Abstract
Objective:
To test the hypothesis that effects of estrogen-containing hormone therapy on cognitive abilities differ between postmenopausal women near to, and further from, menopause.
Methods:
In this randomized, double-blind, placebo-controlled trial, healthy women within 6 years of menopause or 10+ years after menopause were randomly assigned to oral 17β-estradiol 1 mg/d or placebo. Women with a uterus received cyclic micronized progesterone vaginal gel or placebo. The primary outcome assessed at 2.5 and 5 years, compared between treatment groups, was change in a standardized composite of neuropsychological test scores assessing verbal episodic memory. Secondary outcomes assessed executive functions and global cognition.
Results:
A total of 567 women were included in modified intention-to-treat analyses after a mean treatment duration of 57 months. For verbal memory, the mean estradiol minus placebo standardized difference in composite scores (−0.06, 95% confidence interval −0.22 to 0.09) was not significant (2-tailed p = 0.33). Differences were similar in early and late postmenopause groups (2-tailed interaction p = 0.88). Interactions between postmenopause groups and differences between treatment groups were not significant for executive functions or global cognition.
Conclusions:
Estradiol initiated within 6 years of menopause does not affect verbal memory, executive functions, or global cognition differently than therapy begun 10+ years after menopause. Estradiol neither benefits nor harms these cognitive abilities regardless of time since menopause.
Classification of evidence:
This study provides Class I evidence that estradiol initiated within 6 years of menopause does not affect cognition at 2.5 years differently than estradiol initiated 10+ years after menopause.
With menopause, the cyclic production of estradiol and progesterone ceases. Serum concentrations of these hormones decline steeply and have the potential to affect brain processes associated with cognition and age-related disorders, such as Alzheimer disease.1 Menopausal hormone therapy (MHT)—a systemic estrogen with or without a progestogen, usually prescribed for the alleviation of moderate to severe vasomotor symptoms—is used by millions of women,2 despite declines in MHT prescriptions following the Women's Health Initiative trials.3,4 It is controversial whether MHT benefits or harms memory and other cognitive skills.5 Clinical trials have failed to discern consistent, clinically meaningful effects on cognition.5 However, with one exception,6 large, long-duration trials7 have been restricted to older postmenopausal women. Some hormone effects on cognition are postulated to vary by age or by timing in relation to the menopause. MHT, when used by younger postmenopausal women close to time of menopause, is proposed to enhance cognition, whereas no cognitive benefit accrues from use by healthy older postmenopausal women.8,9 However, the timing, or critical window, hypothesis of cognitive benefit has not been directly examined within the context of a randomized clinical trial.
The Early vs Late Intervention Trial with Estradiol (ELITE), designed to test the timing hypothesis, was conducted in 2 postmenopausal strata: women near to menopause and further from menopause. The randomized interventions were oral estradiol or placebo, and the goal was to determine if time since menopause as represented by postmenopause group modifies the effect of estradiol on specified health outcomes. For cognitive endpoints (ELITE-Cog), the primary hypothesis is that the change in verbal episodic memory would differ between postmenopause groups, with better performance predicted for women in the early postmenopause group randomized to estradiol compared to placebo but not for women in the late postmenopause group.
METHODS
Design and setting.
ELITE is a randomized, double-blind, parallel-groups trial of oral 17β-estradiol (1 mg/d) or identically appearing placebo, conducted at a single academic medical center site (University of Southern California). Women with a uterus were assigned cyclic micronized progesterone (45 mg) as a 4% vaginal gel or matched placebo gel, one daily application for 10 days per 30-day cycle. The endpoint for the parent ELITE trial is the effect of estradiol compared to placebo on progression of subclinical atherosclerosis.10 For ELITE-Cog, the primary research question is whether estradiol initiated within 6 years of menopause affects verbal memory differently than estradiol initiated 10 or more years after menopause.11 Full protocols for ELITE10 and ELITE-Cog11 are published.
Standard protocol approvals, registrations, and patient consents.
The study was approved by institutional review boards of the University of Southern California and Stanford University and was monitored by an external Data and Safety Monitoring Board. Participants provided written informed consent. The protocol is registered at clinicaltrials.gov (NCT00114517).
Participants.
Healthy women were recruited into early and late postmenopause groups. Postmenopausal status was defined by absence of vaginal bleeding for at least 6 months (natural menopause) or bilateral oophorectomy (surgical menopause) and serum total estradiol <25 pg/mL. Early-group women were within 6 years of a final menstrual period (natural menopause) or bilateral oophorectomy (surgical menopause). Late-group women were at least 10 years beyond natural or surgical menopause. Other exclusion criteria are published.10,11
Randomization.
Within each postmenopausal stratum, assignment to treatment group in a 1:1 ratio used concealed stratified blocked randomization. Other stratification factors were carotid artery intima-media thickness (<0.75 mm, ≥0.75 mm) defined by high-resolution B-mode ultrasonography10 and presence or absence of a uterus. The computer-generated randomization sequence was prepared by the study statistician (SAS statistical software) before trial initiation. Other investigators, participants, and staff were masked to treatment assignment. Stratified randomization lists were used to prepare blinded study products. After determining a participant's eligibility, clinic staff pulled the next study product in sequence from the appropriate stratum, recorded the product identification number, and dispensed the product.
Endpoints.
Recruitment was based on a 5-year trial with planned treatment of 2–4.5 years. With supplemental funding, the trial was extended before blinding was unmasked,11 allowing a third cognitive assessment as participants completed the trial. Cognitive skills were assessed at baseline, at about 2.5 years (mean 33 months, SD 2.5, range 29–50), and at about 5 years (mean 57 months, SD 5.8, range 36–77). Cognitive assessment used a comprehensive neuropsychological battery12 that emphasized standardized tests sensitive to age-related change.
