Abstract
Aims
The ratio of aspartate aminotransferase (AST) to alanine aminotransferase (ALT) is of great interest as a possible novel marker of metabolic syndrome. However, longitudinal studies emphasizing the incremental predictive value of the AST-to-ALT ratio in diagnosing individuals at higher risk of developing metabolic syndrome are very scarce. Therefore, our study aimed to evaluate the AST-to-ALT ratio as an incremental predictor of new onset metabolic syndrome in a population-based cohort study.
Material and Methods
The population-based cohort study included 2276 adults (903 men and 1373 women) aged 40–70 years, who participated from 2005–2008 (baseline) without metabolic syndrome and were followed up from 2008–2011. Metabolic syndrome was defined according to the harmonized definition of metabolic syndrome. Serum concentrations of AST and ALT were determined by enzymatic methods.
Results
During an average follow-up period of 2.6-years, 395 individuals (17.4%) developed metabolic syndrome. In a multivariable adjusted model, the odds ratio (95% confidence interval) for new onset of metabolic syndrome, comparing the fourth quartile to the first quartile of the AST-to-ALT ratio, was 0.598 (0.422–0.853). The AST-to-ALT ratio also improved the area under the receiver operating characteristic curve (AUC) for predicting new cases of metabolic syndrome (0.715 vs. 0.732, P = 0.004). The net reclassification improvement of prediction models including the AST-to-ALT ratio was 0.23 (95% CI: 0.124–0.337, P<0.001), and the integrated discrimination improvement was 0.0094 (95% CI: 0.0046–0.0143, P<0.001).
Conclusions
The AST-to-ALT ratio independently predicted the future development of metabolic syndrome and had incremental predictive value for incident metabolic syndrome.
Introduction
Metabolic syndrome is a constellation of several cardiovascular risk factors, including hyperglycemia, obesity, high blood pressure, hypertriglyceridemia, and low HDL cholesterol [1]. It is well reported that metabolic syndrome escalates the risk of type 2 diabetes and cardiovascular disease [2–4]. The deadly consequences and elevated prevalence of metabolic syndrome motivates interest in understanding the causes and risk factors in population-based cohort studies. Besides the well-accepted metabolic syndrome components, diverse risk factors also have been recognized as non-traditional components, such as hyperuricemia, microalbuminuria, and non-alcoholic fatty liver disease (NAFLD) [5–7].
A reduced ratio of aspartate aminotransferase (AST) to alanine aminotransferase (ALT) might be a surrogate measure of NAFLD, and is considered to be another aspect of hyperinsulinemia and insulin resistance [6, 8]. An increased AST-to-ALT ratio is strongly suggestive of alcoholic liver disease, whereas a reduced ratio specifies non-alcoholic steatohepatitis [9]. Recent studies have reported that an increased AST-to-ALT ratio is inversely associated with the future development of metabolic syndrome [10, 11]. However, the clinical utility and ability of the AST-to-ALT ratio in predicting metabolic syndrome and its individual components is not well understood. It is also speculated that this ratio could be used as a predictor of incident metabolic syndrome beyond the information contributed by each component of metabolic syndrome among healthy subjects. We hypothesized that a higher AST-to-ALT ratio would serve as a negative predictor of developing metabolic syndrome.
Hence, we studied the prospective association of the AST-to-ALT ratio with the risk of new-onset of metabolic syndrome and its individual components, as well as the discrimination value of the AST-to-ALT ratio in identifying participants who are likely to develop metabolic syndrome in the future. In addition, we calculated net reclassification improvement (NRI) and integrated discrimination index (IDI) to examine the incremental predictive value of the AST-to-ALT ratio as a novel biomarker for predicting future metabolic syndrome.
Material and Methods
Study population
Our study data were collected from the Korean Genome and Epidemiology Study (KoGES), an ongoing multicenter prospective cohort study designed to estimate the prevalence, incidence, and risk factors related with many disorders such as diabetes, hypertension, and cardiovascular disease [12–14]. The study enrolled adults in the rural region of Wonju and Pyeongchang in South Korea.
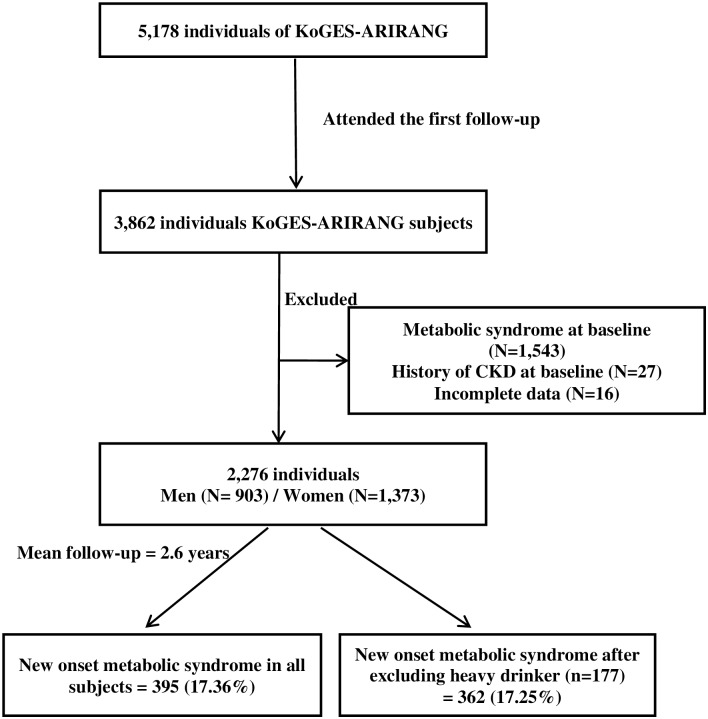
The baseline period of this study was from November 2005 to January 2008, and involved 5178 adults (2127 men and 3051 women) aged 40–70 years. The first follow-up study was carried out from April 2008–January 2011 and 3862 (74.6%) participants attended (mean time between appointments = 2.6 years). Participants with missing data (n = 16) and diagnosed with metabolic syndrome at baseline (n = 1543) were excluded. We also excluded 27 subjects with a history of cardiovascular disease at baseline. A total of 2276 individuals were involved in the current analysis (903 men and 1373 women) (Fig 1).
Fig 1. Description of the study population.
Ethics statement
Written consent was obtained from each participant before the commencement of the study. The protocol was approved by the institutional review board (IRB; CR105024-026) of Wonju Severance Christian Hospital.
