Abstract
Objective:
To explore longitudinal changes in brain activity in patients with Parkinson disease (PD), multiple system atrophy (MSA), and progressive supranuclear palsy (PSP) using task-based functional MRI (fMRI).
Methods:
A total of 112 individuals were scanned 1 year apart while performing a unimanual grip force task: 46 PD, 13 MSA, 19 PSP, and 34 healthy controls. The outcome measure was percent signal change in prespecified regions of interest: putamen, primary motor cortex (M1), supplementary motor area (SMA), and superior motor regions of the cerebellum (lobules V–VI).
Results:
Patients with PD showed a decline in functional activity over the course of 1 year in the putamen and M1 compared to controls. Changes after 1 year in MSA were exclusively extrastriatal, and included a reduction in functional activity in M1, SMA, and superior cerebellum. In PSP, all regions of interest were less active at 1 year compared to baseline. The functional activity of these regions did not change in the control group.
Conclusions:
We provide evidence using task-based fMRI for cortical and striatal functional deterioration in PD over a 1-year period of time. Results also describe more widespread and unique patterns of functional changes in MSA and PSP compared to PD, suggesting distinct rates of disease progression in parkinsonian disorders that may assist in future clinical studies testing the potential efficacy of disease-modifying therapies.
Major efforts are focused on treatments that slow the progression of Parkinson disease (PD) and atypical parkinsonian syndromes.1,2 To facilitate this effort, it is important to gain a better understanding of the rate of progression of functional brain changes in these disorders. In vivo radiotracer imaging has been used to study the progression of the nigrostriatal circuit and shown that the rate of dopaminergic loss in the striatum is greater in patients with PD than in normal aging, but with time it becomes less prominent.3,4 Furthermore, patients with early-stage hemiparkinsonism have a steeper decline in the putamen contralateral to the affected side than in the putamen contralateral to the unaffected side.5,6 As for atypical parkinsonian syndromes, the literature is sparse, with one study showing a greater decline in striatal β-CIT binding of atypical parkinsonian disorders than PD.7 Although these studies provide key insights into progression of dopaminergic degeneration in parkinsonism, they are focused exclusively on the nigrostriatal circuit.8 Since the annual loss of cortical D2 receptors in PD was estimated to be up to 3 times faster than the rate previously reported in putamen,9 objective markers for disease progression may also be found in the cerebral cortex.
Motor control studies using functional MRI (fMRI) engage an extensive task-related network including the basal ganglia, cerebellum, and motor cortex in healthy individuals, and abnormal activation of these structures in PD, multiple system atrophy (MSA), and progressive supranuclear palsy (PSP).10–15 Although the use of MRI in parkinsonian disorders has increased in recent years,16–20 there are no longitudinal studies that monitor and compare the progression of cortical and subcortical function between these disorders. The purpose of this study was to assay functional changes over the course of 1 year in key regions of the basal ganglia, cerebellum, and motor cortex in PD, MSA, and PSP using a unimanual grip force fMRI protocol.10–15 We hypothesized that the basal ganglia and motor cortex would have reduced fMRI signal in PD, MSA, and PSP when compared with the control group over 1 year, and that both MSA and PSP would have widespread and more pronounced cortical changes than PD.
METHODS
Participants.
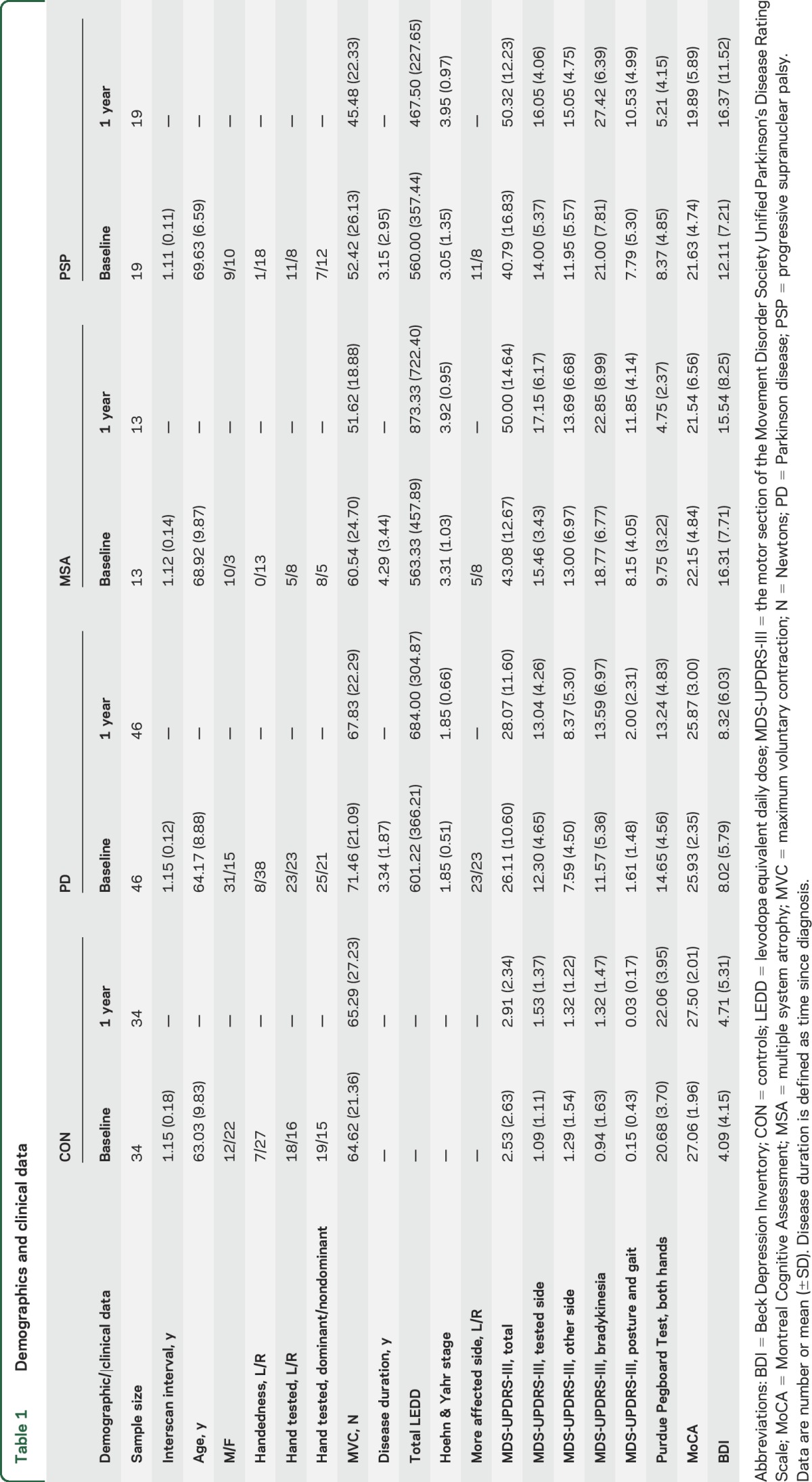