Demographic and clinical variables.
Structured questionnaires were used to collect demographic information, medical history, and medication use. Women used a daily diary to record the number of hot flashes (mean duration of recording prior to randomization of 40 days, SD 21 days). Physical activity and alcohol intake during the preceding week were assessed with standardized interviews. The Center for Epidemiologic Studies depression scale was used to assess mood.13 After an overnight fast, blood was obtained for estradiol assays,11 and DNA was extracted to determine APOE gene isoforms.11
Statistical analysis.
The change from baseline for each cognitive endpoint was calculated at both postrandomization cognitive visits. We used generalized estimating equation models to analyze repeated measures of change in cognitive endpoints. Independent variables were treatment group and postmenopause group; covariates included randomization stratification variables and baseline value of the cognitive endpoint. Differences in cognitive change were tested by treatment group, by postmenopause group, and by time (2.5 vs 5 years). We tested our hypothesis with the interaction of treatment group by postmenopause group, which assessed whether treatment effects on cognitive change (at 2.5 and 5 years) differed in early and late groups. We assessed whether treatment effects were consistent in early and late groups across the 2 test intervals through the treatment-by-postmenopause group-by-time interaction. No interim analyses were performed.
Sample size requirements for the parent ELITE trial were estimated from the projected mean treatment group difference in the rate of change in carotid artery intima-media thickness, as described.10 Sample size estimates to test this interaction (80% power, 2-tailed α level 0.05, allowing for 25% dropout) yielded a sample size of 126 in each of the 4 strata. Supplemental funding was obtained before outcomes were examined to extend the trial and to increase sample size to enhance power.10,11 For analyses based on verbal memory, we estimated 82% power (2-sided α of 0.05) to detect differences in the estradiol treatment effect between postmenopause groups if the early group had a treatment effect size of 0.5, as suggested by some clinical trials in relatively younger women,14–17 and the late group had a treatment effect size of 0, as suggested by clinical trials in relatively older women.18–21 In the absence of interaction by postmenopause group, we had 80% power to detect a treatment effect size of 0.22 in early and late groups combined.11
Primary analyses were conducted separately within each postmenopause stratum. To guard against a type I error, we tested our hypothesis with endpoints derived from composite neuropsychological measures. Verbal episodic memory is reported to benefit from estrogen,14–17 and for this reason the primary endpoint was a composite score of 4 measures of verbal memory based on word list recall and paragraph story recall.11 The secondary endpoint was a global composite of all cognitive tests.11 Composites were calculated as weighted sums of component standardized scores weighted by the inverse inter-test correlation matrix.12 To evaluate estradiol treatment effects in other cognitive domains, we examined change in an executive functions composite measure11 and changes in individual neuropsychological test scores, with Bonferroni adjustment for multiple comparisons.