Data collection and measurements
The study participants completed a health examination including the lifestyle questionnaire, and a medical history. Body weight and height were measured, with the participants barefooted and lightly dressed, and from these measures body mass index (BMI) was calculated. Waist circumference was measured using tape (SECA-200; SECA, Hamburg, Germany). Blood pressure was monitored from the right arm using a mercury sphygmomanometer (Baumanometer, Copiague, NY, USA) after the participant had rested for at least 5 min in a quiet room. Two consecutive measurements of systolic and diastolic pressure were taken at an interval of at least 5 min, and the average of the readings was used. The data for baseline information on smoking status and current alcohol intake was collected using a self-reported questionnaire (yes/no). The heavy drinker group was defined as those whose alcohol consumption exceeded 30 g/day [15].
After fasting for more than 12 h or overnight, venous blood samples were collected from each participant. Blood glucose, serum HDL-cholesterol, triglyceride, LDL-cholesterol (LDL-C), very low density lipoprotein (VLDL) cholesterol, aspartate transaminase (AST), and alanine transaminase (ALT) were measured by enzymatic methods (ADVIA 1650, Siemens, Tarrytown, NY, USA). High-sensitivity C-reactive protein (hs-CRP) was measured using the Denka Seiken (Tokyo, Japan) assay. Fasting insulin and homeostasis model assessment of insulin resistance (HOMA-IR) were also measured [16].
Definition of metabolic syndrome
The end-point of the study was new onset of metabolic syndrome at the follow-up. The harmonized definition was used to diagnose metabolic syndrome in the participants [17, 18].
Statistical methods
Data in this study are expressed as frequencies with percentage, means with standard deviation, or medians with interquartile range. It has been noted that the ratio of AST-to-ALT is used to classify fatty liver disease as alcoholic or non-alcoholic. In our study, we also analyzed the data with the exclusion of heavy drinkers (n = 177) to reveal the association between heavy drinking and liver injury. AST-to-ALT ratio was evaluated as a prediction tool for different components of metabolic syndrome over 2.6 years of follow-up by logistic regression. The odds ratio with 95% confidence intervals (95% CI) was estimated according to increasing quartiles of the AST-to-ALT ratio. The independent association between the baseline AST-to-ALT ratio and the onset of metabolic syndrome was analyzed by multivariate logistic regression. We utilized three models for the adjustment. First, we executed an age-related analysis. Second, we adjusted for age, BMI, LDL cholesterol, regular exercise, and smoking. Third, we adjusted for the factors in the second analysis with the addition of hs-CRP levels and HOMA-IR.
To calculate the added discrimination or incremental effect contributed by the AST-to-ALT ratio to predict future participants diagnosed with metabolic syndrome beyond the information furnished by the different components of the metabolic syndrome, our study analyzed the areas under the receiver operating characteristic curve (AUC) in models that contained HDL-cholesterol, waist circumference, systolic and diastolic blood pressure, triglycerides, and fasting glucose, with and without the AST-to-ALT ratio. Additionally, we calculated the category-free NRI and IDI for models with and without the AST-to-ALT ratio, to measure the improvement of corrected reclassification and sensitivity based on the addition of the serum AST-to-ALT ratio to the logistic model. NRI represents the difference between the proportion of participants moving up and the proportion of participants moving down for the development of metabolic syndrome, as well as the corresponding difference in proportions for those who did not have metabolic syndrome, by obtaining the difference of these two differences [19]. IDI represents the difference between the integrated difference in sensitivity and the integrated difference in one minus specificity for models with and without the new biomarker [20]. A P-value less than 0.05 was considered statistically significant, and all statistical analyses were accomplished using SAS, version 9.2 (SAS Institute, Cary, NC, USA).
Results
Patient characteristics
After an average follow-up of 2.6 years, 395 (17.4%) participants developed metabolic syndrome. The incidence of metabolic syndrome was similar after excluding heavy drinkers from the study. Baseline characteristics of the study subjects, categorized according to whether or not they had metabolic syndrome at follow-up, are shown in Table 1. Subjects who developed metabolic syndrome at follow-up were older and had significantly higher waist circumference, BMI, fasting blood glucose, blood pressure, LDL cholesterol, triglycerides, and HOMA-IR values than the non-metabolic syndrome participants.
Table 1. Baseline characteristics of the study subjects stratified by incident metabolic syndrome, and evaluation of the AST-to-ALT ratio in relation to the number of metabolic parameters used to define metabolic syndrome at follow-up.
| Variables | All subjects | Excluding heavy drinkers | |||||
|---|---|---|---|---|---|---|---|
| Metabolic syndrome (n = 395) | Non-metabolic syndrome (n = 1881) | p-value | Metabolic syndrome (n = 362) | Non-metabolic syndrome (n = 1737) | p-value | ||
| Age (y) | 55.40 ± 7.89 | 53.91 ± 8.24 | 0.001 | 55.34 ± 7.87 | 53.88 ± 8.2 | 0.0019 | |