Participants in this cohort study included 46 patients with PD, 13 patients with MSA, 19 patients with PSP, and 34 controls (table 1). All participants were tested at baseline and 1 year. Patients were referred from the University of Florida Center for Movement Disorders and Neurorestoration and were diagnosed by a movement disorder specialist.21–23 Control participants were recruited from surrounding communities. At the time of recruitment, average diagnosis period was stable for over 3 years, and patients' diagnoses did not change over the course of the study. Most patients were taking medication, but all testing was performed 12–14 hours after overnight withdrawal of antiparkinsonian medication.24 The total levodopa equivalent daily dose (LEDD)25 for each group is listed in table 1, and the treatment regimen for each patient is described in table e-1 on the Neurology® Web site at Neurology.org.
Table 1.
Demographics and clinical data
Standard protocol approvals, registrations, and patient consents.
The study was approved by the Institutional Review Board at the University of Florida, and written informed consent to participate in the study was obtained from all participants.
Clinical assessment.
Participants were administered the motor section of the Movement Disorder Society Unified Parkinson's Disease Rating Scale (MDS-UPDRS-III),26 Purdue Pegboard Test (PPB),27 Montreal Cognitive Assessment (MoCA),28 and Beck Depression Inventory (BDI).29 MDS-UPDRS-III was used to calculate total motor severity, hemibody motor scores (side-specific items for limbs only: 3–8, 15–17), total bradykinesia (items 4–8, 14), and posture and gait (items 9–13) (table 1). Hemibody motor scores at baseline were contrasted in order to select the hand patients had to use inside the MRI scanner. The hand corresponding to the side of the body with a higher motor score was defined as the more affected hand and was tested at baseline and 1 year.
Force acquisition.
Participants produced force against a custom-designed MRI-compatible fiber optic transducer with a resolution of 0.025 N (Neuroimaging Solutions, Gainesville, FL). Force data were sampled at 125 Hz by an SM130 Fiber Optic Interrogator (Micron Optics, Atlanta, GA), and recorded by a LabVIEW program (National Instruments, Austin, TX).
Force task.
Prior to MRI, participants were trained on the task and the grip maximum voluntary contraction (MVC) was measured using a Jamar pinch gauge. The target force level was set at 15% of MVC for each visit. Patients were required to produce force with the more affected hand, while for controls we balanced hand use (table 1). The protocol consisted of a block design that alternated force and rest blocks: 30 seconds rest, 30 seconds force with feedback, 12.5 seconds rest, and 30 seconds force without feedback.10–15 This sequence was repeated 4 times and there was an additional 30-second rest period at the end. Throughout the scan, 2 bars were displayed on an LCD monitor that participants could see through a mirror mounted on the head coil: a target bar and a force bar. A change in color of the force bar cued participants to either push or release the force sensor. Green was a go signal for producing and sustaining force (2 seconds), while red indicated a rest period (1 seconds). Force was produced in the presence of feedback as well as in the absence of feedback. In the feedback condition, the target bar was stationary at 15% MVC, while the force bar moved in the vertical plane according to the force output. Instructions were to produce force in order to bring the force bar on top of the target bar for each 2-second period. In the no-feedback condition, both target and force bars were stationary. Participants were required to produce and maintain 15% MVC during each 2-second period without feedback, and the timing of the force contractions was controlled by the same green and red bars.
Force analysis.
Force was filtered using a 10th-order Butterworth filter with a cutoff of 15 Hz.10,14,15 Custom algorithms in MATLAB (The MathWorks, Inc., Natick, MA) were used to calculate mean force during the 2-second hold period as %MVC.
MRI acquisition.
Data were collected on a 3T Philips system (Best, the Netherlands) with a 32-channel SENSE head coil. The protocol included a T2*-weighted, single-shot, echoplanar pulse sequence (repetition time [TR] = 2,500 ms, echo time [TE] = 30 ms, flip angle = 80°, field of view = 240 mm2, voxel size = 3 mm isotropic) and an anatomical 3D T1-weighted sequence (TR = 8.2 ms, TE = 3.7 ms, flip angle = 8°, field of view = 240 mm2, voxel size = 1 mm isotropic).