We specified 2 subgroup analyses.11 The first compared treatment differences on composite cognitive change scores in women who experienced a natural vs surgical menopause.22 The second compared treatment differences based on the presence or absence of hot flash symptoms at baseline. In trials of MHT that included women with hot flashes, women assigned to active treatment had better cognitive outcomes.23 Unspecified analyses excluded nonadherent women or early-group women not within 3 years of menopause, and we examined subgroups defined by APOE genotype, prior use of MHT, or hysterectomy status.
We determined unused portions of the study drug at each visit. Adherence was calculated as the number of pills taken divided by the amount that should have been taken. Serum concentrations of total estradiol11 at baseline and at 6-month intervals provided an ancillary measure of adherence.
Classification of evidence.
This study provides Class I evidence that oral estradiol 1 mg/d initiated within 6 years of menopause does not significantly affect verbal memory, executive functions, or global cognition differently at 2.5 years than estradiol initiated 10+ years after menopause. More than 20% of randomized women were not analyzed at 5 years, and for this reason the level of evidence for 5-year outcomes is Class II.
RESULTS
Participants.
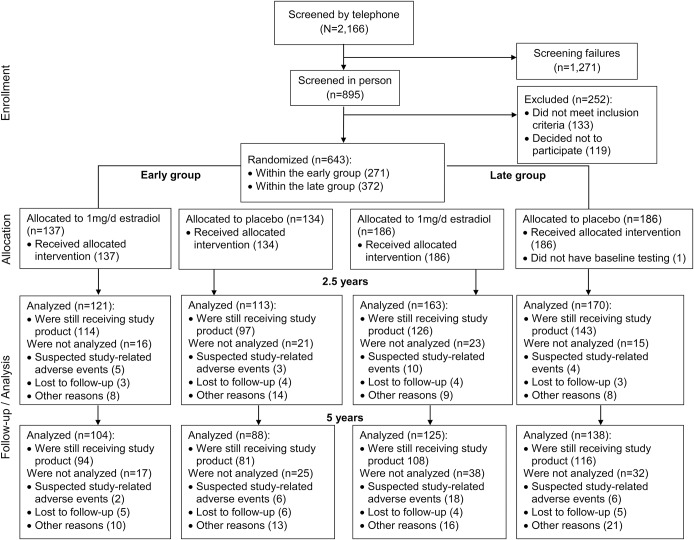
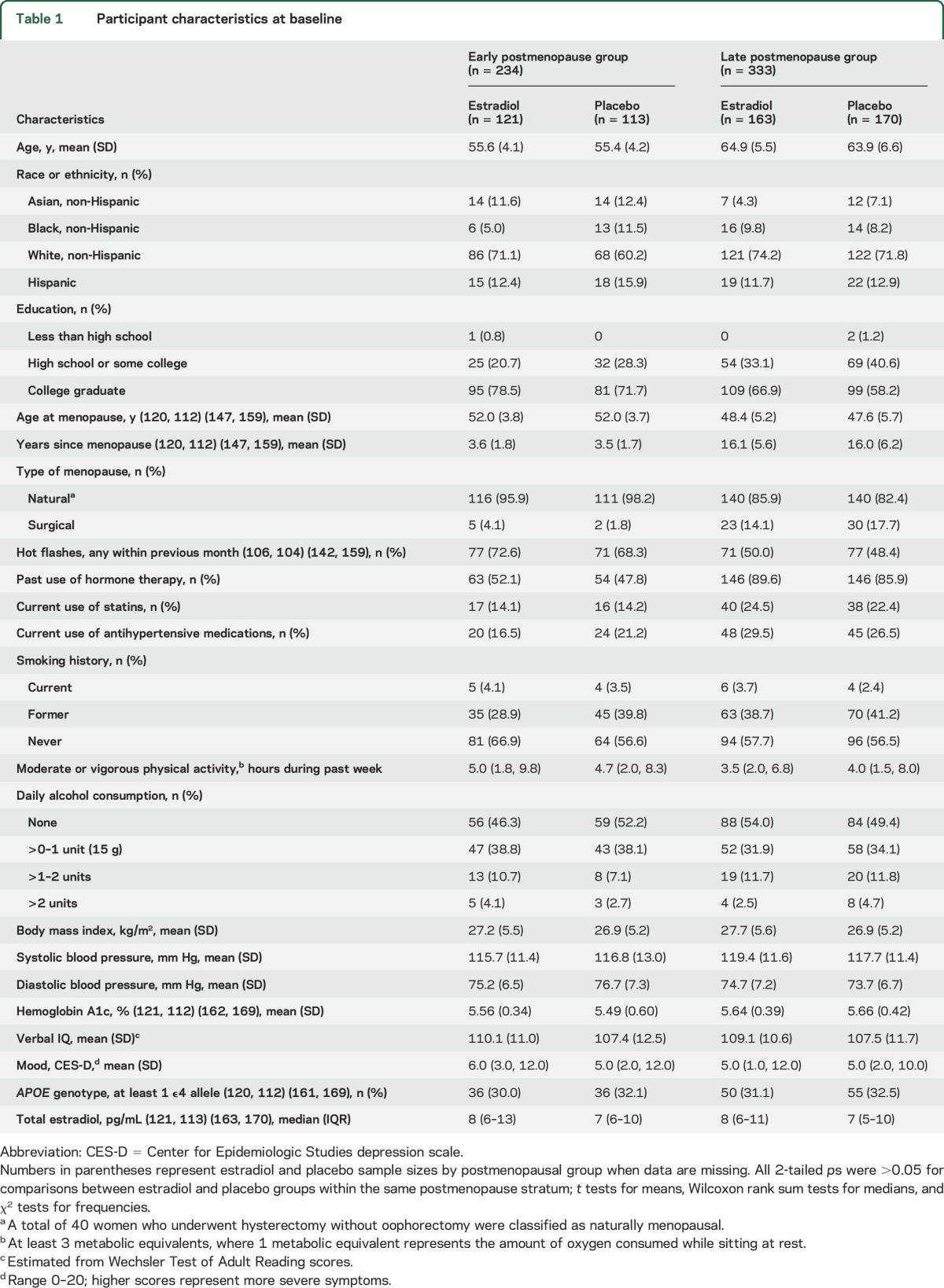
We screened 2,166 women for eligibility. A total of 643 were recruited into early and late postmenopausal groups. A total of 567 underwent cognitive assessment at baseline and on at least one other occasion and were included in modified intent-to-treat analysis (figure). A total of 567 women (88%) provided cognitive outcomes at 2.5 years, and 455 (71%) provided outcomes at 5 years (e-Results). Participant ages ranged from 41 to 84 years. Within early and late strata, estradiol and placebo groups were similar on most baseline characteristics (table 1). Women not contributing to this analysis were similar to other women in most, but not all, comparisons (table e-1 on the Neurology® Web site at Neurology.org).
Figure. Flowchart of study enrollment and follow-up.
Suspected study-related adverse events and other reasons for participant discontinuation are given in the e-Results.
Table 1.
Participant characteristics at baseline
Adherence.
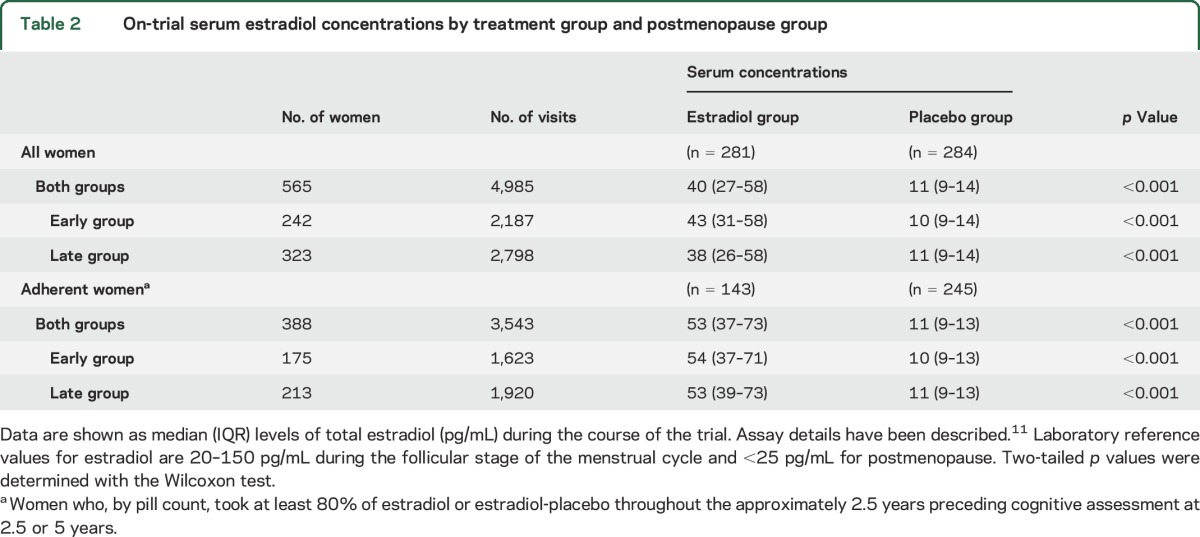
The median adherence for estradiol or placebo was ≥98% for early- and late-group women. Eighty-seven women (44 estradiol group, 43 placebo group) who discontinued study medications before 2.5 years and 56 women (27 estradiol group, 29 placebo group) who discontinued after 2.5 years but before 5 years contributed cognitive outcomes for analysis. As expected, median on-trial serum estradiol levels were significantly higher for women assigned to active treatment (table 2).