| Gender | Men | 164 (18.16%) | 739 (81.84%) | 0.41 | 135 (18.05%) | 613 (81.95%) | 0.469 |
| Women | 231 (16.82%) | 1142 (83.18%) | 227 (16.8%) | 1124 (83.2%) | |||
| Waist circumference (cm) | 83.93 ± 7.0 | 79.28 ± 7.23 | <0.0001 | 83.92 ± 7.04 | 78.98 ± 7.72 | <0.0001 | |
| BMI (kg/m2) | 24.99 ± 2.53 | 23.21 ± 2.66 | <0.0001 | 25.08 ± 2.56 | 23.21 ± 2.68 | <0.0001 | |
| Fasting glucose (mg/dL) | 95.03 ± 18.39 | 90.3 ± 12.6 | <0.0001 | 94.92 ± 18.92 | 90.08 ± 12.78 | <0.0001 | |
| SBP (mmHg) | 131.9 ± 18.52 | 124.2 ± 16.61 | <0.0001 | 132 ± 18.46 | 123.9 ± 16.55 | <0.0001 | |
| DBP (mmHg) | 82.82 ± 11.35 | 79.44 ± 11.21 | <0.0001 | 82.75 ± 11.27 | 79.2 ± 11.15 | <0.0001 | |
| HDL cholesterol (mg/dL) | 45.95 ± 9.9 | 50.06 ± 11.09 | <0.0001 | 45.76 ± 9.79 | 49.74 ± 10.66 | <0.0001 | |
| LDL cholesterol (mg/dL) | 121 ± 30.33 | 114.6 ± 30.62 | 0.0002 | 122.4 ± 29.27 | 115 ± 30.78 | <0.0001 | |
| Triglyceride (mg/dL) | 132.7 ± 73.49 | 106.2 ± 54.79 | <0.0001 | 127.5 ± 61.7 | 104.2 ± 52.57 | <0.0001 | |
| hs-CRP (mg/L) | 2.13 ± 5.66 | 1.75 ± 5.04 | 0.228 | 2.17 ± 5.84 | 1.76 ± 5.15 | 0.2204 | |
| HOMA-IR | 1.95 ± 0.99 | 1.69 ± 0.96 | <0.0001 | 1.98 ± 1.02 | 1.7 ± 0.96 | <0.0001 | |
| AST (units/L) | 28.07 ± 15.89 | 26.79 ± 14.09 | 0.1376 | 27.35 ± 15.7 | 26.14 ± 11.79 | 0.1662 | |
| ALT (units/L) | 22.31 ± 14.97 | 26.34 ± 17.86 | <0.0001 | 25.72 ± 17.45 | 21.75 ± 13.6 | <0.0001 | |
| AST/ALT ratio | 1.18 ± 0.35 | 1.32 ± 0.43 | <0.0001 | 1.18 ± 0.36 | 1.32 ± 0.43 | <0.0001 | |
| Smoker (%) | 127 (32.23%) | 511 (27.52%) | 0.0456 | 104 (28.81%) | 415 (23.97%) | 0.0531 | |
| Exercise (%) | 134 (33.92%) | 573 (30.59%) | 0.1939 | 122 (33.7%) | 529 (30.6%) | 0.2458 | |
| Drinking (%) | 187 (47.46%) | 821 (43.72%) | 0.1737 | 154 (42.66%) | 677 (39.04) | 0.201 | |
| No. of components | AST/ALT ratio | AST/ALT ratio | |||||
| 0 | 1.38 ± 0.48 | <0.0001 | 1.38 ± 0.49 | <0.0001 | |||
| 1 | 1.33 ± 0.43 | 1.34 ± 0.44 | |||||
| 2 | 1.26 ± 0.37 | 1.27 ± 0.37 | |||||
| 3 | 1.19 ± 0.37 | 1.2 ± 0.37 | |||||
| ≥4 | 1.14 ± 0.3 | 1.13 ± 0.29 | |||||
Data are expressed as mean ± standard deviation or number (%). BMI, Body mass index; SBP, Systolic blood pressure; DBP, Diastolic blood pressure; hs-CRP, high-sensitivity C-reactive protein; HOMA-IR, homeostasis model assessment-estimated insulin resistance; AST, aspartate aminotransferase; ALT, alanine aminotransferase
HDL-cholesterol and the AST-to-ALT ratio were significantly lower in participants who developed metabolic syndrome than in those who did not (all P<0.0001). The prevalence of smoking was significantly higher in the metabolic syndrome group (P<0.05), while there were no significant differences in regular exercise or current alcohol intake between the two groups. The estimates were similar when we excluded heavy drinkers from our analysis, except for smoking status, which nevertheless was found to not be significant. The elevated level of hs-CRP did not show any statistically significant difference between the metabolic syndrome group and the non-metabolic syndrome group. The AST-to-ALT ratio at baseline constantly decreased with the number of metabolic syndrome components developed in study subjects over the 2.6 years of follow-up (P for trend <0.0001). Moreover, we analyzed the data in a cross-sectional manner and the data corroborated the results of the prospective study analysis after 2.6 years of follow-up (Table A and B in S1 Tables).
Multivariate Analysis for Predicting the Risk of Metabolic Syndrome According to Quartile Increment of AST-to-ALT ratio
We divided the study population into quartiles of AST-to-ALT ratios with cut-offs of 1.04, 1.25, and 1.5, and evaluated the association between baseline AST-to-ALT ratio and incident metabolic syndrome and its individual components at the follow-up visit. The incidence of metabolic syndrome in the first quartile of the AST-to-ALT ratio was 25.87%, which was reduced significantly to 18.68%, 12.5%, and 12.2% in the successive quartiles (Table 2). The crude odds ratio for the development of metabolic syndrome, comparing individuals in the fourth quartile with those in the first quartile of the AST-to-ALT ratio, was 0.398 (0.288–0.551, P for trend <0.0001). When the highest quartile of the AST-to-ALT ratio was compared with the first quartile, the age-adjusted odds ratio (95% CI) of metabolic syndrome was 0.364 (0.262–0.505) in all subjects and 0.367 (0.261–0.517) after excluding heavy drinkers. In the multivariate-adjusted model (Table 2), when the highest quartile of the AST-to-ALT ratio was compared to the lowest quartile, the odds ratio for the onset of metabolic syndrome was 0.598 (0.420–0.853, P = 0.0004). The analysis was repeated with the exclusion of heavy drinkers, as shown in Table 2, and the results were consistent. We calculated the odds ratio and 95% CI for incident metabolic syndrome based on the serial change of AST-to -ALT ratio. After adjustment for several confounding factors (i.e., model 3), the odds ratio for incident metabolic syndrome in the highest quartile of serial change of AST-to-ALT ratio compared with the lowest quartile of change in AST-to-ALT ratio was 0.796 (0.570–1.113), P = 0.1751. The study did not observe any significant difference for onset of metabolic syndrome stratified by the serial changes of AST-to-ALT ratio in all participants as well as after excluding heavy drinkers (Table C in S1 Tables). The study also analyzed the odds ratio for incident metabolic syndrome based on the quartiles of ALT and GGT levels (Table D in S1 Tables).