MRI analysis.
Processing steps were based on previous studies of grip force in parkinsonian disorders.10–15 The outcome measure was percent signal change during force production. This was calculated for a 15-second period (spanning 6 TRs) towards the end of each force block,10 in the following prespecified regions of interest (ROIs): contralateral putamen, ispilateral putamen, contralateral primary motor cortex (M1), contralateral supplementary motor area (SMA), and ipsilateral superior cerebellum (lobules V-VI), where contralateral and ipsilateral are defined with respect to the hand tested. The putamen was extracted from the Basal Ganglia Human Area Template,30 M1 and SMA from the Human Motor Area Template,31 and the cerebellum ROI was defined based on the Spatially Unbiased Infratentorial Template and spanned lobules V–VI (figure e-1).32
Statistics.
First, we examined group differences at baseline. Differences in age and sex were assessed using one-way analysis of variance (ANOVA) and Pearson χ2. Next, age and sex were included as covariates in a multivariate ANOVA (MANOVA) for continuous data. Pearson χ2 was used in the remaining categorical data. Imaging data were compared among the 4 groups using MANOVA, with age, sex, and MoCA as covariates.
Longitudinal changes in outcome measures were calculated by subtracting baseline values from 1-year follow-up values. One-year changes in clinical and force measures were compared between groups using ANOVA, while adjusting for sex, baseline differences in age, and the tested measure. We utilized a similar approach with imaging data. For each ROI, we ran ANOVA on the 1-year difference in percent signal change, while accounting for differences in sex, and baseline differences in age, MoCA, and percent signal change in that particular ROI. Group effects were followed up with post hoc comparisons. Also, a separate univariate analysis was performed on the data from the control group. Results were corrected for multiple comparisons using the Benjamini-Hochberg false discovery rate (FDR), and considered significant whenever pFDR < 0.05.33
RESULTS
Baseline clinical and force data.
We found group differences at baseline in age (pFDR = 0.028) and sex (pFDR = 0.044). Overall, mean age of patients with PSP was greater than that of controls and patients with PD (pFDR < 0.05), but not different from that of patients with MSA (pFDR = 0.827) (table 1). There were more men than women in the PD and MSA groups, but more women than men in the control and PSP groups. The ratios for handedness, and whether the hand tested was the left/right hand, or the dominant/nondominant hand did not differ across groups (pFDR > 0.05). No group differences were found between scans (pFDR = 0.407).
Of note, upcoming results are adjusted for differences in age and sex. We found a group effect for MVC (pFDR = 0.027), with patients with MSA and patients with PSP having a lower MVC than controls (pFDR < 0.05), and no differences in MVC between patients with PD and controls, or among the 3 patient groups (pFDR > 0.05). Further group effects were found for PPB (pFDR = 0.002), MoCA (pFDR = 0.002), and BDI (pFDR = 0.002). Post hoc comparisons revealed that all patients placed fewer pegs than controls (pFDR > 0.05). When comparing the 3 groups of patients, results showed that patients with PD performed better than patients with MSA and patients with PSP (pFDR < 0.05), with no difference in performance between MSA and PSP (pFDR = 0.400). MoCA scores did not differ between controls and patients with PD (pFDR = 0.096), but were lower in patients with MSA and patients with PSP as compared to the other 2 groups (pFDR < 0.05). Scores did not differ between patients with MSA and patients with PSP (pFDR = 0.947). BDI scores were higher in patients as compared to controls (pFDR < 0.05), and differed across patient groups such that patients with PD had the lowest scores, patients with PSP the highest, while patients with MSA had intermediate scores. The 3 patient groups differed on the Hoehn & Yahr stage (pFDR = 0.006) and the following MDS-UPDRS-III scores: total motor severity (pFDR = 0.010), total bradykinesia (pFDR = 0.003), and posture and gait (pFDR = 0.003). Specifically, we found that patients with PD were overall less impaired than patients with patients with MSA and patients with PSP (pFDR < 0.05), and that there were no differences in motor symptoms between patients with MSA and patients with PSP (pFDR > 0.05). Disease duration, total LEDD, and severity of symptoms on the tested side/other side did not differ among patients with PD, patients with MSA, and patients with PSP (pFDR > 0.05). Mean force during the 2-second hold period did not differ between groups (pFDR = 0.151): controls = 13.26%MVC, patients with PD = 12.15%MVC, patients with MSA = 16.01%MVC, and patients with PSP = 13.15%MVC.
Baseline imaging data.
