The occurrence of the first NMSC predicts the early development of subsequent neoplasms.17,18 In this series, the increase in the CI of subsequent NMSCs was more pronounced within the first 3 years following the initial NMSC, reaching 53%. In a study from the Netherlands, the CI of subsequent NMSCs at the first and third years was 32% and 59%, respectively. These incidences are higher than in the general population, wherein the cumulative risk of subsequent SCC is 18% at 3 years.17 Moreover, it has been demonstrated that the first NMSC's histological type predicts the subsequent type of neoplasm, as we observed in this study.17,18 This may occur due to immune tolerance to the first type of malignancy.17
v) Ratio SCC:BCC
While BBCs predominate over SCCs in the general population at a ratio of 4:1, in solid organ transplant recipients this ratio is inverted and becomes more pronounced with time, because the increase in risk is exponential for SCCs, but linear for BCCs.7,19
We found a SCC:BCC ratio of 1.1:1. Higher ratios (4:1) were described in Nordic countries. Ratios of between 1.3:1 and 1:1 were recently reported in Portugal and are closer to those observed in Spain and Italy, where there is a predominance of BCCs over SCCs, at a ratio of 1.4:1 to 2.2:1.10,14,20 The SCC:BCC ratio seems to decline at low latitudes, which may be explained by differences in sun exposure habits and genetic susceptibility to these cancers.10,16 It is speculated that the SCC:BCC ratio may be lower in Mediterranean countries due to the population's phenotypes (higher phototypes), which may be more protective against SCC than BCC in the first years after transplantation.21
vi) Clinical and histological high-risk features of SCC
SCC is the NMSC associated with higher morbimortality, especially when it requires complex and disfiguring surgical procedures on sun-exposed areas like the face, and given the inherent risk of metastases.15 Several clinical and histological risk features have been proposed as predictors of a higher risk of recurrence, metastases, or death, in line with the definition of "high-risk features of SCC" from the American Joint Committee on Cancer (AJCC), the National Comprehensive Cancer Network and the International Transplant Skin Cancer Collaborative.22-24 Due to the lack of harmony in defining these characteristics, we analyzed risk features reviewed by Jennings that globally overlap with those considered primarily by the above-mentioned entities.25
In locations such as the ear and non-glabrous lower lip - the most prevalent high-risk clinical locations in this population - the estimated risk of lymph node metastasis is between 9-10% and 5-14%, respectively.22,25 Among the histological features associated with poor outcomes, tumor depths of over 4mm (30.23%) and the presence of desmoplasia/infiltration (23.26%) were the most commonly found risk factors. In a prospective study, depths greater than 4mm were linked to a metastasis rate of 9%, increasing to 16% when depths were over 6mm.22 On the other hand, desmoplastic SCCs entail an increased risk of recurrence and nodal metastases (up to 6 times higher) and this rises with growing depths.25 Only 7% of SCCs were larger than 2cm, which is currently the most important factor for T staging of AJCC.24 When diameter weas neglected, we verified that 40% of SCCs presented at least two other high-risk features, which overlap with those defined by the AJCC. One of the current indications for immunosuppression revision is the presence of an SCC with at least two high-risk AJCC features (Chart 2).26
Chart 2.
Indication of Colegio et al. for revising the immunosuppression regimen (26)
| Indications for immunosuppression revision |
|---|
| 5-10 well differentiated SCCs per year |
| 1 SCC with 2 high-risk AJCC features* |
| Recurrent SCC |
| SCC with in-transit metastasis |
| Metastatic SCC |
Breslow tumor thickness greater than 2mm, Clark level > IV (into the reticular dermis), perineural invasion, anatomic site (ear, nonglabrous lip) and degree of histologic differentiation (poorly differentiated or undifferentiated).
Nevertheless, the occurrence of nodal metastases in 5% of cases - lower than the 8% previously reported in the KTR population - and the absence of deaths attributable to NMSC, may have resulted from early referral and treatment.3
vii) Age and gender
Older ages at transplantation and at the first consultation were associated with a higher risk of NMSC. Solid organ transplant recipients typically experience cancer rates similar to those of members of the general population who are 20-30 years older.1 In a KTR series, Otley observed that the relative risk of NMSC increased from 5 to 12, when compared individuals in the age group of 34-55 years with those over 55 years.27 Although males had a higher risk of NMSC in several studies, this was not reflected in our predominantly male population.2,27
viii) Role of immunosuppressive drugs
The impact of immunosuppressive drug use on the development of NMSC is well known.2,6 However, it has been difficult to establish a risk hierarchy of NMSC among the various drugs used in the KTR population.