Table 2. Odds ratio and 95% confidence interval (CI) for new-onset metabolic syndrome according to different quartiles of AST-to-ALT ratios.
| Q1 | Q2 | Q3 | Q4 | p-value | |
|---|---|---|---|---|---|
| All subjects | N = 576 | N = 562 | N = 630 | N = 508 | |
| AST-to-ALT ratio | ~ 1.04 | 1.04–1.25 | 1.25–1.5 | 1.5 ~ | |
| Incidence | 149 (25.87%) | 105 (18.68%) | 79 (12.54%) | 62 (12.2%) | <0.0001 |
| Crude OR | 1 | 0.658 (0.496–0.873) | 0.411 (0.304–0.555) | 0.398 (0.288–0.551) | <0.0001 |
| Model 1 | 1 | 0.633 (0.476–0.842) | 0.385 (0.284–0.522) | 0.364 (0.262–0.505) | <0.0001 |
| Model 2 | 1 | 0.755 (0.56–1.018) | 0.505 (0.367–0.694) | 0.571 (0.403–0.81) | 0.0001 |
| Model 3 | 1 | 0.778 (0.575–1.051) | 0.522 (0.378–0.719) | 0.598 (0.42–0.853) | 0.0004 |
| Excluding heavy drinkers | N = 527 | N = 528 | N = 578 | N = 466 | |
| AST-to-ALT ratio | ~ 1.04 | 1.04–1.26 | 1.26–1.5 | 1.5 ~ | |
| Incidence | 137 (26%) | 99 (18.75%) | 69 (11.94%) | 57 (12.23%) | <0.0001 |
| Crude OR | 1 | 0.657 (0.49–0.88) | 0.386 (0.281–0.53) | 0.397 (0.283–0.557) | <0.0001 |
| Model 1 | 1 | 0.632 (0.47–0.848) | 0.365 (0.265–0.503) | 0.367 (0.261–0.517) | <0.0001 |
| Model 2 | 1 | 0.759 (0.556–1.035) | 0.494 (0.352–0.692) | 0.595 (0.413–0.857) | 0.0003 |
| Model 3 | 1 | 0.786 (0.574–1.075) | 0.513 (0.365–0.721) | 0.627 (0.434–0.905) | 0.0011 |
Data are OR (95% CI) or n (%). Model 1: adjusted for age. Model 2: Model 1, plus additional adjustment for baseline BMI, LDL cholesterol, smoking, regular exercise, and alcohol drinking. Model 3: Model 2, plus additional adjustment for baseline hs-CRP and HOMA-IR
Odds Ratio for Each Component of Metabolic Syndrome Classified by the Baseline AST-to-ALT ratio
Table 3 displays the odds ratios for each component of metabolic syndrome, stratified by the baseline AST-to-ALT ratio. Subjects in the lowest AST-to-ALT ratio quartile had significantly higher odds for abnormal components of metabolic syndrome than those in the other three quartiles. Upon comparison with subjects in the first quartile of the AST-to-ALT ratio, subjects in the fourth quartile had significantly lower odds ratios for metabolic abnormalities, i.e., waist circumference, blood pressure, triglycerides, and blood glucose, in spite of not having any significant differences in HDL cholesterol. The crude odds ratios (95% CI) for large waist circumference, high triglycerides, low HDL cholesterol, elevated blood pressure, and elevated blood glucose were 0.331 (0.25–0.44), 0.450 (0.332–0.611), 0.889 (0.7–1.129), 0.814 (0.631–1.05), and 0.618 (0.451–0.847), respectively. Similar results were obtained after repeating the logistic analysis for subjects who were heavy alcohol drinkers.
Table 3. Odds ratios for individual components of metabolic syndrome according to baseline AST-to-ALT ratio.
| Q1 | Q2 | Q3 | Q4 | p-value | ||
|---|---|---|---|---|---|---|
| All subjects | N = 576 | N = 562 | N = 630 | N = 508 | ||
| Large waist circumference | Incidence | 225 (39.06%) | 163 (29%) | 150 (23.81%) | 89 (17.52%) | <0.0001 |
| Odds ratio | 1 | 0.637 (0.498–0.816) | 0.487 (0.38–0.625) | 0.331 (0.25–0.44) | <0.0001 | |
| High triglycerides | Incidence | 160 (27.78%) | 136 (24.2%) | 116 (18.41%) | 75 (14.76%) | <0.0001 |
| Odds ratio | 1 | 0.83 (0.636–1.082) | 0.587 (0.447–0.77) | 0.45 (0.332–0.611) | <0.0001 | |
| Low HDL cholesterol | Incidence | 280 (48.61%) | 270 (48.04%) | 294 (46.67%) | 232 (45.67%) | 0.7615 |
| Odds ratio | 1 | 0.977 (0.775–1.233) | 0.925 (0.738–1.16) | 0.889 (0.7–1.129) | 0.7619 | |
| High blood pressure | Incidence | 203 (35.24%) | 193 (34.34%) | 174 (27.62%) | 156 (30.71%) | 0.0173 |
| Odds ratio | 1 | 0.961 (0.753–1.227) | 0.701 (0.549–0.895) | 0.814 (0.631–1.05) | 0.0176 | |
| High glucose | Incidence | 126 (21.88%) | 93 (16.55%) | 94 (14.92%) | 75 (14.76%) | 0.0037 |
| Odds ratio | 1 | 0.708 (0.526–0.953) | 0.626 (0.466–0.841) | 0.618 (0.451–0.847) | 0.0039 | |
| Excluding heavy drinkers | N = 527 | N = 528 | N = 578 | N = 466 | ||
| Large waist circumference | Incidence | 209 (39.66%) | 156 (29.55%) | 136 (23.53%) | 82 (17.6%) | <0.0001 |
| Odds ratio | 1 | 0.638 (0.494–0.824) | 0.468 (0.361–0.607) | 0.325 (0.242–0.437) | <0.0001 | |
| High triglycerides | Incidence | 138 (26.19%) | 123 (23.3%) | 105 (18.17%) | 64 (13.73%) | <0.0001 |
| Odds ratio | 1 | 0.856 (0.647–1.133) | 0.626 (0.47–0.834) | 0.449 (0.323–0.623) | <0.0001 | |
| Low HDL cholesterol | Incidence | 264 (50.09%) | 263 (49.81%) | 279 (48.27%) | 223 (47.85%) | 0.8592 |
| Odds ratio | 1 | 0.989 (0.777–1.259) | 0.93 (0.734–1.177) | 0.914 (0.712–1.173) | 0.8593 | |
| High blood pressure | Incidence | 182 (34.54%) | 177 (33.52%) | 158 (27.34%) | 144 (30.9%) | 0.0465 |
| Odds ratio | 1 | 0.956 (0.741–1.233) | 0.713 (0.552–0.921) | 0.848 (0.65–1.106) | 0.0471 | |
| High blood glucose | Incidence | 112 (21.25%) | 79 (14.96%) | 80 (13.84%) | 65 (13.95%) | 0.0021 |
| Odds ratio | 1 | 0.652 (0.475–0.895) | 0.595 (0.434–0.816) | 0.601 (0.43–0.84) | 0.0023 |
Data are OR (95% CI) or frequency (%). P values show the trend. A P value <0.05 was considered statistically significant.