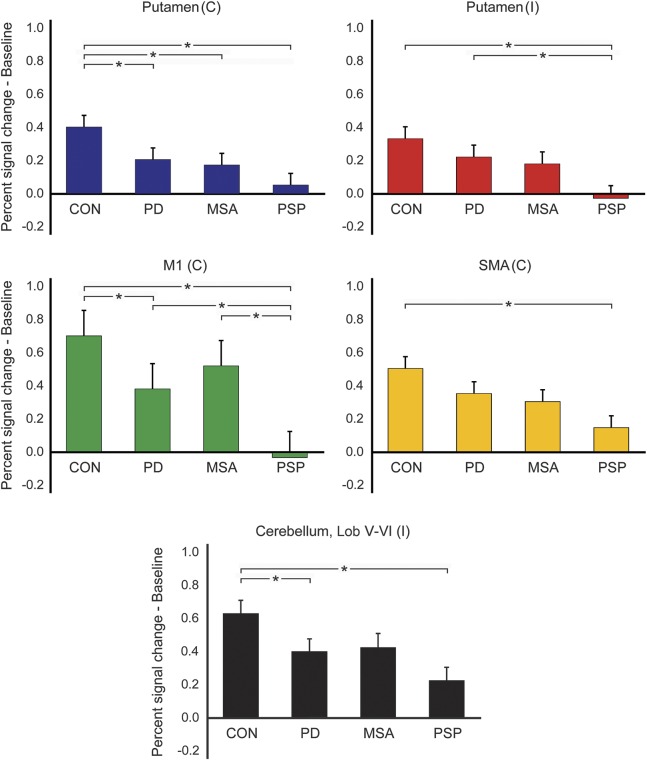
Between-group differences in percent signal change at baseline adjusted for age, sex, and MoCA were found in all ROIs: contralateral putamen (pFDR = 0.002), ipsilateral putamen (pFDR = 0.003), contralateral M1 (pFDR = 0.002), contralateral SMA (pFDR = 0.006), and ipsilateral superior cerebellum (pFDR = 0.002). Patients with PD had reduced percent signal change compared to controls in the contralateral putamen, contralateral M1, and ipsilateral superior cerebellum (pFDR < 0.05), but not in the ipsilateral putamen and contralateral SMA (pFDR > 0.05) (figure 1). In MSA, we found reduced percent signal change compared to controls in the contralateral putamen (pFDR = 0.042), but not other ROIs (pFDR > 0.05). The PSP group had reduced percent signal change compared to controls in all ROIs, and reduced percent signal change compared to patients with PD in the ipsilateral putamen and contralateral M1 (pFDR < 0.05). When comparing the 2 forms of atypical parkinsonism, patients with PSP had lower percent signal change than patients with MSA in M1 only (pFDR = 0.027).
Figure 1. Group differences in baseline percent signal change.
Mean (±SE) percent signal change at baseline adjusted for age, sex, and Montreal Cognitive Assessment Test (MoCA). Significant between-group differences in percent signal change are marked by an asterisk. C = contralateral; CON = controls; I = ipsilateral; Lob = lobule; M1 = primary motor cortex; MSA = multiple system atrophy; PD = Parkinson disease; PSP = progressive supranuclear palsy; SMA = supplementary motor area.
Longitudinal clinical and force data.
The longitudinal analysis revealed no group differences after 1 year in the following measures: MVC, MoCA, and mean force during hold (pFDR > 0.05). Also, we found no changes in MVC over time in any of the groups (pFDR > 0.05). The 1-year change in PPB performance differed across groups (pFDR = 0.005). Bimanual coordination deteriorated in all patient groups as compared to controls, and even further in patients with MSA and patients with PSP as compared to patients with PD (pFDR < 0.05). The decline in patients with MSA and patients with PSP was comparable (pFDR = 0.195). The change in BDI was different between groups (pFDR = 0.048). Overall, patients with PSP had a greater BDI increase than controls and patients with PD (pFDR < 0.05). We found between-group differences in MDS-UPDRS-III (pFDR = 0.006), severity of symptoms on the other side (pFDR = 0.010), total bradykinesia (pFDR = 0.004), and posture and gait (pFDR = 0.003). There was a greater increase in motor severity (all measures) in PSP as compared to PD (pFDR < 0.05), but no difference in these measures with respect to the MSA group (pFDR > 0.05). Patients with MSA had a greater increase in total motor severity and decline of posture and gait than patients with PD (pFDR < 0.05). Finally, changes in the severity of symptoms on the tested side did not differ between patient groups (pFDR = 0.059).
Longitudinal imaging data.
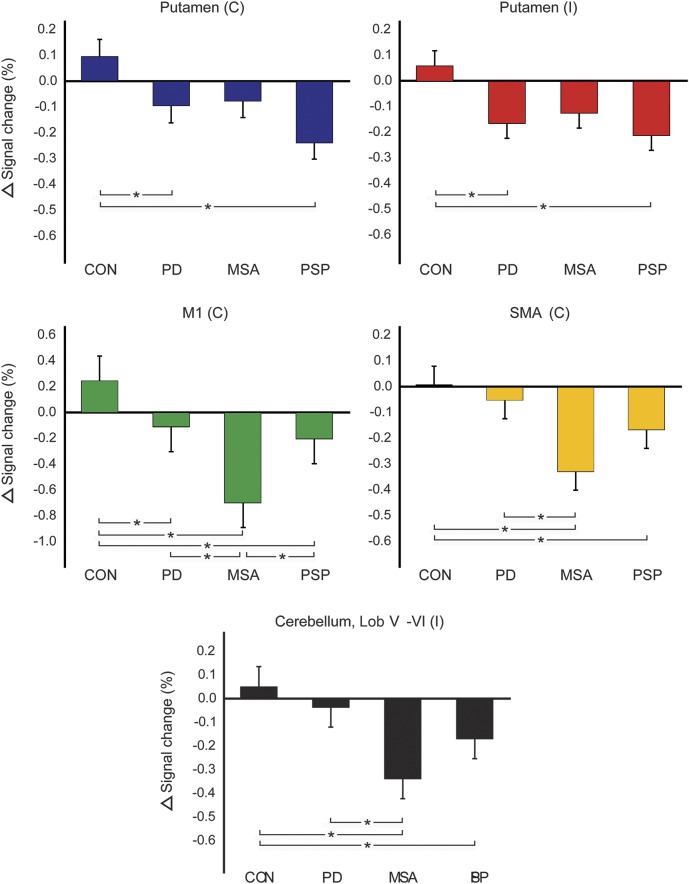
Table 2 lists mean percent signal change at baseline and 1 year in all ROIs (unadjusted for other measures). Table 3 lists p values corresponding to group statistics for baseline and longitudinal imaging data. Figure 2 shows the difference from baseline to 1 year in percent signal change for each group and ROI, adjusted for the following measures at baseline: age, sex, MoCA, and percent signal change in each ROI. For all ROIs, we found a group effect on the 1-year change in percent signal change: contralateral putamen (pFDR = 0.005), ipsilateral putamen (pFDR = 0.005), contralateral M1 (pFDR = 0.003), contralateral SMA (pFDR = 0.003), and ipsilateral superior cerebellum (pFDR = 0.003). The within-group analysis in controls revealed no change in percent signal change after 1-year in any of the ROIs (pFDR > 0.05). In PD, patients had a reduction in percent signal change compared to controls in the contralateral putamen, ipsilateral putamen, and contralateral M1 (pFDR < 0.05). Patients with MSA had a decrease in percent signal change compared to controls in M1, SMA, and superior cerebellum, whereas patients with PSP had a decline in functional activity compared to controls in all 5 ROIs (pFDR\ < 0.05). The decline in M1 was greater in patients with MSA than in patients with PD and patients with PSP (pFDR < 0.05), while the decline in SMA was greater in MSA than in PD (pFDR = 0.003), but not PSP (pFDR = 0.056).