Cyclosporine is the calcineurin inhibitor that most increases the risk of cancer in the general population - by 200 times for NMSC and 65 times for SCC. Tacrolimus seems to entail a lower increment in risk, especially when used in young recipients. The increase in risk with azathioprine is three times lower than with cyclosporine, while mycophenolate is the antimetabolite associated with the lowest risk.28
In a prospective study by Wisgerhof, KTRs chronically treated with azathioprine presented a significantly higher risk of SCC than those treated with mycophenolate or cyclosporine.17 Although it is less frequently used nowadays, azathioprine was the immunosuppressant of choice in the 80s and 90s.29 In this study, azathioprine was the only drug, which, when evaluated alone or in combination with cyclosporine or prednisolone (regimen C), was associated with an increased risk of NMSC. Azathioprine has been recognized as a skin photosensitizing agent but it also provokes direct DNA damage through UVA radiation.17 Hofbauer demonstrated that in KTRs, the photochemical damage previously inflicted on DNA is attenuated - but not completely reversed - in the long-term following discontinuation of azathioprine.30
Sirolimus and everolimus are immunosuppressive drugs that work by inhibiting mTOR, which blocks the cellular response to IL-2 and does not allow for T- and B-lymphocyte activation.19 We did not identify any protective effect on NMSC development in KTRs initially treated with sirolimus. In a recent meta-analysis, however, sirolimus was associated with a reduced risk of malignancy, including NMSC, in KTRs. The benefit was most pronounced in patients who converted from an established immunosuppressive regimen to sirolimus. Nevertheless, its use was linked to a significantly higher risk of death in both conversion and de novo trial subgroups.31
The discussion of indications for immunosuppression revision overcomes the goals of this study. However, chart 2 lists the current recommendations of Colegio et al.26
CONCLUSION
The overall incidence of NMSC found in this population, as well as the estimated cumulative incidence of the first and subsequent malignancies, are a cause for concern. Furthermore, the significant proportion of small-sized but high-risk invasive SCCs is also worrying. All physicians who follow up on these patients should be aware of risk factors for these neoplasms, especially for the oncogenic potential of different immunosuppressive drugs. Only strong articulation between dermatology departments and kidney transplant units can ensure early detection, treatment and secondary prevention of these cancers.
ACKNOWLEDGMENTS
The authors wish to thank Dr. Margarida Marques for their support in the statistical analysis.
Footnotes
Study conducted at the Dermatology Department, Hospitais da Universidade de Coimbra, Centro Hospitalar e Universitário de Coimbra, Coimbra, Portugal.
Financial support: None.
Conflict of interest: None.
REFERENCES
- 1.Chapman JR, Webster AC, Wong G. Cancer in the transplant recipient. Cold Spring Harb Perspect Med. 2013;3: doi: 10.1101/cshperspect.a015677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Navarro MD, López-Andréu M, Rodríguez-Benot A, Agüera ML, Del Castillo D, Aljama P. Cancer incidence and survival in kidney transplant patients. Transplant Proc. 2008;40:2936–2940. doi: 10.1016/j.transproceed.2008.09.025. [DOI] [PubMed] [Google Scholar]
- 3.Zwald FO, Brown M. Skin cancer in solid organ transplant recipients: advances in therapy and management: part II. Management of skin cancer in solid organ transplant recipients. J Am Acad Dermatol. 2011;65:263–279. doi: 10.1016/j.jaad.2010.11.063. quiz 280. [DOI] [PubMed] [Google Scholar]