The Additional Contribution of AST-to-ALT ratio to Predicting Risk of High Metabolic Syndrome
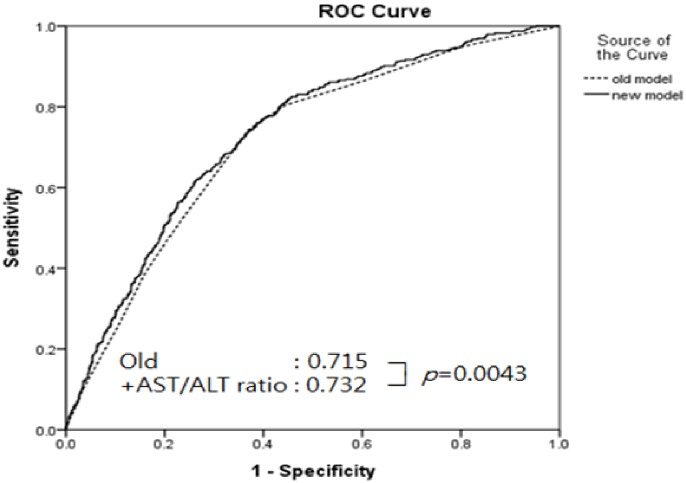
The consistency observed in the association between the AST-to-ALT ratio and the risk of metabolic syndrome in the further analysis centered on all subjects in this cohort study. First, we measured the AUC of the AST-to-ALT ratio, ALT, GGT, and AST levels to predict the development of metabolic syndrome (Table E in S1 Tables). The AUCs for AST-to-ALT, ALT, and GGT were 0.611 (0.580–0.642), 0.604 (0.573–0.635), and 0.601(0.571–0.630), respectively. The baseline risk factors of metabolic syndrome and additional AST-to-ALT ratio model are shown in Table 4 to predict the future development of metabolic syndrome obtained with an AUC. The addition of the AST-to-ALT ratios to the models of individual components of metabolic syndrome significantly increased the AUCs. We also evaluated the prediction of new cases of metabolic syndrome by baseline variables of AST-to-ALT ratio over and above the information contributed by individual component of metabolic syndrome. The AUC predicts incident metabolic syndrome employing each component of metabolic syndrome (large waist circumference, high triglycerides, low HDL cholesterol, high blood pressure, and high blood glucose) to be 0.715 (0.688–0.741). After the AST-to-ALT ratio was added to this model, the resulting AUC was 0.732 (95% CI: 0.706–0.758, P = 0.0043) (Fig 2). We also calculated the additional predictive ability of other enzymes ALT, GGT, and AST to predict the future risk of developing metabolic syndrome beyond the information presented by different components of metabolic syndrome (Table F in S1 Tables).
Table 4. Comparison of AUC of each component of metabolic syndrome and additional AST-to-ALT ratio model.
| Each component’s AUC (95% CI) | AST/ALT additional model AUC (95%CI) AUC (95% CI) | p-value | |
|---|---|---|---|
| All subjects | |||
| Large waist circumference | 0.569 (0.546–0.592) | 0.641 (0.546–0.592) | <0.0001 |
| High triglycerides | 0.543 (0.521–0.564) | 0.623 (0.591–0.654) | <0.0001 |
| Low HDL cholesterol | 0.537 (0.51–0.564) | 0.615 (0.584–0.645) | <0.0001 |
| High blood pressure | 0.563 (0.536–0.59) | 0.627 (0.597–0.657) | <0.0001 |
| High blood glucose | 0.549 (0.528–0.569) | 0.639 (0.608–0.669) | <0.0001 |
| 5 components | 0.715 (0.688–0.741) | 0.732 (0.706–0.758) | 0.0043 |
AUC, area under the ROC curve. P value is for the comparison of AUC between the model with each component of metabolic syndrome and the model with the addition of the AST-to-ALT ratio.
Fig 2. The additional contribution of the AST-to-ALT ratio to predicting the risk of metabolic syndrome.
To measure the ability of the baseline AST-to-ALT ratio to predict the future onset of metabolic syndrome, we also calculated the ideal cut-off value of the AST-to-ALT ratio to define a discretional component of metabolic syndrome by the Youden index, which was 1.15 (data not shown). The NRI and IDI for prediction models including AST-to-ALT ratio were 0.23 (95% CI: 0.124–0.337, P<0.0001) and 0.0094 (95% CI: 0.0046–0.0143, p<0.0001).
Discussion
In this longitudinal cohort study, we observed that the serum AST-to-ALT ratio was an independent negative predictor of the onset of metabolic syndrome and its individual components, except for HDL cholesterol, in the general Korean population. This independent relationship between the AST-to-ALT ratio and metabolic syndrome was not gender-biased and was unaffected by the exclusion of heavy drinkers. Moreover, our study showed that the serum AST-to-ALT ratio may improve the predictive power to accurately identify participants with risk for incident metabolic syndrome, beyond the information contributed by each of its components.
The serum AST-to-ALT ratio serves as a proxy measure for NAFLD and was shown to be inversely associated with metabolic syndrome and insulin resistance in clinical and epidemiological studies [9, 21, 22]. Despite strong evidence about the association between the AST-to-ALT ratio and obesity-related metabolic disorders, there have been little data from prospective studies based on the incremental predictive value of the serum AST-to-ALT ratio for the onset of metabolic syndrome [10, 23]. In our study, an increasing AST-to-ALT ratio was correlated with a consistent reduction in the onset of metabolic syndrome and its components. The prospective design, dose-dependent relationship and robustness of the association imply that the AST-to-ALT ratio may play a major role in the future diagnosis of metabolic syndrome.
We found that AUC was improved in the model in which the AST-to-ALT ratio was added to the metabolic syndrome components (0.715 to 0.732, P<0.0043). This indicates that the AST-to-ALT ratio enables the identification of incident metabolic syndrome independent of conventional risk assessment. We also used NRI and IDI to evaluate the prediction performance when the new biomarker (AST-to-ALT ratio) was added to a conventional metabolic syndrome risk model. The category-free NRI was 23% in our prospective cohort, which means that 23% of individuals in our study population were classified in the correct direction. Indeed, both NRI and IDI are more sensitive than AUC for stabilizing improvement in the predictive value [14, 24]. Improvements in the NRI, IDI, and c-statistics revealed that the AST-to-ALT ratio could have clinical importance in screening for the risk of metabolic syndrome, beyond the information suggested by traditional risk factors.