Table 2.
Imaging data
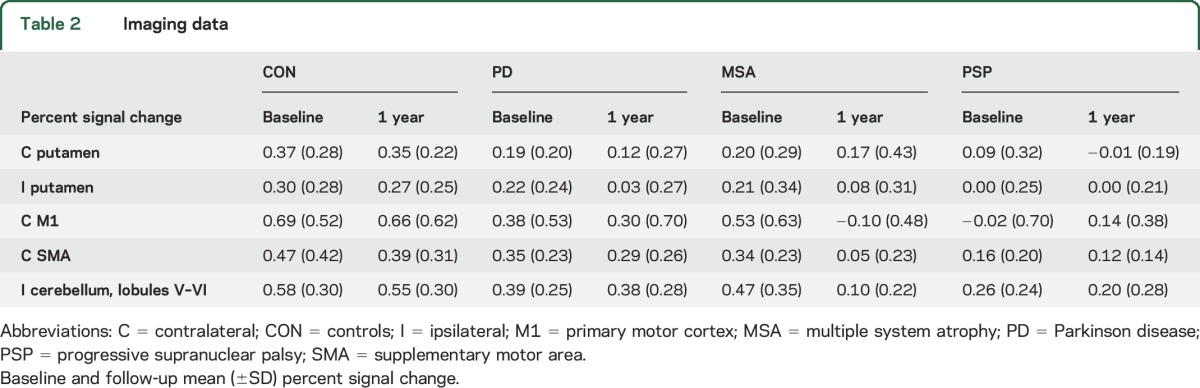
Table 3.
Group statistics
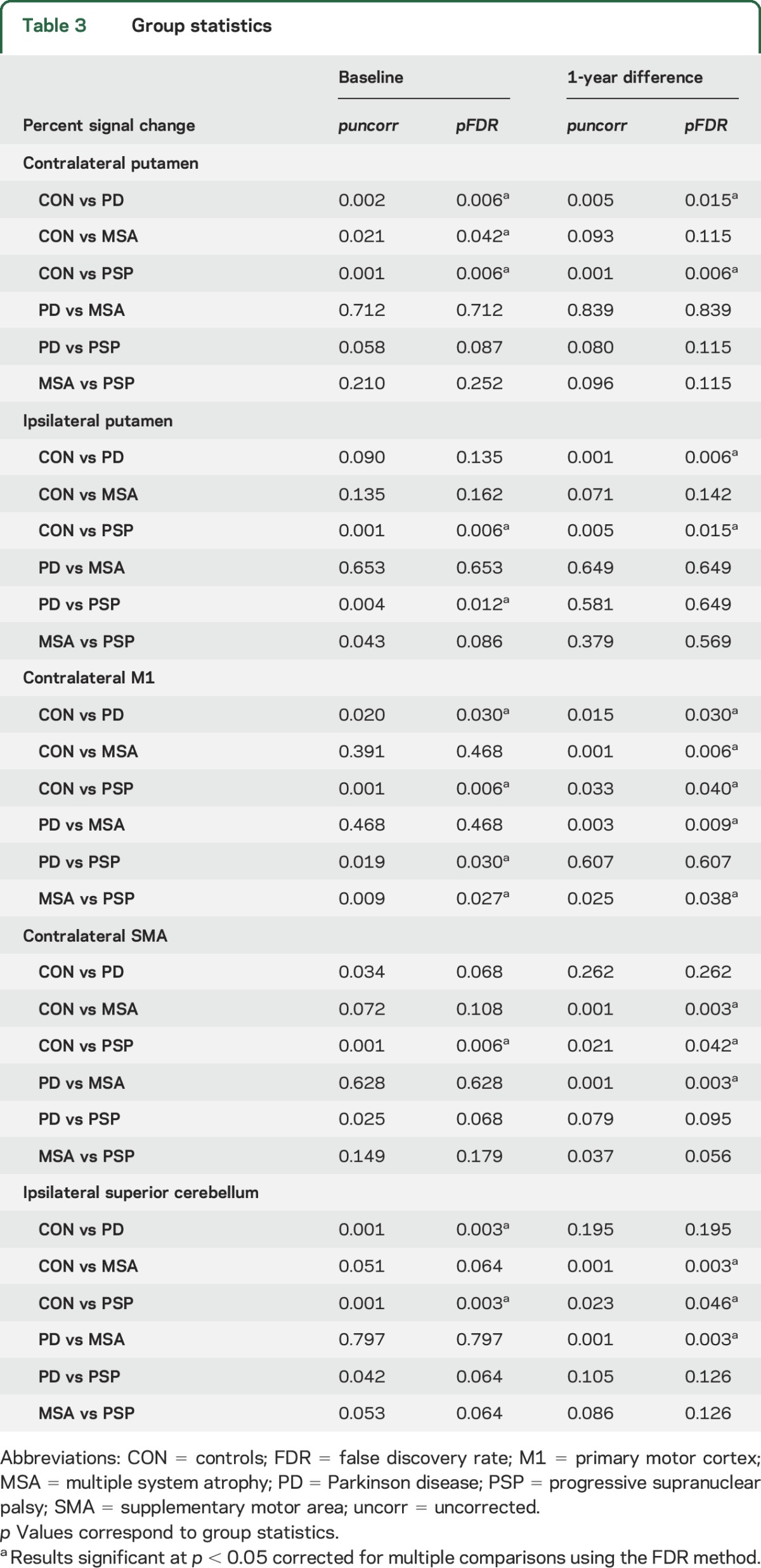
Figure 2. The 1-year change in percent signal change.