Table 2.
On-trial serum estradiol concentrations by treatment group and postmenopause group
Cognitive composite scores.
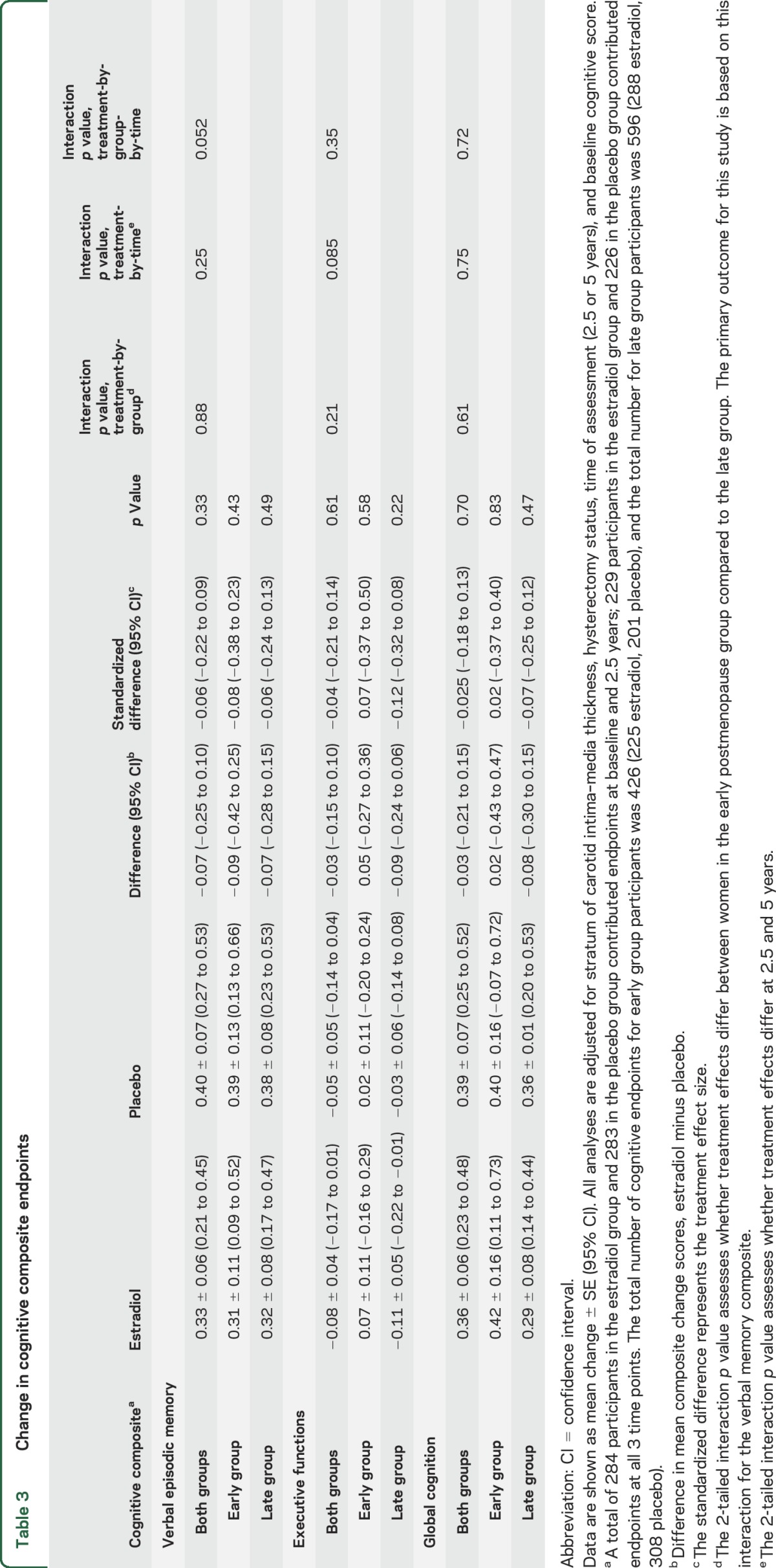
Compared to baseline, verbal memory and global cognition composite scores improved in both treatment groups (practice effect); performance was stable on the composite measure of executive functions (table 3). For verbal memory, the mean difference in composite scores of −0.07 between treatment groups—corresponding to a standardized difference of −0.06 (95% confidence interval [CI] −0.22 to 0.09)—was not significant. Between-groups standardized differences were similar in early and late groups (2-tailed interaction p = 0.88). Interactions between postmenopause groups and mean standardized differences between treatment groups were also not significant for executive functions (−0.04, 95% CI −0.21 to 0.14) and global cognition (−0.025, 95% CI −0.18 to 0.13) (table 3). Interactions were not significant when time since menopause was analyzed as a continuous variable (2-tailed interaction p values: verbal memory 0.80, executive functions, 0.60, global cognition 0.76). Expressed in terms of baseline composite scores, the magnitude of the estradiol–placebo treatment difference was no more than 1/20th of a SD (verbal memory, 0.05 SD; executive functions, 0.02 SD; global cognition, 0.02 SD). Treatment-by-time interactions and treatment-by-group-by-time interactions for composite outcomes were not significant (table 3), indicating that treatment effects did not differ at 2.5 and 5 years.