- 4.Krynitz B, Edgren G, Lindelöf B, Baecklund E, Brattström C, Wilczek H, et al. Risk of skin cancer and other malignancies in kidney, liver, heart and lung transplant recipients 1970 to 2008-A Swedish population-based study. Int J Cancer. 2013;132:1429–1438. doi: 10.1002/ijc.27765. [DOI] [PubMed] [Google Scholar]
- 5.Athar M, Walsh SB, Kopelovich L, Elmets CA. Pathogenesis of nonmelanoma skin cancers in organ transplant recipients. Arch Biochem Biophys. 2011;508:159–163. doi: 10.1016/j.abb.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burke MT, Isbel N, Barraclough KA, Jung JW, Wells JW, Staatz CE. Genetics and nonmelanoma skin cancer in kidney transplant recipients. Pharmacogenomics. 2015;16:161–172. doi: 10.2217/pgs.14.156. [DOI] [PubMed] [Google Scholar]
- 7.Asch WS, Bia MJ. Oncologic Issues and Kidney Transplantation: A Review of Frequency, Mortality, and Screening. Adv Chronic Kidney Dis. 2014;21:106–113. doi: 10.1053/j.ackd.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 8.Tessari G, Girolomoni G. Nonmelanoma skin cancer in solid organ transplant recipientes: update on epidemiology, risk factors, and management. Dermatol Surg. 2012;38:1622–1630. doi: 10.1111/j.1524-4725.2012.02520.x. [DOI] [PubMed] [Google Scholar]
- 9.Gómez MP, Pérez B, Manyalich M. International Registry in Organ Donation and Transplantation-2013. Transplant Proc. 2014;46:1044–1048. doi: 10.1016/j.transproceed.2013.11.138. [DOI] [PubMed] [Google Scholar]
- 10.Borges-Costa J, Vasconcelos JP, Travassos AR, Guerra J, Santana A, Weigert A, et al. Skin Cancer in Kidney Transplant Recipients: Incidence and Association with Clinical and Demographic Factors. Acta Med Port. 2013;26:123–126. [PubMed] [Google Scholar]
- 11.Fernandes S, Carrelha AS, Marques Pinto G, Nolasco F, Barroso E, Cardoso J. Skin Disease in Liver and Kidney Transplant Recipients Referred to the Department of Dermatology and Venereology. Acta Med Port. 2013;26:555–563. [PubMed] [Google Scholar]
- 12.Irodat.org International Registry of Organ Donation and Transplantation. [2015 Jun 12]. [2015-06-20]. internet. Available from: http://www.irodat.org/?p=database&c=PT&year=2009#data.
- 13.Correia M, Sereijo M, Domingues JC, Couto JC, Martins R, Alves R. Patologia cutânea em transplantados renais. Acta Med Port. 1992;5:359–364. [PubMed] [Google Scholar]
- 14.Bernat García J, Morales Suárez-Varela M, Vilata JJ, Marquina A, Pallardó L, Crespo J. Risk factors for non-melanoma skin cancer in kidney transplant patients in a Spanish population in the Mediterranean region. Acta Derm Venereol. 2013;93:422–427. doi: 10.2340/00015555-1525. [DOI] [PubMed] [Google Scholar]
- 15.Tessari G, Naldi L, Boschiero L, Minetti E, Sandrini S, Nacchia F, et al. Incidence of primary and second cancers in renal transplant recipientes: a multicenter cohort study. Am J Transplant. 2013;13:214–221. doi: 10.1111/j.1600-6143.2012.04294.x. [DOI] [PubMed] [Google Scholar]
- 16.Ulrich C, Schmook T, Sachse MM, Sterry W, Stockfleth E. Comparative epidemiology and pathogenic factors for nonmelanoma skin cancer in organ transplant patients. Dermatol Surg. 2004;30:622–627. doi: 10.1111/j.1524-4725.2004.30147.x. [DOI] [PubMed] [Google Scholar]
- 17.Wisgerhof HC, Edelbroek JR, de Fijter JW, Haasnoot GW, Claas FH, Willemze R, et al. Subsequent squamous- and basal-cell carcinomas in kidney-transplant recipients after the first skin câncer: cumulative incidence and risk factors. Transplantation. 2010;89:1231–1238. doi: 10.1097/TP.0b013e3181d84cdc. [DOI] [PubMed] [Google Scholar]