The difference from baseline to 1 year in percent signal change for each group and region of interest (ROI), adjusted for the following measures at baseline: age, sex, Montreal Cognitive Assessment Test (MoCA), and percent signal change in each ROI. Error bars represent the SE. Group comparisons significant at p < 0.05 (false discovery rate–corrected) are marked by an asterisk. C = contralateral; CON = controls; I = ipsilateral; Lob = lobule; M1 = primary motor cortex; MSA = multiple system atrophy; PD = Parkinson disease; PSP = progressive supranuclear palsy; SMA = supplementary motor area.
DISCUSSION
Our results demonstrate that task-related fMRI signal reflects different patterns of disease progression in PD, MSA, and PSP. Using a motor control paradigm,10–15 we found a decline in fMRI signal over the course of 1 year of putamen and M1 in patients with PD compared with controls. Further, when compared with controls, a reduction in functional activity was observed in M1, SMA, and superior cerebellum in MSA, and a decline in all ROIs in PSP. Importantly, functional brain activity in controls did not change over 1 year. Collectively, these findings point to disease-specific noninvasive progression markers of sensorimotor brain regions in parkinsonian disorders.
Results in PD demonstrate ongoing functional deterioration over the course of 1 year in the basal ganglia, with a bilateral reduction in percent signal change in the putamen, and a slightly greater effect in the putamen ipsilateral to the tested side. These findings are consistent with a previous PET study in PD with a comparable level of disability, which identified a greater decline in [18F]-CIT uptake in the putamen ipsilateral to the more affected side.34 We extend the existing literature on PD by showing that functional activity of the primary motor cortex, which has been consistently shown to be reduced in these patients,10–15 continues to deteriorate with disease progression. Thus, it appears that defective basal ganglia signaling in PD leads to long-term changes that propagate along the basal ganglia-thalamo-cortical loop, suggesting modulation of cortical motor output as an additional biomarker for treatment of PD.35
The progression of functional brain changes in atypical parkinsonian syndromes has been scarcely documented. A longitudinal β-CIT SPECT study found a greater decline in striatal binding over the course of 2 years in a heterogeneous group of atypical patients than in patients with PD.7 Here, we extend existing work, and further characterize MSA and PSP as having a more symmetric functional loss in the putamen than PD, which may be linked to the fact that these patients often present with bilateral symptoms of similar severity (table 1).36 In addition, we show different patterns of functional decline in MSA and PSP across subcortical and cortical structures (figure 2). For instance, despite a low functional activity at baseline in all cortical and subcortical regions in PSP, this group continued to decline over time. By contrast, patients with MSA had a steeper decline in functional activity of M1, SMA, and superior cerebellum than patients with PSP, regions where initially patients with MSA had greater fMRI signal than patients with PSP. Together, these findings suggest the motor system is differentially affected with disease progression in PD, MSA, and PSP. Since both MSA and PSP involve extensive pathology in extranigral structures including the brainstem and cerebellum,2,37 which is also detected using diffusion MRI,38 the more rapid decline of functional activity in these disorders may be partly modulated by abnormalities outside the investigated brain networks. For example, it could be that the steeper decline in functional activity in atypical patients is related to progression of brain atrophy. A previous longitudinal structural MRI study has shown that 1 year of disease progression in patients with MSA is associated with increased brain atrophy in several extrastriatal regions including the primary sensorimotor cortex, premotor cortex, and cerebellum, and this is more prominent in those patients with longer disease duration.39 By contrast, PSP has a different pattern of structural changes at the level of the rostral midbrain and superior cerebellar peduncle14,40 that may affect brain networks and could relate to the current progression findings. Furthermore, the lack of change in functional activity in the control group suggests that the pattern of fMRI changes observed cannot be explained by normal aging. Although the groups produced a similar level of force, this does not rule out the possibility of results being influenced by other factors such as apathy, which is commonly observed in atypical parkinsonism.2
Overall, we provide evidence using task-based fMRI for distinct patterns of motor-related changes across the basal ganglia and cerebello-thalamo-cortical loops in PD, MSA, and PSP. These findings could provide a platform to evaluate therapeutic strategies aimed at slowing the progression in parkinsonian disorders. It is essential, however, that future studies expand current research by further evaluating progression of clinical subtypes of PD (tremor-dominant/non-tremor-dominant), or atypical parkinsonian syndromes (MSA-P/MSA-C), which in turn may prompt the assessment of additional progression markers.