Table 3.
Change in cognitive composite endpoints
Individual neuropsychological test scores.
There was no significant treatment effect on any neuropsychological task after Bonferroni correction (2-tailed ps > 0.0026) (tables e-2 and e-3).
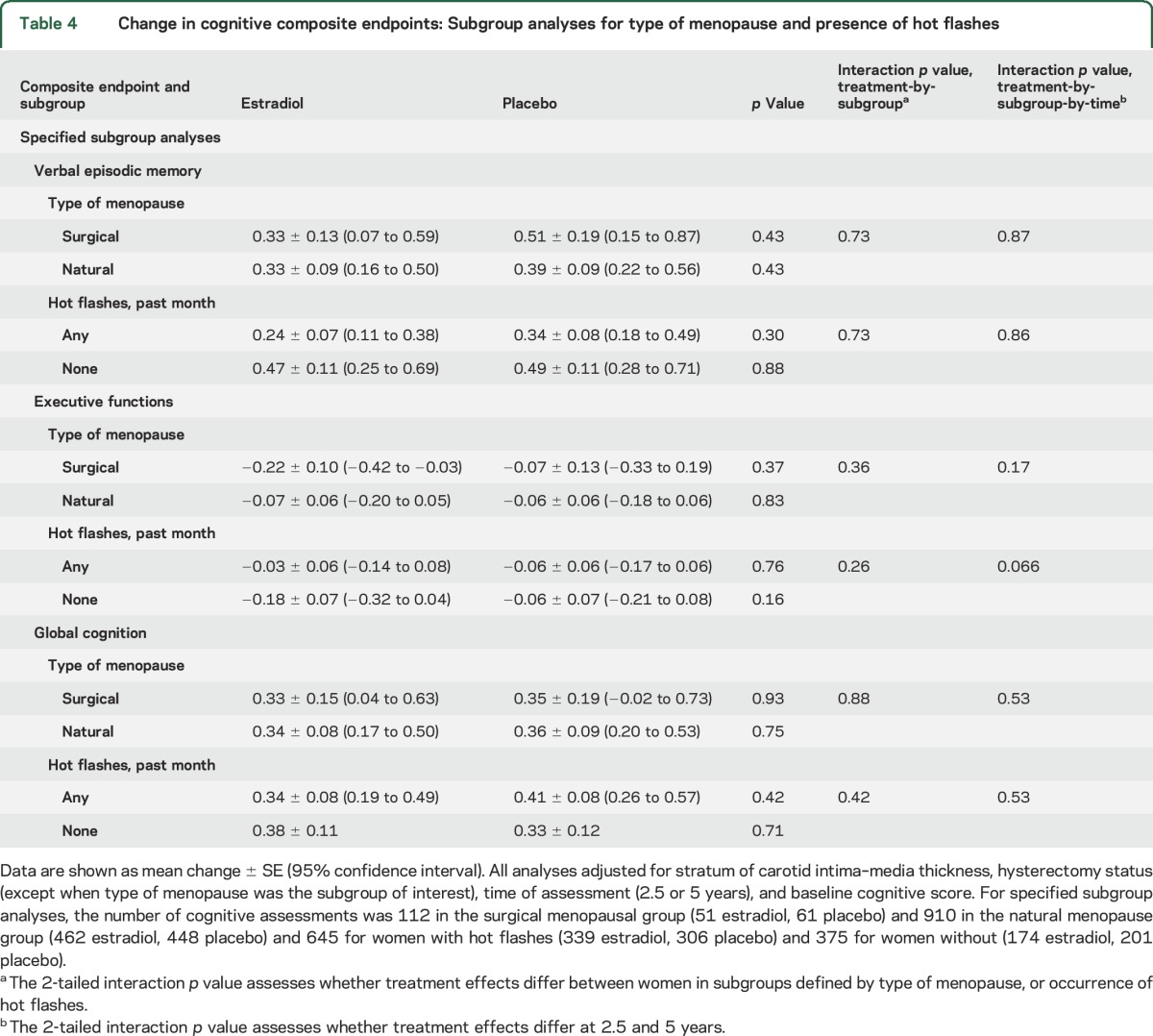
Type of menopause and presence of hot flashes.
Treatment effects on verbal memory, executive functions, and global cognition did not differ between women who had a surgical menopause compared to natural menopause or between women with hot flashes within the preceding month compared to women without (table 4).
Table 4.
Change in cognitive composite endpoints: Subgroup analyses for type of menopause and presence of hot flashes
Other cognitive endpoints.
Adherent analyses that excluded women who took less than 80% of oral estradiol or placebo, and analyses that excluded early-group women not within 3 years of menopause, did not alter the significance of findings on cognitive composite outcomes (tables e-4 and e-5). Unspecified subgroup analyses stratified by the presence of at least one APOE ε4 allele, by use of MHT before trial enrollment, or by hysterectomy status (women without a uterus were not exposed to vaginal progesterone gel) showed no effect of estradiol treatment on composite neuropsychological outcomes (table e-6).
Safety.
There was one death in the estradiol group and one in the placebo group. The number of other serious adverse events was similar in each treatment group (43 estradiol, 45 placebo) (e-Results).