- 18.Tessari G, Naldi L, Boschiero L, Nacchia F, Fior F, Forni A, et al. Incidence and clinical predictors of a subsequent nonmelanoma skin cancer in solid organ transplant recipients with a first nonmelanoma skin câncer: a multicenter cohort study. Arch Dermatol. 2010;14:294–299. doi: 10.1001/archdermatol.2009.377. [DOI] [PubMed] [Google Scholar]
- 19.Brin L, Zubair AS, Brewer JD. Optimal management of skin cancer in immunosuppressed patients. Am J Clin Dermatol. 2014;15:339–356. doi: 10.1007/s40257-014-0085-5. [DOI] [PubMed] [Google Scholar]
- 20.Fuente MJ, Sabat M, Roca J, Lauzurica R, Fernández-Figueras MT, Ferrándiz C. A prospective study of the incidence of skin cancer and its risk factors in a Spanish Mediterranean population of kidney transplant recipients. Br J Dermatol. 2003;149:1221–1226. doi: 10.1111/j.1365-2133.2003.05740.x. [DOI] [PubMed] [Google Scholar]
- 21.Proby CM, Wisgerhof HC, Casabonne D, Green AC, Harwood CA, Bavinck JNB. Stockfleth E, Ulrich C. Skin Cancer after Organ Transplantation. New York: Springer; 2009. The Epidemiology of Transplant-Associated Keratinocyte Cancers in Different Geographical Regions; pp. 75–95. [DOI] [PubMed] [Google Scholar]
- 22.Breuninger H, Brantsch K, Eigentler T, Häfner HM. Comparison and evaluation of the current staging of cutaneous carcinomas. J Dtsch Dermatol Ges. 2012;10:579–586. doi: 10.1111/j.1610-0387.2012.07896.x. [DOI] [PubMed] [Google Scholar]
- 23.Jambusaria-Pahlajani A, Kanetsky PA, Karia PS, Hwang WT, Gelfand JM, Whalen FM, et al. Evaluation of AJCC tumor staging for cutaneous squamous cell carcinoma and a proposed alternative tumor staging system. JAMA Dermatol. 2013;149:402–410. doi: 10.1001/jamadermatol.2013.2456. [DOI] [PubMed] [Google Scholar]
- 24.Chu MB, Slutsky JB, Dhandha MM, Beal BT, Armbrecht ES, Walker RJ, et al. Evaluation of the definitions of "high-risk" cutaneous squamous cell carcinoma using the american joint committee on cancer staging criteria and national comprehensive cancer network guidelines. J Skin Cancer. 2014;2014:154340–154340. doi: 10.1155/2014/154340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jennings L, Schmults CD. Management of high-risk cutaneous squamous cell carcinoma. J Clin Aesthet Dermatol. 2010;3:39–48. [PMC free article] [PubMed] [Google Scholar]
- 26.Colegio OR, Hanlon A, Olasz EB, Carucci JA. Sirolimus reduces cutaneous squamous cell carcinomas in transplantation recipients. J Clin Oncol. 2013;31:3297–3298. doi: 10.1200/JCO.2013.50.6840. [DOI] [PubMed] [Google Scholar]
- 27.Otley CC, Cherikh WS, Salasche SJ, McBride MA, Christenson LJ, Kauffman HM. Skin cancer in organ transplant recipientes: effect of pretransplant end-organ disease. J Am Acad Dermatol. 2005;53:783–790. doi: 10.1016/j.jaad.2005.07.061. [DOI] [PubMed] [Google Scholar]
- 28.Kuschal C, Thoms KM, Schubert S, Schäfer A, Boeckmann L, Schön MP, et al. Skin cancer in organ transplant recipientes: effects of immunosuppressive medications on DNA repair. Exp Dermatol. 2012;21:2–6. doi: 10.1111/j.1600-0625.2011.01413.x. [DOI] [PubMed] [Google Scholar]
- 29.Mahmud N, Klipa D, Ahsan N. Antibody immunosuppressive therapy in solidorgan transplant: Part I. MAbs. 2010;2:148–156. doi: 10.4161/mabs.2.2.11159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hofbauer GF, Attard NR, Harwood CA, McGregor JM, Dziunycz P, Iotzova-Weiss G, et al. Reversal of UVA skin photosensitivity and DNA damage in kidney transplant recipients by replacing azathioprine. Am J Transplant. 2012;12:218–225. doi: 10.1111/j.1600-6143.2011.03751.x. [DOI] [PubMed] [Google Scholar]
- 31.Knoll GA, Kokolo MB, Mallick R, Beck A, Buenaventura CD, Ducharme R, et al. Effect of sirolimus on malignancy and survival after kidney transplantation: systematic review and meta-analysis of individual patient data. BMJ. 2014;349: doi: 10.1136/bmj.g6679. [DOI] [PMC free article] [PubMed] [Google Scholar]