The relationship between metabolic syndrome and NAFLD or liver enzyme levels has been well established by liver biopsies, which are considered the gold standard for diagnosing NAFLD, although we did not use liver biopsies to confirm NAFLD-associated liver damage. Still, AST and ALT have been used as noninvasive surrogate markers of liver damage in epidemiological studies [25, 26] and the serum AST-to-ALT ratio is independently associated with metabolic syndrome and its components, consistent with the results of other studies in people of different ethnic origins [23, 27]. Moreover, some studies have reported that a possible mechanism for the association of the AST-to-ALT ratio with metabolic syndrome could be increased hepatic fat content [28], which adversely affects each component of metabolic syndrome. Another mechanism may involve an inflammatory effect in the liver that impairs insulin signaling, leading to a failure to suppress glucose production, and ultimately hyperglycemia [29–31]. Indeed, our results also indicate that the quartiles of AST-to-ALT ratios significantly predict hyperglycemia.
Recently, one study proposed that the liver enzyme ratio is the best surrogate marker of insulin resistance among non-obese Japanese adults [32]. Our study agrees with studies of particular adjusted models in the Korean and Chinese populations, wherein the liver enzyme ALT was significantly associated with metabolic syndrome, independent of insulin resistance measured by HOMA-IR [33, 34]. However, the above studies were focused on ALT (not AST), so we cannot confirm whether a similar relationship would exist with AST. Here, we used the HOMA index to estimate insulin resistance rather than the hyperinsulinemic-euglycemic clamp, which is invasive and requires a prolonged testing time, although many clinical trials have reported a good correlation between the two procedures for the assessment of insulin resistance [35, 36]. Additionally, we observed an independent association of the AST-to-ALT ratio and new onset of metabolic syndrome after adjustment for the potential inflammatory marker, hs-CRP. A possible explanation for the above result is the interaction between insulin resistance and hs-CRP. Insulin resistance is known to be involved with chronic inflammation, which is distinguished by elevated cytokine release and the activation of pro-inflammatory pathways [37, 38], indicating that insulin resistance may precede elevated hs-CRP by attenuating insulin-induced suppression of hepatic acute-phase plasma protein [39]. The exact mechanism by which insulin resistance, hs-CRP, and metabolic syndrome are related is still not clear in epidemiological studies. The above results and further adjustments suggest that the AST-to-ALT ratio has subsidiary physiopathology, distinct from other established risk factors. Our study clearly defines this uniqueness of the NAFLD marker, the AST-to-ALT ratio, as a prerequisite for diagnosing metabolic syndrome, but further verification is needed from long-term epidemiological studies.
Our study has several limitations. First, this study included middle-aged and elderly people living in rural settings with proportionately higher cases of metabolic syndrome [14]. Nonetheless, our cohort observed a similar pattern in the prevalence of metabolic syndrome to that of the Korean National Health and Nutrition Examination Survey (KNHANES) [12, 40, 41]. Moreover, the lifestyle change in Korea towards the Western pattern seems to be the primary cause for the increasing risk of metabolic syndrome. The time period of our study denoted a rapid increase in the prevalence rate of metabolic syndrome; therefore, it may not be extrapolated to other ethnic populations. Second, our study follow-up period was short, and thus we could not evaluate whether the association between the AST-to-ALT ratio and the onset of metabolic syndrome would endure long-term. Third, we could not eliminate the probability of confounding influences by viral liver disease. However, in previous reports from the third KNHANES, the prevalence of hepatitis B virus (HBV) infection among Korean adults over 40 years of age was 4% (4.2% for men and 3.8% for women [42]). Therefore, this could not have been a major confounder in our study population. Finally, a single assessment of serum AST and ALT is not adequate to examine the extent of liver inflammation, and thus we may have underemphasized the strength of the association.
In conclusion, the results of this study confirm the existence of an independent relationship between the AST-to-ALT ratio (a liver injury marker) and incident metabolic syndrome, as well as its components, in a prospective cohort study. Our findings suggest that the AST-to-ALT ratio should be considered clinically important for the evaluation of future risk of developing metabolic syndrome. This marker may be a useful tool with which clinicians can stratify cardiometabolic risk in population-based studies.
Supporting Information
(XLSX)
Table A. Baseline characteristics of the study subjects stratified by metabolic syndrome, and evaluation of the AST-to-ALT ratio in relation to the number of individual components of metabolic syndrome at baseline; Table B. Odds ratio and 95% confidence interval (CI) for the prevalence of metabolic syndrome according to the different quartiles of AST-to-ALT ratio; Table C. Odds ratio and 95% confidence interval (CI) for new-onset metabolic syndrome according to serial change quartiles of AST-to-ALT ratios; Table D. Odds ratio and 95% confidence interval (CI) for new-onset of metabolic syndrome according to different quartiles of ALT and GGT levels; Table E. The AUC curve of AST-to-ALT ratio, ALT, GGT, and AST levels to predict the development of metabolic syndrome; Table F. Comparison of the AUC curves for 5 components and additional predictive ability of the AST-to-ALT ratio, ALT, GGT, and AST for the future risk of metabolic syndrome.
(DOCX)
Acknowledgments
We are very grateful to all the participants in the KoGES-ARIRANG study for their continuing interest and participation in the study.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was supported in part by a grant of the Korea Centers for Disease Control and Prevention (2005-E71013-00, 2006-E71002-00, 2007-E71013-00, 2008-E71004-00, 2009- E71006-00, 2010-E71003-00). There was no additional external funding received for this study.