DISCUSSION
Results of this randomized, double-blind, placebo-controlled trial fail to confirm the timing hypothesis for cognitive outcomes in healthy postmenopausal women. Estradiol initiated within 6 years of menopause did not affect verbal memory, executive functions, or global cognition differently than estradiol begun 10 or more years after menopause. During a mean treatment period of 57 months, 1 mg oral 17β-estradiol daily compared to placebo had no effect on cognitive composite change scores in either postmenopausal stratum. The randomized design minimized risk of bias, and the internal validity of trial results is supported by a low rate of participant withdrawal and by high adherence. These findings are consistent with inferences derived from much lower endogenous exposures in study participants, where baseline serum levels of estradiol were unassociated with baseline cognitive composite scores in early- or late-group women.11 Very small mean differences in cognitive composite change scores (observed effect sizes ranged from −0.025 to −0.06 SDs) suggest that estradiol does not have a clinically meaningful effect on verbal memory, executive functions, or global cognition regardless of time since menopause. Safety profiles were similar in estradiol and placebo treatment groups.
We did not identify subgroups of women in which estradiol improved or impaired cognitive function. There was limited power to detect small treatment effects in subgroup analyses, but cognitive outcomes were similar for naturally and surgically menopausal women, women with and without hot flashes, women with and without the APOE ε4 allele, women who had and had not used MHT in the past, and women with and without a uterus. Findings were similar when we examined a narrower early window that included only women within 3 years of menopause.
In some trials of younger postmenopausal women, estrogen improved cognitive skills, particularly verbal episodic memory.15,17,24 External validity is challenged, however, by small sample sizes and short treatment durations, and results from larger, longer-duration trials6,18–21,25–29 imply the absence of meaningful cognitive effects.5,7 Prior trials, however, were conducted within restricted age ranges that failed to accommodate both younger and older postmenopausal women,6,18–21,25–29 thus precluding the ability to identify possible differences in cognitive outcomes based on age or timing. Moreover, generalizability is limited by the exclusion of women most likely to consider MHT for approved clinical indications, the use of estrogens other than estradiol, and the use of continuously administered synthetic progestogens.
The primary indication for MHT is treatment of vasomotor symptoms, more common among younger postmenopausal women closer to menopause. With one exception,6 larger, longer-duration trials have been conducted principally or exclusively in women aged 65 and older.18–21,25–29 Recent myocardial infarction and stroke are contraindications to MHT; 2 trials were restricted to women with coronary heart disease18 or cerebrovascular disease.19 For early-group women, ELITE-Cog results are supported by null findings in a 2.85-year trial of younger postmenopausal women using relatively low doses of conjugated estrogens or transdermal estradiol6 and by follow-up analyses from the Women's Health Initiative, which found no residual cognitive effect of conjugated estrogens allocated to women aged 50–55 years, approximately 7 years after trial termination.30
In ELITE-Cog, women with a uterus allocated to estradiol received cyclic micronized vaginal progesterone for uterine protection. Most larger trials studied cognitive outcomes of conjugated estrogens,18,20,21,25–27 whose effects may differ from those of estradiol,31 the principal estrogen produced by the ovaries during a woman's reproductive years. Progesterone receptor subtypes are broadly expressed throughout the brain, and progestogens influence neural function.32 Most larger MHT trials that included a progestational agent for endometrial protection used medroxyprogesterone acetate exclusively, often given continuously rather than cyclically.18,20,26,27 Behavioral effects of this compound differ from those of progesterone,33 and sustained use may impair cognition.34,35
No prior trial has directly addressed the timing hypothesis in both younger and older postmenopausal women. This hypothesis is best developed for coronary heart disease outcomes10 and has been considered for dementia risk36 and cognition.8,9 For cognition, the critical-window theory finds support from rodent models, where hippocampal plasticity and memory performance are affected differently, depending on the age of the animal or timing of estradiol replacement after ovariectomy.37,38 Our results, however, suggest that these findings do not extend to postmenopausal women.
Our trial has strengths and limitations. To test the timing hypothesis, we compared younger and older postmenopausal women within the framework of a large, long-duration trial, where women in both groups were randomly allocated to the same estrogen and assessed with the same comprehensive neuropsychological battery. We demonstrated that prespecified cognitive outcomes in early and late groups did not differ. The experimental intervention was estradiol, the estrogen most often used in animal studies showing cognitive benefit, at a dose commonly used in clinical practice. On-trial serum levels of estradiol were above those associated with meaningful clinical outcomes in other studies, such as vasomotor symptom reduction and fracture prevention.
Results, however, pertain to healthy postmenopausal women with characteristics similar to those in our research cohort. Results do not generalize to women of reproductive age or in the menopausal transition, to women with primary ovarian insufficiency or premature menopause induced by surgery or cancer chemotherapy,39,40 or women with mild cognitive impairment or dementia. The study lacked power to exclude small treatment effects in participant subgroups. It was not designed to assess short-term cognitive effects of estradiol or effects on risks of mild cognitive impairment or Alzheimer disease.
ELITE-Cog findings fail to confirm an early critical window during which estradiol benefits verbal memory, executive functions, or global cognition. These results indicate that postmenopausal women near the time of menopause—in addition to women further from the time of menopause—should not expect MHT to enhance cognition. Healthy younger postmenopausal women considering MHT for approved indications need not be overly concerned that treatment adversely affects these cognitive abilities over a 5-year period.