Supplementary Material
ACKNOWLEDGMENT
The authors thank the participants for their time and commitment to this research and the NIH for funding this study.
GLOSSARY
- ANOVA
analysis of variance
- BDI
Beck Depression Inventory
- FDR
false discovery rate
- fMRI
functional MRI
- LEDD
levodopa equivalent daily dose
- M1
primary motor cortex
- MANOVA
multivariate analysis of variance
- MDS-UPDRS-III
Movement Disorder Society Unified Parkinson's Disease Rating Scale
- MoCA
Montreal Cognitive Assessment
- MSA
multiple system atrophy
- MVC
maximum voluntary contraction
- PD
Parkinson disease
- PPB
Purdue Pegboard Test
- PSP
progressive supranuclear palsy
- ROI
region of interest
- SMA
supplementary motor area
- TE
echo time
- TR
repetition time
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Roxana G. Burciu: research project execution, data processing and statistical analysis, manuscript preparation. Jae Woo Chung: data processing. Priyank Shukla: data processing. Edward Ofori: manuscript preparation. Hong Li: revision of statistical analysis. Nikolaus R. McFarland: subject recruitment, manuscript preparation. Michael S. Okun: subject recruitment, manuscript preparation. David E. Vaillancourt: study concept and design, manuscript preparation.
STUDY FUNDING
Supported by NIH (R01 NS52318, R01 NS75012, T32 NS082169).
DISCLOSURE
R. Burciu, J. Chung, P. Shukla, E. Ofori, and H. Li report no disclosures relevant to the manuscript. N. McFarland reports grants from the NIH and the Michael J. Fox Foundation. He has received personal honoraria from the NIH and the American Academy of Neurology. M. Okun serves as consultant for the National Parkinson's Foundation and has received research grants from the NIH, National Parkinson's Foundation, Michael J. Fox Foundation, Parkinson Alliance, Smallwood Foundation, Bachmann-Strauss Foundation, Tourette Syndrome Association, and UF Foundation; has previously received honoraria, but in the past >60 months has received no support from industry; has received royalties for publications with Demos, Manson, Amazon, Smashwords, Books4Patients, and Cambridge (movement disorders books); is an associate editor for New England Journal of Medicine, Journal Watch Neurology; and has participated in CME and educational activities on movement disorders (in the last 36 months) sponsored by PeerView, Prime, Quantia, Henry Stewart, and the Vanderbilt University. The institution and not Dr. Okun receives grants from Medtronic, Abbvie, and ANS/St. Jude, and the PI has no financial interest in these grants. Dr. Okun has participated as a site PI and/or co-I for several NIH, foundation, and industry-sponsored trials over the years but has not received honoraria. D. Vaillancourt reports grants from NIH, Bachmann-Strauss, and Tyler's Hope Foundation during the conduct of the study, and personal honoraria from NIH, National Parkinson's Foundation, UT Southwestern Medical Center, and Northwestern University unrelated to the submitted work. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Seibyl J, Jennings D, Tabamo R, Marek K. Neuroimaging trials of Parkinson's disease progression. J Neurol 2004;251(suppl 7):VII/9–VII/13. [DOI] [PubMed] [Google Scholar]
- 2.Brooks DJ. Diagnosis and management of atypical parkinsonian syndromes. J Neurol Neurosurg Psychiatry 2002;72(suppl 1):i10–i16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marek K, Innis R, van Dyck C, et al. . [123I]beta-CIT SPECT imaging assessment of the rate of Parkinson's disease progression. Neurology 2001;57:2089–2094. [DOI] [PubMed] [Google Scholar]
- 4.Morrish PK, Sawle GV, Brooks DJ. An [18F]dopa-PET and clinical study of the rate of progression in Parkinson's disease. Brain 1996;119:585–591. [DOI] [PubMed] [Google Scholar]
- 5.Sawle GV, Playford ED, Brooks DJ, Quinn N, Frackowiak RSJ. Asymmetrical pre-synaptic and post-synaptic changes in the striatal dopamine projection in dopa naïve parkinsonism. Brain 1993;116:853–867. [DOI] [PubMed] [Google Scholar]
- 6.Marek KL, Seibyl JP, Zoghbi SS, et al. . [sup 123 I] beta-CIT/SPECT imaging demonstrates bilateral loss of dopamine transporters in hemi-Parkinson's disease. Neurology 1996;46:231–237. [DOI] [PubMed] [Google Scholar]
- 7.Pirker W, Djamshidian S, Asenbaum S, et al. . Progression of dopaminergic degeneration in Parkinson's disease and atypical parkinsonism: a longitudinal beta-CIT SPECT study. Mov Disord 2002;17:45–53. [DOI] [PubMed] [Google Scholar]
- 8.Politis M. Neuroimaging in Parkinson disease: from research setting to clinical practice. Nat Rev Neurol 2014;10:708–722. [DOI] [PubMed] [Google Scholar]
- 9.Kaasinen V, Aalto S, Nagren K, Hietala J, Sonninen P, Rinne JO. Extrastriatal dopamine D(2) receptors in Parkinson's disease: a longitudinal study. J Neural Transm 2003;110:591–601. [DOI] [PubMed] [Google Scholar]