References
- 1.Grundy SM, Brewer HB Jr, Cleeman JI, Smith SC Jr, Lenfant C. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109(3):433–8. Epub 2004/01/28. 10.1161/01.cir.0000111245.75752.c6 . [DOI] [PubMed] [Google Scholar]
- 2.Isomaa B, Almgren P, Tuomi T, Forsen B, Lahti K, Nissen M, et al. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes care. 2001;24(4):683–9. Epub 2001/04/24. . [DOI] [PubMed] [Google Scholar]
- 3.Lorenzo C, Okoloise M, Williams K, Stern MP, Haffner SM. The metabolic syndrome as predictor of type 2 diabetes: the San Antonio heart study. Diabetes care. 2003;26(11):3153–9. Epub 2003/10/28. . [DOI] [PubMed] [Google Scholar]
- 4.Hunt KJ, Resendez RG, Williams K, Haffner SM, Stern MP. National Cholesterol Education Program versus World Health Organization metabolic syndrome in relation to all-cause and cardiovascular mortality in the San Antonio Heart Study. Circulation. 2004;110(10):1251–7. Epub 2004/08/25. 10.1161/01.cir.0000140762.04598.f9 . [DOI] [PubMed] [Google Scholar]
- 5.Invitti C, Maffeis C, Gilardini L, Pontiggia B, Mazzilli G, Girola A, et al. Metabolic syndrome in obese Caucasian children: prevalence using WHO-derived criteria and association with nontraditional cardiovascular risk factors. Int J Obes (Lond). 2006;30(4):627–33. Epub 2006/03/30. 10.1038/sj.ijo.0803151 . [DOI] [PubMed] [Google Scholar]
- 6.Marchesini G, Brizi M, Bianchi G, Tomassetti S, Bugianesi E, Lenzi M, et al. Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes. 2001;50(8):1844–50. Epub 2001/07/27. . [DOI] [PubMed] [Google Scholar]
- 7.Zhang ML, Gao YX, Wang X, Chang H, Huang GW. Serum uric acid and appropriate cutoff value for prediction of metabolic syndrome among Chinese adults. J Clin Biochem Nutr. 2013;52(1):38–42. Epub 2013/01/24. 10.3164/jcbn.12-65 ; PubMed Central PMCID: PMCPmc3541417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cortez-Pinto H, Camilo ME, Baptista A, De Oliveira AG, De Moura MC. Non-alcoholic fatty liver: another feature of the metabolic syndrome? Clin Nutr. 1999;18(6):353–8. Epub 2000/01/15. 10.1054/clnu.1999.0047 . [DOI] [PubMed] [Google Scholar]
- 9.Sorbi D, Boynton J, Lindor KD. The ratio of aspartate aminotransferase to alanine aminotransferase: potential value in differentiating nonalcoholic steatohepatitis from alcoholic liver disease. The American journal of gastroenterology. 1999;94(4):1018–22. Epub 1999/04/14. 10.1111/j.1572-0241.1999.01006.x . [DOI] [PubMed] [Google Scholar]
- 10.Lee K, Yang JH. Which liver enzymes are better indicators of metabolic syndrome in adolescents: the Fifth Korea National Health and Nutrition Examination Survey, 2010. Metab Syndr Relat Disord. 2013;11(4):229–35. Epub 2013/03/05. 10.1089/met.2012.0153 . [DOI] [PubMed] [Google Scholar]
- 11.Perera S, Lohsoonthorn V, Jiamjarasrangsi W, Lertmaharit S, Williams MA. Association between elevated liver enzymes and metabolic syndrome among Thai adults. Diabetes & Metabolic Syndrome: Clinical Research & Reviews. 2008;2(3):171–8. 10.1016/j.dsx.2008.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koh SB, Park JK, Yoon JH, Chang SJ, Oh SS, Kim JY, et al. Preliminary report: a serious link between adiponectin levels and metabolic syndrome in a Korean nondiabetic population. Metabolism: clinical and experimental. 2010;59(3):333–7. Epub 2009/10/03. 10.1016/j.metabol.2009.07.031 . [DOI] [PubMed] [Google Scholar]
- 13.Koh SB, Yoon J, Kim JY, Yoo BS, Lee SH, Park JK, et al. Relationships between serum adiponectin with metabolic syndrome and components of metabolic syndrome in non-diabetic Koreans: ARIRANG study. Yonsei medical journal. 2011;52(2):234–41. Epub 2011/02/15. 10.3349/ymj.2011.52.2.234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim JY, Ahn SV, Yoon JH, Koh SB, Yoon J, Yoo BS, et al. Prospective study of serum adiponectin and incident metabolic syndrome: the ARIRANG study. Diabetes care. 2013;36(6):1547–53. Epub 2013/01/01. 10.2337/dc12-0223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosell M, De Faire U, Hellenius ML. Low prevalence of the metabolic syndrome in wine drinkers—is it the alcohol beverage or the lifestyle? European journal of clinical nutrition. 2003;57(2):227–34. Epub 2003/02/07. 10.1038/sj.ejcn.1601548 . [DOI] [PubMed] [Google Scholar]
- 16.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–9. Epub 1985/07/01. . [DOI] [PubMed] [Google Scholar]
- 17.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120(16):1640–5. Epub 2009/10/07. 10.1161/circulationaha.109.192644 . [DOI] [PubMed] [Google Scholar]
- 18.Yadav D, Lee ES, Kim HM, Choi E, Lee EY, Lim JS, et al. Prospective study of serum uric acid levels and incident metabolic syndrome in a Korean rural cohort. Atherosclerosis. 2015;241(1):271–7. Epub 2015/05/11. 10.1016/j.atherosclerosis.2015.04.797 . [DOI] [PubMed] [Google Scholar]
- 19.Pencina MJ, D'Agostino RB Sr., Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Statistics in medicine. 2011;30(1):11–21. Epub 2011/01/05. 10.1002/sim.4085 ; PubMed Central PMCID: PMCPmc3341973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steyerberg EW, Vickers AJ, Cook NR, Gerds T, Gonen M, Obuchowski N, et al. Assessing the performance of prediction models: a framework for traditional and novel measures. Epidemiology (Cambridge, Mass). 2010;21(1):128–38. Epub 2009/12/17. 10.1097/EDE.0b013e3181c30fb2 ; PubMed Central PMCID: PMCPmc3575184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Targher G, Day CP, Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. The New England journal of medicine. 2010;363(14):1341–50. Epub 2010/10/01. 10.1056/NEJMra0912063 . [DOI] [PubMed] [Google Scholar]
- 22.Sidorenkov O, Nilssen O, Grjibovski AM. Metabolic syndrome in Russian adults: associated factors and mortality from cardiovascular diseases and all causes. BMC public health. 2010;10:582 Epub 2010/10/06. 10.1186/1471-2458-10-582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hanley AJ, Williams K, Festa A, Wagenknecht LE, D'Agostino RB Jr., Haffner SM. Liver markers and development of the metabolic syndrome: the insulin resistance atherosclerosis study. Diabetes. 2005;54(11):3140–7. Epub 2005/10/27. . [DOI] [PubMed] [Google Scholar]