Supplementary Material
ACKNOWLEDGMENT
The authors thank the study participants and members of the ELITE Research Group (primary trial investigators denoted by an asterisk): Chair, Howard N. Hodis*; Clinical Center Staff, Liny Zurbrugg (clinic coordinator), Esther Bhimani, Martha Charlson, Irma Flores, Martha Huerta, Thelma LaBree, Sonia Lavender, Violetta McElreath, Janie Teran, Philip Zurbrugg; Ultrasound Image Acquisition and Processing Laboratory, Robert H. Selzer* (director), Yanjie Li (technical director), Mei Feng, Lora Whitfield-Maxwell, Ming Yan; Data Coordinating Center, Wendy J. Mack* (director), Stanley P. Azen,* Farzana Choudhury, Carlos Carballo, Laurie Dustin, Adrian Herbert, Naoko Kono, George Martinez, Olga Morales; Atherosclerosis Research Unit Core Lipid/Lipoprotein Laboratory, Juliana Hwang-Levine* (director), Gail Izumi, Arletta Ramirez, Luci Rodriguez; Gynecology and Mammography, Donna Shoupe,* Juan C. Felix, Pulin Sheth, Mary Yamashita; USC Endocrinology Laboratory, Carole Spencer; Cognition and Mood, Victor W. Henderson* (director), Carol A. McCleary, Jan A. St. John; Kaiser Permanente Medical Center Recruitment Site, Malcolm G. Munro; Cardiac Computed Tomography Core Center, Matthew J. Budoff (director), Lily Honoris, Chris Dailing, Sivi Carson; APOE Genotyping, Hooman Allayee; and Data Safety Monitoring Board, Leon Speroff (chair), Robert H. Knopp (deceased), Richard H. Karas, Joan Hilton, Judy Hannah (ex-officio, National Institute on Aging).
GLOSSARY
- ELITE
Early vs Late Intervention Trial with Estradiol
- ELITE-Cog
Early vs Late Intervention Trial with Estradiol cognitive endpoints
- MHT
menopausal hormone therapy
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
V.W. Henderson, W.J. Mack, and H.N. Hodis conceptualized and designed the trial and obtained funding. J.A. St. John, F.Z. Stanczyk, D. Shoupe, C.A. McCleary, V.W. Henderson, N. Kono, and H. Allayee collected the data and provided technical support. W.J. Mack performed the statistical analysis. V.W. Henderson and W.J. Mack interpreted the data. V.W. Henderson wrote the manuscript. All authors critically revised and approved the final manuscript.
STUDY FUNDING
Supported by NIH grant R01AG024154 for initial and supplemental funding of ELITE and ELITE-Cog and by P01AG026572. Study drugs and placebo were supplied without charge or restriction by Teva Pharmaceuticals, Watson Pharmaceuticals, Inc., and Abbott Laboratories.
DISCLOSURE
V. Henderson receives research support from the NIH and has received travel expense reimbursement from the NIH, the American Academy of Neurology, and the International Menopause Society. J. St. John reports no disclosures relevant to the manuscript. H. Hodis receives research support from the NIH and has received travel reimbursement from the North American Menopause Society. C. McCleary, F. Stanczyk, D. Shoupe, N. Kono, and L. Dustin report no disclosures relevant to the manuscript. H. Allayee receives research support from the NIH. W. Mack receives research support from the NIH. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Vest RS, Pike CJ. Gender, sex steroid hormones, and Alzheimer's disease. Horm Behav 2013;63:301–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steinkellner AR, Denison SE, Eldridge SL, Lenzi LL, Chen W, Bowlin SJ. A decade of postmenopausal hormone therapy prescribing in the United States: long-term effects of the Women's Health Initiative. Menopause 2012;19:616–621. [DOI] [PubMed] [Google Scholar]
- 3.Rossouw JE, Anderson GL, Prentice RL, et al. . Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative randomized controlled trial. JAMA 2002;288:321–333. [DOI] [PubMed] [Google Scholar]
- 4.Anderson GL, Limacher M, Assaf AR, et al. . Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women's Health Initiative randomized controlled trial. JAMA 2004;291:1701–1712. [DOI] [PubMed] [Google Scholar]
- 5.Lethaby A, Hogervorst E, Richards M, Yesufu A, Yaffe K. Hormone replacement therapy for cognitive function in postmenopausal women. Cochrane Database Syst Rev 2008;CD003122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gleason CE, Dowling NM, Wharton W, et al. . Effects of hormone therapy on cognition and mood in recently postmenopausal women: findings from the randomized, controlled KEEPS-cognitive and affective study. PLoS Med 2015;12:e1001833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Henderson VW, Popat RA. Effects of endogenous and exogenous estrogen exposures in midlife and late-life women on episodic memory and executive functions. Neuroscience 2011;191:129–138. [DOI] [PubMed] [Google Scholar]
- 8.Sherwin BB. Estrogen therapy: is time of initiation critical for neuroprotection? Nat Rev Endocrinol 2009;5:620–627. [DOI] [PubMed] [Google Scholar]
- 9.Maki PM. Critical window hypothesis of hormone therapy and cognition: a scientific update on clinical studies. Menopause 2013;20:695–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hodis HN, Mack WJ, Shoupe D, et al. . Methods and baseline cardiovascular data from the Early versus Late Intervention Trial with Estradiol testing the menopausal hormone timing hypothesis. Menopause 2014;22:391–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henderson VW, St. John JA, Hodis HN, et al. . Cognition, mood, and physiological concentrations of sex hormones in the early and late postmenopause. Proc Natl Acad Sci USA 2013;110:20290–20295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Henderson VW, St John JA, Hodis HN, et al. . Long-term soy isoflavone supplementation and cognition in women: a randomized, controlled trial. Neurology 2012;78:1841–1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andresen EM, Malmgren JA, Carter WB, Patrick DL. Screening for depression in well older adults: evaluation of a short form of the CES-D. Am J Prev Med 1994;10:77–84. [PubMed] [Google Scholar]