- 10.Spraker MB, Prodoehl J, Corcos DM, Comella CL, Vaillancourt DE. Basal ganglia hypoactivity during grip force in drug naïve Parkinson's disease. Hum Brain Mapp 2010;31:1928–1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prodoehl J, Spraker M, Corcos D, Comella C, Vaillancourt D. Blood oxygenation level–dependent activation in basal ganglia nuclei relates to specific symptoms in de novo Parkinson's disease. Mov Disord 2010;25:2035–2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neely KA, Kurani AS, Shukla P, et al. . Functional brain activity relates to 0–3 and 3–8 Hz force oscillations in essential tremor. Cereb Cortex 2015;25:4191–4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prodoehl J, Planetta PJ, Kurani AS, Comella CL, Corcos DM, Vaillancourt DE. Differences in brain activation between tremor- and nontremor-dominant Parkinson disease. JAMA Neurol 2013;70:100–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burciu RG, Ofori E, Shukla P, et al. . Distinct patterns of brain activity in progressive supranuclear palsy and Parkinson's disease. Mov Disord 2015;30:1248–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Planetta PJ, Kurani AS, Shukla P, et al. . Distinct functional and macrostructural brain changes in Parkinson's disease and multiple system atrophy. Hum Brain Mapp 2015;36:1165–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Du G, Lewis MM, Sen S, et al. . Imaging nigral pathology and clinical progression in Parkinson's disease. Mov Disord 2012;27:1636–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu T, Wang J, Wang C, et al. . Basal ganglia circuits changes in Parkinson's disease patients. Neurosci Lett 2012;524:55–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Helmich RC, Derikx LC, Bakker M, Scheeringa R, Bloem BR, Toni I. Spatial remapping of cortico-striatal connectivity in Parkinson's disease. Cereb Cortex 2010;20:1175–1186. [DOI] [PubMed] [Google Scholar]
- 19.Pyatigorskaya N, Gallea C, Garcia-Lorenzo D, Vidailhet M, Lehericy S. A review of the use of magnetic resonance imaging in Parkinson's disease. Ther Adv Neurol Disord 2014;7:206–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Festini SB, Bernard JA, Kwak Y, et al. . Altered cerebellar connectivity in Parkinson's patients ON and OFF L-DOPA medication. Front Hum Neurosci 2015;9:214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hughes AJ, Ben-Shlomo Y, Daniel SE, Lees AJ. What features improve the accuracy of clinical diagnosis in Parkinson's disease: a clinicopathologic study. 1992. Neurology 2001;57(suppl 3):S34–S38. [PubMed] [Google Scholar]
- 22.Gilman S, Wenning GK, Low PA, et al. . Second consensus statement on the diagnosis of multiple system atrophy. Neurology 2008;71:670–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Litvan I, Agid Y, Calne D, et al. . Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP international workshop. Neurology 1996;47:1–9. [DOI] [PubMed] [Google Scholar]
- 24.Langston JW, Widner H, Goetz CG, et al. . Core assessment program for intracerebral transplantations (CAPIT). Mov Disord 1992;7:2–13. [DOI] [PubMed] [Google Scholar]
- 25.Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Mov Disord 2010;25:2649–2653. [DOI] [PubMed] [Google Scholar]
- 26.Goetz CG, Tilley BC, Shaftman SR, et al. . Movement Disorder Society-sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord 2008;23:2129–2170. [DOI] [PubMed] [Google Scholar]
- 27.Desrosiers J, Hébert R, Bravo G, Dutil E. The Purdue Pegboard Test: normative data for people aged 60 and over. Disabil Rehabil 1995;17:217–224. [DOI] [PubMed] [Google Scholar]
- 28.Nasreddine ZS, Phillips NA, Bédirian V, et al. . The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005;53:695–699. [DOI] [PubMed] [Google Scholar]
- 29.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry 1961;4:561–571. [DOI] [PubMed] [Google Scholar]
- 30.Prodoehl J, Yu H, Little DM, Abraham I, Vaillancourt DE. Region of interest template for the human basal ganglia: comparing EPI and standardized space approaches. NeuroImage 2008;39:956–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mayka MA, Corcos DM, Leurgans SE, Vaillancourt DE. Three-dimensional locations and boundaries of motor and premotor cortices as defined by functional brain imaging. NeuroImage 2006;31:1453–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Diedrichsen J. A spatially unbiased atlas template of the human cerebellum. NeuroImage 2006;33:127–138. [DOI] [PubMed] [Google Scholar]
- 33.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B Methodol 1995;57:289–300. [Google Scholar]
- 34.Nurmi E, Bergman J, Eskola O, et al. . Progression of dopaminergic hypofunction in striatal subregions in Parkinson's disease using [18F]CFT PET. Synapse 2003;48:109–115. [DOI] [PubMed] [Google Scholar]
- 35.Udupa K, Chen R. Motor cortical plasticity in Parkinson's disease. Front Neurol 2013;4:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Djaldetti R, Ziv I, Melamed E. The mystery of motor asymmetry in Parkinson's disease. Lancet Neurol 2006;5:796–802. [DOI] [PubMed] [Google Scholar]
- 37.Dickson DW. Parkinson's disease and parkinsonism: neuropathology. Cold Spring Harb Perspect Med 2012;2:a009258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Planetta PJ, Ofori E, Pasternak O, et al. . Free-water imaging in Parkinson's disease and atypical parkinsonism. Brain 2015;139:495–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brenneis C, Egger K, Scherfler C, et al. . Progression of brain atrophy in multiple system atrophy: a longitudinal VBM study. J Neurol 2007;254:191–196. [DOI] [PubMed] [Google Scholar]
- 40.Kato N, Arai K, Hattori T. Study of the rostral midbrain atrophy in progressive supranuclear palsy. J Neurol Sci 2003;210:57–60. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.