- 24.Pencina MJ, D'Agostino RB Sr., D'Agostino RB Jr., Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Statistics in medicine. 2008;27(2):157–72; discussion 207–12. Epub 2007/06/15. 10.1002/sim.2929 . [DOI] [PubMed] [Google Scholar]
- 25.Clark JM, Diehl AM. Nonalcoholic fatty liver disease: an underrecognized cause of cryptogenic cirrhosis. Jama. 2003;289(22):3000–4. Epub 2003/06/12. 10.1001/jama.289.22.3000 . [DOI] [PubMed] [Google Scholar]
- 26.Clark JM, Brancati FL, Diehl AM. The prevalence and etiology of elevated aminotransferase levels in the United States. The American journal of gastroenterology. 2003;98(5):960–7. Epub 2003/06/18. 10.1111/j.1572-0241.2003.07486.x . [DOI] [PubMed] [Google Scholar]
- 27.Tzima N, Pitsavos C, Panagiotakos DB, Chrysohoou C, Polychronopoulos E, Skoumas J, et al. Adherence to the Mediterranean diet moderates the association of aminotransferases with the prevalence of the metabolic syndrome; the ATTICA study. Nutrition & metabolism. 2009;6:30 Epub 2009/08/01. 10.1186/1743-7075-6-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology (Baltimore, Md). 2004;40(6):1387–95. Epub 2004/11/27. 10.1002/hep.20466 . [DOI] [PubMed] [Google Scholar]
- 29.Malnick SD, Beergabel M, Knobler H. Non-alcoholic fatty liver: a common manifestation of a metabolic disorder. QJM: monthly journal of the Association of Physicians. 2003;96(10):699–709. Epub 2003/09/23. . [DOI] [PubMed] [Google Scholar]
- 30.Hotamisligil GS. Inflammatory pathways and insulin action. International journal of obesity and related metabolic disorders: journal of the International Association for the Study of Obesity. 2003;27 Suppl 3:S53–5. Epub 2004/01/06. 10.1038/sj.ijo.0802502 . [DOI] [PubMed] [Google Scholar]
- 31.Hsueh WA, Quinones MJ. Role of endothelial dysfunction in insulin resistance. The American journal of cardiology. 2003;92(4A):10J–7J. Epub 2003/09/06. . [DOI] [PubMed] [Google Scholar]
- 32.Kawamoto R, Kohara K, Kusunoki T, Tabara Y, Abe M, Miki T. Alanine aminotransferase/aspartate aminotransferase ratio is the best surrogate marker for insulin resistance in non-obese Japanese adults. Cardiovascular diabetology. 2012;11:117 Epub 2012/10/02. 10.1186/1475-2840-11-117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yun JE, Kim SY, Kang HC, Lee SJ, Kimm H, Jee SH. Alanine aminotransferase is associated with metabolic syndrome independently of insulin resistance. Circulation journal: official journal of the Japanese Circulation Society. 2011;75(4):964–9. Epub 2011/02/10. . [DOI] [PubMed] [Google Scholar]
- 34.Xu Y, Bi YF, Xu M, Huang Y, Lu WY, Gu YF, et al. Cross-sectional and longitudinal association of serum alanine aminotransaminase and gamma-glutamyltransferase with metabolic syndrome in middle-aged and elderly Chinese people. Journal of diabetes. 2011;3(1):38–47. Epub 2011/01/05. 10.1111/j.1753-0407.2010.00111.x . [DOI] [PubMed] [Google Scholar]
- 35.Bonora E, Targher G, Alberiche M, Bonadonna RC, Saggiani F, Zenere MB, et al. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes care. 2000;23(1):57–63. Epub 2000/06/17. . [DOI] [PubMed] [Google Scholar]
- 36.Emoto M, Nishizawa Y, Maekawa K, Hiura Y, Kanda H, Kawagishi T, et al. Homeostasis model assessment as a clinical index of insulin resistance in type 2 diabetic patients treated with sulfonylureas. Diabetes care. 1999;22(5):818–22. Epub 1999/05/20. . [DOI] [PubMed] [Google Scholar]
- 37.Wellen KE, Hotamisligil GS. Obesity-induced inflammatory changes in adipose tissue. The Journal of clinical investigation. 2003;112(12):1785–8. Epub 2003/12/18. 10.1172/jci20514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ryu SY, Kim KS, Park J, Kang MG, Han MA. The association between circulating inflammatory markers and metabolic syndrome in Korean rural adults. Journal of preventive medicine and public health = Yebang Uihakhoe chi. 2008;41(6):413–8. Epub 2008/11/28. 10.3961/jpmph.2008.41.6.413 . [DOI] [PubMed] [Google Scholar]
- 39.Campos SP, Baumann H. Insulin is a prominent modulator of the cytokine-stimulated expression of acute-phase plasma protein genes. Molecular and cellular biology. 1992;12(4):1789–97. Epub 1992/04/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim Y. The Korea National Health and Nutrition Examination Survey (KNHANES): current status and challenges. Epidemiology and health. 2014;36:e2014002 Epub 2014/05/20. 10.4178/epih/e2014002 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lim S, Shin H, Song JH, Kwak SH, Kang SM, Won Yoon J, et al. Increasing prevalence of metabolic syndrome in Korea: the Korean National Health and Nutrition Examination Survey for 1998–2007. Diabetes care. 2011;34(6):1323–8. Epub 2011/04/21. 10.2337/dc10-2109 ; PubMed Central PMCID: PMCPmc3114326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ministry of Health & Welfare. The Third Korea National Health and Nutrition Examination Survey (KNHANES III), 2005: Health Examination. Seoul, Korea: Ministry of Health & Welfare; 2006. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Table A. Baseline characteristics of the study subjects stratified by metabolic syndrome, and evaluation of the AST-to-ALT ratio in relation to the number of individual components of metabolic syndrome at baseline; Table B. Odds ratio and 95% confidence interval (CI) for the prevalence of metabolic syndrome according to the different quartiles of AST-to-ALT ratio; Table C. Odds ratio and 95% confidence interval (CI) for new-onset metabolic syndrome according to serial change quartiles of AST-to-ALT ratios; Table D. Odds ratio and 95% confidence interval (CI) for new-onset of metabolic syndrome according to different quartiles of ALT and GGT levels; Table E. The AUC curve of AST-to-ALT ratio, ALT, GGT, and AST levels to predict the development of metabolic syndrome; Table F. Comparison of the AUC curves for 5 components and additional predictive ability of the AST-to-ALT ratio, ALT, GGT, and AST for the future risk of metabolic syndrome.
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.