- 14.Sherwin BB, Tulandi T. “Add-back” estrogen reverses cognitive deficits induced by a gonadotropin-releasing hormone agonist in women with leiomyomata uteri. J Clin Endocrinol Metab 1996;81:2545–2549. [DOI] [PubMed] [Google Scholar]
- 15.Phillips SM, Sherwin BB. Effects of estrogen on memory function in surgically menopausal women. Psychoneuroendocrinology 1992;17:485–495. [DOI] [PubMed] [Google Scholar]
- 16.Woo J, Lau E, Ho SC, et al. . Comparison of Pueraria lobata with hormone replacement therapy in treating the adverse health consequences of menopause. Menopause 2003;10:352–361. [DOI] [PubMed] [Google Scholar]
- 17.Shaywitz SE, Naftolin F, Zelterman D, et al. . Better oral reading and short-term memory in midlife, postmenopausal women taking estrogen. Menopause 2003;10:420–426. [DOI] [PubMed] [Google Scholar]
- 18.Grady D, Yaffe K, Kristof M, Lin F, Richards C, Barrett-Connor E. Effect of postmenopausal hormone therapy on cognitive function: the Heart and Estrogen/progestin Replacement Study. Am J Med 2002;113:543–548. [DOI] [PubMed] [Google Scholar]
- 19.Viscoli CM, Brass LM, Kernan WN, Sarrel PM, Suissa S, Horwitz RI. Estrogen therapy and risk of cognitive decline: results from the Women's Estrogen for Stroke Trial (WEST). Am J Obstet Gynecol 2005;192:387–393. [DOI] [PubMed] [Google Scholar]
- 20.Resnick SM, Maki PM, Rapp SR, et al. . Effects of combination estrogen plus progestin hormone treatment on cognition and affect. J Clin Endocrinol Metab 2006;91:1802–1810. [DOI] [PubMed] [Google Scholar]
- 21.Resnick SM, Espeland MA, An Y, et al. . Effects of conjugated equine estrogens on cognition and affect in postmenopausal women with prior hysterectomy. J Clin Endocrinol Metab 2009;94:4152–4161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Henderson VW, Sherwin BB. Surgical versus natural menopause: cognitive issues. Menopause 2007;14:572–579. [DOI] [PubMed] [Google Scholar]
- 23.LeBlanc ES, Janowsky J, Chan BKS, Nelson HD. Hormone replacement therapy and cognition: systematic review and meta-analysis. JAMA 2001;285:1489–1499. [DOI] [PubMed] [Google Scholar]
- 24.Sherwin BB. Estrogen and/or androgen replacement therapy and cognitive functioning in surgically menopausal women. Psychoneuroendocrinology 1988;13:345–357. [DOI] [PubMed] [Google Scholar]
- 25.Espeland MA, Rapp SR, Shumaker SA, et al. . Conjugated equine estrogens and global cognitive function in postmenopausal women: Women's Health Initiative Memory Study. JAMA 2004;291:2959–2968. [DOI] [PubMed] [Google Scholar]
- 26.Greenspan SL, Resnick NM, Parker RA. The effect of hormone replacement on physical performance in community-dwelling elderly women. Am J Med 2005;118:1232–1239. [DOI] [PubMed] [Google Scholar]
- 27.Rapp SR, Espeland MA, Shumaker SA, et al. . The effect of estrogen with progestin treatment on global cognitive function in postmenopausal women: results from the Women's Health Initiative Memory Study. JAMA 2003;289:2663–2672. [DOI] [PubMed] [Google Scholar]
- 28.Tierney MC, Oh P, Moineddin R, et al. . A randomized double-blind trial of the effects of hormone therapy on delayed verbal recall in older women. Psychoneuroendocrinology 2009;37:1065–1074. [DOI] [PubMed] [Google Scholar]
- 29.Yaffe K, Vittinghoff E, Ensrud KE, et al. . Effects of ultra-low-dose transdermal estradiol on cognition and health-related quality of life. Arch Neurol 2006;63:945–950. [DOI] [PubMed] [Google Scholar]
- 30.Espeland MA, Shumaker SA, Leng I, et al. . Long-term effects on cognitive function of postmenopausal hormone therapy prescribed to women aged 50 to 55 years. JAMA Intern Med 2013;173:1429–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barha CK, Galea LA. The hormone therapy, Premarin, impairs hippocampus-dependent spatial learning and memory and reduces activation of new granule neurons in response to memory in female rats. Neurobiol Aging 2013;34:986–1004. [DOI] [PubMed] [Google Scholar]
- 32.Brinton RD, Thompson RF, Foy MR, et al. . Progesterone receptors: form and function in brain. Front Neuroendocrinol 2008;29:313–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lowry NC, Pardon LP, Yates MA, Juraska JM. Effects of long-term treatment with 17 beta-estradiol and medroxyprogesterone acetate on water maze performance in middle aged female rats. Horm Behav 2010;58:200–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sherwin BB, Grigorova M. Differential effects of estrogen and micronized progesterone or medroxyprogesterone acetate on cognition in postmenopausal women. Fertil Steril 2011;96:399–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maki PM. Minireview: effects of different HT formulations on cognition. Endocrinology 2012;153:3564–3570. [DOI] [PubMed] [Google Scholar]
- 36.Resnick SM, Henderson VW. Hormone therapy and risk of Alzheimer disease: a critical time. JAMA 2002;288:2170–2172. [DOI] [PubMed] [Google Scholar]
- 37.Daniel JM. Estrogens, estrogen receptors, and female cognitive aging: the impact of timing. Horm Behav 2013;63:231–237. [DOI] [PubMed] [Google Scholar]
- 38.Smith CC, Vedder LC, Nelson AR, Bredemann TM, McMahon LL. Duration of estrogen deprivation, not chronological age, prevents estrogen's ability to enhance hippocampal synaptic physiology. Proc Natl Acad Sci USA 2010;107:19543–19548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rocca WA, Bower JH, Ahlskog JE, Grossardt BR, de Andrade M, Melton LJ III. Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology 2007;69:1074–1083. [DOI] [PubMed] [Google Scholar]
- 40.Ryan J, Scali J, Carriere I, et al. . Impact of a premature menopause on cognitive function in later life. BJOG 2014;121:1729–1739. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.